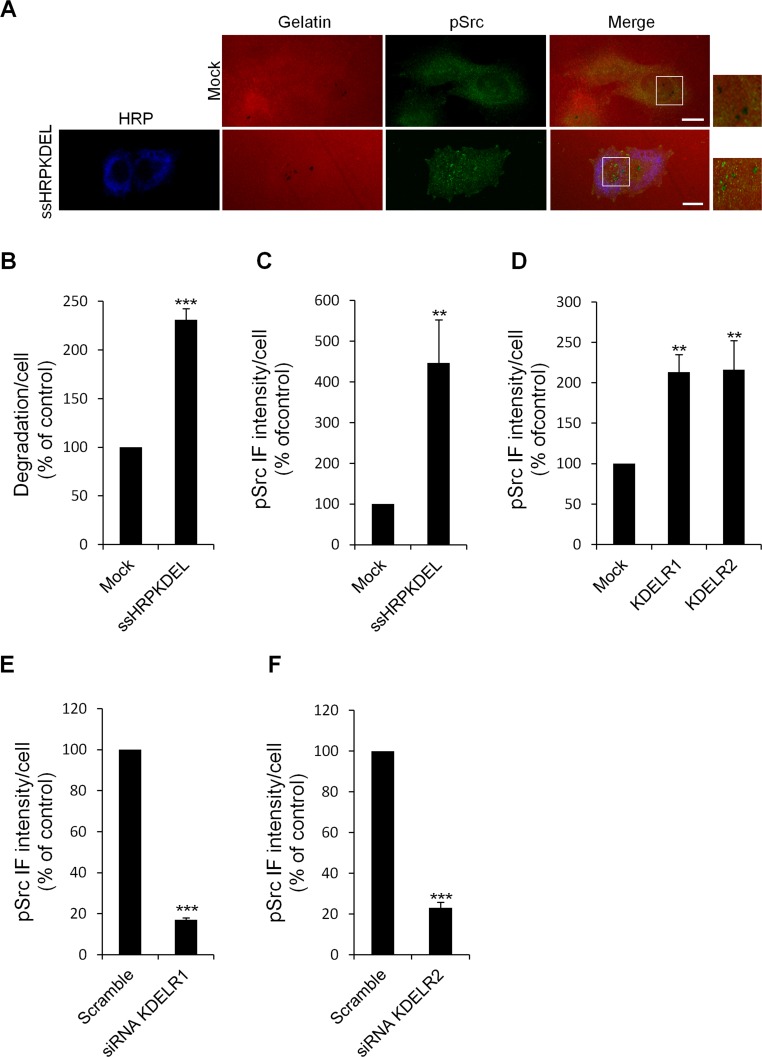
Figure 6. KDELR stimulation increases the levels of active Src in the cell regions overlapping the ECM degradation patches.
(A) A375MM cells were transfected with the empty vector (Mock) or ssHRPKDEL and grown on rhodamine-conjugated crosslinked gelatine (red) for 16 h in the presence of BB94. Following BB94 wash-out, the cells were incubated for a further 3 h, and then fixed and stained for pSrc (pTyr 419, green). An anti-HRP antibody (blue) was used to visualise ssHRPKDEL-transfected cells. Merged images of red and green (Mock) and red, green and blue signals (ssHRPKDEL) are also shown (Merge). pSrc immunofluorescence overlapping the degradation patches are shown in the enlargements of the boxed regions (small right panels: red and green signals). Scale bars, 10 μm. The images are representative of three independent experiments. (B) Quantification of the degradation area per cell. Data are degradation area per cell (% of control), as means ±SEM of three independent experiments, with at least 100 cells quantified per experiment. (C) Quantification of pSrc immunofluorescence levels in the cell regions overlapping the ECM degradation patches. Data are means ±SEM of pSrc immunofluorescence per cell (% of control), from three independent experiments, with at least 100 cells quantified per experiment.. (D) A375MM cells were transfected with the empty vector (Mock) or the myc-tagged KDELR isoforms, KDELR1 and KDELR2, and grown on rhodamine-conjugated crosslinked gelatine for 16 h in the presence of BB94. Following BB94 wash-out, the cells were incubated for a further 3 h, and then fixed and labelled for pSrc. Data are means ±SEM, as indicated for C. (E-F) A375MM cells were treated without (Scramble) or with siRNA targeting KDELR1 or KDELR2 (siRNA KDELR) for 96 h. Seventy-two hours post interference, the cells were plated for 24 h on rhodamine-conjugated gelatine in the presence of BB94. Following the wash-out of the BB94, the cells were incubated for a further 3 h, then fixed and labelled for pSrc. Data are means ±SEM, as indicated for C. (B-E) ***p <0.001, **p <0.01 compared to Mock cells (t-test).