Abstract
AIM
To compare the intraocular pressure (IOP) measurements obtained with the rebound tonometry (RT), dynamic contour tonometry (DCT) and Goldmann applanation tonometry (GAT) in normal and glaucomatous eyes and investigate the effects of central corneal thickness (CCT) and corneal curvature (CC) on IOP measurements.
METHODS
One hundred and twenty-four eyes of 124 subjects were enrolled in this cross-sectional study. Fifty-six of participants were healthy individuals and 68 of them were glaucomatous patients. IOP was measured on each subject always in the same order, ICare RT-Pascal DCT-GAT, after a minimum interval of 10min between measurements. CCT and CC were measured using a rotating Scheimpflug camera before the IOP measurements in all subjects. One way repeated measures ANOVA, Pearson correlation coefficient and regression analysis, and Bland-Altman analysis was used for the statistical assessment.
RESULTS
Mean IOP for all enrolled eyes was 16.00±3.80 mm Hg for GAT, 16.99±4.91 mm Hg for RT, and 20.40±4.44 mm Hg for DCT. Mean differences between GAT and RT was -1.75±3.41 mm Hg in normal (P<0.001) and -0.37±3.00 mm Hg in glaucomatous eyes (P=0.563). Mean differences between GAT and DCT was -4.06±3.42 mm Hg in normal (P<0.001) and -4.67±3.12 mm Hg in glaucomatous eyes (P<0.001). GAT and RT were significantly positive correlated with CCT in normal (r=0.317, P=0.017 and r=0.576, P<0.001, respectively) and glaucomatous eyes (r=0.290, P=0.016 and r=0.351, P=0.003, respectively). DCT was also significantly positive correlated with CCT in normal eyes (r=0.424, P=0.001) but not in glaucomatous eyes (r=0.170, P=0.165). All tonometers were unaffected by CC.
CONCLUSION
IOP measurements by RT and DCT were significantly higher than GAT. DCT has highest IOP measurements among these tonometers. RT was most influenced tonometer from CCT although all tonometers were significantly positive correlated with CCT except DCT in glaucomatous eyes. CC did not influence IOP measurements.
Keywords: central corneal thickness, corneal curvature, glaucoma, tonometry
INTRODUCTION
Accurate determination of intraocular pressure (IOP) is fundamental in the diagnosis and follow-up of glaucoma because IOP remains the only treatable risk factor in the management of glaucoma. The Goldmann applanation tonometer (GAT) is currently the most widely used device in clinical setting, and is considered the gold standard for IOP measurement. However, it is known to be affected by changes in corneal thickness, structure, and curvature[1]. This has led to the development of new tonometers that attempt to measure IOP independently of these corneal properties.
The ICare rebound tonometer (RT) is a portable handheld tonometer, which does not require any topical anesthetic. It records IOP by detecting the deceleration of a rod probe as it is bounced off the cornea. As the IOP increases, the rod probe bounced off the cornea faster. This movement is detected by a solenoid inside the instrument. RT also minimizes corneal injury and avoids the risk of cross infection through the use of disposable probes[2]. RT has been shown to correlate well with GAT and is generally accepted to be dependent on corneal parameters. RT readings are, however, on average, higher than GAT readings in previous studies[3]–[9].
The Pascal dynamic contour tonometer (DCT) claims to be relatively unaffected by corneal biomechanical properties. It is a slit-lamp mounted, nonapplanation, contour-matching contact tonometry. The tip of the tonometry has a concave surface and measures IOP when the cornea of patient matches the tip of the tonometry. It produces minimal distortion of the cornea and the IOP is the result of a direct measurement by a sensor integrated into the center of the tip. The IOP and quality of the data (Q1-5) are reported on a digital display[10]. DCT also evaluate ocular pulse amplitude (OPA) which is the difference between the average systolic and diastolic IOPs. DCT would be theoretically unaffected by neither central corneal thickness (CCT) nor corneal curvature (CC)[10]–[12].
The purpose of this study was to compare IOP measurements obtained by GAT, RT, and DCT and to assess relationship between IOP measurements and CCT, CC in normal and glaucomatous eyes.
SUBJECTS AND METHODS
This cross-sectional study was conducted at the Department of Ophthalmology, Eskişehir Osmangazi University School of Medicine. The study was performed in accordance with Declaration of Helsinki principles and the local Medical Ethics Committee approved the study. Informed consent was obtained from all participants before the study.
One hundred and twenty-four eyes of 124 subjects were enrolled in the study. Fifty-six of participants were healthy individuals and 68 of them were glaucomatous patients. Diagnostic classification of the patients with glaucoma: 36 primary open angle glaucoma (POAG), 16 pseudoexfoliation glaucoma (PEG), and 16 primary angle closure glaucoma (PACG). The definition of glaucoma in eyes was based on the following criteria: Open anterior chamber angle in gonioscopy for POAG (an occludable angle in gonioscopy for PACG), optic nerve damage (such as focal notching or diffuse thinning of neuroretinal rim, asymmetry of the vertical cup-disk ratio of more than 0.2 between the eyes, visible nerve fiber layer defects, peripapillary atrophy, optic disk hemorrhages) in fundus examination with 78 diopter lens with congruent glaucomatous visual field defects. An occludable angle was defined if the posterior trabecular meshwork was invisible on gonioscopy for at least 270° of the angle circumference in the primary position without indentation. Pseudoexfoliation was diagnosed by the observation of the complete or partial peripheral band and/or a central shield of characteristic grayish-white exfoliative material on the anterior lens capsule and/or at the pupillary margin during the slit-lamp examination following dilatation of the pupil.
Exclusion criteria were: a spherical refractive error >3 D or >1.5 D astigmatism. We also excluded subjects who had active ocular inflammation, ocular surface infection or ocular surface disease such as corneal scarring, pterygium, keratoconus, and recent ocular surgery. If both eyes of a subject fulfilled all the inclusion and exclusion criteria, the right eye was selected for statistical analysis.
All study participants underwent detailed ophthalmologic examination including best-corrected visual acuity, slit-lamp biomicroscopy, and fundoscopy. IOP was measured on each subject in a sitting position and always in the same order, RT-DCT-GAT, after a minimum interval of 10min between measurements[13]. All measurements were taken by one experienced examiner.
ICare RT (TA01i, Tiolat Oy, Helsinki, Finland) is conducted by positioning the tip of the probe in front of the central cornea at a distance of 4 to 8 mm before the measurement. The RT software is preprogrammed for six measurements. After the sixth measurement, the letter P appears in the display, followed by the IOP reading. The software discards the highest and lowest IOP readings automatically and calculates the average IOP value from the rest.
Pascal DCT (SMT Swiss Microtechnology AG, Port, Switzerland) is a self-calibrating device mounted on the slit-lamp. It consist of a sensor tip with a 10.5 mm radius of curvature, a concave surface, and a miniaturized pressure sensor integrated into the centre of the contact surface. The device displays the IOP value accompanied by a quality control (Q1-5). Mean of tree qualified IOP value (Q result is 1 or 2) were considered for statistical analysis in this study.
GAT (AT900, Haag-Streit, Koeniz, Switzerland) measurement was performed using a slit-lamp with topical anaesthesia and fluorescein under cobalt blue filtered light. Three consecutive readings were obtained moving the probeaway from the cornea after each measurement and a mean IOP value was calculated.
In all subjects, CCT and CC were measured using a rotating Scheimpflug camera (Oculus Pentacam, Wetzlar, Germany) before the IOP measurements.
The normality of the continuous variables was evaluated with the Shapiro-Wilk test. The differences between IOP readings were compared with the one way repeated measures ANOVA. The relationship between CCT, CC, and IOP readings were evaluated by Pearson correlation coefficient and regression analysis. Bland-Altman analysis was used to assess the clinical agreement of IOP measurements between the tonometers. P values lower than 0.05 were considered as statistically significant. All analyses were performed by IBM SPSS Statistics version 21 and MedCalc version 12.7.5.0.
RESULTS
Demographic data of the all subjects are given in Table 1. Mean IOP measurements obtained by each tonometer, mean CCT, and mean CC values are shown in Table 2. Comparison of the GAT, RT, and DCT derived IOP measurements are shown in Table 3. DCT measurements were significantly higher than GAT measurements in both normal (P<0.001) and glaucomatous eyes (P<0.001). RT measurements were significantly higher than GAT measurements in normal eyes (P<0.001) but not significantly different in glaucomatous eyes (P=0.563). OPA measurements was not significantly different between normal and glaucomatous eyes (P=0.302).
Table 1. Demographic data of the subjects.
| Parameters | All subjects n=124 | Normal n=56 | All glaucoma n=68 |
| Age (a) | |||
| x±s | 59.57±13.08 | 55.02±11.60 | 63.32±13.11 |
| Range | 20-88 | 20-81 | 28-88 |
| Gender | |||
| M | 45 | 17 | 28 |
| F | 79 | 39 | 40 |
M: Male, F: Female.
Table 2. Mean±SD IOP readings obtained by each tonometer, CCT, and CC values.
| Parameters | All subjects n=124 | Normal n=56 | All glaucoma n=68 |
| GAT (mmHg) | 16.00±3.80 | 16.14±3.81 | 15.88±3.82 |
| RT (mm Hg) | 16.99±4.91 | 17.89±4.82 | 16.25±4.89 |
| DCT (mm Hg) | 20.40±4.44 | 20.20±4.06 | 20.57±4.76 |
| OPA (mm Hg) | 3.37±1.32 | 3.51±1.24 | 3.25±1.40 |
| CCT (µm) | 534.10±41.55 | 541.71±39.86 | 527.84±42.16 |
| CC (mm) | 7.69±0.29 | 7.69±0.28 | 7.69±0.30 |
GAT: Goldmann applanation tonometer; RT: Rebound tonometer; DCT: Dynamic contour tonometer; OPA: Ocular pulse amplitude; IOP: Intraocular pressure; CCT: Central corneal thickness; CC: Corneal curvature; SD: Standard deviation.
Table 3. Comparison of the GAT, RT, and DCT derived IOP readings.
| Parameters (mm Hg) | All subjects n=124 | Normal n=56 | All glaucoma n=68 | |||
| GAT | 16.00±3.80 | 16.14±3.81 | 15.88±3.82 | |||
| Dif | P | Dif | P | Dif | P | |
| RT | +0.99 | 0.002 | +1.75 | <0.001 | +0.37 | 0.563 |
| DCT | +4.40 | <0.001 | +4.06 | <0.001 | +4.69 | <0.001 |
GAT: Goldmann applanation tonometer; RT: Rebound tonometer; DCT: Dynamic contour tonometer; IOP: Intraocular pressure; Dif: Difference from GAT readings.
Figure 1 shows correlation analyses between CCT and IOP measurements (Table 4). We found significantly positive correlation between CCT and IOP measurements in all type tonometers. However, DCT did not significantly correlated with CCT in glaucomatous eyes. GAT and RT were significantly positive correlated with CCT in normal (r2=0.101, P=0.017 and r2=0.331, P<0.001, respectively) and glaucomatous eyes (r2=0.084, P=0.016 and r2=0.123, P=0.003, respectively). DCT was also significantly positive correlated with CCT in normal eyes (r2=0.179, P=0.001) but not in glaucomatous eyes (r2=0.029, P=0.165). RT is the most affected and DCT is the least affected tonometer from CCT in all groups. Figure 2 shows correlation analyses between CC and IOP readings (Table 5). There was no correlation between CC and IOP measurements in all type tonometers in all groups.
Figure 1. Correlation analyses between CCT and IOP readings in all type tonometers.
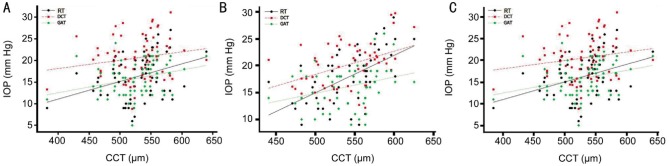
A: All subjects; B: Normal; C: All glaucoma.
Table 4. Statistical results of correlation analyses between CCT and IOP readings in all type tonometers.
| CCT | RT |
DCT |
GAT |
||||||
| Regression coefficient | r2 | P | Regression coefficient | r2 | P | Regression coefficient | r2 | P | |
| Figure 1A (n=124) | 0.055 | 0.215 | <0.001 | 0.028 | 0.068 | 0.004 | 0.028 | 0.092 | 0.001 |
| Figure 1B (n=56) | 0.070 | 0.331 | <0.001 | 0.043 | 0.179 | 0.001 | 0.030 | 0.101 | 0.017 |
| Figure 1C (n=68) | 0.041 | 0.123 | 0.003 | 0.019 | 0.029 | 0.165 | 0.026 | 0.084 | 0.016 |
CCT: Central corneal thickness; IOP: Intraocular pressure; GAT: Goldmann applanation tonometer; RT: Rebound tonometer; DCT: Dynamic contour tonometer
Figure 2. Correlation analyses between CC and IOP readings in all type tonometers.
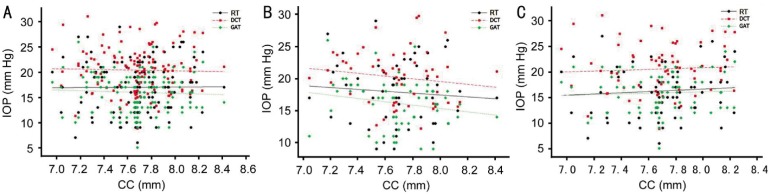
A: All subjects; B: Normal; C: All glaucoma.
Table 5. Statistical results of correlation analyses between CC and IOP readings in all type tonometers.
| CC | RT |
DCT |
GAT |
||||||
| Regression coefficient | r2 | P | Regression coefficient | r2 | P | Regression coefficient | r2 | P | |
| Figure 2A (n=124) | 0.137 | 0.000065 | 0.929 | -0.405 | 0.001 | 0.771 | -0.644 | 0.002 | 0.558 |
| Figure 2B (n=56) | -1.447 | 0.007 | 0.542 | -2.160 | 0.022 | 0.278 | -2.579 | 0.035 | 0.166 |
| Figure 2C (n=68) | 1.181 | 0.005 | 0.554 | 0.815 | 0.003 | 0.675 | 0.681 | 0.003 | 0.662 |
CC: Corneal curvature; IOP: Intraocular pressure; GAT: Goldmann applanation tonometer; RT: Rebound tonometer; DCT: Dynamic contour tonometer
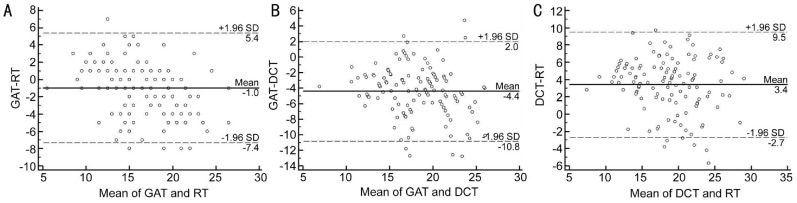
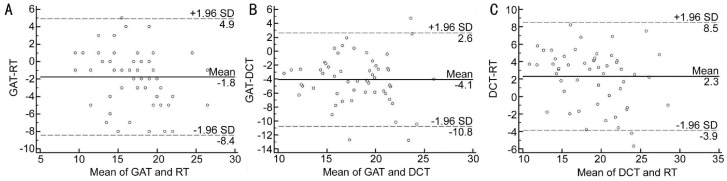
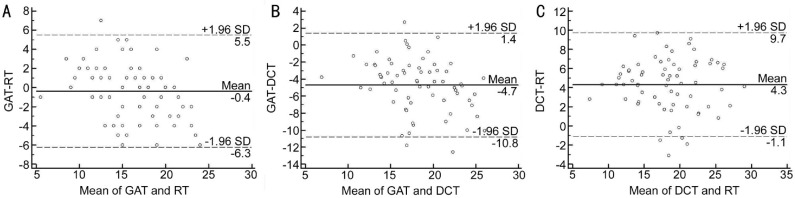
Figures 3, 4, 5 show Bland-Altman plots of the agreement between GAT, RT and DCT in all, normal and glaucomatous eyes. The mean±SD differences and 95% limits of agreement between GAT, RT and DCT are shown in Table 6.
Figure 3. Bland-Altman plot showing the agreement between GAT, RT and DCT in all eyes.
A: GAT-RT; B: GAT-DCT; C: DCT-RT.
Figure 4. Bland-Altman plot showing the agreement between GAT, RT and DCT in normal eyes.
A: GAT-RT; B: GAT-DCT; C: DCT-RT.
Figure 5. Bland-Altman plot showing the agreement between GAT, RT and DCT in glaucomatous eyes.
A: GAT-RT; B: GAT-DCT; C: DCT-RT.
Table 6. Results of Bland-Altman analyses of the agreement between GAT, RT, and DCT.
| Parameters | x±s difference (mm Hg) | 95% LoA (mm Hg) |
| All subjects | ||
| GAT-RT | -0.99±3.25 | -7.4 to 5.4 |
| GAT-DCT | -4.40±3.26 | -10.8 to 2.8 |
| RT-DCT | -3.41±3.10 | -2.7 to 9.5 |
| Normal eyes | ||
| GAT-RT | -1.75±3.41 | -8.4 to 4.9 |
| GAT-DCT | -4.06±3.42 | -10.8 to 2.6 |
| RT-DCT | -2.31±3.16 | -3.9 to 8.5 |
| Glaucomatous eyes | ||
| GAT-RT | -0.37±3.00 | -6.3 to 5.5 |
| GAT-DCT | -4.67±3.12 | -10.8 to 1.4 |
| RT-DCT | -4.32±2.77 | -1.1 to 9.7 |
GAT: Goldmann applanation tonometer; RT: Rebound tonometer; DCT: Dynamic contour tonometer; LoA: Limits of agreement.
DISCUSSION
The GAT is the most widely used method of measuring the IOP, but corneal parameters, especially corneal thickness, affect the accuracy of this tonometer. GAT underestimates IOP in thin corneas and overestimates IOP in thick corneas[14]. So, newer devices such as RT, DCT have been developed. The initial reports on these new tonometers are promising and the reproducibility or reliability of data is being evaluated.
RT has recently appeared in clinical practice after being used for some time in animal research. Its relatively low cost, portability, lack of need for topical anesthesia, being independent of a slit lamp and ease of use make it ideal for routine clinical practice[15]. Previous comparative studies of IOP measurements recorded with RT and GAT have shown clinical agreement between the two devices, with a slight overestimation of readings with RT when compared with GAT. Most studies have been conducted in normal subjects, and there is limited literature in glaucoma patients[16]–[18]. Salim et al[17] reported that 2.45 mm Hg overestimation of IOP by RT compared with GAT in glaucoma patients. Kim et al[18] reported that RT and GAT have good correlation and RT measurements 1.92 mm Hg higher than GAT measurements in patients with glaucoma. RT and GAT have good clinical agreement in our study and RT measurements 1.75 mm Hg higher than GAT measurements in normal eyes and 0.37 mm Hg higher than GAT measurements in glaucomatous eyes. This difference may be explained by RT is the most affected tonometer from CCT and glaucomatous eyes having the lower CCT than normal eyes in this study.
DCT is a method to measure IOP by using a pressure-sensitive tip that is closely shaped following the corneal curvature to minimize the corneal deformation. The forces of both sides of the cornea are meant to be nearly equal during the measurement[19]. According to studies using human cadaver eyes, IOP values measured by DCT were significantly closer to the manometric reference pressure than the GAT measurements[20]–[21]. Previous studies have demonstrated an excellent agreement between GAT and DCT, although DCT readings tended to be generally higher in healthy eyes and glaucomatous eyes by an average of 4 mm Hg[2],[11],[12],[22]. Parallel to previous studies we determined DCT measurements 4.06 mm Hg higher than GAT measurements in normal eyes and 4.69 mm Hg higher than GAT measurements in glaucomatous eyes.
Today, many studies have shown that CCT is variable and is major source of error in GAT, so the common practice of relying on unadjusted GAT results in misdiagnosis and mismanagement[1],[14],[23]. We considered two corneal parameters (CCT and CC) to effect on IOP measurements in this study. We found significantly positive correlation between CCT and IOP measurements in all type tonometers except DCT in glaucomatous eyes. RT is the most affected tonometer and DCT is the least affected tonometer from CCT in all groups. Similarly our previous study has reported that the IOP measured by RT increased 8 mm Hg for every 100-micron increase in CCT[6]. On the other hand there is no consensus in the literature about the relation between the CCT and IOP measurements by RT; some of the studies CCT affect the IOP measurements by RT[6]–[9],[17] but the others did not report this relationship[4],[5],[18]. DCT did not significantly correlate with CCT in glaucomatous eyes in our study. DCT has been proposed to measure IOP irrespective of the corneal thickness because DCT does not flatten the cornea, which allows the cornea to maintain its shape provoking minimal distortion during the measurement[2],[10]–[12],[19]–[22]. RT and GAT also have significantly positive correlated with CCT in normal and glaucomatous eyes but these relationship is weak in glaucomatous eyes according to normal eyes. Topical glaucoma medications affects ocular surface, tear film and also affects corneal biomechanical properties therefore, relationship between the CCT and DCT in glaucomatous eyes may have weak than the normal eyes[24],[25]. It would be better we evaluate corneal biomechanical properties in this study.
Many published studies have suggested correction factors based on CCT and GAT, but the effects of CC on IOP measurements by GAT remain uncertain. In 1973, Mark[26] suggested that a flatter cornea might lead to lower GAT measurements. Orssengo and Pye[27] discussed the deformation of a central cornea flattened by pressure of the prism and bulging outward from the middle to the peripheries due to the inner pressure of the eye. However, other studies could not find any significant correlation between CC and IOP[28],[29]. Gunvant et al[30] reported that an increase of 1 mm of mean CC was accompanied by a rise in IOP of 1.14 mm Hg measured by GAT, but this effect was weak and not statistically significant. Similarly, we found a weak and statistically insignificant correlation between CC and IOP measurements in all type tonometers in all groups.
In conclusion, DCT measurements were significantly higher than GAT measurements in both normal and glaucomatous eyes. RT measurements were significantly higher than GAT measurements in normal eyes but not significantly different in glaucomatous eyes. Highest IOP measurements were recorded by DCT in all groups. We also found significantly positive correlation between CCT and IOP measurements in all tonometers but there was no correlation between CC and IOP measurements in all tonometers in all groups. RT is the most affected and DCT is the least affected tonometer from CCT however, DCT did not significantly correlated with CCT in glaucomatous eyes.
Acknowledgments
Conflicts of Interest: Ozcura F, None; Yildirim N, None; Sahin A, None; Colak E, None.
REFERENCES
- 1.Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol. 1993;38(1):1–30. doi: 10.1016/0039-6257(93)90053-a. [DOI] [PubMed] [Google Scholar]
- 2.ElMallah MK, Asrani SG. New ways to measure intraocular pressure. Curr Opin Ophthalmol. 2008;19(2):122–1206. doi: 10.1097/ICU.0b013e3282f391ae. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-de-la-Casa JM, Garcia-Feijoo J, Castillo A, Garcia-Sanchez J. Reproducibility and clinical evaluation of rebound tonometry. Invest Ophthalmol Vis Sci. 2005;46(12):4578–4580. doi: 10.1167/iovs.05-0586. [DOI] [PubMed] [Google Scholar]
- 4.Iliev ME, Goldblum D, Katsoulis K, Amstutz C, Frueh B. Comparison of rebound tonometry with Goldmann applanation tonometry and correlation with central corneal thickness. Br J Ophthalmol. 2006;90(7):833–835. doi: 10.1136/bjo.2005.089870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chui WS, Lam A, Chen D, Chiu R. The influence of corneal properties on rebound tonometry. Ophthalmology. 2008;115(1):80–84. doi: 10.1016/j.ophtha.2007.03.061. [DOI] [PubMed] [Google Scholar]
- 6.Sahin A, Niyaz L, Yildirim N. Comparison of the rebound tonometer with the Goldmann applanation tonometer in glaucoma patients. Clin Experiment Ophthalmol. 2007;35(4):335–339. doi: 10.1111/j.1442-9071.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 7.Poostchi A, Mitchell R, Nicholas S, Purdie G, Wells A. The iCare rebound tonometer: comparisons with Goldmann tonometry, and influence of central corneal thickness. Clin Experiment Ophthalmol. 2009;37(7):687–691. doi: 10.1111/j.1442-9071.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-de-la-Casa JM, Jimenez-Santos M, Saenz-Frances F, Matilla-Rodero M, Mendez-Hernandez C, Herrero-Vanrell R, Garcia-Feijoo J. Performance of the rebound, noncontact and Goldmann applanation tonometers in routine clinical practice. Acta Ophthalmol. 2011;89(7):676–680. doi: 10.1111/j.1755-3768.2009.01774.x. [DOI] [PubMed] [Google Scholar]
- 9.Rao A, Kumar M, B P, Varshney G. Relationship of central corneal thickness and intraocular pressure by iCare rebound tonometer. J Glaucoma. 2012;23(6):380–384. doi: 10.1097/IJG.0b013e318279b819. [DOI] [PubMed] [Google Scholar]
- 10.Kanngiesser HE, Kniestedt C, Robert YC. Dynamic contour tonometry: presentation of a new tonometer. J Glaucoma. 2005;14(5):344–350. doi: 10.1097/01.ijg.0000176936.16015.4e. [DOI] [PubMed] [Google Scholar]
- 11.Herndon LW. Measuring intraocular pressure-adjustments for corneal thickness and new technologies. Curr Opin Ophthalmol. 2006;17(2):115–119. doi: 10.1097/01.icu.0000193093.05927.a1. [DOI] [PubMed] [Google Scholar]
- 12.Carbonaro F, Andrew T, Mackey DA, Spector TD, Hammond CJ. Comparison of three methods of intraocular pressure measurement and their relation to central corneal thickness. Eye(Lond) 2010;24(7):1165–1170. doi: 10.1038/eye.2010.11. [DOI] [PubMed] [Google Scholar]
- 13.Recep OF, Hasiripi H, Vayisoglu E, Kalayci D, Sarikatipoglu H. Accurate time interval in repeated tonometry. Acta Ophthalmol Scand. 1998;76(5):603–605. doi: 10.1034/j.1600-0420.1998.760518.x. [DOI] [PubMed] [Google Scholar]
- 14.Brown KE, Congdon NG. Corneal structure and biomechanics: impact on the diagnosis and management of glaucoma. Curr Opin Ophthalmol. 2006;17(4):338–343. doi: 10.1097/01.icu.0000233951.01971.5b. [DOI] [PubMed] [Google Scholar]
- 15.Cervino A. Rebound tonometry: new opportunities and limitations of non-invasive determination of intraocular pressure. Br J Ophthalmol. 2006;90(12):1444–1446. doi: 10.1136/bjo.2006.102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent SJ, Vincent RA, Shields D, Lee GA. Comparison of intraocular pressure measurement between rebound, non-contact and Goldmann applanation tonometry in treated glaucoma patients. Clin Experiment Ophthalmol. 2012;40(4):163–170. doi: 10.1111/j.1442-9071.2011.02670.x. [DOI] [PubMed] [Google Scholar]
- 17.Salim S, Du H, Wan J. Comparison of intraocular pressure measurements and assessment of intraobserver and interobserver reproducibility with the portable ICare rebound tonometer and Goldmann applanation tonometer in glaucoma patients. J Glaucoma. 2013;22(4):325–329. doi: 10.1097/IJG.0b013e318237caa2. [DOI] [PubMed] [Google Scholar]
- 18.Kim KN, Jeoung JW, Park KH, Yang MK, Kim DM. Comparison of the new rebound tonometer with Goldmann applanation tonometer in a clinical setting. Acta Ophthalmol. 2013;91(5):392–396. doi: 10.1111/aos.12109. [DOI] [PubMed] [Google Scholar]
- 19.Punjabi OS, Kniestedt C, Stamper RL, Lin SC. Dynamic contour tonometry: principle and use. Clin Experiment Ophthalmol. 2006;34(9):837–840. doi: 10.1111/j.1442-9071.2006.01389.x. [DOI] [PubMed] [Google Scholar]
- 20.Kniestedt C, Nee M, Stamper RL. Accuracy of dynamic contour tonometry compared with applanation tonometry in human cadaver eyes of different hydration states. Graefes Arch Clin Exp Ophthalmol. 2005;243(4):359–366. doi: 10.1007/s00417-004-1024-6. [DOI] [PubMed] [Google Scholar]
- 21.Kniestedt C, Nee M, Stamper RL. Dynamic contour tonometry: a comparative study on human cadaver eyes. Arch Ophthalmol. 2004;122(9):1287–1293. doi: 10.1001/archopht.122.9.1287. [DOI] [PubMed] [Google Scholar]
- 22.Yoo C, Eom YS, Kim YY. Goldmann applanation tonometry and dynamic contour tonometry in eyes with elevated intraocular pressure (IOP): comparison in the same eyes after subsequent medical normalization of IOP. Graefes Arch Clin Exp Ophthalmol. 2010;248(11):1611–1616. doi: 10.1007/s00417-010-1462-2. [DOI] [PubMed] [Google Scholar]
- 23.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44(5):367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 24.Anwar Z, Wellik SR, Galor A. Glaucoma therapy and ocular surface disease: current literature and recommendations. Curr Opin Ophthalmol. 2013;24(2):136–143. doi: 10.1097/ICU.0b013e32835c8aba. [DOI] [PubMed] [Google Scholar]
- 25.Tsikripis P, Papaconstantinou D, Koutsandrea C, Apostolopoulos M, Georgalas I. The effect of prostaglandin analogs on the biomechanical properties and central thickness of the cornea of patients with open-angle glaucoma: a 3-year study on 108 eyes. Drug Des Devel Ther. 2013;7:1149–1156. doi: 10.2147/DDDT.S50622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mark HH. Corneal curvature in applanation tonometry. Am J Ophthalmol. 1973;76(2):223–224. doi: 10.1016/0002-9394(73)90164-5. [DOI] [PubMed] [Google Scholar]
- 27.Orssengo GJ, Pye DC. Determination of the true intraocular pressure and modulus of elasticity of the human cornea in vivo. Bull Math Biol. 1999;61(3):551–572. doi: 10.1006/bulm.1999.0102. [DOI] [PubMed] [Google Scholar]
- 28.Paranhos A, Jr, Paranhos FR, Prata JA, Jr, Omi CA, Mello PA, Shields MB. Influence of keratometric readings on comparative intraocular pressure measurements with Goldmann, Tono-Pen, and noncontact tonometers. J Glaucoma. 2000;9(3):219–223. doi: 10.1097/00061198-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Eysteinsson T, Jonasson F, Sasaki H, Arnarsson A, Sverrisson T, Sasaki K, Stefánsson E, Reykjavik Eye Study Group Central corneal thickness, radius of the corneal curvature and intraocular pressure in normal subjects using non-contact techniques: Reykjavik Eye Study. Acta Ophthalmol Scand. 2002;80(1):11–15. doi: 10.1034/j.1600-0420.2002.800103.x. [DOI] [PubMed] [Google Scholar]
- 30.Gunvant P, Baskaran M, Vijaya L, Joseph IS, Watkins RJ, Nallapothula M, Broadway DC, O'Leary DJ. Effect of corneal parameters on measurements using the pulsatile ocular blood flow tonograph and Goldmann applanation tonometer. Br J Ophthalmol. 2004;88(4):518–522. doi: 10.1136/bjo.2003.019331. [DOI] [PMC free article] [PubMed] [Google Scholar]