Abstract
AIM
To investigate the cytotoxic effect on human corneal epithelial cells (HCECs) and the ability to faciliate corneal epithelial wound healing of carboxymethylcellulose (CMC) and hyaluronic acid (HA).
METHODS
HCECs were exposed to 0.5% CMC (Refresh plus®, Allergan, Irvine, California, USA) and 0.1% and 0.3% HA (Kynex®, Alcon, Seoul, Korea, and Hyalein mini®, Santen, Osaka, Japan) for the period of 30min, and 4, 12, and 24h. Methyl thiazolyl tetrazolium (MTT)-based calorimetric assay was performed to assess the metabolic activity of cellular proliferation and lactate dehydrogenase (LDH) leakage assay to assess the cytotoxicity. Apoptotic response was evaluated with flow cytometric analysis and fluorescence staining with Annexin V and propiodium iodide. Cellular morphology was evaluated by inverted phase-contrast light microscopy and electron microscopy. The wound widths were measured 24h after confluent HCECs were scratch wounded.
RESULTS
The inhibitory effect of human corneal epithelial proliferation and cytotoxicity showed the time-dependent response but no significant effect. Apoptosis developed in flow cytometry and apoptotic cells were demonstrated in fluorescent micrograph. The damaged HCECs were detached from the bottom of the dish and showed the well-developed vacuole formations. Both CMC and HA stimulated reepithehlialization of HCECs scratched, which were more observed in CMC.
CONCLUSION
CMC and HA, used in artificial tear formulation, could be utilized without any significant toxic effect on HCECs. Both significantly stimulated HCEC reepithelialization of corneal wounds.
Keywords: carboxymethylcellulose, corneal wound healing, human corneal epithelial cells, hyaluronic acid, cytotoxicity
INTRODUCTION
The corneal epithelium forms an integral part of the ocular surface and is necessary for maintaining a clear and proper functioning cornea. When compromised, it is therefore important that it is rapidly regenerated. Dry eye syndrome is a common disorder that affects approximately 10% to 20% of the adult population worldwide[1],[2]. Dry eye is associated with a decrease in tear aqueous production and abnormalities of the lipid, protein, and mucin profiles. These changes cause desiccation of the ocular surface, leading to epithelial damage as a result of inflammation on the surface. Although dry eye develops from multiple etiologies, it is most often treated with artificial tear supplementation, which promotes epithelial healing[3],[4].
Carboxymethylcellulose (CMC), a high-molecular-weight polysaccharide, is one of the most common viscous polymers used in artificial tears to achieve their prolonged residence efficacious in the treatment of aqueous tear-deficient dry eye symptoms and ocular surface staining[5],[6]. It is generally understood that it is the physical properties of CMC, such as its viscous and mucoadhesive properties, that contributes to its prolonged retention time in the ocular surface. CMC can also effectively reduce the incidence of epithelial defects through stimulating epithelial cell migration by its binding to matrix proteins[6]–[8].
Hyaluronic acid (HA) is naturally occurring glycosaminoglycan of the extracellular matrix that plays an important role in development, would healing, and inflammation[4]. Its viscoelastic properties have rendered it ideally suited for use in ophthalmic practice to protect the corneal endothelium and to maintain the anterior chamber depth during intraocular surgery[9],[10]. It has also been used in the treatment of dry eyes because of its long ocular surface residence time[9],[11]. Recent experiment have shown sodium hyaluronate promotes migration on human corneal epithelial cells (HCECs) in vitro and has beneficial effect in corneal wound healing by rapid migration of cells leading to rapid wound closure[12].
This study was undertaken to compare the cytotoxicity on human corneal epithelium which is easily damaged in dry eye syndrome and the effectiveness on corneal epithelial wound healing of CMC versus HA.
SUBJECTS AND METHODS
Human Corneal Epithelial Cell Culture
This study was performed according to the tenets of the Declaration of Helsinki. Primary cultures of human corneal epithelium were obtained using human donor corneas that had been discarded before transplantation due to low endothelial cell counts as previously described[13]. Briefly, under aseptic conditions and using the dissecting microscope, donor corneoscleral rim was divided into six equal pieces. The endothelial and posterior stromal layer was carefully peeled off and each explants was placed separately, with the epithelial surface facing downwards. These were left covered in a laminar flow cabinet at room temperature for 5min and then covered in growth medium [DMEM (Gibco BRL, Life Technologies, CA, USA)] supplemented with FBS (5%) (Gibco); DMSO (0.5%) (Sigma-Aldrich, MO, USA); getamicin (5 µL/mL) (Sigma); epidermal growth factor (10 ng/mL) (Gibco); bovine insulin (5 µg/mL) (Gibco); and cholera toxin (0.1 µg/mL) (Gibco). Cultures were incubated at 37°C in 5% carbon dioxide in humid air. The epithelial cell morphology of the cultures was evaluated daily by phase contrast microscopy.
Methyl Thiazolyl Tetrazolium Assay
HCECs' viability was determined using the methyl thiazolyl tetrazolium (MTT) assay. One hundred microliters of the cells was plated in 96 well tissue-culture plates at a concentration of 5×104 cell/mL, and incubated at 37°C in 5% CO2 for 24 to 48h until the cultures were subconfluent. One hundred microliters of preservative free artificial tear drops [0.5% CMC (Refresh plus®, Allergan, Irvine, California, USA) and 0.1% and 0.3% HA (Kynex®, Alcon, Seoul, Korea, and Hyalein mini®, Santen, Osaka, Japan)] was added to each of 96 well plate and incubated for 30min, and 4, 12, and 24h. DMEM was added to the control tube in 100 µL aliquots. After 30min, and 4, 12, and 24h, the plate was washed three times with PBS to remove the drugs. The cell viability was evaluated after 24h of incubation. One hundred microliters of ten fold diluted PBS containing MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; thiazoyl blue, Sigma-Aldrich, St. Louis, MO, USA] (5 mg/mL) was added to each well. In the absence of light, the samples were incubated for 4h at 37°C, and the medium was removed. The precipitates were resuspended in 100 µL dimethyl sulfoxide (DMSO, Sigma-Aldrich). The absorbance was measured on a plate reader at a wavelength of 570 nm. Each experiment was performed in triplicate.
Lactate Dehydrogenase Assay
In the lactate dehydrogenase (LDH) assay, leakage of the cytoplasm-located enzyme LDH into the extracellular medium was measured. The presence of the exclusive cytosolic enzyme, LDH, in the cell culture medium represents the cell membrane damage. For the LDH assay, 4.0×103 HCECs/mL were seeded per well of 96-microtiter plates. Twenty-four hours after cell seeding, cells were exposed to 0.5 % CMC, 0.3% HA and 0.1% HA, respectively. The LDH titer of CMC and HA was assessed at 0, 30min, and 4, 12 and 24h after the addition of each these agents. After 24h later, the supernatants were collected from each well. Cell monolayer was then treated with a cell lysis solution for 30min at room temperature to lyse. The cells and the lysate were collected. LDH activity was measured in both the supernatants and the cell lysate fractions using CytoTox 96, a non-radioactive cytotoxicity assay kit (Promega, WI, USA), in accordance with the manufacturer's instruction. The color intensity is proportional to the LDH activity. The absorbance was determined at 490 nm with 96-well plate ELISA reader. The LDH activity was expressed as optical density. The effect of balanced salt solution-treated group was used as a control. Each drug, as well as the control, was tested 3 times.
Apoptosis Assay by Flow Cytometric Analysis
HCECs were either left untreated or were treated with 0.5% CMC, 0.3% HA, and 0.1% HA for 24h. Cells were incubated with annexin V-FITC (Caltag Laboratories, Burlingame, CA, USA), in buffer containing PI, and analyzed by flow cytometry[14].
Morphologic Assay
HCECs were treated with 0.5% CMC, 0.3% HA and 0.1% HA for 24h and photographed using phase-contrast microscopy. For transmission electron microscopy, the cells that had been grown to confluence in 24 well plates were then incubated in DMEM containing 0.5% CMC and 0.1% and 0.3% HA and phosphate buffer control for 4 and 8h under 5% CO2 at 37°C. The cells were then incubated at 37°C for 24h after rinsing with PBS. The cells were fixed with 2.5% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.4) for 12h, and postfixed with 0.1% osmium tetroxide for 2h. After rinsing with 0.1 mol/L of a phosphate buffer and dehydrating in a graded series of ethanol, the specimens were embedded in an Epon 812 mixture. An ultrathin section of 60-80 nm was cut, stained with uranyl acetate and lead citrate, and examined by transmission electron microscopy (JEOL1200EX: Jeol Ltd., Tokyo, Japan).
Analysis of Drug Component
The ionic composition of formulation was assessed by LX-20 (Beckman Coulter, Fullerton, CA, USA). The pH was measured by using Metrohn 780 (Metrohn, Switzerland), and the osmolarity of three agents was measured by using the Micro sample Osmometer (Fiske Associates, Norwood, MA, USA).
Scratch Wound Healing Assay
A scratch-wound assay with HCECs was used to determine whether CMC and HA could promote wound closure. HCECs were cultured to a confluent monolayer on eight-well chamber slides coated with collagen I (10 µg/cm2; Auspep, Parkville, VIC, Australia) before being wounded by scratching with a 100 µL pipette tip. The scratch wounded HCECs were washed with fresh medium to remove detached cells before incubation in the medium in the absence or presence of CMC and HA for 24h. To ensure that the wounds with the same wound area were compared, multiple positioning marks were made at the center of the denuded surface with a small needle, and the mean distance between the wound edge was measured. Twenty-four hours after wounding, the monolayers were fixed and stained, and the wound areas in a marked field of view were imaged. The mean distance between the migrated cell edge (measurements in three separate samples) was determined using an image analysis system (Image J 1.33o; available by ftp at zippy.nimh.nih.gov/ or at http://rsb.info.nih.gov/nih-imageJ; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD, USA) and the percentage of wound closure by HCECs in response to CMC and HA was compared.
Statistical Analysis
Statistical analyses significance was determined by ANOVA followed by Tukey post hoc analysis (Prism; Graph Pad Software). A value of P less than 0.05 was considered significant.
RESULTS
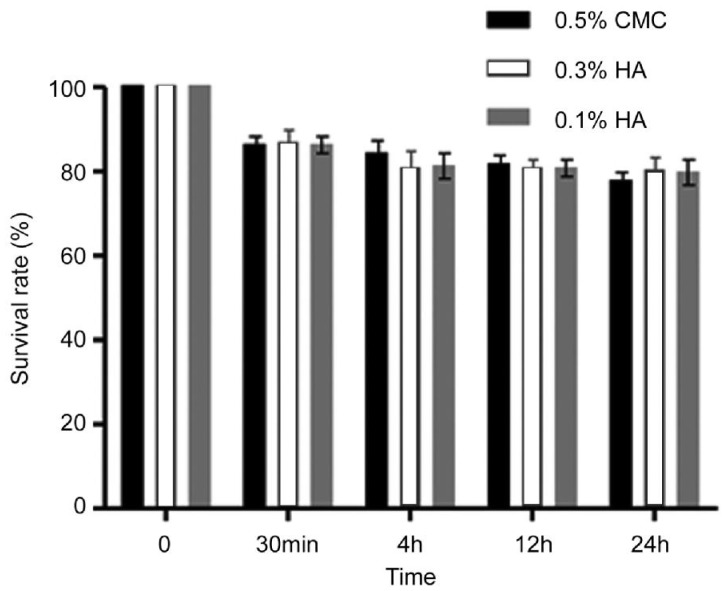
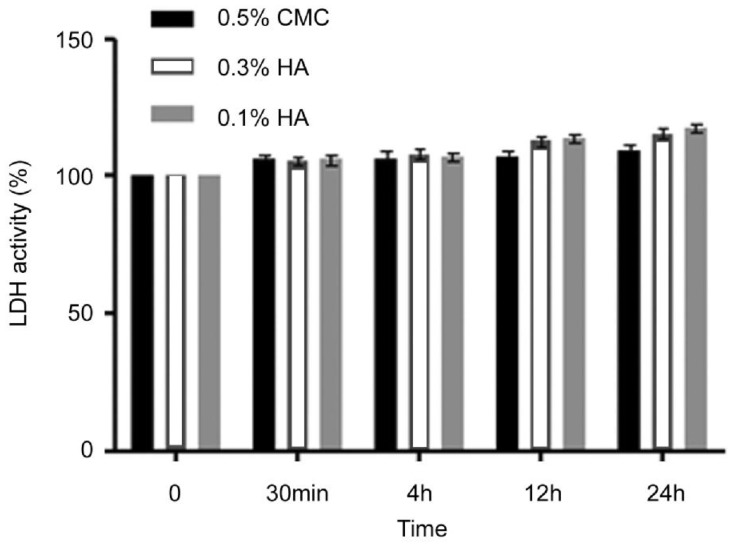
The level of cell death increased with increasing exposure time (Figure 1). LDH activity was also time depedent and continued to increase until 24h (Figure 2). However, no significant differences after treatment with 0.5% CMC, 0.3% HA and 0.1% HA were noted in MTT and LDH activity regardless of exposure time.
Figure 1. Metabolic activities of HCECs measured with MTT assays showed the time-dependant response by the exposure time of 0.5% CMC, 0.3% HA and 0.1% HA.
There was no significant decrease in the proliferation of corneal epithelial cells comparing with control.
Figure 2. With time, the LDH acitivity of HCECs in of 0.5% CMC, 0.3% HA and 0.1% HA showed time-dependant response for cytotoxicity.
There was no significant increase in the cytotoxicity of corneal epithelail cells comparing with control.
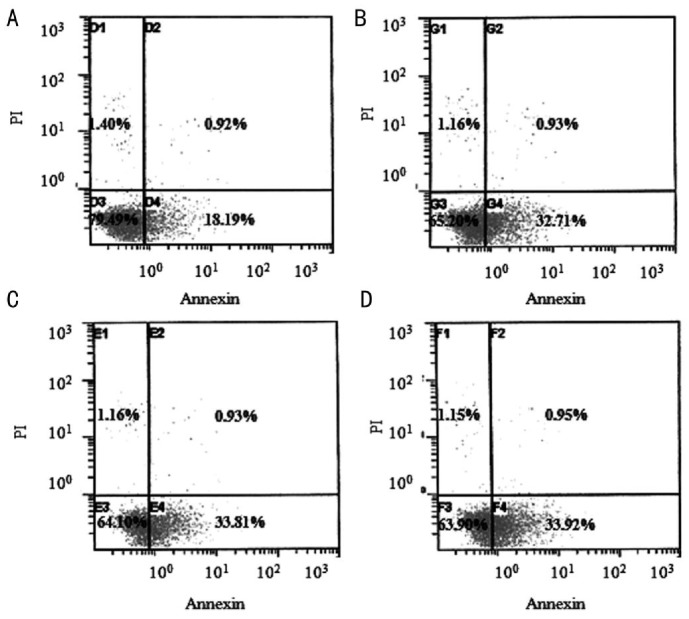
In flow cytometric analysis of apoptotic cells using annexin V-FITC, untreated cells (Figure 3A) were primarily annexin V-FITC and PI negative, indicating viability and no apoptosis. After treatment with 0.5% CMC, 0.3% HA and 0.1% HA for 24h, a significant number of cells were annexin V-FITC positive and PI negative (Figure 3B-D), signifying that cells were in the stages of apoptosis. Apoptotic cells were visible (Figure 4B-D) in the fluorescent microscope, which represented fluorescence in the cytoplasm after binding DNA flowing into cytoplasm due to disruption of nuclear membrane.
Figure 3. Flow cytometric analysis of apoptotic cells using annexin V-FITC.
HCECs were left untreated or were treated for 24h with 0.5% CMC, 0.3% HA and 0.1% HA. Cells were incubated with annexin V-FITC in a buffer containing PI and analyzed by flow cytometry. Untreated cells were primarily annexin V-FITC and PI negative (A, bottom left quadrant) indicating that the cells were viable and not undergoing apoptosis. After treatment with 0.5% CMC (B), 0.3% HA (C) and 0.1% HA (D), a significant number of cells were annexin V-FITC positive and PI negative (B-D, bottom right quadrant), indicating that the cells were in the stage of apoptosis.
Figure 4. Fluorescent micrograph of HCECs after exposure to 0.5% CMC, 0.3% HA and 0.1% HA (original magnification, ×400).
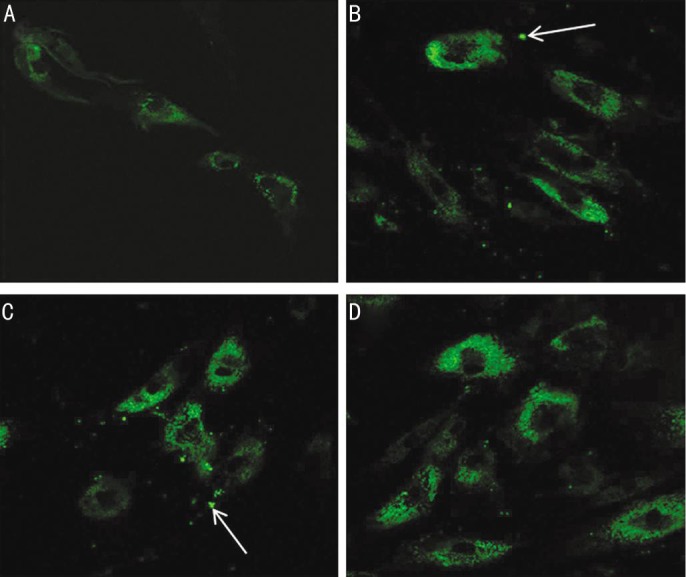
Compared with untreated cells (A), apoptotic cells were visible after treatment with 0.5% CMC (B), 0.3% HA (C) and 0.1% HA (D), which represented fluorescence after binding DNA flowing into cytoplasm due to disruption of nuclear membrane. There were visible apoptotic bodies (white arrow) in extra-cytoplasmic portion (B&C).
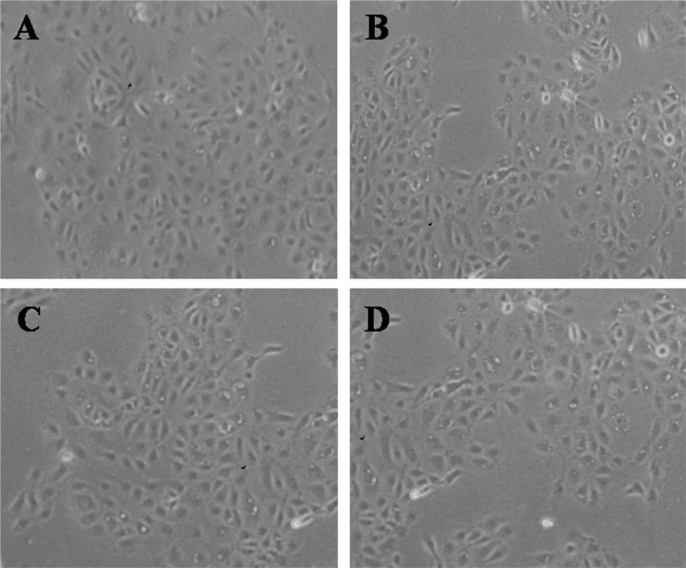
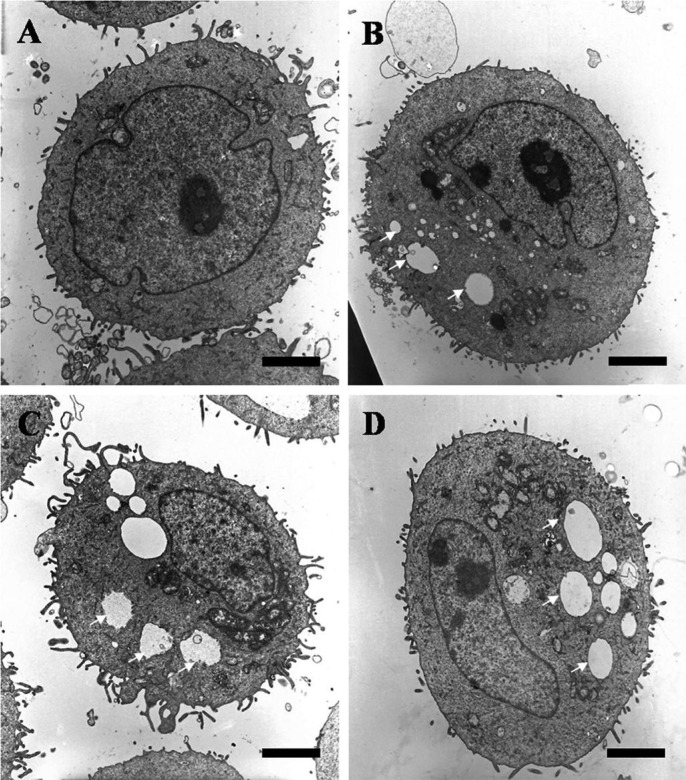
Using phase-contrast microscope, many epithelial cells were visible in control culture media (Figure 5A). HCECs became more detached from the dishes and less visible with rather than the control, in the cases of exposure to 0.5% CMC, 0.3% HA and 0.1% HA (Figure 5B-D). Also, the electron microscopy examination showed that cells treated with 0.5% CMC, 0.3% HA and 0.1% HA demonstrated only some cytoplasmic bleb formation (Figure 6B-D) compared with a normal corneal epithelial cell showing microvilli, homogenous cytoplasm and intact cell and nuclear membrane (Figure 6A).
Figure 5. Inverted phase-contrast micrographs of HCECs after exposure to 0.5% CMC, 0.3% HA and 0.1% HA (original magnification, ×200).
Many epithelial cells are visible in control culture media (A). The number of HCECs were decreased and more detached from the culture media, in the case of exposure to 0.5% CMC (B), 0.3% HA (C) and 0.1% HA (D) compared with control.
Figure 6. Transmission electron micrographs of HCECs after exposure to 0.5% CMC, 0.3% HA and 0.1% HA (bar length 2 µm: original magnification, ×3000-4000).
A normal corneal epithelial cell (A) showed microvilli, homogenous cytoplasm and intact cell and nuclear membrane. When exposed to 0.5% CMC (B), 0.3% HA (C) and 0.1% HA (D), the corneal epithelial cells revealed well-developed vacuole formations (white arrows).
The concentrations of Na+ and Cl− were lower and higher with 0.1% and 0.3% HA, compared to 0.5% CMC respectively, and the concentrations of K+ were higher in all agents. The pH values were same and the osmolarity was 276 mOsm/kg for 0.1% HA, 284 mOsm/kg for 0.3% HA, and 303 mOsm/kg for 0.5% CMC (Table 1).
Table 1. Electrolyte composition, pH, and osmolarity of the agents.
| Agents | Na+ (mEq/L) | K+ (mEq/L) | Cl− (mEq/L) | pH | Osmolarity (mOsm/kg) |
| 0.5% Carboxymethylcellulose | 155.8 | 17.94 | 124.9 | 5.5 | 303 |
| 0.3% Hyaluronic acid | 133.6 | 15.43 | 131.9 | 5.5 | 284 |
| 0.1% Hyaluronic acid | 128.9 | 13.37 | 133.2 | 5.5 | 276 |
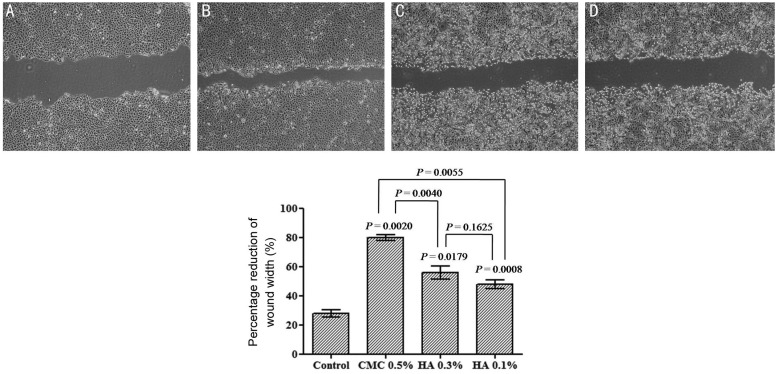
Twenty-four hours after wounding the monolayers of HCLE, 0.5% CMC, 0.3% HA and 0.1% HA treated HCECs had closed the wound significantly more than the control medium treated wounds, indicating that CMC and HA promoted HCEC wound closure. Especially CMC stimulation was significant compared with the HA (Figure 7).
Figure 7. The closure of scratched HCEC wounds in response to 0.5% CMC, 0.3% HA and 0.1% HA stimulation.
The effect of CMC and HA on wound closure was visualized and also represented as the percentage reduction of the average wound width 24h after confluent HCECs were scratch wounded and incubated in the culture medium in the absence (A) or presence of CMC (B) or HA (C&D) (2 mg/mL). Data represent the mean±SD (n=3) of percentage reduction of the wound width which was measured as the average width of the 10 measurements along the wound edge. CMC and HA stimulation was significant compared with the control and CMC showed more wound healing effect than HA.
DISCUSSIOIN
Although CMC and HA is widely used in artificial tears for the treatment of dry eye, few studies concerned with the comparison of ocular surface toxicity and wound heaing effect of these agents have been reported[15],[16]. One of the aims of the present study was to compare the cellular cytotoxicity of commercially available preservative free topical artificial tear drops on HCECs in vitro. According to our results, the longer the duration of exposure, the more decreased the cell survival and increased the cell lysis. However, there were no significant differences in all groups regardless of the exposure time, and the concentration of drugs did not cause any difference in cell survival and lysis. Also, there was no noticeable damage to the cytoplasmic membrane or to the cytoplasm of corneal epithelial cells except vacuole formations. This means that HA and CMC with different concentrations do not induce any time-dependent cell dysfunction and cytotoxicity in corneal epithelial cells, which was supported by our annexin V binding assays demonstrating that cell loss by CMC and HA was due to apoptosis, but not cytotoxicity such as necrosis.
Electrolytes, pH, osmolarity, and viscosity of commercially available topical ocular agents may induce ocular surface damage when they are used for a long period of time or when overdosed. Thus, this study also evaluated the difference of electrolyte composition, pH, and osmolarity among 0.5% CMC, 0.3% HA, and 0.1% HA. The electrolyte composition of these agents usually involves Na+, Cl−, K+, and Ca2+. Based on the results of our study, there was no significant difference of K+ concentration in all three agents, although the ideal value of extracellular fluid ranged from 4.3 to 4.6 mEq/L. However, for the concentration of Na+, 0.3% HA and 0.1% HA were lower and for Cl−, they were higher than 0.5% CMC, compared with the ideal value of Na+ in the range 142-152.7 mEq/L and Cl− in 104.0-117.4 mEq/L[17]. This means that low and high concentrations of Na+ and Cl− may induce cellular dysfunction of the ocular surface. The unbalance of osmolarity may also induce the damage of cellular function. It was reported that hypertonic stress induced HCEC shrinkage and apoptosis using in vitro cell culture models[18]. The ideal value of osmolar pressure of 260-320 mosm/kg is generally acceptable, not to damage the cellular function[19]. The values of osmolarity of all three agents were similar, and they fell into the normal range and were unlikely to impact on cellular function. The pH of all agents was determined to be same but out of the ideal value ranged from 7.0 to 7.7[20]. Therefore, all three agents have a mildly acidic pH, and acidic conditions increase corneal epithelial permeability[18]. Additionally, the neutralization of the pH caused by tear fluid may trigger the white crystalline precipitation or may induce the unbalance of the ocular surface condition[21],[22].
Although it is not yet understood how CMC and HA stimulate cell migration in vivo, our in vitro wound-healing model had its advantages in providing information on the basic principles involved and is widely used to assess exogenous agents that may modify the healing process[6],[23]–[25]. In vitro, closure of wounds is a result of cell migration from the wound's edge as well as cell proliferation[12],[23]. CMC and HA showed a potent stimulatory effect on HCLE cell migration, and the effect of 0.5% CMC on wound closure was significantly better than that of 0.3% or 0.1% HA. We suggest that there may be two possible reasons for 0.5% CMC to have better effect on wound healing. One is that CMC has higher concentration of active ingredient compared with HA. It has been reported that the active ingredient is not the only important aspect of an artificial tear. Concentration, molecular weight, tonicity, preservatives and salts have to be taken into account[26]. The differences in the wound healing effect might be related in part to the concentration of the drugs. The other reason is that the electrolyte composition of CMC is much closer to the ideal value than that of HA. Electrolytes and osmolarity are considered to be the most important factors to induce cellular dysfunction of the ocular surface[18], and the different value of electrolyte of all three agents was likely to impact on cellular function.
CMC and HA are widely used first treatment options of dry eye syndrome. The present results demonstrated that both CMC and HA showed no differences in cytotoxicity without any significant toxic effect and in wound healing effect on HECEs. CMC showed more wound healing effect, presumably due to relatively high concentration and more balanced electrolytes. Although the results of the in vitro test may not be always applicable to the in vivo activities, it is believed that this in vitro study will be a valuable guide for determining the optimal drug in clinical use. So the future studies investigating the results of clinical trial should be followed. In conclusion, the properties of these agents with different active ingredient and concentration may form the basis for the observed long-lasting benefits of clinical use of CMC and HA.
Acknowledgments
Foundation: Supported by Biomedical Research Institute Grant (No.2009-39), Pusan National University Hospital.
Conflicts of Interest: Lee JS, None; Lee SU, None; Che CY, None; Lee JE, None.
References
- 1.Uchino M, Nishiwaki Y, Michikawa T, Shirakawa K, Kuwahara E, Yamada M, Dogru M, Schaumberg DA, Kawakita T, Takebayashi T, Tsubota K. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology. 2011;118(12):2361–2367. doi: 10.1016/j.ophtha.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 2.Galor A, Feuer W, Lee DJ, Florez H, Carter D, Pouyeh B, Prunty WJ, Perez VL. Prevalence and risk factors of dry eye syndrome in a United States veterans affairs population. Am J Ophthalmol. 2011;152(3):377–384. doi: 10.1016/j.ajo.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JE, Kim NM, Yang JW, Kim SJ, Lee JS, Lee JE. A randomised controlled trial comparing a thermal massager with artificial teardrops for the treatment of dry eye. Br J Ophthalmol. 2014;98(1):46–51. doi: 10.1136/bjophthalmol-2013-303742. [DOI] [PubMed] [Google Scholar]
- 4.Dogru M, Nakamura M, Shimazaki J, Tsubota K. Changing trends in the treatment of dry-eye disease. Expert Opin Investig Drugs. 2013;22(12):1581–1601. doi: 10.1517/13543784.2013.838557. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Ahn HS, Kim EK, Kim TI. Efficacy of sodium hyaluronate and carboxymethylcellulose in treating mild to moderate dry eye disease. Cornea. 2011;30(2):175–179. doi: 10.1097/ICO.0b013e3181e9adcc. [DOI] [PubMed] [Google Scholar]
- 6.Garrett Q, Simmons PA, Xu S, Vehige J, Zhao Z, Ehrmann K, Willcox M. Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2007;48(4):1559–1567. doi: 10.1167/iovs.06-0848. [DOI] [PubMed] [Google Scholar]
- 7.Ahee JA, Kaufman SC, Samuel MA, Bogorad D, Wee C. Decreased incidence of epithelial defects during laser in situ keratomileusis using intraoperative nonpreserved carboxymethylcellulose sodium 0.5% solution. J Cataract Refract Surg. 2002;28(9):1651–1654. doi: 10.1016/s0886-3350(01)01348-7. [DOI] [PubMed] [Google Scholar]
- 8.Albietz JM, Lenton LM, McLennan SG, Earl ML. A comparison of the effect of refresh plus and bion tears on dry eye symptoms and ocular surface health in myopic LASIK patients. CLAO J. 2002;28(2):96–100. [PubMed] [Google Scholar]
- 9.Storr-Paulsen A, Norregaard JC, Farik G, Tarnhoj J. The influence of viscoelastic substances on the corneal endothelial cell population during cataract surgery: a prospective study of cohesive and dispersive viscoelastics. Acta Ophthalmol Scand. 2007;85(2):183–187. doi: 10.1111/j.1600-0420.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 10.Miyata K, Yamagami S, Nejima R, Miyai T, Shimizu K, Amano S. Corneal endothelial cell protection with Viscoat and Healon or Healon alone during penetrating keratoplasty. Cornea. 2005;24(8):962–966. doi: 10.1097/01.ico.0000159757.28607.ae. [DOI] [PubMed] [Google Scholar]
- 11.Aragona P, Pata V, Micali A, Santocono M, Milazzo G. Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye. Br J Ophthalmol. 2002;86(2):181–184. doi: 10.1136/bjo.86.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes JA, Amankwah R, Powell-Richards A, Dua HS. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br J Ophthalmol. 2004;88(6):821–825. doi: 10.1136/bjo.2003.027573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JE, Oum BS, Choi HY, Yu HS, Lee JS. Cysticidal effect on acanthamoeba and toxicity on human keratocytes by polyhexamethylene biguanide and chlorhexidine. Cornea. 2007;26(6):736–741. doi: 10.1097/ICO.0b013e31805b7e8e. [DOI] [PubMed] [Google Scholar]
- 14.Kim TI, Tchah H, Lee SA, Sung K, Cho BJ, Kook MS. Apoptosis in keratocytes caused by mitomycin C. Invest Ophthalmol Vis Sci. 2003;44(5):1912–1917. doi: 10.1167/iovs.02-0977. [DOI] [PubMed] [Google Scholar]
- 15.Vehige JG, Simmons PA, Anger C, Graham R, Tran L, Brady N. Cytoprotective properties of carboxymethyl cellulose (CMC) when used prior to wearing contact lenses treated with cationic disinfecting agents. Eye Contact Lens. 2003;29(3):177–180. doi: 10.1097/01.ICL.0000074106.82322.17. [DOI] [PubMed] [Google Scholar]
- 16.Aragona P, Di Stefano G, Ferreri F, Spinella R, Stilo A. Sodium hyaluronate eye drops of different osmolarity for the treatment of dry eye in Sjögren's syndrome patients. Br J Ophthalmol. 2002;86(8):879–884. doi: 10.1136/bjo.86.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park YS. Physiology of body fluid. In: Kang DH, editor. Physiology. 5th ed. Seoul: Sin-Kwang; 2000. pp. 585–606. [Google Scholar]
- 18.Garrett Q, Khandekar N, Shih S, Flanagan JL, Simmons P, Vehige J, Willcox MD. Betaine stabilizes cell volume and protects against apoptosis in human corneal epithelial cells under hyperosmotic stress. Exp Eye Res. 2013;108(1):33–41. doi: 10.1016/j.exer.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Lee JS, Lee JE, Kim N, Oum BS. Comparison of the conjunctival toxicity of topical ocular antiallergic agents. J Ocul Pharmacol Ther. 2008;24(6):557–562. doi: 10.1089/jop.2008.0071. [DOI] [PubMed] [Google Scholar]
- 20.Kim K. Acid-base balance. In: Sung H, Kim KH, editors. Physiology. 3rd ed. Seoul: Eui-hak; 2006. pp. 326–327. [Google Scholar]
- 21.Wilhelmus KR, Abshire RL. Corneal ciprofloxacin precipitation during bacterial keratitis. Am J Ophthalmol. 2003;136(6):1032–1037. doi: 10.1016/s0002-9394(03)00636-6. [DOI] [PubMed] [Google Scholar]
- 22.Szentmáry N, Kraszni M, Nagy ZZ. Interaction of indomethacin and ciprofloxacin in the cornea following phototherapeutic keratectomy. Graefes Arch Clin Exp Ophthalmol. 2004;242(7):614–616. doi: 10.1007/s00417-004-0889-8. [DOI] [PubMed] [Google Scholar]
- 23.Ronot X, Doisy A, Tracqui P. Quantitative study of dynamic behavior of cell monolayers during in vitro wound healing by optical flow analysis. Cytometry. 2000;41(1):19–30. [PubMed] [Google Scholar]
- 24.Gottrup F, Agren MS, Karismark T. Models for use in wound healing research: a survey focusing on in vitro and in vivo adult soft tissue. Wound Repair Regen. 2000;8(2):83–96. doi: 10.1046/j.1524-475x.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- 25.Movahedan A, Majdi M, Afsharkhamseh N, Sagha HM, Saadat NS, Shalileh K, Milani BY, Ying H, Djalilian AR. Notch inhibition during corneal epithelial wound healing promotes migration. Invest Ophthalmol Vis Sci. 2012;53(12):7476–7483. doi: 10.1167/iovs.12-10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez MA, Torralbo-Jimenez P, Giron N, de la Heras B, Herrero Vanrell R, Arriola-Villalobos P, Diaz-Valle D, Alvarez-Barrientos A, Benitez-Del-Castillo JM. Comparative analysis of carmellose 0.5% versus hyaluronate 0.15% in dry eye: A flow cytometric study. Cornea. 2010;29(2):167–171. doi: 10.1097/ICO.0b013e3181b11648. [DOI] [PubMed] [Google Scholar]