Abstract
AIM
To evaluate the therapeutic effect of netrin-4 on the early acute phase of inflammation in the alkali-burned eye.
METHODS
Eye drops containing netrin-4 or phosphate buffered saline (PBS) were administered to a alkali-burn-induced corneal acute inflammatory model four times daily. The clinical evaluations, including fluorescein staining and inflammatory index, were performed on day 1, 4 and 7 using slit lamp microscopy. Global specimens were collected on day 7 and processed for immunofluorescent staining. The levels of inflammatory mediators in the corneas were determined by real-time polymerase chain reaction (PCR).
RESULTS
Exogenous netrin-4 administered on rat ocular surfaces showed more improvements in decreasing fluorescein staining on day 4 and 7, and resolved alkali burn-induced corneal inflammation index on day 7 (P<0.01). The levels of IL-1β, IL-6, intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), monocyte chemotactic protein-1 (MCP-1) and macrophage inflammatory protein-1 (MIP-1) in corneas were decreased in netrin-4-treated groups (P<0.05). In addition, netrin-4 significantly reduced the expression of leukocyte common antigen 45 (CD45) in the alkali-burn cornea (P<0.001).
CONCLUSION
Topical netrin-4 accelerated wound healing and reduced the inflammation on alkali-burn rat model, suggesting a potential as an anti-inflammatory agent in the clinical to treat the acute inflammation.
Keywords: netrin-4, inflammation, alkali burn, cornea
INTRODUCTION
Alkali injury to the eye is one of the most common and devastating ophthalmic emergencies. The corneal alkali burn model is a well-established severe ocular surface disease model that causes corneal epithelial defects, prominent corneal acute inflammation, corneal neovascularization, and reduced corneal transparency[1],[2]. It is widely used to study the mechanism and therapies of acute inflammation and angiogenesis, due to the easy local administration of medicine, well accessible position for observation, and the relatively immune-privileged status of the cornea[3].
Netrins are laminin-like secreted proteins, initially identified as axonal guidance molecules[4],[5]. The netrin system comprises at least five ligands (netrins 1, 2, 4, G1a, and G1b) and seven receptors (neogenin, deleted in colorectal cancer, Unc5A, Unc5B, Unc5C, Unc5D, and A2b)[6],[7]. Netrin-4 is a recently identified member of the netrin family[8],[9]. Netrin-4 is expressed in both neural and nonneural tissues[10]–[13]. Netrin-4 promotes neurite outgrowth and regulates vasculogenesis[14]. Netrin-4 is an pro-angiogenesis[15]–[18] or anti-angiogenesis factor[19]–[21]. Netrin-4 has also been shown to regulate epithelial branching morphogenesis in the breast, lung and pancreas and endothelial proliferation[22]–[25]. And recent studies found that netrin-4 expression was down-regulated in tumors[26],[27]. Netrin-4 induces lymphangiogenesis in vivo, whereas it may act as a negative regulator of corneal epithelial cell proliferation and retinal branching in ocular tissues[28],[29]. Moreover, endothelial cell-derived netrin-4 supports adhesion and differentiation of pancreatic epithelial cells[30]. Another netrins family member netrin-1 in acute inflammation models in tissues such as the cornea[31], colon[32], lungs[33]–[35], or kidneys[36]. Recently, netrin-1 was found to be a negative guidance cue for leukocyte migration[33],[35], which indicates the anti-inflammatory potential of netrin-1. Several in vivo studies have been conducted to evaluate the effect of netrin-1 on animal disease models, such as subcutaneous application for experimental colitis, intraperitoneal injection for peritonitis, and inhalation or intravenous injection for lipopolysaccharide-induced pulmonary injury[32],[34],[37]. These studies have shown the potent effect of netrin-1 on reducing neutrophil infiltration, while the mechanism is variously dependent on receptor A2BAR[32],[34],[37] or UNC5B[35]. But until now, nothing is known about the roles of netrin-4 in the inflammation.
Here, we provide evidence that netrin-4 functions as an anti-inflammatory factor in the acute phase of inflammation. We show that netrin-4 promotes ocular surface wound healing and alleviates the early inflammatory index on alkali-burn rat model. We demonstrate that netrin-4 suppresses mRNA levels of proinflammatory cytokines, and chemokines in the alkali-burn cornea. Moreover, netrin-4 significantly decreased leukocyte infiltration in the alkali-burn cornea. Taken together, the data demonstrate that netrin-4 functions as an anti-inflammatory factor in the acute phase of inflammation.
MATERIALS AND METHODS
Animal Model of a Corneal Alkali Burn and Treatment with the Ophthalmic Solutions
Wistar rats (180-220 g, 2 months old, male, n=12 per time point) were used in the study. Animal experiments were performed in accordance with the guidelines of the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research, and the animal experimental procedures were approved by the Experimental Animal Committee of Xiamen University. All rats were confirmed as being free of ocular diseases before the experiments. Rat corneal alkali burns were conducted as previously reported[30],[31]. The procedure was performed unilaterally (right eye) in each rat. In brief, the animals were anesthetized with intraperitoneal ketamine (60 mg/kg), and a filter paper disc (3 mm in diameter) incubated with 1 mol NaOH for 60s was then placed on the central cornea of the right eye for 30s. The ocular surface was then rinsed with 30 mL of phosphate buffered saline (PBS).
After the alkali burn injury, the animals were randomly divided into two groups of equal size. Rats in one group were topically administered with PBS (four times per day, 10 μL each), and the rats in the other group were topically applied with recombinant mouse netrin-4 (R&D Systems, Minneapolis, MN, USA) using pipette (four times per day, 10 μL each, at the concentration of 5.0 μg/mL in PBS). The treatments were administered for 7 consecutive days. Normal rats without alkali burn injuries were used as controls. After different durations, the animals were euthanized and their eyes were enucleated. The corneas were then dissected and stored at -80°C for histologic studies or used for RNA or protein extraction.
Slit-lamp Microscopic Observation
Animals were examined daily using a slit-lamp microscope after the alkali burns were applied. Corneal images were obtained by an experienced researcher (Shao Y). Corneal epithelial defects were determined by staining the ocular surface with 0.1% fluorescein sodium and observation under cobalt blue light. Images were processed using image-processing software (Image Pro Plus V6.0; Media Cybernetics, Silver Spring, MD, USA). The inflammatory index was analyzed based on parameters including ciliary hyperemia, central corneal edema, and peripheral corneal edema as previously described[32].
Ribonucleic Acid Isolation and Real-time Polymerase Chain Reaction
Total RNA of the corneas was extracted using the Trizol reagent (Invitrogen), and cDNA was synthesized using a reverse transcription kit (RR047A; TaKaRa, Shiga, Japan). Real-time polymerase chain reaction (PCR) was performed on a StepOne real-time PCR system (Applied Biosystems, Alameda, CA, USA) using a synergy brands (SYBR) Premix Ex Taq Kit (RR420A; TaKaRa), and the primer sequences are summarized in Table 1. The amplification program included an initial denaturation step at 95°C for 10min, followed by 40 cycles of 95°C for 10s, and 60°C for 30s, after which a melt curve analysis was conducted to check amplification specificity. The results of quantitative PCR were analyzed by the comparative threshold cycle (Ct) method, normalized with β−actin as an endogenous reference, and calibrated against the normal control group.
Table 1. Rat primer sequences used for qRT-PCR.
| Gene | Sense primer | Antisense primer | PCR product (bp) |
| IL-6 | CACAAGTCCGGAGAGGAGAC | ACAGTGCATCATCGCTGTTC | 168 |
| IL-1β | CTGTGACTCGTGGGATGATG | GGGATTTTGTCGTTGCTTGT | 210 |
| ICAM-1 | ACGCAGTCCTCGGCTTCTG | GGTTCTTGCCCACCTGCTG | 97 |
| VCAM-1 | ACAAAACGCTCGCTCAGATT | GTCCATGGTCAGAACGGACT | 152 |
| Ccl2 (MCP-1) | ATGCAGTTAATGCCCCACT | TTCCTTATTGGGGTCAGCAC | 167 |
| Ccl3 (MIP-1α) | TGCCCTTGCTGTTCTTCTCT | AAAGGCTGCTGGTCTCAAAA | 152 |
| GAPDH | GCAAGTTCAACGGCACAG | GCCAGTAGACTCCACGACAT | 140 |
PCR: Polymerase chain reaction; ICAM-1: Intercellular cell adhesion molecule-1; VCAM-1: Vascular cell adhesion molecule-1; MCP-1: Monocyte chemotactic protein-1; MIP-1α: Macrophage inflammatory protein-1α.
Immunofluorescent Staining
Immunofluorescent staining was performed in cryosections (6 μm thick) of the eyeballs. Sections were fixed in acetone at -20°C, blocked, and then incubated at 4°C overnight with mouse anti-common antigen 45 (CD45) antibody (1:100; eBioscience, San Diego, CA, USA). After incubation with Alexa Fluor 594 donkey anti-mouse IgG (1:500; Invitrogen, Carlsbad, CA, USA), sections were counterstained with DAPI (Vector, Burlingame, CA, USA), mounted, and photographed using the Leica upright microscope (DM2500; Leica Microsystems, Wetzlar, Germany).
Statistical Analysis
Summary data were reported as mean±SD. The Student's t-test was applied in the analysis of all experimental data using the GraphPad Prism 5.0 software. Differences with P value <0.05 were considered statistically significant.
RESULTS
Effect of Local Administration of Netrin-4 on Corneal Epithelial Wound Healing
To investigate the mechanism that netrin-4 implements its effect on alkali burn-induced corneal inflammation, we first investigated the corneal epithelial wound healing after the alkali burns. The fluorescein staining showed that corneal epithelial defects were completely healed on day 7, when the corneas were treated with 5.0 mg/mL netrin-4, while the corneas treated with PBS did not heal (Figure 1A).
Figure 1. Netrin-4 promotes corneal epithelial wound healing after alkali burns.
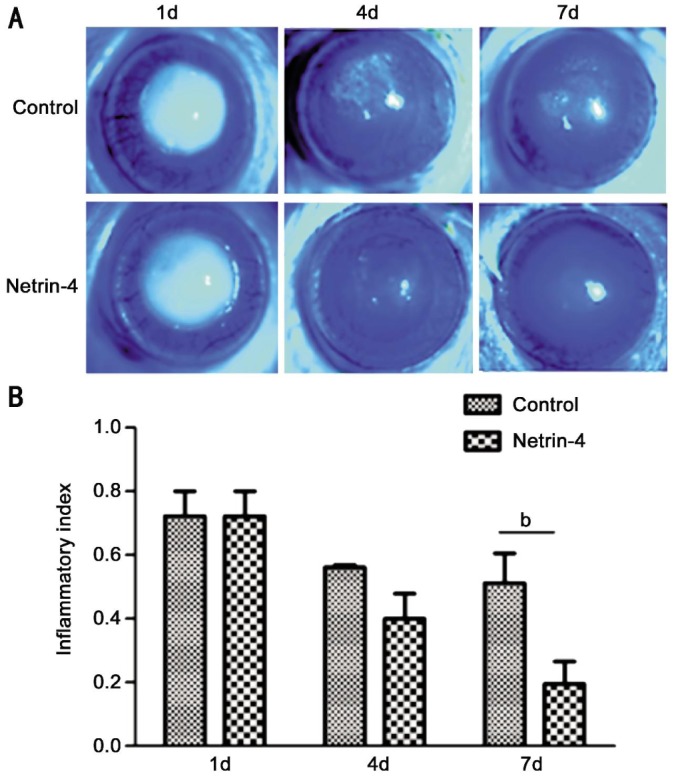
A: Fluorescein staining showed central corneal epithelial defects at day 1 after the alkali burns, and there was gradual decrease of the epithelial defect areas at day 4 and 7 in both groups. The epithelial defects were completely healed on day 7, if the corneas were treated with netrin-4, while the corneas treated with PBS did not heal. Images at different time points are sequential pictures from the same rat eye treated with either PBS or netrin-4. B: The inflammatory index of the ocular surface declined from day 1 to 7 in both groups. However, it was significantly lower in eyes treated with netrin-4 at day 7 (bP<0.01). The figure is a summary of three experiments with n=3 corneas per time point per group per experiment.
Effect of Local Administration of Netrin-4 on Corneal Inflammatory Index
Alkali burn can cause severe corneal inflammation. One day after the alkali burns, the central stroma of the rat cornea appeared opaque and edematous (Figure 1A). In the PBS group, the central cornea maintained opaque appearance and there was scar formation on day 7 (Figure 1A). However, there was only mild edema and no scar formation in corneas treated with netrin-4 for 7d (Figure 1A). The inflammatory index showed slight decrease from day 1 to 7 in PBS group, while there was dramatic reduction in the netrin-4 group, and there was significant difference between the two groups at day 7 (Figure 2B).
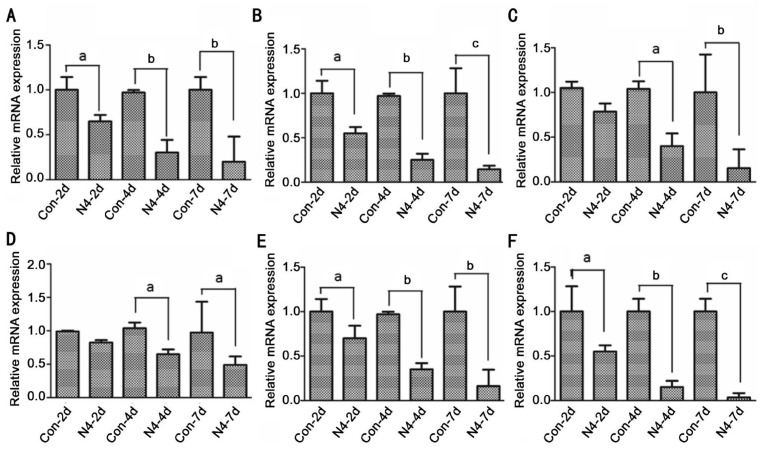
Figure 2. Netrin-4 inhibits cornea inflammation, adhesion and chemotactic factors induced by alkali-burn.
Corneal inflammation evaluated by real-time PCR for the mRNA expression of IL-1β (A), IL-6 (B), MIP-1 (C), MCP-1 (D), ICAM-1 (E) and VACM-1 (F) in the corneas of each group. The figure is a summary of three experiments with n=3 corneas per time point per group per experiment. Data are shown as mean±SD. aP<0.05, bP<0.01, cP<0.001. Scale bar: 20 mm.
Effect of Netrin-4 on Corneal Inflammation Factors mRNA Expression
To evaluate the effect of netrin-4 on the corneal inflammation factors mRNA expression caused by alkali-burn after day 2, 4 and 7, real-time PCR for the levels of proinflammatory cytokines, chemokines, and adhesion molecules in the corneas were performed. The data of real-time PCR revealed the increases of mRNA expression of IL-1β, IL-6, intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), monocyte chemotactic protein-1 (MCP-1) and macrophage inflammatory protein-1 (MIP-1) in the alkali burn-induced corneas, which were dramatically decreased after the netrin-4 treatment after day 2, 4 and 7 induced by alkali-burn (Figure 2).
Effect of Local Administration of Netrin-4 on Corneal Inflammatory Cell Infiltration after Corneal Alkali Burns
To further illustrate the mechanism that netrin-4 inhibits corneal inflammation, we conducted immunostaining of CD45 antibodies to detect macrophage infiltration after alkali burns after day 7. No macrophages were infiltrated in the normal corneal stroma, while CD45 positive cells were increased significantly in the control group (Figure 3A-C). However, there were less CD45 positive cells in netrin-4-treated groups (Figure 3E). The CD45 positive cells quantification showed a significant difference between groups at day 7 (Figure 3G).
Figure 3. Netrin-4 inhibits cornea inflammatory cell infiltration after corneal alkali burns.
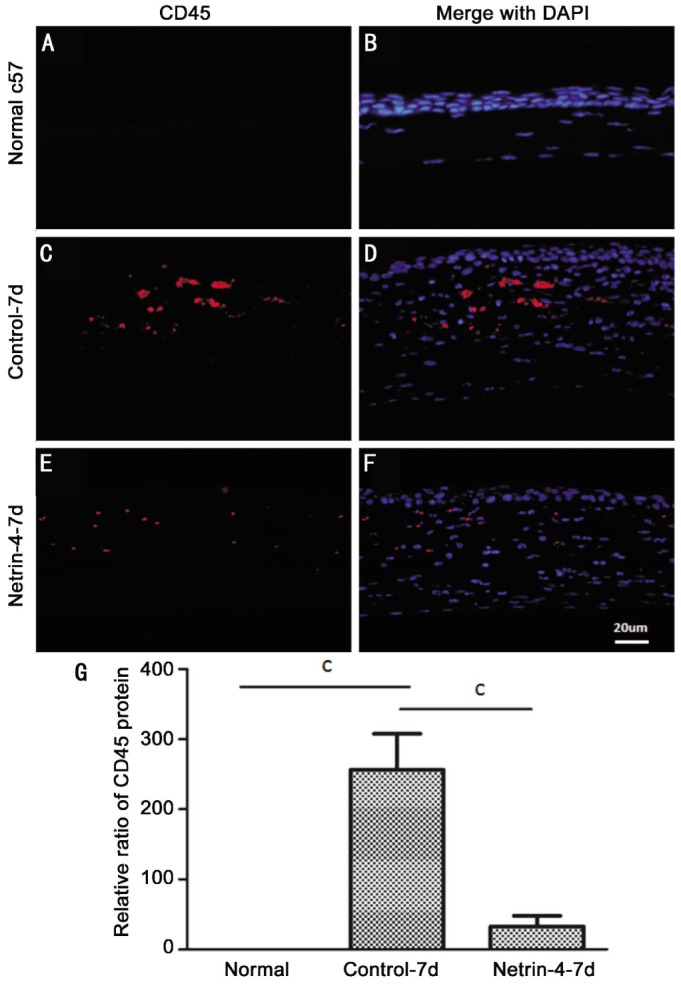
Corneal inflammation evaluated by immunofluorescent staining of CD45 for infiltrating macrophages in the corneal stroma (as red indicated) of the normal c57 (A), control group (C) and netrin-4-treated (E) groups. G: Six representative slices from homologous positions of each sample were selected (three samples for each group). Data are shown as mean±SD. cP<0.001. Scale bar: 20 µm.
DISCUSSION
The corneal epithelium plays important roles in the maintenance of corneal function and integrity. Corneal epithelial defects resulting from corneal injury such as a chemical burn may heal inappropriately and lead to corneal opacification, neovascularization, infection, and visual loss[33]. Alkali burn can cause severe acute corneal inflammation. For the first time, our study evaluated the effects of netrin-4 using an in vivo acute wounding model, in the early stage of the rat corneal alkali burns, which presents severe inflammation. We found that netrin-4 could reduce corneal inflammation, and meanwhile, promote wound healing in corneal alkali burns.
It has been recognized widely that the ocular surface inflammation has a critical role in the pathogenesis of alkali-burn eye. Increased proinflammatory cytokines and inflammatory cell infiltration are found in the ocular surface and lacrimal tissues of alkali-burn patients. Therefore, the efficacy of a variety of anti-inflammatory agents for the treatment of alkali-burn disease has been evaluated. To this day, the standard anti-inflammatory treatment of ocular inflammation, despite its many side effects, remains corticosteroid therapy, and has been proven to decrease the production of inflammatory cytokines, chemokines[34]. In the alkali burn-induced corneal acute inflammatory model, the expression of pro-inflammatory cytokines IL-1β, IL-6, chemokines MCP-1, MIP-1, adhesion molecules ICAM-1, and VCAM-1, as well as infiltration of CD45-positive macrophages in the corneas were elevated, mimicking the inflammatory features of human alkali-burn eye. After the netrin-4 treatments, the expression of proinflammatory cytokines, chemokines, and macrophage infiltration were decreased.
Our study clearly demonstrated that netrin-4 could dampen alkali burn-induced the early acute phase of inflammation. Netrin-4 strongly inhibits macrophage infiltration in the intermediate phase of the wounding. Macrophages act in concert with neutrophils to phagocytose debris and invading pathogenic microorganisms and are a source of growth factors that promote resolution of inflammation as well as cell migration and proliferation for wound healing. Macrophages also secrete abundant inflammatory cytokines, chemokines which contribute to scar formation of the wounded tissue. Therefore, exaggerated or constant influx and presence of macrophage is detrimental[36],[37]. In our study, macrophage infiltration was significantly reduced approximately 7d post injury, which may have a major impact on the resolution of corneal inflammation after alkali burns. Consistent with another netrins family member netrin-1in other acute inflammation models in tissues such as the cornea[31], colon[32], lungs[33]–[35], or kidneys[36]. Recently, netrin-1 was found to be a negative guidance cue for leukocyte migration[33],[35], which indicates the anti-inflammatory potential of netrin-1. Several in vivo studies have been conducted to evaluate the effect of netrin-1 on animal disease models, such as subcutaneous application for experimental colitis[32], intraperitoneal injection for peritonitis[37], and inhalation or intravenous injection for lipopolysaccharide-induced pulmonary injury[34]. These studies have shown the potent effect of netrin-1 on reducing neutrophil infiltration, while the mechanism is variously dependent on receptor A2BAR[32],[34],[37] or UNC5B[35].
In summary, our study clearly demonstrated that exogenous netrin-4 application to the ocular surface could dampen inflammation, and accelerate epithelial wound healing of alkali burn-induced corneal damage. The effects of netrin-4 on the entire orchestration of the disease mechanisms need to further investigate. The multifunction features of netrin-4 may shed new light on the treatment of inflammatory disease of ocular surface as well as other organs.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81300729, No.81160118); the Clinical Medicine Research Special Purpose Foundation of China (No.L2012052); Education Department Youth Scientific Research Foundation of Jiangxi Province (No.GJJ14170); the Shanhai Foundation of Xiamen University (No.2013SH008)
Conflicts of Interest: Han Y, None; Shao Y, None; Liu TT, None; Li SM, None; Li W, None; Liu ZG, None.
REFERENCES
- 1.Liu X, Lin Z, Zhou T, Zong R, He H, Liu Z, Ma JX, Liu Z, Zhou Y. Anti-angiogenic and anti-inflammatory effects of SERPINA3K on corneal injury. PLoS One. 2011;6(1):e16712. doi: 10.1371/journal.pone.0016712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saika S, Miyamoto T, Yamanaka O, Kato T, Ohnishi Y, Flanders KC, Ikeda K, Nakajima Y, Kao WW, Sato M, Muragaki Y, Ooshima A. Therapeutic effect of topical administration of SN50, an inhibitor of nuclear factor-kappaB, in treatment of corneal alkali burns in mice. Am J Pathol. 2005;166(5):1393–1403. doi: 10.1016/s0002-9440(10)62357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen M, Matsuda H, Wang L, Watanabe T, Kimura MT, Igarashi J, Wang X, Sakimoto T, Fukuda N, Sawa M, Nagase H. Pretranscriptional regulation of Tgf-beta1 by PI polyamide prevents scarring and accelerates wound healing of the cornea after exposure to alkali. Mol Ther. 2010;18(3):519–527. doi: 10.1038/mt.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78(3):409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 5.Cirulli V, Yebra M. Netrins: beyond the brain. Nat Rev Mol Cell Biol. 2007;8(4):296–306. doi: 10.1038/nrm2142. [DOI] [PubMed] [Google Scholar]
- 6.Dumartin L, Quemener C, Laklai H, Herbert J, Bicknell R, Bousquet C, Pyronnet S, Castronovo V, Schilling MK, Bikfalvi A, Hagedorn M. Netrin-1 mediates early events in pancreatic adenocarcinoma progression, acting on tumor and endothelial cells. Gastroenterology. 2010;138(4):1595–1606, 1606e1–1606e8. doi: 10.1053/j.gastro.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 7.Koch M, Murrell JR, Hunter DD, Olson PF, Jin W, Keene DR, Brunken WJ, Burgeson RE. A novel member of the netrin family, beta-netrin, shares homology with the beta chain of laminin: identification, expression, and functional characterization. J Cell Biol. 2000;151(2):221–234. doi: 10.1083/jcb.151.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin Y, Sanes JR, Miner JH. Identification and expression of mouse netrin-4. Mech Dev. 2000;96(1):115–119. doi: 10.1016/s0925-4773(00)00369-5. [DOI] [PubMed] [Google Scholar]
- 9.Lejmi E, Leconte L, Pedron-Mazoyer S, Ropert S, Raoul W, Lavalette S, Bouras I, Feron JG, Maitre-Boube M, Assayag F, Feumi C, Alemany M, Jie TX, Merkulova T, Poupon MF, Ruchoux MM, Tobelem G, Sennlaub F, Plouet J. Netrin-4 inhibits angiogenesis via binding to neogenin and recruitment of Unc5B. Proc Natl Acad Sci U S A. 2008;105(34):12491–12496. doi: 10.1073/pnas.0804008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez-Bolado G, Eichele G. Analysing the developing brain transcriptome with the GenePaint platform. J Physiol. 2006;575(Pt2):347–352. doi: 10.1113/jphysiol.2006.112763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoang S, Liauw J, Choi M, Choi M, Guzman RG, Steinberg GK. Netrin-4 enhances angiogenesis and neurologic outcome after cerebral ischemia. J Cereb Blood Flow Metab. 2009;29(2):385–397. doi: 10.1038/jcbfm.2008.128. [DOI] [PubMed] [Google Scholar]
- 12.Lambert E, Coissieux MM, Laudet V, Mehlen P. Netrin-4 acts as a pro-angiogenic factor during zebrafish development. J Biol Chem. 2012;287(6):3987–3999. doi: 10.1074/jbc.M111.289371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange J, Yafai Y, Noack A, Yang XM, Munk AB, Krohn S, Iandiev I, Wiedemann P, Reichenbach A, Eichler W. The axon guidance molecule netrin-4 is expressed by Muller cells and contributes to angiogenesis in the retina. Glia. 2012;60(10):1567–1578. doi: 10.1002/glia.22376. [DOI] [PubMed] [Google Scholar]
- 14.Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, Urness LD, Suh W, Asai J, Kock GA, Thorne T, Silver M, Thomas KR, Chien CB, Losordo DW, Li DY. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313(5787):640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nacht M, St Martin TB, Byrne A, Klinger KW, Teicher BA, Madden SL, Jiang Y. Netrin-4 regulates angiogenic responses and tumor cell growth. Exp Cell Res. 2009;315(5):784–794. doi: 10.1016/j.yexcr.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Eveno C, Contreres JO, Hainaud P, Nemeth J, Dupuy E, Pocard M. Netrin-4 overexpression suppresses primary and metastatic colorectal tumor progression. Oncol Rep. 2013;29(1):73–78. doi: 10.3892/or.2012.2104. [DOI] [PubMed] [Google Scholar]
- 17.Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Breant C, Claes F, De Smet F, Thomas JL, Autiero M, Carmeliet P, Tessier-Lavigne M, Eichmann A. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432(7014):179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Stein E, Oliver T, Li Y, Brunken WJ, Koch M, Tessier-Lavigne M, Hogan BL. Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr Biol. 2004;4(10):897–905. doi: 10.1016/j.cub.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, Murphy KJ, Kuo CJ, Li DY. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci U S A. 2004;101(46):16210–16215. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneiders FI, Maertens B, Bose K, Li Y, Brunken WJ, Paulsson M, Smyth N, Koch M. Binding of netrin-4 to laminin short arms regulates basement membrane assembly. J Biol Chem. 2007;282(33):23750–23758. doi: 10.1074/jbc.M703137200. [DOI] [PubMed] [Google Scholar]
- 21.Latil A, Chene L, Cochant-Priollet B, Mangin P, Fournier G, Berthon P, Cussenot O. Quantification of expression of netrins, slits and their receptors in human prostate tumors. Int J Cancer. 2003;103(3):306–315. doi: 10.1002/ijc.10821. [DOI] [PubMed] [Google Scholar]
- 22.Esseghir S, Kennedy A, Seedhar P, Nerurkar A, Poulsom R, Reis-Filho JS, Isacke CM. Identification of NTN4, TRA1, and STC2 as prognostic markers in breast cancer in a screen for signal sequence encoding proteins. Clin Cancer Res. 2007;13(11):3164–3173. doi: 10.1158/1078-0432.CCR-07-0224. [DOI] [PubMed] [Google Scholar]
- 23.Larrieu-Lahargue F, Welm AL, Thomas KR, Li DY. Netrin-4 induces lymphangiogenesis in vivo. Blood. 2010;115(26):5418–5426. doi: 10.1182/blood-2009-11-252338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li YN, Pinzon-Duarte G, Dattilo M, Claudepierre T, Koch M, Brunken WJ. The expression and function of netrin-4 in murine ocular tissues. Exp Eye Res. 2012;96(1):24–35. doi: 10.1016/j.exer.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yebra M, Diaferia GR, Montgomery AM, Kaido T, Brunken WJ, Koch M, Hardiman G, Crisa L, Cirulli V. Endothelium-derived Netrin-4 supports pancreatic epithelial cell adhesion and differentiation through integrins alpha2beta1 and alpha3beta1. PLoS One. 2011;6(7):e22750. doi: 10.1371/journal.pone.0022750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Planck SR, Rich LF, Ansel JC, Huang XN, Rosenbaum JT. Trauma alkali burns induce distinct patterns of cytokine gene expression in the rat cornea. Ocul Immunol Inflamm. 1997;5(2):95–100. doi: 10.3109/09273949709085057. [DOI] [PubMed] [Google Scholar]
- 27.Laria C, Alio JL, Ruiz-Moreno JM. Combined non-steroidal therapy in experimental corneal injury. Ophthalmic Res. 1997;29(3):145–153. doi: 10.1159/000268009. [DOI] [PubMed] [Google Scholar]
- 28.Reinach PS, Pokorny KS. The corneal epithelium: clinical relevance of cytokine-mediated responses to maintenance of corneal health. Arq Bras Oftalmol. 2008;71(6Suppl):80–86. doi: 10.1590/s0004-27492008000700016. [DOI] [PubMed] [Google Scholar]
- 29.Aksoy MO, Li X, Borenstein M, Yi Y, Kelsen SG. Effects of topical corticosteroids on inflammatory mediator-induced eicosanoid release by human airway epithelial cells. J Allergy Clin Immunol. 1999;103(6):1081–1091. doi: 10.1016/s0091-6749(99)70183-1. [DOI] [PubMed] [Google Scholar]
- 30.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330(6002):362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 31.Han Y, Shao Y, Lin Z, Qu YL, Wang H, Zhou Y, Chen W, Chen Y, Chen WL, Hu FR, Li W, Liu Z. Netrin-1 simultaneously suppresses corneal inflammation and neovascularization. Invest Ophthalmol Vis Sci. 2012;53(3):1285–1295. doi: 10.1167/iovs.11-8722. [DOI] [PubMed] [Google Scholar]
- 32.Aherne CM, Collins CB, Masterson JC, Tizzano M, Boyle TA, Westrich JA, Parnes JA, Furuta GT, Rivera-Nieves J, Eltzschig HK. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut. 2012;61(5):695–705. doi: 10.1136/gutjnl-2011-300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10(2):195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 34.Mirakaj V, Thix CA, Laucher S, Mielke C, Morote-Garcia JC, Schmit MA, Henes J, Unertl KE, Kohler D, Rosenberger P. Netrin-1 dampens pulmonary inflammation during acute lung injury. Am J Respir Crit Care Med. 2010;181(8):815–824. doi: 10.1164/rccm.200905-0717OC. [DOI] [PubMed] [Google Scholar]
- 35.Ly NP, Komatsuzaki K, Fraser IP, Tseng AA, Prodhan P, Moore KJ, Kinane TB. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci U S A. 2005;102(41):14729–14734. doi: 10.1073/pnas.0506233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tadagavadi RK, Wang W, Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J Immunol. 2010;185(6):3750–3758. doi: 10.4049/jimmunol.1000435. [DOI] [PubMed] [Google Scholar]
- 37.Mirakaj V, Gatidou D, Potzsch C, Konig K, Rosenberger P. Netrin-1 signaling dampens inflammatory peritonitis. J Immunol. 2011;186(1):549–555. doi: 10.4049/jimmunol.1002671. [DOI] [PubMed] [Google Scholar]