Abstract
Over the past 10 years, researchers have studied the effects of recreational football training as a health-promoting activity for participants across the lifespan. This has important public health implications as over 400 million people play football annually. Results from the first randomised controlled trial, published in the BJSM in January 2009, showed that football increased maximal oxygen uptake and muscle and bone mass, and lowered fat percentage and blood pressure, in untrained men, and since then more than 70 articles about football for health have been published, including publications in two supplements of the Scandinavian Journal of Medicine and Science in Sports in 2010 and 2014, prior to the FIFA World Cup tournaments in South Africa and Brazil. While studies of football training effects have also been performed in women and children, this article reviews the current evidence linking recreational football training with favourable effects in the prevention and treatment of disease in adult men.
The physiology of recreational football—why might training confer health benefits?
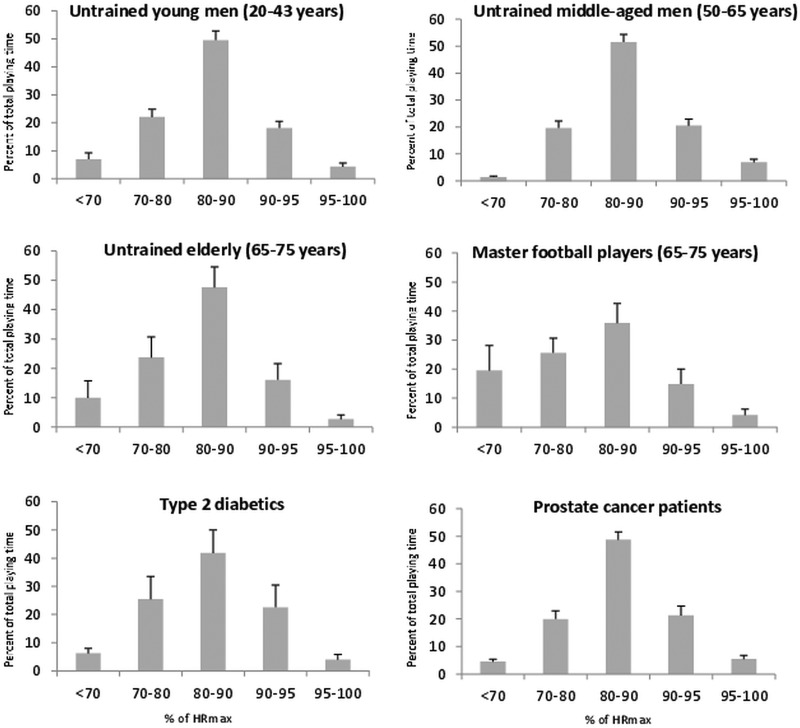
Recreational football training conducted as small-sided games (4v4 to 6v6) has broad-ranging physiological effects, with more pronounced changes achieved than through recreational running, interval running and fitness training.1–4 Its marked effect on the cardiovascular system may, in part, be a result of average heart rates being around 80% of maximal heart rate (HRmax) during training, with substantial time spent at 80–90% and above 90% HRmax during a 1 h training session, irrespective of age, fitness status and previous experience of football training (figure 1).
Figure 1.
Heart rate distribution, expressed as a percentage of maximum heart rate, during football training consisting of small-sided games for various study groups. Data are presented as means±SEM. HRmax, maximal heart rate.
Notably, overweight men with type 2 diabetes mellitus (T2DM), 65–75-year-old men with no prior experience of football and men with prostate cancer, were all able to perform football training with much time spent above 80% HRmax.5–7 These groups carried out the training at an intensity as high as lifelong-trained veteran (masters) football players.8 Generally, the participants conducted more than 100 high-intensity runs and specific intense actions such as dribbles, shots, tackles, turns and jumps per training session. Importantly, despite the high heart rates during training, recreational football training had the lowest score in perceived exertion (3.9 of 10) in comparison with other activities such as jogging, interval running and fitness training.9 10 This may be one reason why participants in the training studies usually found the game enjoyable and maintained their interest in football training even after the intervention study period was over.11–13
Cardiovascular effects of recreational football
Blood pressure and heart rate at rest
Many studies have shown that a period of recreational football training lowers blood pressure in normotensive untrained participants (table 1). Systolic blood pressure in middle-aged men was typically reduced by 7–8 mm Hg after a 3-month training period, higher than the 3–4 mm Hg reduction often seen with other types of exercise modalities with the same duration and frequency.14 Also, diastolic pressure was lowered (5–7 mm Hg) significantly after a period of recreational football (table 1). It should be noted, however, that in some studies, blood pressure was not reduced by a period of football training, which may be due, in part, to the inclusion of healthy participants with low baseline values in these studies (table 1).
Table 1.
Changes in cardiovascular variables in untrained men as a result of a period of recreational F training compared to R or inactive C
| Study | Activity, target group, gender | Age (years) | Training intervention; Duration (weeks), intensity (%), frequency (per week), session duration (min) |
VO2max (mL/kg/min or %) | VO2max (L/min or %) | HR sub-max (bpm) | HR rest (bpm) | BPsys rest, (mmHg) | BPdia rest, (mmHg) | MAP rest (mmHg) | RV systolic function, TAPSE (cm) | LV diastolic function, E/A ratio (%) | Arterial stiffness (AI) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Krustrup et al1
2
10 |
F, UT, M, | 29 | 12; 82%HRmax; 2.3; 60 | 13%↑* | 11%↑* | 20↓*† | 6↓* | 8↓* | 5↓* | 6↓* | – | – | – |
| R, UT, M | 31 | 12; 82%HRmax; 2.5; 60 | 8%↑* | 7%↑* | 21↓*† | 6↓* | 7↓* | 5↓* | 6↓* | – | – | – | |
| C, UT, M | 31 | No intervention | 1%↓NS | 1%↓NS | 0↔NS | 1↑NS | 2↓NS | 2↑NS | 1↑NS | – | – | – | |
| Randers et al8 41 | F, UT, M | 31 | 64; 82%HRmax; 1.3; 60 | 8%↑* | 6%↑* | 22↓*† | 8↓*‡ | 8↓*‡ | 3↓NS | 5↓* | – | – | – |
| C, UT, M | 32 | No intervention | – | – | – | 2↓NS | 2↓NS | 3↑NS | 1↑NS | – | – | – | |
| Schmidt et al26 | F, UT, M | 68 | 52; 82%HRmax; 1.7; 45–60 | – | 18%↑*‡ | – | 8↓* | NS | NS | NS | 0.5↑*‡ | 25%↑*‡ | – |
| S, UT, M | 69 | 52; 8–20RM; 1.9; 45–60 | – | 3%↑NS | – | 2↓NS | NS | NS | NS | 0.0↔NS | NS | – | |
| C, UT, M | 67 | No intervention | – | 1%↑NS | – | 2↓NS | NS | NS | NS | 0.2 ↓NS | NS | – | |
| Andersen et al6 | F, UT, M | 68 | 16; 84%HRmax; 1.6; 45–60 | 3.8↑* | – | 8↓* | – | – | – | – | – | – | – |
| S, UT, M | 69 | 16; 8–20RM; 1.5; 45–60 | 0.8↑NS | – | 9↓NS | – | – | – | – | – | – | – | |
| C, UT, M | 67 | No intervention | 0.7↓NS | 7↓NS | |||||||||
| Krustrup et al15 | F, UT, Ma, | 46 | 24; 85%HRmax; 1.7; 60 | 8%↑* | – | 12↓NS | 8↓* | 13↓*‡ | 8↓*‡ | 10↓*‡ | – | – | 7%↓* |
| DAG, UT, Ma | 47 | DA on CRF | 3%↓NS | – | 4↓NS | 3↓NS | 8↓* | 3↓* | 5↓* | – | – | ↔NS | |
| Andersen et al6 | F, UT, Ma | 46 | 24; 83%HRmax; 1.7; 60 | 8%↑* | – | – | 8↓* | – | – | 10↓*‡ | 0.4↑*‡ | 34%↑*‡ | – |
| DAG, UT, Ma | 47 | DA on CRF | 2%↓NS | – | – | 3↓NS | – | – | 5↓* | NS | NS | – | |
| Knoepfli-Lenzin et al22 | F, UT, Mmh | 37 | 12; 80%HRmax; 2.4; 60 | 9%↑* | 6%↑* | – | 7↓* | 11↓* | 9↓*‡ | 10↓*† | – | – | – |
| R, UT, Mmh | 36 | 12; 79%HRmax; 2.5; 60 | 12%↑* | 11%↑* | – | 9↓* | 7↓* | 6↓* | 6↓* | – | – | – | |
| C, UT, Mmh | 38 | No intervention | 1%↑NS | 1%↑NS | – | 6↓* | 7↓* | 4↓* | 6↓* | – | – | – | |
| Schmidt et al17 | F, UT, Mt | 51 | 24; 82%HRmax; 1.2; 60 | 12%↑* | 11%↑* | – | 6↓NS | 9↓* | 8↓* | 8↓* | 0.4↑*‡ | 18%*↑ | 0.1%↑NS |
| C, UT, Mt | 49 | No intervention | 2%↑NS | 2%↑NS | – | 2↑NS | 3↑NS | 0↔NS | 1↑NS | 0.1↓NS | NS | 0.6%↑NS | |
| De Sousa et al39 | F, UT, Mt+Wt | 61 | 12; 83%HRmax; 3; 40; F+D | 10%↑*‡ | – | – | – | – | – | – | – | – | – |
| C, UT, Mt+Wt | 61 | Diet group | 3%↓ NS | – | – | – | – | – | – | – | – | – | |
| Uth et al37 | F, UT, Mp | 67 | 12; 85%HRmax; 2–3; 45–60 | 1.5↑* | – | – | – | – | – | – | – | – | – |
| C, UT, Mp | 67 | No intervention | 0.3↑ NS | – | – | – | – | – | – | – | – | – | |
| Faude et al56 | F, UT, OCh | 11 | 12; 80%HRmax; 4.5; 60 | 7%↓NS | 5%↓NS | 7↓* | – | – | – | – | – | – | – |
| SP, UT, OCh | 11 | 12; 77%HRmax; 4.5; 60 | 7%↓NS | 3%↓NS | 7↓* | – | – | – | – | – | – | – | |
| Hansen et al55 | F, UT, OCh | 10 | 12; >80%HRmax; 4; 60–90 | – | – | – | – | – | – | – | 0.26↑*‡ | NS | 1.9%↑NS |
| C, UT, OCh | 11 | No intervention | – | – | – | – | – | – | – | NS | NS | 2.7%↓NS |
Changes between pretraining and post-training intervention (unless otherwise stated).
*Significant difference from 0 weeks.
†Significant group difference compared to control.
‡Significant group difference.
a, hypertensive participants; AI, augmentation index; BPdia, diastolic blood pressure; BPsys, systolic blood pressure; C, controls; CRF, cardiovascular risk factors; DAG, doctor's advice group; E/A ratio, ratio of early (E) to late (A) ventricular filling velocities; F, football; F+D, football + diet group; HRmax, maximal heart rate; LV left ventricular; M, men; MAP, mean arterial pressure; mh, mildly hypertensive participants; NS, not significant; OC, overweight children; p, prostate cancer patients; R, running; RV, right ventricular; S, strength training; SP, standard physical activity; t, type 2 diabetics; TAPSE, tricuspid annular plane systolic excursion; UT, untrained; W, women.
Recreational football training lowers blood pressure remarkably in patients with hypertension. Thus, football training twice a week for 24 weeks led to men's systolic blood pressure falling from 151 to 139 mm Hg, and diastolic pressure, from 92 to 84 mm Hg.15 Three quarters of the participants reached systolic and diastolic blood pressure values below 140 and 90 mm Hg, respectively.
Football training also lowers blood pressure in patients with T2DM. Approximately 80% of patients with T2DM are hypertensive, which nearly doubles the risk of adverse cardiovascular events.16 In patients with T2DM, systolic and diastolic blood pressure was reduced by 9 and 8 mm Hg, respectively, through 12 weeks of football training, with no further change in the following 12 weeks of football training.17 These reductions in blood pressure are more pronounced than those reported for other exercise interventions with hypertensive and patients with T2DM, where reductions in resting mean blood pressure of 3–5 mm Hg are observed after 3 months of training and compares favourably with commonly used medication such as β-blockers.18 19
In addition, in a randomised study of prostate cancer, patients receiving androgen deprivation therapy (gonadotropin-releasing hormone agonists with or without anti-androgens), systolic and diastolic blood pressures were 3 mm Hg lower after 12 weeks of football training. This was, however, not significantly different from changes in the control group.7 The lack of change in blood pressure observed in these patients, where approximately 50% received antihypertensive therapy and most had been treated for prostate cancer for more than 3 years, may have been due to bias associated with low blood pressures and optimal blood pressure control at baseline owing to long-term medical surveillance of cardiovascular risk factors associated with androgen deprivation therapy.20
The mechanisms behind the larger blood pressure reduction after a period of recreational football training compared with other training modalities are not clear. In almost all studies, heart rate at rest was markedly lowered (4–12 bpm) after a period of football training (table 1), probably mediated by an augmented stroke volume (see below) and modulation of the autonomous nervous system with an increase in parasympathetic activity.21 22 It is unclear, however, whether cardiac output was reduced.
Vascular tone and total peripheral resistance may have been lowered by a reduced sympathetic drive accompanying the period of football training. Although physical exercise can improve endothelium-dependent vasodilation in large conduit arteries, the effects on microvascular endothelial function, a primary determinant of peripheral vascular resistance, are less clear.23–25 Indeed, in four of the more recent football training studies of men with hypertension, T2DM or prostate cancer with androgen deprivation therapy and elderly male participants, there was no change in microvascular reactive hyperaemic index, a measure of microvascular endothelial function determined by peripheral arterial tonometry.15 26
On the other hand, a cross-sectional comparison of veteran football players and untrained elderly healthy men showed a higher reactive hyperaemic index in the football group, while arterial stiffness measured by the augmentation index was reduced by football training in middle-aged hypertensive men.15 27 Furthermore, in men with T2DM, football training was associated with an increased number of capillaries around type I striated skeletal muscle fibres.5 It is therefore possible that reduced arterial stiffness and an increased microvascular bed contribute to blood pressure-lowering effects, but the mechanisms by which football training may reduce arterial blood pressure more than other training modalities warrant further study. Altogether, the pronounced drops in blood pressure and resting heart rate observed in a range of studies are important markers of improved cardiovascular health profile in sedentary individuals. Resting heart rate is an independent risk factor for cardiovascular disease in healthy participants and in patients with established diseases, for example, T2DM and hypertension.28–30
Heart structure and function
Recreational football training has significant effects on myocardial structure and function at rest, as determined by comprehensive transthoracic echocardiography using tissue Doppler, speckle tracking and strain rate analyses (table 1). For example, there were considerable improvements in variables associated with left ventricular systolic and diastolic function, and right ventricular systolic function, after a period of football training in untrained middle-aged hypertensive men, men with T2DM and elderly men.5 17 26 Left ventricular end-diastolic volume was also increased, which, in view of unaltered or increased left ventricular systolic function, suggests increased stroke volume. Interestingly, there were no changes in echocardiographic parameters after football training in patients with prostate cancer undergoing androgen deprivation therapy, raising the intriguing possibility that the latter may counteract the favourable effects of football training on the heart.7
Notably, several of the changes observed after football training, for example, enhanced diastolic function, have not been found in selected studies with other training modalities, for example, cycling, strength training, jogging and walking, suggesting that football may be a more powerful stimulus.31–33 Interestingly, in participants with T2DM, high-intensity interval cycle training improved diastolic function much more than medium-intensity cycle training,34 and it is likely that the underlying mechanisms are similar to the mechanisms behind the marked effects of football training, which includes numerous intense activity periods interspersed by periods with low-intensity activity.
Notwithstanding these considerations, subclinical cardiac systolic and diastolic dysfunction measured by these echocardiographic variables have been associated with increased mortality, and it remains to be determined whether the favourable changes demonstrated after football training, if maintained over time, can improve major end points such as myocardial infarction, stroke or death.35 36
Maximum oxygen uptake
Regular recreational football training increases maximum oxygen uptake (VO2max) in previously untrained participants. Most studies have shown 7–15% increases in VO2max after 12–24 weeks of training, which is comparable to or higher than observed in investigations with running and cycling (table 1). Even more pronounced effects were observed in 65–75-year-old men, that is, 16% and 18% increases in VO2max after 16 and 54 weeks of football training, respectively, which may be related to the low baseline levels.26
In men with T2DM, VO2max was also higher (11%) after 24 weeks of football training, and in hypertensive participants football training increased VO2max by 8% after 3 months of training.15 17 A smaller within-group increase of 4% was observed for a group of patients with cancer conducting 45–60 min football training sessions two to three times a week for 12 weeks, and no significant between-group effect was found.37
Effect of recreational football on blood lipid profile and body composition
Blood lipid profile
A typical finding, though not always significant, is that total plasma cholesterol and low-density lipoprotein (LDL) cholesterol are lower after a period of recreational football training (table 2). For example, in young and middle-aged men, training for 12 weeks led to a significant 15% decrease in LDL cholesterol and a non-significant 8% increase in high-density lipoprotein cholesterol levels.1 In addition, LDL cholesterol levels were lower by 13% in young and middle-aged homeless men playing street football for 3×40 min for 12 weeks.38 These changes may add to the aforementioned favourable cardiovascular effects of football training to reduce the risk of future cardiovascular diseases.14 Also, patients with T2DM aged 48–68 years lowered their total cholesterol and LDL cholesterol levels when combining 3×40 min football sessions a week for 12 weeks with a calorie-restricted diet.39
Table 2.
Changes in blood lipids in untrained men as a result of a period of recreational F training compared to R or inactive C
| Study | Activity, target group, gender | Age (years) | Training intervention; Duration (weeks), intensity (%), frequency (per week), session duration (min) |
Total-Chol rest (mmol/l or %) | HDL-Chol rest (mmol/l or %) | LDL-Chol rest (mmol/l or %) |
|---|---|---|---|---|---|---|
| Krustrup et al1 | F, UT, M | 29 | 12; 82%HRmax; 2.3; 60 | 5%↓NS | 8%↑NS | 15%↓*† |
| R, UT, M | 31 | 12; 82%HRmax; 2.5; 60 | 7%↓NS | 8%↑NS | 4%↓NS | |
| C, UT, M | 31 | No intervention | 0%↔NS | 7%↑NS | 0%↔NS | |
| Randers et al8 41 | F, UT, M | 31 | 64; 82%HRmax; 1.3; 60 | 0%↔NS | 8%↑NS | 7%↓NS |
| C, UT, M | 32 | No intervention | 2%↑NS | 0%↔NS | 7%↑NS | |
| Randers et al38 | F, UT, Mh | 36 | 12; 82%HRmax; 2.2; 60 | 0.1↓NS | 0.0↔NS | 0.4↓*NS† |
| C, UT, Mh | 43 | No intervention | 0.1↑NS | 0.1↓NS | 0.1↑NS | |
| Krustrup et al15 | F, UT, Ma | 46 | 24; 85%HRmax; 1.7; 60 | – | 8%↓NS | 9%↓NS |
| DAG, UT, Ma | 47 | DA on CRF | – | 9%↑NS | 9%↑NS | |
| Knoepfli-Lenzin et al22 | F, UT, Mmh | 37 | 12; 80%HRmax; 2.4; 60 | 5%↓* | 8%↑NS | 3%↓NS |
| R, UT, Mmh | 36 | 12; 79%HRmax; 2.5; 60 | 2%↓NS | 0%↔NS | 0↔NS | |
| C, UT, Mmh | 38 | No intervention | 4%↓NS | 8%↑NS | 3%↓NS | |
| Schmidt et al17 | F, UT, Mt | 51 | 24; 82%HRmax; 1.2; 60 | 5%↓NS | 8%↑NS | 11↑NS |
| C, UT, Mt | 49 | No intervention | 8%↑NS | 9%↑NS | 9%↑NS | |
| De Sousa et al39 | F, UT, Mt+Wt | 61 | 12; 83%HRmax; 3; 40; F+D | 0.6↓*† | 0↔NS | 0.4↓*† |
| C, UT, Mt+Wt | 61 | Diet group | 0.4↑NS | 0↔NS | 0.3↑NS |
Changes between pre and post training intervention (unless otherwise stated).
*Significant difference from 0 weeks.
†Significant group difference compared to control.
a, hypertensive participants; C, controls; Chol, cholesterol; CRF; cardiovascular risk factors; DAG, doctor's advice group; F, football; F+D, football+diet group; HDL, high-density lipoprotein; HRmax, maximal heart rate; LDL, low-density lipoprotein; M, men; mh, mildly hypertensive participants; NS, not significant; R, running; t, type 2 diabetics; Total-chol, total plasma cholesterol; UT, untrained; W, women.
Body fat and lean body mass
Regular recreational football training influences body composition (table 3). Loss of body fat in middle-aged men was in the range of 1–3 kg following 3 months of training, corresponding to a reduction in fat percentage of 1–3%. Specifically, fat mass was lowered by 1.8 kg in young and middle-aged homeless men playing street football for 45 min, two to three times a week for 12 weeks, corresponding to a decrease in body fat percentage from 17.9% to 15.9%.38
Table 3.
Changes in body composition in untrained men as a result of a period of recreational F training compared to R or inactive C
| Study | Activity, target group, gender | Age (years) | Training programme; Duration (weeks), intensity (%), frequency (per week), session duration (min) |
Total fat mass (kg) | Total fat percentage (%) | Lean body mass, whole body (kg) | Lean body mass, legs (kg) | Bone mineral density, left and right proximal femur (%) | Bone mineral density, left and right femoral neck (%) | Bone mineral density (legs) (%) | Bone mineral density, trunk (%) | Bone marker –osteocalcin (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Krustrup et al1 | F, UT, M | 29 | 12; 82%HRmax; 2.3; 60 | 2.7↓*† | 2.9↓*† | 1.7↑*† | 1.1↑*† | – | – | – | – | – |
| R, UT, M | 31 | 12; 82%HRmax; 2.5; 60 | 1.7↓* | 1.7↓* | 0.6↑NS | 0.6↑NS | – | – | – | – | – | |
| C, UT, M | 31 | No intervention | 0.3↓NS | 0.2↓NS | 0.1↑NS | 0.3↓NS | – | – | – | – | – | |
| Randers et al8 41 | F, UT, M | 31 | 64; 82%HRmax; 1.3; 60 | 3.2↓*† | 3.8↓*† | 2.7↑* | 1.1↑*†‡ | – | – | 2%↑* | – | – |
| C, UT, M | 32 | No intervention | 0.2↓NS | 0.6↓NS | 1.2↑* | 0.2↑NS | – | – | 1%↑NS | – | – | |
| Helge et al42 | F, UT, M | 68 | 52; 82%HRmax; 1.7; 45–60 | – | – | – | – | LL:2.4%↑* RL:2.9%↑* | LL:5.4%↑* RL:3.8%↑* | – | – | 46%↑*† |
| S, UT, M | 69 | 52; 8–20RM; 1.9; 45–60 | – | – | – | – | NS | NS | – | – | NS | |
| C, UT, M | 67 | No intervention | – | – | – | – | NS | NS | – | – | NS | |
| Krustrup et al15 | F, UT, Ma | 46 | 24; 85%HRmax; 1.7; 60 | 1.9↓NS | 2.2↓NS | 0.2↑NS | 0.1↑NS | – | – | 2.1%↑NS | – | – |
| DAG, UT, Ma | 47 | DA on CRF | 0.9↓NS | 1.0↓NS | 0.2↑NS | 0.0↔NS | – | – | 0.0% NS | – | – | |
| Knoepfli-Lenzin et al22 | F, UT, Mmh | 37 | 12; 80%HRmax; 2.4; 60 | 2.0↓* | 2.0↓*† | 0.5↑NS | – | – | – | – | – | – |
| R, UT, Mmh | 36 | 12; 79%HRmax; 2.5; 60 | 1.7↓* | 1.4↓NS | 0.0↔NS | – | – | – | – | – | – | |
| C, UT, Mmh | 38 | No intervention | 0.1↑NS | 0.1↑NS | 0.3↓NS | – | – | – | – | – | – | |
| De Sousa et al39 | F, UT, Mt+Wt | 61 | 12; 83%HRmax; 3; 40; F+D | 3.4↓* | 2.4↓NS | 0.2↓NS | – | – | – | – | – | – |
| C, UT, Mt+Wt | 61 | Diet group | 3.7↓* | 2.4↓NS | 1.0↓NS | – | – | – | – | – | – | |
| Andersen et al5 | F, UT, Mt | 51 | 24; 83%HRmax; 1.5; 60 | 1.7↓* | 1.5↓* | 0.7↑NS | 0.5↑NS | – | – | NS | – | – |
| C, UT, Mt | 49 | No intervention | 0.1↓NS | 0.2↓NS | 0.8↑NS | 0.5↓* | – | – | NS | – | – | |
| Helge et al46 | F, UT, Mh | 36 | 12; 82%HRmax; 2.2; 60 | 1.8↓* | – | 0.9↑* | – | – | – | – | 1%↑* | 27%↑*† |
| C, UT, Mh | 43 | No intervention | NS | – | NS | – | – | – | – | NS | NS | |
| Uth et al37 | F, UT, Mp | 67 | 12; 85%HRmax; 2–3; 45–60 | 1.3↓* | 0.9↓* | 0.9↑*† | – | – | – | – | – | – |
| C, UT, Mp | 67 | No intervention | 0.3↓NS | 0.0↔NS | 0.1↑NS | – | – | – | – | – | – |
Changes between pre and post training intervention (unless otherwise stated).
*Significant difference from 0 weeks.
†Significant group difference compared to control.
‡Significant group differences.
a, hypertensive subjects; C, controls; CRF, cardiovascular risk factors; DAG, doctor's advice group; F, football; F+D, football+diet group; h, homeless subjects; HRmax, maximal heart rate; LL, left leg; M, men; mh; mildly hypertensive subjects; NS, not significant; p, prostate cancer patients; R, running; RL, right leg; S, strength training; t, type 2 diabetics; UT, untrained; W, women
In some studies, a period of recreational football training led to higher lean body mass. Total and leg muscle mass were elevated by 1.7 and 1.1 kg, respectively, after 12 weeks of two to three 60 min football training sessions per week.1 A few studies have, however, not been able to demonstrate a significant effect of football training on lean body mass (table 3). The number and length of sprints, and the number of intense actions, depend on the number of players, the degree of man-to-man marking and the pitch size used for small-sided games.8 40 Further studies are warranted to clarify whether the change in muscle mass is related to the way the training is conducted.
Also, marked effects of football training on body composition have been observed in patient groups (table 3). In middle-aged men with T2DM, total fat mass was 1.7 kg lower and android fat percentage reduced by 12.8% after 24 weeks of football training.5 An even more pronounced response was found when another group of T2DM patients conducted 3×40 min football sessions per week for 12 weeks with a caloric-restricted diet, with a loss of fat mass of 3.4 kg.39 Interestingly, a significant increase in muscle mass of 0.9 kg in patients with prostate cancer undergoing antiandrogen treatment was observed after two to three times weekly 45–60 min training sessions over 12 weeks, despite the minimal levels of testosterone in these patients.37
Bone mass and bone mineral density
Participation in small-sided football games also affects the skeleton (table 3). Thus, in 20–43-year-old sedentary men, lower extremity bone mineral content was elevated by 2% after 12 weeks of recreational football training twice a week and was maintained in the following 52 weeks with a reduced frequency to about once a week.1 41 Football training also influenced elderly participants (65–75 years of age), with bone mineral density (BMD) in left and right proximal femur being, respectively, 1.1 and 1.0% higher after 4 months of training.42 Continuing the training for another 8 months led to further marked improvements in the elderly, reaching increases in BMD of 3.8% and 5.4% in the right and left femoral neck, respectively, as well as increases of 2.4% and 2.9% in the left and right proximal femur, respectively42 (table 3). These findings suggest that the osteogenic BMD response in elderly men is not lower, but rather slower, than in their younger counterparts.
The changes in the elderly are markedly higher than what has been observed in other intervention studies examining the skeletal effect of physical activity.43–45 It is likely that the actions in the small-sided football games, with many changes in direction and speed,46 augment BMD, since the osteogenic stimulus from exercise depends on the strain rate and magnitude induced by muscle contraction and ground reaction forces.47–50
Measurements of biochemical bone markers in the elderly suggested that the anabolic response was due to improved bone formation (table 3). Similarly, indication of anabolic effect in bone metabolism was seen in a study of homeless men, where trunk BMD was also elevated (1.0%) after 12 weeks of 2.2 football training sessions a week46 (table 3). Together with the functional improvements in rapid muscle force and postural balance (see below), the higher BMD with regular participation in football training is likely to reduce the risk of fractures due to falling.51 52
Muscle adaptations in recreational football
A few studies have measured changes in muscle oxidative enzymes as a result of a period of recreational football training (table 4). A 14% increase in the maximal activity of leg muscle citrate synthase (CS) occurred after 12 weeks of training, with no further increase during the following 54 weeks, with training frequency reduced from 2.3 to 1.3 times a week.1 41 The change during the first 12 weeks was greater than that observed in a running group performing the same volume of training, suggesting that the intermittent nature of football training had a greater impact on the development of the muscle oxidative system. The maximal activity of 3-hydroxyacyl-CoA dehydrogenase (HAD) was non-significantly elevated by 5% and 16% after 12 and 64 weeks, respectively, of football training,41 which may have been one reason for the elevated fat oxidation during exercise found after a period of football training.53
Table 4.
Changes in muscle enzymatic activity and capillarisation in untrained men as a result of a period of recreational F training compared to R or inactive C
| Study | Activity, target group, gender | Age (years) | Training intervention; Duration (weeks), intensity (%), frequency (per week), session duration (min) |
Capillarisation, cap per fibre (%) | CS activity (%) | HAD activity (%) |
|---|---|---|---|---|---|---|
| Krustrup et al2 10 53 | F, UT, M | 29 | 12; 82%HRmax; 2.3; 60 | 22%↑† | 14%↑† | 5%↑NS |
| R, UT, M | 31 | 12; 82%HRmax; 2.5; 60 | 16%↑ | 7%↑NS | 5%↑NS | |
| C, UT, M | 31 | No intervention | 5%↑NS | 11%↓NS | 11%↓NS | |
| Randers et al8 41 | F; UT; M | 31 | 64; 82%HRmax; 1.3; 60 | – | 18%↑ | 16%↑NS |
| C; UT; M | 32 | No intervention | – | – | – | |
| Andersen et al5 | F, UT, Mt | 51 | 24; 83%HRmax; 1.5; 60 | 7%↑ | 7%↓NS | 5%↓NS |
| C, UT, Mt | 49 | No intervention | 5%↓NS | 9%↑NS | 1%↑NS |
Changes between pre and post training intervention (unless otherwise stated).
†Significant group difference compared to control.
CS, citrate synthase; C, controls; HAD, 3-hydroxyacyl-CoA dehydrogenase; HRmax, maximal heart rate; F, football; M, men; NS, not significant; R, running; t, type 2 diabetics; UT, untrained.
Surprisingly, there was no change in maximal activity of leg muscle CS and HAD in patients with T2DM after 12 and 24 weeks of football training, and the expression of CS was even significantly lowered after 24 week of training.5 On the other hand, the training led to higher expression of muscle actin and Akt-2, as well as a tendency to a higher amount of the GLUT-4 protein. In addition, muscle capillarisation, expressed as number of capillaries per fibre, was increased by 22% in middle-aged men after 12 weeks of recreational football training,1 which was similar to that observed in a running group performing a similar amount of training (table 4). Also, a group of patients with T2DM with an average age of around 50 years increased leg muscle capillarisation during a 24-week recreational football training period, albeit to a lesser degree than observed in the younger men.5
Effect of recreational football on functional capacity
Regular recreational football training has a marked positive effect on the functional capacity of the participants (table 5). In addition to improvements in VO2max (see above), middle-aged male participants, as well as school children, had 25–50% improved performance in Yo-Yo intermittent tests consisting of 2×20-m runs performed repeatedly at progressively increasing speeds and separated by either 5 seconds (Yo-Yo intermittent endurance test level 1 and 2; IE1 and IE2) or 10 s of rest (Yo-Yo intermittent recovery test level 1, IR1).38 53 54 55 Also, elderly men had improved Yo-Yo IE1 performance (43%) after 16 weeks of football training, as well as better sit-to-stand (29%) performance.6
Table 5.
Changes in performance of untrained men as a result of a period of recreational F training compared to R or inactive C
| Study | Activity, target group, gender | Age (years) | Training programme; Duration (weeks), intensity (%), frequency (per week), session duration (min) |
Time to exhaustion, max work (s) | Counter-movement jump (%) | Sprint, 30 m (s) | Max leg strength (kg or %) | Yo-Yo IR1 (m) |
Yo-Yo IE1/IE2 (m or %) |
Postural balance, flamingo test (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Krustrup et al2 10 53 | F, UT, M | 29 | 12; 82%HRmax; 2.3; 60 | 102↑* | – | 0.11↑* | 11%↑*†‡ | – | 420↑*†‡ | – |
| R, UT, M | 31 | 12; 82%HRmax; 2.5; 60 | 101↑* | – | 0.01↓NS | 1%↓NS | – | 195↑* | – | |
| C, UT, M | 31 | No intervention | 25↑* | – | – | 2%↑NS | – | 21↑NS | – | |
| Randers et al8 41 | F, UT, M | 31 | 64; 82%HRmax; 1.3; 60 | 98↑* | 5%↑*† | 0.15↑* | – | – | 382↑* | 49%↑* |
| C, UT, M | 32 | No intervention | – | 0%↔NS | – | – | – | – | 27%↑NS | |
| Jakobsen et al55 | F, UT, M | 29 | 12; 82%HRmax; 2.3; 60 | – | 1%↓NS | – | 0%↔NS | – | – | 41%↑*† |
| R, UT, M | 31 | 12; 82%HRmax; 2.5; 60 | – | 1%↓NS | – | 2%↓NS | – | – | 38%↑*† | |
| C, UT, M | 31 | No intervention | – | 3%↓NS | – | 0%↔NS | – | – | 11%↑NS | |
| Andersen et al6 | F, UT, M | 68 | 16; 84%HRmax; 1.6; 45–60 | 53↑*† | NS | – | – | – | 43%↑*† | – |
| S, UT, M | 69 | 16; 8–20RM; 1.5; 45–60 | 43↓NS | NS | – | – | – | 8%↑NS | – | |
| C, UT, M | 67 | No intervention | 58↓NS | NS | – | – | – | 5%↓NS | – | |
| Krustrup et al15 | F, UT, Ma | 46 | 24; 85%HRmax; 1.7; 60 | 79↑NS | – | – | – | – | – | – |
| DAG, UT, Ma | 47 | DAG on CRF | 19↑NS | – | – | – | – | – | – | |
| Knoepfli-Lenzin et al22 | F, UT, Mmh | 37 | 12; 80%HRmax; 2.4; 60 | 0.9↑*†§ | – | – | – | – | 144↑* | – |
| R, UT, Mmh | 36 | 12; 79%HRmax; 2.5; 60 | 1.1↑*†§ | – | – | – | – | 168↑*† | – | |
| C, UT, Mmh | 38 | No intervention | 0.0↔NS§ | – | – | – | – | 50↑NS | – | |
| Schmidt et al17 | F, UT, Mt. | 51 | 24; 82%HRmax; 1.2; 60 | – | – | – | – | – | 377↑* | – |
| C, UT, Mt | 49 | No intervention | – | – | – | – | – | 52↑NS | – | |
| Helge et al46 | F, UT, Mh | 36 | 12; 82%HRmax; 2.2; 60 | – | – | – | – | – | – | 46%↑* |
| C, UT, Mh | 43 | No intervention | – | – | – | – | – | – | 3%↓NS | |
| Uth et al37 | F, UT, Mp | 67 | 12; 85%HRmax; 2–3; 45–60 | – | – | – | 8.9↑*† | – | – | – |
| C, UT, Mp | 67 | No intervention | – | – | – | 2.2↑NS | – | – | – | |
| Faude et al56 | F, UT, OCh | 11 | 12; 80%HRmax; 4.5; 60 | – | 15%↑* | – | – | – | – | – |
| SP, UT, OCh | 11 | 12; 77%HRmax; 4.5; 60 | – | 14%↑* | – | – | – | – | – | |
| Bendiksen et al54 | F+H, UT, Ch | 9 | 6; 76%HRmax; 2; 30 | – | – | – | – | 148↑*† | – | – |
| C, UT, Ch | 9 | Low-intensity activities | – | – | – | – | 106↓NS | – | – |
Changes are between pre and post training intervention (unless otherwise stated).
*Significant difference from 0 weeks.
†Significant group difference compared to control. ‡Significant group differences.
§Maximal velocity (km/h).
¶Yo-Yo IR1 children.
a, hypertensive participants; C, children; C, controls; CRF, cardiovascular risk factors; DAG, doctor's advice group; F, football; F+H, football+hockey; h, homeless participants; HRmax, maximal heart rate; M, men; mh, mildly hypertensive participants; NS, not significant; OC, overweight children; p, prostate cancer patients; R, running; S, strength training; SP, standard physical activity; t, type 2 diabetics; UT, untrained.
In some studies, maximal leg strength was higher after, rather than before, a period of recreational football training (table 5). In the study by Krustrup et al,53 maximal hamstring power was increased by 11% in combination with a 0.11 s improvement in a 30 m sprint, after 12 weeks of training. Studies have observed that recreational football training led to an increase in counter-movement jump performance for boys56 and young men,41 whereas others did not find any change for young57 and elderly men.6 Nevertheless, a consistent finding has been that recreational football training improves balance (table 5), clearly suggesting that this training also ameliorates the participants’ ability to coordinate movements and thereby potentially reduce accidental injuries in their everyday life.
Synopsis
Recreational football training conducted as small-sided games (4v4 to 7v7) performed for 45–60 min up to three times a week promotes health. Such easy to do training resulted in reduced blood pressure, lowered resting heart rate, favourable adaptations in cardiac structure and function, improved blood lipid profile, elevated muscle mass, reduced fat mass and improved functional capacity. Most changes occurred within the first 3 months, with bone mass density developing further when the training was continued.
For patients with non-communicable diseases (NCDs), such as hypertension and T2DM, even greater effects have been observed on key variables, and the marked improvements of cardiac function and cardiorespiratory fitness are likely to reduce the high risk of cardiovascular diseases in these patient groups. Nevertheless, further studies should examine the value of increasing the volume of recreational football, including a higher frequency of training, and, ultimately, investigate long-term effects of football training on clinical end points and mortality.
Perspectives
Football is by far the most popular sport in the world, with more than 400 million active players, and it is now clear that recreational football promotes health. Thus, football is an attractive way of reducing the risk of increasing the number of individuals becoming overweight and developing NCDs, as well as treating those already affected.
Importantly, recreational football has been associated with positive psychosocial interactions, including increased social capital, improved quality of life, general well-being and motivational status.11–13 The participants in these studies, irrespective of their background, age, weight and whether they are suffering from hypertension, diabetes or cancer, enjoyed playing. As such, football appeals to many and may improve the chances of long-term adherence for individuals who are not motivated to engage in individual exercise otherwise. As an example, a group of middle-aged men with T2DM, introduced to one another during a study, still play football together more than 2 years after the study finished.5 13
Despite these encouraging data, scale-up requires a considerable collaborative effort from volunteers, sports organisations and bodies responsible for health promotion, such as FIFA and the WHO. Indeed, FIFA has taken the first step, promoting information about the health benefits of recreational football and implementing projects around the world; the Danish Football Association has had great success with the Football Fitness concept, recruiting a high number of adults with no previous experience of football.58
Practical applications
The size of the pitch should be adjusted to the number of participants playing football, 80 m2 per participant is recommended.58 Standard football rules, except the offside rule, should be applied. The risk of injury when participating in small-sided football games must be addressed. Generally, in organised football, the number of injuries in training is one-fifth to one-tenth of that occurring during match play, at around eight injuries per 1000 h of training.10 60 This corresponds to one injury every 1.2 years and one severe injury approximately every 13 years per participant, if training is performed for 1 h, twice a week.10 The figures may be less for recreational football, as less than 5% of the participants sustained an injury that kept them away from training (Bangsbo et al, unpublished data). Most injuries occurred in the initial phase of the training period, emphasising that football training should be slowly introduced. Notably, the overall injury rate during recreational football training appears to be reduced with age, which may be due to a reduction in game intensity, speed and forceful contacts.
What are the new findings?
Recreational football training conducted as small-sided games has marked effects on the cardiovascular system with average heart rates being around 80% of maximal heart rate (HRmax) and substantial time is spent above 90%HRmax even for elderly and patient groups.
Recreational football training has broad-ranging physiological effects. It lowers systolic and diastolic blood pressure by typically 7–8 and 5–7 mm Hg, respectively, and even more in hypertensive and patients with type II diabetes.
Recreational football improves left and right ventricular function and increases VO2max by 7–15% and even more in 65–75-year-old men.
Recreational football also lowers body fat, total cholesterol and low-density lipoprotein cholesterol, and increases leg muscle mass and bone mineral content, as well as muscle oxidative enzymes and functional capacity.
Recreational football training produces more pronounced broad-spectrum adaptations than training programmes solely focusing on continuous jogging, interval running or strength training.
Acknowledgments
The authors would like to thank the team at Copenhagen Centre for Team Sport and Health, University of Copenhagen, supported by Nordea-fonden, Denmark, as well as close collaborators in 12 countries. The authors would also like to thank Therese Hornstrup, Henrik Pedersen and Henrik Holm Andersen, for editing and proofreading the article.
Footnotes
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Krustrup P, Nielsen JJ, Krustrup BR, et al. Recreational soccer is an effective health promoting activity for untrained men. Br J Sports Med 2009;43:825–31. 10.1136/bjsm.2008.053124 [DOI] [PubMed] [Google Scholar]
- 2.Krustrup P, Dvorak J, Junge A, et al. Executive summary: the health and fitness benefits of regular participation in small-sided football games. Scand J Med Sci Sports 2010c;20(Suppl 1):132–5. 10.1111/j.1600-0838.2010.01106.x [DOI] [PubMed] [Google Scholar]
- 3.Bangsbo J, Junge A, Dvorak J, et al. Executive summary: Football for health—prevention and treatment of non-communicable diseases across the lifespan through football. Scand J Med Sci Sports 2014;24(S1):147–50. 10.1111/sms.12271 [DOI] [PubMed] [Google Scholar]
- 4.Nybo L, Sundstrup E, Jakobsen MD, et al. High intensity training versus traditional exercise interventions for promoting health. Med Sci Sports Exerc 2010;42:1951–8. 10.1249/MSS.0b013e3181d99203 [DOI] [PubMed] [Google Scholar]
- 5.Andersen TR, Schmidt JF, Thomassen M, et al. A preliminary study: effects of football training on glucose control, body composition, and performance in men with type 2 diabetes. Scand J Med Sci Sports 2014a;24(Suppl 1):43–56. 10.1111/sms.12259 [DOI] [PubMed] [Google Scholar]
- 6.Andersen TR, Schmidt JF, Nielsen JJ, et al. The effect of football or strength training on functional ability and physical performance in elderly untrained men. Scand J Med Sci Sports 2014b;24(S1):76–85. 10.1111/sms.12245 [DOI] [PubMed] [Google Scholar]
- 7.Schmidt JF. Cardiovascular adaptations to recreational football training in men with type 2 diabetes, untrained elderly men and in men with prostate cancer receiving androgen deprivation therapy (PhD thesis). SL grafik, Frederiksberg C, Denmark: ISBN 978-87-7611-801-3. [Google Scholar]
- 8.Randers MB, Nybo L, Petersen J, et al. Activity profile and physiological response to football training for untrained males and females, elderly and youngsters: influence of the number of players. Scand J Med Sci Sports 2010;20(Suppl 1):14–23. 10.1111/j.1600-0838.2010.01069.x [DOI] [PubMed] [Google Scholar]
- 9.Elbe AM, Strahler K, Krustrup P, et al. Experiencing flow in different types of physical activity intervention programs: three randomized studies. Scand J Med Sci Sports 2010;20(Suppl 1):111–17. 10.1111/j.1600-0838.2010.01112.x [DOI] [PubMed] [Google Scholar]
- 10.Krustrup P, Aagaard P, Nybo L, et al. Recreational football as a health promoting activity: a topical review. Scand J Med Sci Sports 2010b;20(Suppl 1):1–13. 10.1111/j.1600-0838.2010.01108.x [DOI] [PubMed] [Google Scholar]
- 11.Ottesen L, Jeppesen RS, Krustrup BR. The development of social capital through football and running: studying an intervention program for inactive women. Scand J Med Sci Sports 2010;20(Suppl 1):118–31. 10.1111/j.1600-0838.2010.01123.x [DOI] [PubMed] [Google Scholar]
- 12.Bruun DM, Bjerre E, Krustrup P, et al. Community-based recreational football: a novel approach to promote physical activity and quality of life in prostate cancer survivors. Int J Environ Res Public Health 2014;11:5567–85. 10.3390/ijerph110605567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen G, Wikman JM, Jensen CJ, et al. Health promotion: the impact of beliefs of health benefits, social relations and enjoyment on exercise continuation. Scand J Med Sci Sports 2014;24(S1):66–75. 10.1111/sms.12275 [DOI] [PubMed] [Google Scholar]
- 14.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 2006;16(Suppl 1):3–63. 10.1111/j.1600-0838.2006.00520.x [DOI] [PubMed] [Google Scholar]
- 15.Krustrup P, Randers MB, Andersen LJ, et al. Soccer improves fitness and attenuates cardiovascular risk factors in hypertensive men. Med Sci Sports Exerc 2013;45:553–60. 10.1249/MSS.0b013e3182777051 [DOI] [PubMed] [Google Scholar]
- 16.Grossman E, Messerli FH, Goldbourt U. High blood pressure and diabetes mellitus: are all antihypertensive drugs created equal? Arch Intern Med 2000;160:2447–52. 10.1001/archinte.160.16.2447 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt JF, Andersen TR, Horton J, et al. Soccer training improves cardiac function in men with type 2 diabetes. Med Sci Sports Exerc 2013;45:2223–33. 10.1249/MSS.0b013e31829ab43c [DOI] [PubMed] [Google Scholar]
- 18.Hayashino Y, Jackson JL, Fukumori N, et al. Effects of supervised exercise on lipid profiles and blood pressure control in people with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2012;98:349–60. 10.1016/j.diabres.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 19.Fagard RH, Cornelissen VA. Effect of exercise on blood pressure control in hypertensive patients. Eur J Cardiovasc Prev Rehabil 2007;14:12–17. 10.1097/HJR.0b013e3280128bbb [DOI] [PubMed] [Google Scholar]
- 20.Levine GN, D'Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and Cardiovascular risk. A science advisory from the American Heart Association, American Cancer Society, and American Urological Association. Circulation 2010;121:833–40. 10.1161/CIRCULATIONAHA.109.192695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenwinkel ET, Bloomfield DM, Arwady MA, et al. Exercise and autonomic function in health and cardiovascular disease. Cardiol Clin 2001;19:369–87. 10.1016/S0733-8651(05)70223-X [DOI] [PubMed] [Google Scholar]
- 22.Knoepfli-Lenzin C, Sennhauser C, Toigo M, et al. Effects of a 12-week intervention period with football and running for habitually active men with mild hypertension. Scand J Med Sci Sports 2010;20(Suppl 1):72–9. 10.1111/j.1600-0838.2009.01089.x [DOI] [PubMed] [Google Scholar]
- 23.Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation 2010;122:1211–38. 10.1161/CIRCULATIONAHA.110.939959 [DOI] [PubMed] [Google Scholar]
- 24.Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation 2012;126:753–67. 10.1161/CIRCULATIONAHA.112.093245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornelissen VA, Onkelinx S, Goetschalckx K, et al. Exercise-base cardiac rehabilitation improves endothelial function assessed by flow-mediated dilation but not by pulse amplitude tonometry. Eur J Prevent Cardiol 2014;21:39–48. 10.1177/2047487312460516 [DOI] [PubMed] [Google Scholar]
- 26.Schmidt JF, Hansen PR, Andersen TR, et al. Cardiovascular adaptations to 4 and 12 months of football and strength training in 65–75-year-old untrained men. Scand J Med Sci Sports 2014;24(S1):86–97. 10.1111/sms.12217 [DOI] [PubMed] [Google Scholar]
- 27.Schmidt JF, Andersen TR, Andersen LJ, et al. Cardiovascular function is better in veteran football players than age-matched untrained elderly men. Scand J Med Sci Sports 2015;25:61–9. 10.1111/sms.12153 [DOI] [PubMed] [Google Scholar]
- 28.Palatini P, Julius S. Heart rate and the cardiovascular risk. J Hypertens 1997;15:3–17. 10.1097/00004872-199715010-00001 [DOI] [PubMed] [Google Scholar]
- 29.Hillis GS, Hata J, Woodward M, et al. Resting heart rate and the risk of microvascular complications in patients with type 2 diabetes mellitus. J Am Heart Assoc 2012;1:e002832 10.1161/JAHA.112.002832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul L, Hastie CE, Li WS, et al. Resting heart rate pattern during follow-up and mortality in hypertensive patients. Hypertension 2010;55:567–74. 10.1161/HYPERTENSIONAHA.109.144808 [DOI] [PubMed] [Google Scholar]
- 31.Stewart KJ, Ouyang P, Bacher AC, et al. Exercise effects on cardiac size and LV diastolic function: relationships to changes in fitness, fatness, blood pressure and insulin resistance. Heart 2006;92:893–8. 10.1136/hrt.2005.079962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guirado GN, Damatto RL, Matsubara BB, et al. Combined exercise training in asymptomatic elderly with controlled hypertension: effects on functional capacity an cardiac diastolic function. Med Sci Monit 2012;18:CR461–5. 10.12659/MSM.883215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hordern MD, Coombes JS, Cooney LM, et al. Effects of exercise intervention on myocardial functions in type 2 diabetes. Heart 2009;95:1343–9. 10.1136/hrt.2009.165571 [DOI] [PubMed] [Google Scholar]
- 34.Hollekim-Strand SM, Bjørgaas MR, Albrektsen G, et al. High-intensity interval exercise effectively improves cardiac function in patients with type 2 diabetes mellitus and diastolic function. J Am Coll Cardiol 2014;64:1758–60. 10.1016/j.jacc.2014.07.971 [DOI] [PubMed] [Google Scholar]
- 35.Møgelvang R, Søgaard P, Pedersen SA, et al. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation 2009;119:2679–85. 10.1161/CIRCULATIONAHA.108.793471 [DOI] [PubMed] [Google Scholar]
- 36.Halley CM, Houghtaling PL, Khalil MK, et al. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med 2011;171:1082–7. 10.1001/archinternmed.2011.244 [DOI] [PubMed] [Google Scholar]
- 37.Uth J, Hornstrup T, Schmidt JF, et al. Football training improves lean body mass in men with prostate cancer undergoing androgen deprivation therapy. Scand J Med Sci Sports 2014;24(S1):105–12. 10.1111/sms.12260 [DOI] [PubMed] [Google Scholar]
- 38.Randers MB, Petersen J, Andersen LJ, et al. Short-term street soccer improves fitness and cardiovascular health of homeless men. Eur J Appl Physiol 2011;112:2097–106. 10.1007/s00421-011-2171-1 [DOI] [PubMed] [Google Scholar]
- 39.De Sousa MV, Fukui R, Krustrup P, et al. Positive effects of football on fitness, lipid profile, and insulin resistance in Brazilian patients with type 2 diabetes. Scand J Med Sci Sports 2014;24(S1):57–65. 10.1111/sms.12258 [DOI] [PubMed] [Google Scholar]
- 40.Hill-Haas SV, Dawson BT, Coutts AJ, et al. Physiological responses and time–motion characteristics of various small-sided soccer games in youth players. J Sports Sci 2009;27:1–8. 10.1080/02640410802206857 [DOI] [PubMed] [Google Scholar]
- 41.Randers MB, Nielsen JJ, Krustrup BR, et al. Positive performance and health effects of a football training program over 12 weeks can be maintained over a 1-year period with reduced training frequency. Scand J Med Sci Sports 2010;20(Suppl 1):80–9. 10.1111/j.1600-0838.2010.01091.x [DOI] [PubMed] [Google Scholar]
- 42.Helge EW, Andersen TR, Schmidt JF, et al. Recreational football improves bone mineral density and bone turnover marker profile in elderly men. Scand J Med Sci Sports 2014a;24(S1):98–104. 10.1111/sms.12239 [DOI] [PubMed] [Google Scholar]
- 43.Bolam KA, van Uffelen JG, Taaffe DR. The effect of physical exercise on bone density in middle-aged and older men: a systematic review. Osteoporos Int 2013;24:2749–62. 10.1007/s00198-013-2346-1 [DOI] [PubMed] [Google Scholar]
- 44.Guadalupe-Grau A, Fuentes T, Guerra B, et al. Exercise and bone mass in adults. Sports Med 2009;39:439–68. 10.2165/00007256-200939060-00002 [DOI] [PubMed] [Google Scholar]
- 45.Vincent KR, Braith RW. Resistance exercise and bone turnover in elderly men and women. Med Sci Sports Exerc 2002;34:17–23. 10.1097/00005768-200201000-00004 [DOI] [PubMed] [Google Scholar]
- 46.Helge EW, Randers MB, Hornstrup T, et al. Street football is a feasible health-enhancing activity for homeless men: biochemical bone marker profile and balance improved. Scand J Med Sci Sports 2014b;24(S1):122–9. 10.1111/sms.12244 [DOI] [PubMed] [Google Scholar]
- 47.Kohrt WM, Barry DW, Schwartz RS. Muscle forces or gravity: what predominates mechanical loading on bone? Med Sci Sports Exerc 2009;41:2050–5. 10.1249/MSS.0b013e3181a8c717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robling AG. Is bone's response to mechanical signals dominated by muscle forces? Med Sci Sports Exerc 2009;41:2044–9. 10.1249/MSS.0b013e3181a8c702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am 1984;66:397–402. [PubMed] [Google Scholar]
- 50.Turner CH, Pavalko FM. Mechanotransduction and functional response of the skeleton to physical stress: the mechanisms and mechanics of bone adaptation. J Orthop Sci 1998;3:346–55. 10.1007/s007760050064 [DOI] [PubMed] [Google Scholar]
- 51.Liu-Ambrose T, Nagamatsu LS, Hsu CL, et al. Emerging concept: ‘central benefit model’ of exercise in falls prevention. Br J Sports Med 2013;47:115–17. 10.1136/bjsports-2011-090725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sherrington C, Henschke N. Why does exercise reduce falls in older people? Unrecognised contributions to motor control and cognition? Br J Sports Med 2013;47:730–1. 10.1136/bjsports-2012-091295 [DOI] [PubMed] [Google Scholar]
- 53.Krustrup P, Christensen JF, Randers MB, et al. Muscle adaptations and performance enchancements of soccer training for untrained men. Eur J Appl Physiol 2010a;108:1247–58. 10.1007/s00421-009-1319-8 [DOI] [PubMed] [Google Scholar]
- 54. doi: 10.1519/JSC.0b013e318270fd0b. Bendiksen M, Ahler T, Clausen H, et al. The use of Yo-Yo intermittent recovery level 1 and Andersen testing for fitness and maximal heart rate assessments of 6- to 10-year-old school children. J Strength Cond Res 2013;27:1583–90. [DOI] [PubMed] [Google Scholar]
- 55. doi: 10.1080/02640414.2013.792951. Hansen PR, Andersen LJ, Rebelo AN, et al. Cardiovascular effects of 3 months of football training in overweight children examined by comprehensive echocardiography: a pilot study. J Sports Sci 2013;31:1432–40. [DOI] [PubMed] [Google Scholar]
- 56.Faude O, Kerper O, Multhaupt M, et al. Football to tackle overweight in children. Scand J Med Sci Sports 2010;20(Suppl 1):103–10. 10.1111/j.1600-0838.2009.01087.x [DOI] [PubMed] [Google Scholar]
- 57.Jakobsen MD, Sundstrup E, Randers MB, et al. The effect of strength training, recreational soccer and running exercise on stretch-shortening cycle muscle performance during countermovement jumping. Hum Mov Sci 2012;31:970–86. 10.1016/j.humov.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 58.Bennike S, Wikman JM, Ottesen LS. Football Fitness—a new version of football? A concept for adult players in Danish football clubs. Scand J Med Sci Sports 2014;24(S1):138–46. 10.1111/sms.12276 [DOI] [PubMed] [Google Scholar]
- 59.Randers MB, Nielsen JJ, Bangsbo J, et al. Physiological response and activity profile in recreational small-sided football: no effect of the number of players. Scand J Med Sci Sports 2014b;24(S1):130–7. 10.1111/sms.12232 [DOI] [PubMed] [Google Scholar]
- 60.Fuller CW, Ekstrand J, Junge A, et al. Consensus statement on injury definitions and data collection procedures in studies of football (soccer) injuries. Clin J Sports Med 2006;16:97–106. 10.1097/00042752-200603000-00003 [DOI] [PubMed] [Google Scholar]