Abstract
FSGS is a heterogeneous fibrosing disease of the kidney, the cause of which remains poorly understood. In most cases, there is no effective treatment to halt or retard progression to renal failure. Increasing evidence points to mitochondrial dysfunction and the generation of reactive oxygen species in the pathogenesis of CKD. Autophagy, a major intracellular lysosomal degradation system, performs homeostatic functions linked to metabolism and organelle turnover. We prevented normal autophagic pathways in nephrons of mice by mutating critical autophagy genes ATG5 or ATG7 during nephrogenesis. Mutant mice developed mild podocyte and tubular dysfunction within 2 months, profound glomerular and tubular changes bearing close similarity to human disease by 4 months, and organ failure by 6 months. Ultrastructurally, podocytes and tubular cells showed vacuolization, abnormal mitochondria, and evidence of endoplasmic reticulum stress, features that precede the appearance of histologic or clinical disease. Similar changes were observed in human idiopathic FSGS kidney biopsy specimens. Biochemical analysis of podocytes and tubules of 2-month-old mutant mice revealed elevated production of reactive oxygen species, activation of endoplasmic reticulum stress pathways, phosphorylation of p38, and mitochondrial dysfunction. Furthermore, cultured proximal tubule cells isolated from mutant mice showed marked mitochondrial dysfunction and elevated mitochondrial reactive oxygen species generation that was suppressed by a mitochondrial superoxide scavenger. We conclude that mitochondrial dysfunction and endoplasmic reticulum stress due to impaired autophagic organelle turnover in podocytes and tubular epithelium are sufficient to cause many of the manifestations of FSGS in mice.
Keywords: Autophagy, mitochondria, focal segmental glomerulosclerosis
FSGS is a heterogeneous renal disease with typically poor outcome due to progressive loss of kidney function. Although FSGS is often associated with other systemic diseases, including morbid obesity, most cases occur in isolation. The cause of primary FSGS remains poorly resolved. Over the last 15 years, several mutations in genes expressed by podocytes that code for proteins of the filtration barrier of the kidney (known as the slit diaphragm) have been identified.1 Mutations in these genes lead to severe life-threatening kidney disease in the neonatal period. Mutations in several other cytoskeletal proteins expressed by many cells, including podocytes, have also been identified and have been associated with later-onset disease.2 Recently, polymorphisms in noncoding regions close to a gene, APOL1, coding for an HDL lipid transfer protein have also been associated with the development of FSGS in patients of African ancestry.3 Nevertheless, most patients with primary FSGS have none of these mutations or polymorphisms, as is the case for those with FSGS when associated with other diseases, including obesity or diabetes mellitus. Although FSGS is characterized by lesions in the glomerulus, particularly podocytes, and although mutations restricted to podocytes cause FSGS, evidence exists that early functional defects in proximal tubule cells, ranging from the isolated glycosuria to multiple solute wasting, known as Fanconi syndrome,4 are found in some patients with FSGS. This finding suggests that pathologic abnormalities may lie in the tubules as well as glomerular cells.
Recent studies from several laboratories have implicated mitochondrial dysfunction, abnormal β-oxidation of fatty acids, and the aberrant production of mitochondrial reactive oxygen species (ROS) in the pathogenesis of tubulointerstitial kidney diseases.5 Moreover, a growing body of evidence indicates that mitochondrial dysfunction may be pathologic in some forms of kidney disease, such as diabetic kidney disease.6,7 In addition, studies from kidney, lung, and other epithelial organs have pointed to stress of the endoplasmic reticulum (ER) as a cause of epithelial ROS generation, cytokine production, and ultimately cell death, which may amplify kidney disease. However, the significance of these changes as inducers of kidney disease has not been systematically tested.
The autophagic degradation pathway is important in many aspects of cell homeostasis and responses to environmental stress. Macroautophagy is a process by which organelles are turned over and disposed of and their building blocks recycled. This pathway involves formation of autophagic vesicles around effete organelles and fusion with lysosomes that mediate enzymatic degradation.8 However, macroautophagy has been identified to play important roles in energy metabolism by transferring triglycerides from stores to mitochondria, and also to play important roles in cell activation and therefore cytokine production. Because of its central role in processes related to metabolism and mitochondrial and ER turnover, we explored its function in the kidney epithelium. Global deletion of the autophagic pathway leads to neonatal mortality as a result many factors, including absence of stress responses necessary for energy mobilization during the physiologic starvation that occurs between parturition and lactation. Therefore, global deletion of autophagy cannot be used to study its role in the adult kidney. To circumvent this problem, we developed methods to delete genes critical to the formation of autophagosomes early in nephrogenesis, only in cells that become the epithelium of the kidney.
RESULTS
Mutation of ATG5 in Kidney Epithelium Results in FSGS
To mutate Atg5 in the kidney epithelium, the locus of the transcription factor Six2 was used to drive production of Cre recombinase in all progenitor cells that become mesenchyme-derived kidney epithelium during nephrogenesis, including podocytes, parietal epithelial cells, proximal tubule, loop of Henle, and distal tubule9 (Supplemental Figure 1, A and B). Six2 is a transcription factor expressed exclusively in cap mesenchyme during nephrogenesis, cells that are fated to become kidney epithelial cells.9 The Six2-Cre BAC transgene was bred to mice with the loxP recombination sites flanking the third exon of both Atg5 alleles. Transcriptional analysis of kidney and skin showed extensive recombination at the Atg5 locus in kidney only, indicating that Atg5 was deleted in kidney epithelium (Figure 1A). Consistent with this, Atg5 protein expression was markedly decreased in whole kidney (Figure 1B). Autophagic flux, detected by degradation of the autophagosome-associated protein, P62, was reduced in whole kidney at 2 months of age and was indicated by accumulation of this protein in mutant kidneys. In addition, autophagic flux as detected by the active form of light-chain microtubule–associated protein-3 (LC3) was markedly decreased in mutant kidney epithelial cells (Figure 1B). By electron microscopy, autophagic vacuoles were detected in normal epithelium but not in mutant epithelium (Figure 1C). Collectively, these findings confirm that autophagy was blocked in the kidney epithelium of mutant mice.
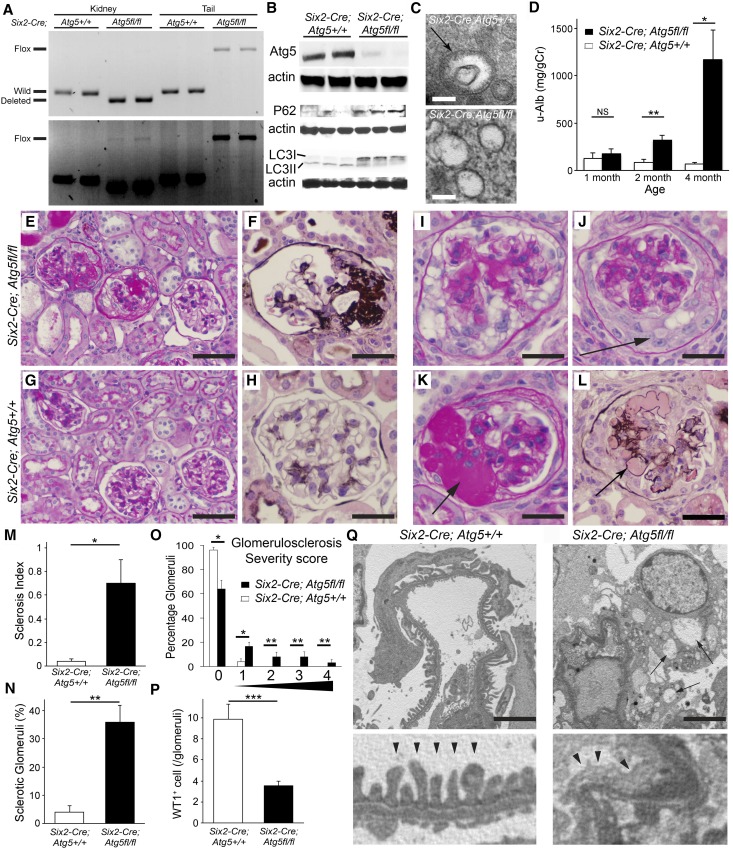
Figure 1.
Mutation of ATG5 in kidney epithelium prevents autophagy and causes FSGS. (A) Ethidium-stained gel showing amplified PCR products of genomic DNA at the Atg5 locus to determine the genotype and deletion of floxed gene of the Atg5 locus. The upper gel represents an image with shorter exposure time, while the lower gel shows the one with longer exposure time. They indicate that in Six2-Cre; Atg5flox/flox whole kidney most genomic DNA has undergone recombination and only a small portion of DNA contains the floxed exon. (B) Western blots of whole kidney at 2 months showing deletion of ATG5 protein, increase of P62 protein, and loss of LC3II (lower band), indicative of loss autophagy in the kidney. (C) Electron microscopic images showing an autophagosome in a Six2-Cre; Atg5fl/fl kidney proximal tubule cell in (bar=100 nm). (D) Graph showing urine albumin-to-creatinine ratio in mice with time after birth. (E–H) Typical glomerular morphology at 4 months after birth, stained by periodic acid-Schiff or silver methenamine. Note the presence of segmental sclerotic lesions (fibrosis and capillary loop destruction) and focal glomerular involvement. (I–L) Examples of additional glomerular changes, including pseudocrescent formation, collapsing lesions, and hyalinosis. (M and N) Quantification of glomerulosclerosis by index or by percentage of glomeruli with sclerosis. (O) Quantification of glomerulosclerosis by severity score. (P) Quantification of WT1+ cells per glomerular cross-section. (Q) Representative electron microscopic images showing capillary loop coated with podocytes in the urinary space. Note in mutant mice that the podocytes show vacuolation (arrows) and there is wrinkling and collapse of the capillary loop structure and extensive foot process effacement (arrowheads). Data are mean±SEM. n=4–7/group. ***P<0.001; **P<0.01; *P<0.05. Bar=100 µm (E, G), 50 µm (F, H, I–L), 2.5 µm (Q).
Mice were born healthy in expected Mendelian ratios and gained weight normally, but by 2 months of age urine from mutants showed albuminuria; by 4 months albuminuria was highly elevated (Figure 1D, Supplemental Figure 1). Histologic examination of the kidneys at 4 months of age showed typical features of FSGS. These features included segmental lesions of scarring with capillary loop obliteration by matrix and hyaline deposition, with resultant collapse of the loop structure, tuft to capsule adhesion, glomeruli with perihilar predominance, lesions predominantly at the tubular pole, and glomeruli with tuft collapse associated with glomerular epithelial cell (GEC) hyperplasia (Figure 1, E–N). Glomeruli with pure endocapillary hypercellularity were not seen. Glomeruli with GEC hypertrophy and hyperplasia frequently showed marked vacuolation of GECs. GEC hyperplasia was identified in 23% of glomeruli. In addition to the presence of glomerulosclerosis, hyalinosis was present in 6% of glomeruli (Figure 1, K and L), another feature typical of human FSGS. Segmental sclerosis was present in 24.0%±2.2% of glomeruli and global glomerulosclerosis in 3.3%±2.0%, whereas 64.7%±5.6% of glomeruli lacked sclerosis (Figure 1, M–O).
Consistent with a primary podocyte disease of glomeruli, whole tissue sections labeled for the podocyte marker WT-1 indicated a 60% reduction in podocytes per glomerular tuft (Figure 1P) compared with controls. Electron microscopy of glomeruli at 4 months showed typical changes of a podocytopathy with extensive effacement of podocyte processes along the glomerular basement membrane, reduction of secondary and even primary podocyte processes, and vacuolation of podocyte cytoplasm (Figure 1Q, Supplemental Figure 1, C–F).
Consistent with the findings of podocytopathy and high-level albuminuria, urinary protein electrophoresis confirmed that albumin was the predominant protein, although additional proteins were detected (Supplemental Figure 1G). In addition to the urinary changes, mutant mice had hypoalbuminemia and hyperlipidemia at 4 months consistent with nephrosis, although mice did not display overt tissue edema or weight gain (Supplemental Figure 1, H and I, and Supplemental Table 1).
Mutation of ATG5 in Kidney Epithelium Results in Tubulointerstitial Disease, Loss of Organ Function, and Death
Human FSGS is frequently closely associated with severe tubulointerstitial disease. The cause of the nonglomerular component in FSGS remains unclear, although current models propose it is predominantly a result of podocyte disease.10 In Six2-Cre; Atg5fl/fl mutant mice, the presence of severe tubulointerstitial disease was prominent at 4 months of age (Figure 2, A–C). These changes included the development of typical tubular injury in the cortex and outer medulla, such as flattening and spreading/simplification of epithelial cells, loss of polarity, apoptotic and necrotic cell death, the formation of protein and cellular debris casts in the lumen, and marked upregulation of the tubular integrin β6 (Supplemental Figure 2). Tubular changes were associated with interstitial inflammation and interstitial fibrosis (Figure 2, D–I). However, there was no significant loss of peritubular capillaries at 4 months. In some cases, the development of tubular cysts was evident. Consistent with the combined effects of glomerular and tubular disease, by 4 months there was an increase in plasma creatinine and BUN levels indicating loss of kidney function, as well as hyperlipidemia and hypoalbuminemia, indicating severe albuminuria (Figure 2J, Supplemental Table 1). From 5 months onward, mutant mice died of kidney failure (Figure 2K).
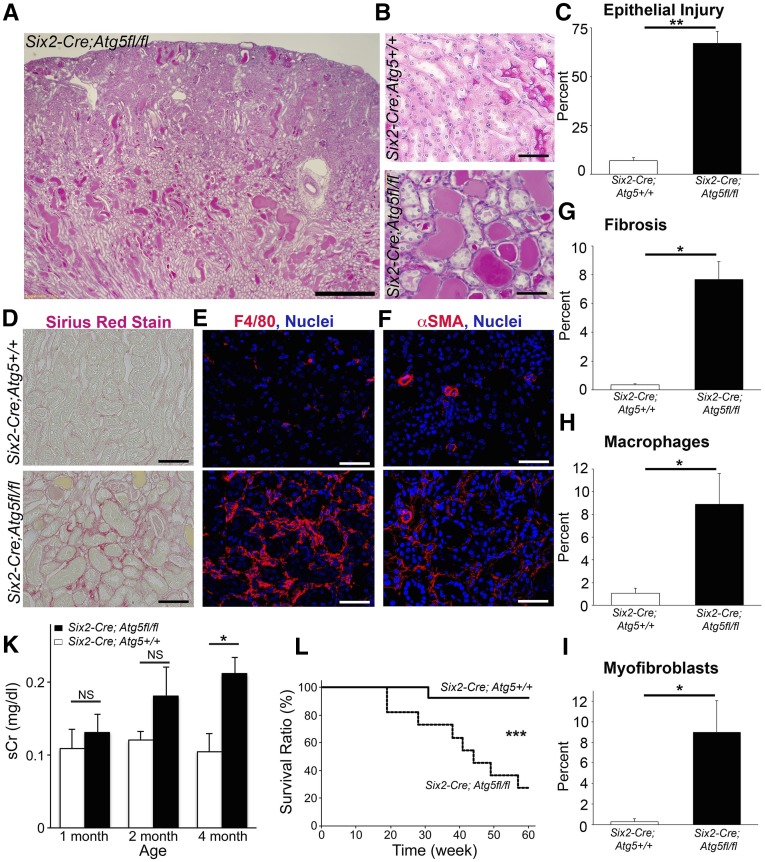
Figure 2.
Mutation of ATG5 in kidney epithelium causes tubulointerstitial disease. (A and B) Lower- and higher-power images showing the extent of tubulointerstitial disease in ATG5 mutant mice at 4 months. (C) Tubular injury score at 4 months. (D–F) Images showing interstitial fibrosis stained by sirius red, macrophage infiltration, and myofibroblast accumulation in the interstitium. (G–I) Quantification of fibrosis, macrophages, and myofibroblasts. (J) Time course of plasma creatinine levels in wild-type and mutant mice. (K) Kaplan–Meier survival curve (P<0.001 by Wilcoxon test). Data are mean±SEM. n=4–13/group. *P<0.05; **P<0.01; ***P<0.001. Bar=500 µm (A) and 100 µm (B–F).
Podocyte Foot Process Effacement Precedes FSGS in Mice with Mutation of ATG5 in Kidney Epithelium
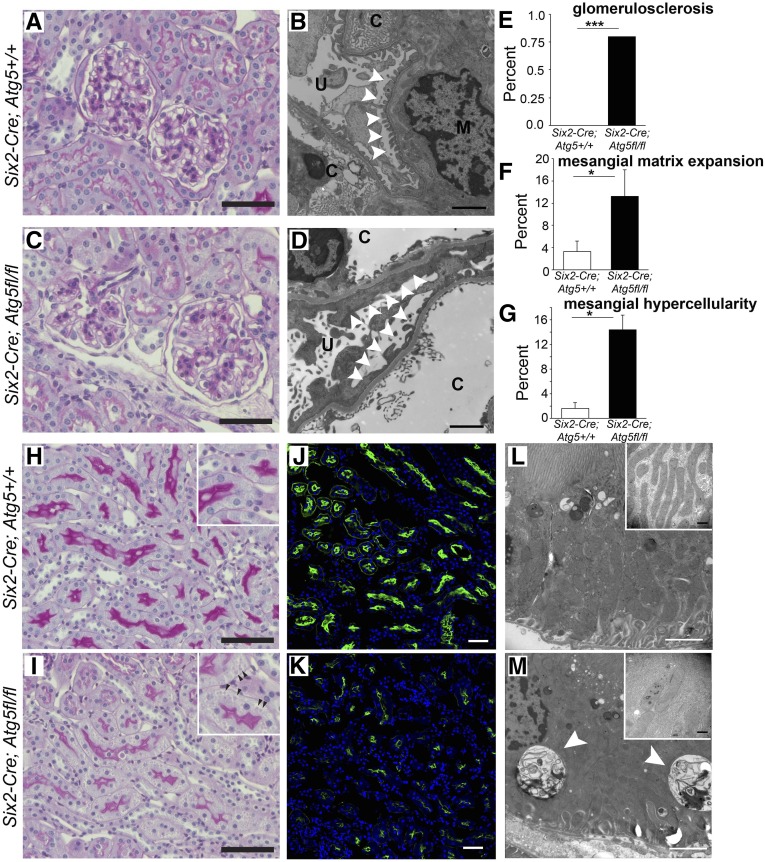
At 2 months of age, Atg5 mutant mice have albuminuria but normal kidney function as assessed by plasma creatinine levels (Figures 1 and 2). Standard histologic examination of glomeruli was essentially normal, and the tubulointerstitium looked essentially normal (Figure 3). Albuminuria in patients who have essentially normal light microscopic features is frequently called minimal-change disease but may be a prelude to FSGS.11 Closer examination of mutant glomeruli, however, showed a subtle but significant increase in mesangial cells at 2 months (Figure 3, C and D), and a few glomeruli had mildly increased mesangial matrix deposition, both recognized features of early FSGS. Glomerulosclerosis was detected rarely (Figure 3, E–G). EM of glomeruli showed widespread effacement of the tertiary foot processes in mutant kidneys at 2 months, also an early characteristic of FSGS (Figure 3, B and D).
Figure 3.
Albuminuria and minor glomerular and tubular changes develop after 2 months which precede the development of FSGS. (A–D) Periodic acid-Schiff–stained light micrographs and electron microscopic images of glomerular capillary loops showing essentially normal glomeruli at 2 months in mutant and wild-type mice, but whereas wild-type podocyte foot processes show normal tertiary structures (B), electron microscopic foot process effacement is readily apparent in mutant glomeruli (D) (arrowheads) (c, capillary; u, urinary space). Bar=100 µm (periodic acid-Schiff) and 2 µm (electron microscopy). (E–G) Quantification of glomerular changes present at 2 months. (H–K) Representative images of periodic acid-Schiff–stained or LTL-fluorescein–stained kidneys showing S3 segment proximal tubules at 2 months. Note reduction in brush border intensity and loss of LTL epitope expression. Also note subtle presence of basolateral inclusion bodies in mutant mice (arrowheads in the inset). Bar=100 µm. (L and M) Electron microscopic images of proximal tubule cells showing presence of inclusions (arrowheads) in mutant cells and the changes in mitochondrial size and shape. Bar=2 µm [in insets=500 nm]). Data are mean±SEM. n=4–6/group. ***P<0.001; *P<0.05.
On closer examination of the tubule, the intensity of brush border staining in proximal tubules was reduced (Figure 3, H and I). Such changes to proximal epithelium were more clearly seen by fluorescence detection of the proximal tubule lotus tetragonolobus lectin (LTL) antigen, revealing evidence of altered cell polarity and reduced intensity of LTL sugar epitopes (Figure 3, J and K). In addition, the basolateral organic anion transporter OAT1, responsible for secretion of uremic toxins, was markedly downregulated (Supplemental Figure 2). These changes occurred in the absence of expression of vimentin, a marker of epithelial to mesenchymal transition(EMT) transcriptional program activity in epithelial cells (Supplemental Figure 2, A and B). In addition, inclusion bodies could be seen prominently in proximal tubule cytoplasm, particularly basally (Figure 3, H and I, Supplemental Figure 3). Electron microscopy of tubules showed large (>2 µm) inclusions that contained proteinaceous or cell organelle material. In addition, proximal tubule cells showed changes to mitochondrial shape, size, and organization of cristae (Figure 3, L and M, Supplemental Figure 3). Mitochondria in diseased tubules at 2 months showed increased diameter, decreased length, and patchy loss of cristae (Supplemental Figure 3). ER was also more prominent as a result of focal dilated cisternae in diseased tubules.
The presence of discrete inclusion bodies containing organelle debris suggested the possible activation of an alternative degradation pathway. Such a pathway has been reported in embryonic fibroblasts through recruitment of membrane to encase organelles, from the trans Golgi network and late endosomes, mediated by the GTPase Rab9, thereby leading to lysosomal fusion.12 A minority of large vesicles was detected in proximal tubules of 2-month-old Six2-Cre; Atg5fl/fl mice expressing Rab9, but these did not colocalize with the lysosomal marker LAMP-2 (Supplemental Figure 4). The extent of Rab9+ inclusions increased in proximal tubules of mutant mice by 4 months, but these again did not coexpress the lysosomal marker LAMP-2 (Supplemental Figure 4). This suggests an attempt by mutant proximal tubules to form vesicles containing effete organelles, but the process appears to be inefficient and does not lead to lysosomal fusion.
Mutation of ATG5 in Kidney Epithelium Results in Enhanced Reactive Oxygen Species Generation, ER Stress, and Mitochondrial Dysfunction
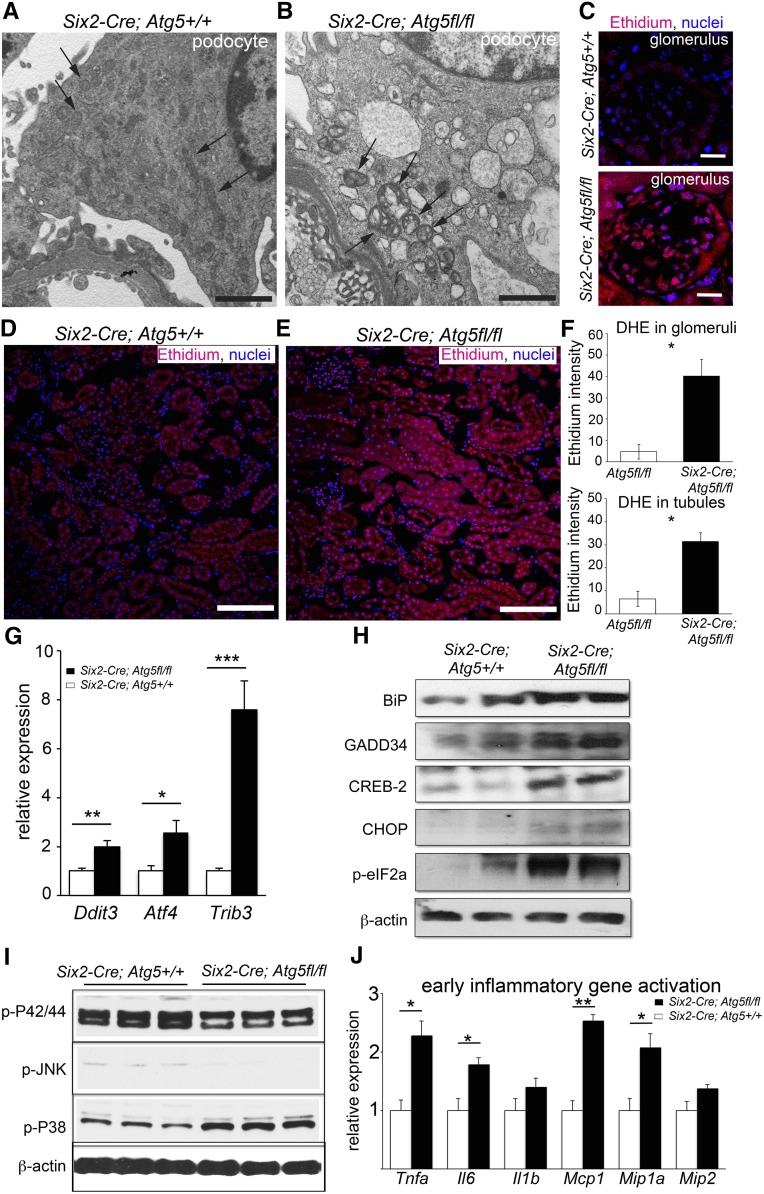
To understand why a lack of autophagy results in profound tubular and podocyte changes, we studied the effect of ATG5 mutation on the kidney at early time points. At 2 months, electron microscopy of proximal tubules showed evidence of abnormal mitochondria and abnormal ER (Figure 3, Supplemental Figure 3). These changes were exaggerated at 4 months, and at the same time podocytes showed overtly abnormal, enlarged mitochondria with loss of cristae (Figure 4, A and B). The presence of disrupted or dysfunctional mitochondria can result in pathologic production of ROS, as can the presence of stressed ER.13,14 We evaluated ROS in mutant kidneys at 2 months, by the detection of dihydroethidium oxidation to ethidium. Both glomerular cells and tubules showed marked increases in ROS production (Figure 4, C–F). Pathologic glomerular staining for ROS was predominantly in podocytes (Figure 4C).
Figure 4.
Increased ROS production and endoplasmic reticulum stress are present before the development of FSGS. (A and B) Electron microscopic images of podocytes at 4 months showing abnormal mitochondria in mutant (Six2Cre;Atg5fl/fl) podocytes (arrows) that are enlarged, are shortened, and feature loss of cristae compared with mitochondria in wild-type (Six2Cre;Atg5+/+) podocytes (arrows). Note also the presence of vacuoles in mutant podocytes. (C–F) Images and quantification of intensity of dihydroethidium-mediated fluorescence (due to oxidation to ethidium) in glomeruli and tubules showing increased levels of ROS in both compartments in mutant mice at 2 months. (G) Increased transcripts for the PERK-dependent ER stress pathway genes in mutant kidney at 2 months. (H) Western blots of proteins from whole kidneys at 2 months showing increased levels of ER-stress pathway proteins, including phospho-eIF2α. (I) Western blots of whole kidney proteins at 2 months probed for stress pathway activation showing increased levels of phospho-P38 mitogen-activated protein kinase. (J) Whole kidney cytokine transcript levels at 2 months. Bar=500 nm (A and B); 50 µm (C), and 100 µm (D and E). Data are means±SEM n=4–6/group. *** P<0.001, ** P<0.01, * P<0.05.
We also observed changes to the ER (Supplemental Figure 3). Failure to turn over ER by autophagy could lead to ER stress as a result of the persistence of unfolded proteins. ER stress in epithelium has been proposed to be pathogenic, resulting in nonmitochondrial ROS generation and cytokine production.15 To evaluate the extent of ER stress, we measured ER stress response genes early in disease. Unfolded proteins in the ER are sensed and regulated by double-stranded RNA activated protein kinase–like endoplasmic reticulum kinase (PERK). The molecular chaperone, binding immunoglobulin protein (BiP/Grp78), which initiates PERK activity, is upregulated (Figure 4, G–H). Regulatory factors activated by PERK, including activating transcription factor-4 (ATF4/CREB-2), C/EBP homologous protein (CHOP/DDIT3), and Tribbles-related protein-3 (TRB3), are all upregulated at 2 months of age (Figure 4, G and H.16 In addition, PERK directly phosphorylates eukaryotic translation initiation factor (eIF)-2α to inhibit protein translation and trigger cell cycle arrest, and this active form (p-eIF-2α) was increased at this timepoint (Figure 4H). GADD34, a regulator of eIF2α activity, was also increased at 2 months of age (Figure 4H). Consistent with increased activation of the PERK ER stress pathway, and potentially increased mitochondrial ROS production, phosphorylation of P38 mitogen-activated protein kinases was increased at this early timepoint (Figure 4I). Interestingly, the stress pathways regulated by pJNK and p42/44 were not increased at this time.
Increasing evidence points to cell stress from mitochondrial dysfunction and ER dysfunction as direct activators of inflammatory pathways.17 To evaluate this, we tested 2-month-old kidneys for the generation of inflammatory cytokines and chemokines (Figure 4J). Small but significant increases in Tnfa, Il6, and Mcp1 transcripts were apparent despite a lack of inflammation in the kidney at this time by histologic examination.
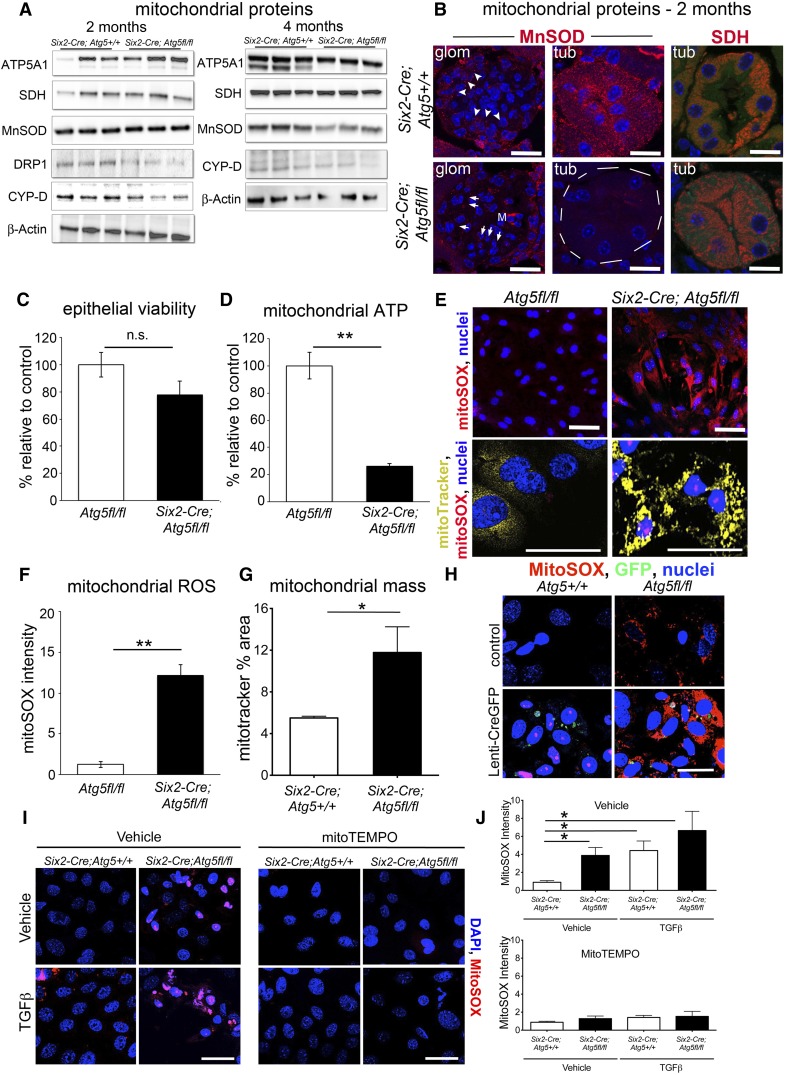
Because of the changes to mitochondrial morphology and excessive ROS generation early in the disease course, expression of mitochondrial proteins involved in energy production, antioxidant homeostasis, and mitochondrial homeostasis were evaluated. In whole kidney, the regulator of mitochondrial fission and dynamics, Dynamin related protein-1 (DRP1) and the transition pore regulator α-cyclophilin-D were downregulated at 2 months whereas the mitochondrial antioxidant enzyme Mn-SOD was downregulated later in disease. Electron transport enzymes were unchanged or modestly upregulated (Figure 5A). In tissue sections at 2 months, individual mutant tubules lacked Mn-SOD but retained other mitochondrial markers, whereas MnSOD was upregulated in interstitial cells, including mesangial cells, explaining why whole kidney levels appeared unchanged. Other markers, such as succinate dehydrogenase, showed loss of normal basolateral polarity in the tubule (Figure 5B). To evaluate the direct effect of ATG5 mutation on kidney epithelial mitochondrial morphology and function, primary cultures of proximal tubule cells (PTECs) were generated from 2-month-old mice. PTECs were evaluated for viability, mitochondrial ATP, and mitochondrial ROS generation, as well as mitochondrial morphology (Figure 5, C–G). Although viability was similar, epithelial cell mitochondrial size was increased whereas ATP generation was markedly reduced and mitochondrial ROS generation markedly increased in Atg5 mutant PTECs. To determine whether this was a direct response to loss of Atg5, primary PTECs were generated from healthy Atg5fl/fl or Atg5+/+ mice. These cells were transduced with a virus, resulting in stable expression green fluorescent protein (GFP) and Cre recombinase that recombines at loxP sites (Figure 5H). Ten days later, PTECs were evaluated for mitochondrial ROS generation. Atg5fl/fl PTECs expressing the Cre recombinase now produced high levels of ROS. Furthermore, an ROS scavenger, MitoTEMPO, which specifically acts in mitochondria, was applied to PTECs derived from 2-month-old Six2Cre; Atg5fl/fl and control kidneys. In quiescent conditions or in conditions of TGF-β–induced epithelial stress, the production of mitochondrial ROS was completely suppressed (Figure 5, I and J), confirming the specificity of our observations.
Figure 5.
Kidney epithelium shows specific defects in mitochondrial function resulting in abnormal levels of superoxide generation. (A) Western blots showing levels of mitochondrial proteins in wild-type and mutant kidneys at 2 and 4 months. (B) Images of kidneys at 2 months showing selective loss of MnSOD in tubules and also GECs (arrowhead=expressed; arrow=reduced expression), whereas SDH expression remains in mutant tubules but basolateral cellular distribution of SDH is lost. (C and D) Viability, mitochondrial ATP generation in primary PTEC cultures from control and mutant 2-month-old kidneys. (E–G) Images and results showing mitochondrial ROS generation by mitoSOX fluorescence and mitochondrial content/morphology by mitoTracker fluorescence in live cultures of primary PTEC cultures from control and mutant 2-month-old kidneys. (H) Images of PTECs from wild-type and Atg5fl/fl kidneys 10 days after treatment with Lenti-GFP-Cre virus, labeled with mitoSOX to detect mitochondrial ROS. Successful transduction of cells to activate recombination of Atg5 at loxP sites can be detected by the presence of GFP. Note only Atg5fl/fl cells that have undergone recombination produce high levels of mitochondrial ROS. (I and J) Images and quantification of mitochondrial ROS generation in wild-type and mutant PTECs from 2-month-old kidneys showing spontaneous ant TGF-β–induced ROS generation, which is completely inhibited by incubation of mitoTEMPO. Bar=25 µm. Data are mean±SEM. n=3–6/group. **P<0.01; *P<0.05.
Mutation of ATG7 in Kidney Epithelium Also Results in FSGS
As further supportive evidence that FSGS can be caused by deficiency of autophagy, the Six2-Cre BAC transgene was bred to mice with the loxP recombination sites flanking the 14th exon of both Atg7 alleles.18 Atg7 is also required for autophagosome formation.19 Six2-Cre; Atg7fl/fl mice also developed typical features of FSGS by 4 months with moderate albuminuria (Supplemental Figure 5, A–G). The extent of sclerosis in glomerular lesions was similar to Atg5 mutant mice (Supplemental Figure 5, H and I), but the presence of GEC hypertrophy and hyperplasia were significantly lower (Supplemental Figure 5J) and hyalinosis was not seen. In addition, the severity of tubular injury was significantly lower (Supplemental Figure 5K). After 6 months there was no change in kidney function as assessed by plasma creatinine levels, and mice survived to 1 year.
Patients with Primary (Idiopathic) FSGS Have Mitochondrial Abnormalities
To determine the relevance of our observations to human FSGS, we retrospectively evaluated archival renal biopsy specimens from a cohort of patients with primary FSGS (not otherwise specified) for evidence of changes to mitochondria in podocytes and tubular epithelium by electron microscopy. The ultrastructural findings in 10 renal biopsy specimens of human FSGS and 10 normal kidney biopsy specimens were compared using mitochondrial morphometry. Podocyte mitochondria in FSGS glomeruli were more numerous with modestly increased diameter. In tubules from patients with FSGS, mitochondria showed a range of morphologic changes, including increased diameter, dysmorphic shape with reduced length, and focal loss of cristae (Supplemental Figure 6).
DISCUSSION
These studies show that preventing autophagy in the kidney epithelium by a single gene mutation is sufficient to recapitulate the characteristic features of FSGS seen in humans, with a pattern of disease evolution more typical of the development of primary human FSGS in adolescents and adults rather than neonates. Strikingly, some of the systemic manifestations of FSGS observed in humans are found in mice with mutations in the autophagy pathway. The deletion of autophagy in kidney epithelium therefore constitutes a new model of FSGS in mice, but it also provides new insights into the pathogenesis of human FSGS.
Although global mutation of the autophagic pathway is neonatally lethal, it remains possible that global dysfunction of the normal autophagic flux may be critically involved in the pathogenesis of kidney disease in humans, and possible therefore that strategies to enhance or modify autophagic flux will limit development or progression of FSGS in humans. The studies presented here provide rationale for studying autophagic flux in patients with FSGS. Autophagy, however, plays important roles in cellular metabolism and homeostasis in other tissues, and these studies show they are disrupted in the nephron by the lack of autophagy. Therefore, therapeutic strategies targeting these metabolic and homeostatic pathways may also prove beneficial for kidney disease. The proximal tubule is a site of high metabolic demand, rich in β-oxidation of fatty acid; it has high numbers of mitochondria and peroxisomes, which are important in detoxification and secretion, as well as other energy-demanding functions, including maintenance of solute gradients.
Excessive generation of ROS by dysfunctional mitochondria has been implicated in kidney diseases, especially diabetic kidney disease.20 Failure to remove aged mitochondria by "‘mitophagy" appears to be a central problem when autophagy is disrupted in kidney epithelium. Therefore, aged mitochondria persist and, instead of generating ATP efficiently, they produce oxygen radicals in excess with limited ATP production. Mitochondria may respond to such a stress by changing shape and fusing, and by inhibiting the mitochondrial permeability transition pore to limit metabolic activity by the action of α-cyclophilin-D.
These studies identified DRP1, which regulates mitochondrial size, as downregulated early in disease, consistent with morphologic data showing increased mitochondrial size and shortening of length. Such morphologic changes are seen in human FSGS, but further studies are required to characterize mitochondria in greater detail in human disease. In addition, the normal complement of mitochondrial antioxidants is downregulated early in disease in some tubules and podocytes and is globally downregulated later in disease, consistent with mitochondria representing potent sources of ROS early in this disease model. Although podocytes are not rich in mitochondria, abnormal podocyte mitochondria can also be seen early in this model of kidney disease, as well as human FSGS. In the disease model, podocytes generate elevated ROS at these early times in disease development, suggesting mitochondrial dysfunction is central to both podocyte and tubular malfunction. In parallel, the development of stressed ER due to persistence of misfolded proteins is sufficient to trigger ROS generation and activation of the cell stress signaling pathway regulated by P38. Autophagy would be expected to remove stressed ER, but in its absence, ER processing of abnormal proteins persists, leading to areas of abnormal ER which generate pathologic signals. Similar to dysfunctional mitochondria, our studies provide evidence for the development of ER stress at early timepoints in disease evolution, although the current studies do not discriminate between ROS generated in the ER compared with mitochondria. Finally, autophagy has recently been linked to the delivery of free fatty acids to mitochondria and peroxisomes from triglyceride-rich vesicles in epithelial and mesenchymal cells, a process known as lipophagy.21 It will be important to understand whether lipophagy is similarly disrupted in this model.
Although further studies will be required, it seems that mitochondrial dysfunction and ROS generation are central problems that result from the lack of normal epithelial autophagy. It is interesting therefore that previous investigations identified antioxidant enzymes in mitochondria that when mutated can result in a proteinuric kidney disease similar to FSGS.22,23 Moreover, rare mutations in mitochondrial genes have been reported in families that develop proteinuria and FSGS.24 Collectively, therefore, these studies place mitochondrial dysfunction as a potentially central problem in primary human FSGS.
Other studies reporting mutation of ATG5 only in podocytes resulted in no significant histologic disease but the presence of very mild albuminuria after 1 year of aging.25 It is interesting to speculate why deletion of podocyte ATG5 resulted in such a minor phenotype whereas deletion in epithelium and podocytes produced a much greater phenotype. One possibility is that the somatic recombination resulting in mutation of Atg5 in podocytes was incomplete in their studies, but more likely is that the evolution of FSGS requires disease in both the tubule and glomerulus, in that if the tubule is stressed, crosstalk between tubule and glomerulus serves to amplify glomerular injury. Consistent with this potential inter-relationship between tubular disease and glomerular disease, recent studies showed ablation of tubule cells led lead to chronic tubulointerstitial disease but also the late development of glomerulosclerosis.26 Further investigation will be required to identify the tubule-glomerular signaling pathways that may regulate such a codependence.
The studies indicate that mutation of ATG7 results in a milder form of disease despite similar levels of glomerulosclerosis after 4 months of age. Although many potential explanations for the difference in disease severity merit exploration, including minor differences in strain, it is striking that GEC hyperplasia and tubular injury were much milder, suggesting that severity of tubular injury may be a major determinant in renal demise.
The studies highlight the importance of mitochondrial dysfunction and the unfolded protein ER stress response in kidney epithelium in the pathogenesis of renal inflammation, fibrosis, and functional demise. It is increasingly recognized that epithelial disease may manifest in several distinct ways to stimulate a fibrosing response. These include cell death, active TGF-β release, DNA damage, cell cycle arrest, and EMT.27 The current studies emphasize that additional upstream changes to epithelial cells, are of themselves sufficient to trigger all manifestations of kidney disease, and that epithelial disease is present at a cellular level in advance of cell death and EMT type phenotypic changes. In fact, autophagy-deficient tubular epithelium failed to express the EMT marker vimentin during disease progression indicating epithelial failure can occur in the absence of the EMT transcriptional program.
It is also noteworthy that genome-wide association studies of CKD progression consistently show that single-nucleotide polymorphisms in or around major secreted proteins of the kidney tubule, including uromodulin, and also major metabolic enzymes, including regulatory subunits of energy sensing mitochondrial enzyme AMP-activated protein kinase.28 It is possible that ER stress caused by dysregulation of the production and export of major synthetic proteins by the kidney epithelium and metabolic dysregulation involving mitochondria contribute to progression in many CKDs.
In conclusion, prevention of autophagic flux in the kidney epithelium and podocytes is sufficient to trigger a degenerative disease of the kidney with many of the manifestations of human FSGS. Mitochondrial dysfunction and ER stress result as a consequence of failure of autophagy, and these are major factors in driving disease progression.
CONCISE METHODS
Animal Studies
Mice containing loxP sites inserted in the Atg5 and Atg7 loci (Atg5flox/flox and Atg7flox/flox) were kindly provided by Dr. Noboru Mizushima and Dr. Masaaki Komatsu, respectively.29 Six2-GFPCre (Six2-Cre) BAC transgenic mice were generated as previously described.9 They were crossed to produce Six2-CreTg; Atg5flox/flox (referred to as Six2-Cre; Atg5fl/fl mice), Six2-CreTg; Atg5+/+ (Six2-Cre;Atg5+/+), Six2-CreTg Atg7flox/flox (Six2-Cre;Atg7fl/fl), and Six2-CreTg; Atg7+/+ (Six2-Cre;Atg7+/+). Six2-Cre mice were also crossed with Rs26R (Gt(ROSA)26Sortm1Sor/J) or Z/red (Tg(CAG-Bgeo-DsRed*MST)1Nagy/J) mice as reported9 to demonstrate recombination. Genotyping was performed by PCR of genomic DNA as described using the following primers: GAATATGAAGGCACACCCCTGAAATG, GTACTGCATAATGGTTTAACTCTTGC, ACAACGTCGAGCAC AGCTGCGCAAGG, and CAGGGAATGGTGTCTCCCAC for Atg5; TGGCTGCTACTTCTGCAATGATGT and CAGGACAGAGACCATCA GCTCCAC for Atg7; and CTAATCGCCATCTTCCAGCAGG and AGGTGTAGAGAAGGCACTTAGC for Six2-Cre.29 Studies were performed under approved Institutional Animal Care and Use Committees protocols at the University of Washington Medical School and Biogen Idec, Inc.
Human Studies
Human biopsy specimens (n=10) with primary idiopathic FSGS (not otherwise specified) and human control biopsy specimens (n=10) with no pathologic alterations were identified retrospectively from the renal pathology database of Columbia University (2010–2014). De-identified archival renal tissues were examined by transmission electron microscopy using standard techniques in a blinded manner according to Columbia institutional review board protocol for research with archival tissues. Digital electron microscopic images were taken using a JEOL 1011 electron microscope (magnification range, ×5000–×15,000). Representative images of podocytes and tubular epithelial cells were evaluated for mitochondrial content and by morphometry. More than 50 podocyte bodies were evaluated per patient, and more than 50 cortical tubule cells per patient were evaluated. Tubule cell subtype was not assessed. All podocyte cell bodies (containing a visible nucleus) were quantified for number of mitochondria per cell and the mitochondrial diameter. All tubules were quantified for mitochondrial diameter. Diameter was defined as the smallest diameter of each mitochondrial cross section.
SDS-PAGE and Western Blotting
SDS-PAGE and Western blotting were performed as described.30 Briefly, kidneys were homogenized in ice-cold MT Cell lysis buffer (Sigma-Aldrich) with proteinase inhibitors (Roche) and 1 mM phenylmethanesulfonyl fluoride (Sigma-Aldrich). Thereafter, tissue lysate was further homogenized by 10 passes through an 18-G needle fitted to a 1-ml syringe. Samples were centrifuged for 10 minutes at 13000 rpm and the protein concentration determined. Cell cultures were lysed in RIPA buffer by similar methods to whole kidney lysis. Aliquots of cell extracts containing 20–50 µg of protein were prepared in SDS-sample buffer and subjected to SDS-PAGE. Proteins were transferred to nitrocellulose paper. After transfer, immunodetection was performed as described.30 Antibodies were diluted at 1:1000 in blocking buffer. Bands were detected by the enhanced chemiluminescence method (Pierce) as recommended by the manufacturer and luminescence captured by FluorChem-Q (Alpha Innotech). Primary antibodies against the following antigens were used: LC3 (Novus Biologicals), p62 (BD Transduction Laboratories), p-eIF2α (Cell Signaling Technology), CHOP (Cell Signaling Technology), GADD34 (Santa Cruz Biotechnology), BiP (Cell Signaling Technology), CREB-2/ATF4 (Santa Cruz Biotechnology), OAT-1 (LifeSpan Biosciences), DRP-1 (BD Biosciences), SDH, ATPA5, MnSOD (Abcam, Inc.), MPV17L (Dr. Erwin Bottinger), Actin (Santa Cruz Biotechnology), p-P42/44, p-JNK, and p-P38 (Cell Signaling Technology). To detect urinary proteins, 3 µl of urine from timed collections was prepared in SDS-sample buffer and separated on a 5%–15% gradient gel. The gel was fixed and stained using a commercial kit for silver stain (Pierce) for the presence of protein.
Urine and Plasma Analysis
Urine albumin was measured using Albuwell M kit (Exocell, Philadelphia, PA). Urine and plasma creatinine levels were measured using Creatinine Liquid Reagents Assay (DIAZYME, San Diego, CA). Plasma albumin, BUN, triglycerides, cholesterol, lipoprotein fractions, and free fatty acids were measured from 50-µl aliquots using an Olympus au640 Chemistry Analyzer.
Histologic Analysis
Kidneys were resected after systemic perfusion with ice-cold PBS. Paraffin-embedded sections of kidney fixed with neutral-buffered formalin were used for periodic acid-Schiff, methenamine silver, and sirius red staining. Sclerosis index was evaluated according to the literature. Briefly, in observer-blinded analysis, the extent of sclerosis in 50 sequential glomeruli per animal in silver-stained kidney sections was determined (scarring plus capillary loop destruction) and scored from grade 0 to 4, where 0=none, 1=0%–25%, 2=25%–50%, 3=50%–75%, and 4=>75%. Sclerosis index is the average. Glomerular hypercellularity and mesangial matrix expansion were evaluated in 50 sequential glomeruli where mesangial cells per pole were counted and mesangial areas with ≥4 mesangial cells were considered hypercellular and matrix expansion was given a score of 1–4 as reported.31
Electron Microscopy
The protocol for tissue preparation and staining for transmission electron microscopy has been described previously.32 Grids were scanned using a Philips 410 electron microscope for mouse studies (Philips Export BV, Eindhoven, The Netherlands) and a JEOL 1011 electron microscope (Japan) for human studies.
Immunofluorescence
Kidneys were fixed with periodate-lysine-paraformaldehyde fixative. After immersion in PBS, including 18% sucrose at 4°C overnight, they were embedded and frozen in optimal cutting temperature compound (Sakura Finite, Torrance, CA). Immunofluorescence detection of antigens was performed using methods described30 using the following antibodies or lectins: anti-WT1 (sc-192; 1:100; Santa Cruz Biotechnology), F4/80 (1:400; Life Technologies), anti-CD31 (1:200; BD Biosciences), anti–α−smooth muscle actin-Cy3 (1:400; Sigma-Aldrich), fluorescein conjugated Lotus Tetragonolobus Lectin (Vector Laboratories, Burlingame, CA), anti-Rab9 (1:100; Cell Signaling Technology), anti-LAMP2-FITC (1:200; eBioscience), anti-vimentin (1:500; Abcam, Inc.), and anti-LC3 (1:200; Novus Biologicals). Where appropriate affinity purified FITC or Cy3 conjugated secondary antibodies were applied (1:400–1:1000; Jackson ImmunoResearch Laboratories, West Grove, PA). Images were captured by confocal fluorescence microscopy (Nikon A1R Confocal; Nikon, Japan). Captured images were quantified morphometrically to measure area of fluorescent signal as described.30 ROS generation was detected in snap-frozen kidneys by immersing freshly prepared cryosections in 2 μg/ml of dihydroethidium (Calbiochem, Nottingham, UK) in PBS using identical conditions for control and diseased tissue sections. Images were captured by confocal fluorescence microscopy (Nikon A1R Confocal; Nikon, Japan) and quantified by NIH ImageJ.33
Epithelial Cell Culture and Assays
Two-month-old kidneys were harvested and digested to release tubules as described.34 Whole tubules were cultured in tubule medium30 for 7 days until confluent monolayers were generated. Purity of epithelial cells of proximal tubule origin has been validated previously.34 PTECs were lysed with protein lysis buffer or evaluated for mitochondrial ROS production or mitochondrial ATP production using Mitosox (Invitrogen) and Mitochondrial ToxGlo (Promega) assays, respectively, according to manufacturer instructions, whereas live mitochondrial staining was achieved using MitoTracker (Life Technologies). Viability was determined before lysing cells following Mitochondrial ToxGlo protocol. In some experiments, TGF-β (3 ng/ml) (Peprotech) was added 6 hours before analysis, and in other experiments mitoTEMPO (100 µM) (Santa Cruz Biotechnology) was applied for 2 hours before analysis. Intensity of signal was determined by confocal live imaging and quantitated by ImageJ software as described.35 In some experiments, primary cell cultures with treated with Lenti-eGFP-cre (1×107TU/mL; Biogenova Corporation) with 0.2 µg/ml of polybrene and cell transduction was enhanced by centrifugation for 30 minutes at 1600 rpm.
Quantitative RT-PCR
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA). cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative RT-PCR assay was performed using an ABI7900HT (Applied Biosystems, Carlsbad, CA) and iTaq SYBR Green Supermix with ROX (Bio-Rad). Sequences of the primers are as follows: Ddit3-forward; AAGATGAGCGGGTGGCAGCG, -reverse; TGCACGTGGACCAG GTTCTGC, Trb3-forward; CAGGACAAGATGCGAGCTAC, -reverse; TAGGACTGGACACTTGGCAT, Atf4-forward; TCGATGGTCT GTTTCGAATG; Reverse; GCCAATTGGGTTCACTGTCT. Primer pairs for Tnfa, Il1b, Il6, Nos2, Ccl2, Ccr5, Mip1a, Mip2, and Gapdh were used as previously reported.36 Specificity of each primer pair was confirmed by verification of single PCR product with expected size in agarose gel electrophoresis. Target genes were normalized by glyceraldehyde 3-phosphate dehydrogenase expression. The mRNA expression was calculated using the 2−ΔΔCt method and expressed as a fold difference relative to the control group.
Statistical Analyses
Data are presented as mean±SEM. Differences between groups were assessed by two-tailed t test or one-way ANOVA. Differences in survival were assessed by Wilcoxon test using the Kaplan–Meier analysis. A P value<0.05 was considered to represent statistically significant differences.
DISCLOSURES
J.S.D. is employed by and holds stock in Biogen Idec, Inc., is on the scientific advisory board for Promedior, Inc. and Regulus Therapeutics, has stock options with Promedior, Inc., is a cofounder of Muregen LLC, and has recently consulted for Abbvie, Bristol-Myers Squibb, and Boehringer Ingelheim.
Supplementary Material
Acknowledgments
We thank Dr. William Altemeier (University of Washington), Dr. Didier Portilla (University of Arkansas for Medical Sciences), Dr. Shelia Violette (Biogen Idec, Inc.), and Dr. Erwin Bottinger (Mount Sinai School of Medicine) for reagents and advice; Dr. Noburu Mizushima (University of Tokyo) and Dr. Masaaki Komatsu (Tokyo Metropolitan Institute of Medical Science) for mice; and the Lynn and Mike Garvey Microscopy Core Facility.
This work was supported in part by the Institute for Stem Cell and Regenerative Medicine at University of Washington; a Nephcure Foundation career development grant; National Institutes of Health grants DK94768, DK93493, DK84077, and TR000504; and American Heart Association grant 12040023 (J.S.D.), as well as an Overseas Research Grant from Mochida Memorial Foundation for Medical and Pharmaceutical Research (T.K.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Could Autophagic Exhaustion Be a Final Common Pathway for Podocytopathy in FSGS?,” on pages 999–1001.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013111202/-/DCSupplemental.
REFERENCES
- 1.Löwik MM, Groenen PJ, Levtchenko EN, Monnens LA, van den Heuvel LP: Molecular genetic analysis of podocyte genes in focal segmental glomerulosclerosis—a review. Eur J Pediatr 168: 1291–1304, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollak MR: The Genetic Basis of FSGS and Steroid-Resistant Nephrosis. Seminars in Nephrology, New York, Elsevier, 2003, pp 141–146 [DOI] [PubMed] [Google Scholar]
- 3.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McVicar M, Exeni R, Susin M: Nephrotic syndrome and multiple tubular defects in children: an early sign of focal segmental glomerulosclerosis. J Pediatr 97: 918–922, 1980 [DOI] [PubMed] [Google Scholar]
- 5.Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, Li J, Tran PT, Kaimal V, Huang X: MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Translat Med 4: 121ra118–121ra118, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bock F, Shahzad K, Wang H, Stoyanov S, Wolter J, Dong W, Pelicci PG, Kashif M, Ranjan S, Schmidt S, Ritzel R, Schwenger V, Reymann KG, Esmon CT, Madhusudhan T, Nawroth PP, Isermann B: Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66Shc. Proc Natl Acad Sci U S A 110: 648–653, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes JM, Coughlan MT, Cooper ME: Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57: 1446–1454, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Lieberthal W: Macroautophagy: A mechanism for mediating cell death or for promoting cell survival? Kidney Int 74: 555–557, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV: The podocyte’s response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol 304: F333–F347, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Cho MH, Hong EH, Lee TH, Ko CW: Pathophysiology of minimal change nephrotic syndrome and focal segmental glomerulosclerosis. Nephrology (Carlton) 12[Suppl 3]: S11–S14, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Carroll KS, Hanna J, Simon I, Krise J, Barbero P, Pfeffer SR: Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science 292: 1373–1376, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Cardaioli E, Malfatti E, Battisti C, Da Pozzo P, Rubegni A, Gallus GN, Malandrini A, Federico A: Sporadic myopathy, myoclonus, leukoencephalopathy, neurosensory deafness, hypertrophic cardiomyopathy and insulin resistance associated with the mitochondrial 8306 T>C MTTK mutation. J Neurol Sci 321: 92–95, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Inagi R: Endoplasmic reticulum stress as a progression factor for kidney injury. Curr Opin Pharmacol 10: 156–165, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Kim H-R, Lee G-H, Cho EY, Chae S-W, Ahn T, Chae H-J: Bax inhibitor 1 regulates ER-stress-induced ROS accumulation through the regulation of cytochrome P450 2E1. J Cell Sci 122: 1126–1133, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Brewer JW, Diehl JA: PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci U S A 97: 12625–12630, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotamisligil GS: Inflammation and metabolic disorders. Nature 444: 860–867, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T: Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 169: 425–434, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y: A protein conjugation system essential for autophagy. Nature 395: 395–398, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Kuroki T, Isshiki K, King GL: Oxidative stress: The lead or supporting actor in the pathogenesis of diabetic complications. J Am Soc Nephrol 14[Suppl 3]: S216–S220, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Eid N, Ito Y, Maemura K, Otsuki Y: Elevated autophagic sequestration of mitochondria and lipid droplets in steatotic hepatocytes of chronic ethanol-treated rats: An immunohistochemical and electron microscopic study. J Mol Histol 44: 311–326, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Weiher H, Noda T, Gray DA, Sharpe AH, Jaenisch R: Transgenic mouse model of kidney disease: insertional inactivation of ubiquitously expressed gene leads to nephrotic syndrome. Cell 62: 425–434, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Zwacka RM, Reuter A, Pfaff E, Moll J, Gorgas K, Karasawa M, Weiher H: The glomerulosclerosis gene Mpv17 encodes a peroxisomal protein producing reactive oxygen species. EMBO J 13: 5129–5134, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emma F, Montini G, Salviati L, Dionisi-Vici C: Renal mitochondrial cytopathies. Int J Nephrol 2011: 609213, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstädt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB: Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120: 1084–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grgic I, Duffield JS, Humphreys BD: The origin of interstitial myofibroblasts in chronic kidney disease. Pediatr Nephrol 27: 183–193, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman SL, Sheppard D, Duffield JS, Violette S: Therapy for fibrotic diseases: Nearing the starting line. Sci Translat Med 5: 167sr161–167sr161, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Köttgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Chen YD, de Boer IH, Haritunians T, Lumley T, Sarnak M, Siscovick D, Benjamin EJ, Levy D, Upadhyay A, Aulchenko YS, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, Chasman DI, Paré G, Ridker PM, Kao WH, Witteman JC, Coresh J, Shlipak MG, Fox CS: Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41: 712–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N: Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441: 885–889, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Ren S, Johnson BG, Kida Y, Ip C, Davidson KC, Lin S-L, Kobayashi A, Lang RA, Hadjantonakis A-K, Moon RT, Duffield JS: LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proc Natl Acad Sci U S A 110: 1440–1445, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.el Nahas AM, Bassett AH, Cope GH, Le Carpentier JE: Role of growth hormone in the development of experimental renal scarring. Kidney Int 40: 29–34, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Li B, Castano AP, Hudson TE, Nowlin BT, Lin S-L, Bonventre JV, Swanson KD, Duffield JS: The melanoma-associated transmembrane glycoprotein Gpnmb controls trafficking of cellular debris for degradation and is essential for tissue repair. FASEB J 24: 4767–4781, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nijmeh J, Moldobaeva A, Wagner EM: Role of ROS in ischemia-induced lung angiogenesis. Am J Physiol Lung Cell Mol Physiol 299: L535–L541, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C-F, Chiang W-C, Lai C-F, Chang F-C, Chen Y-T, Chou Y-H, Wu T-H, Linn GR, Ling H, Wu K-D, Tsai TJ, Chen YM, Duffield JS, Lin SL: Transforming growth factor β-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol 182: 118–131, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Duffield JS, et al. : Anti-microRNA-21 prevents kidney failure in Alport syndrome by stimulating metabolic pathways. J Clin Invest, in press, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campanholle G, Mittelsteadt K, Nakagawa S, Kobayashi A, Lin S-L, Gharib SA, Heinecke JW, Hamerman JA, Altemeier WA, Duffield JS: TLR-2/TLR-4 TREM-1 signaling pathway is dispensable in inflammatory myeloid cells during sterile kidney injury. PLoS ONE 8: e68640, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.