Abstract
Vascular endothelial growth factor A (VEGFA) production by podocytes is critical for glomerular endothelial health. VEGFA is also expressed in tubular epithelial cells in kidney; however, its physiologic role in the tubule has not been established. Using targeted transgenic mouse models, we found that Vegfa is expressed by specific epithelial cells along the nephron, whereas expression of its receptor (Kdr/Vegfr2) is largely restricted to adjacent peritubular capillaries. Embryonic deletion of tubular Vegfa did not affect systemic Vegfa levels, whereas renal Vegfa abundance was markedly decreased. Excision of Vegfa from renal tubules resulted in the formation of a smaller kidney, with a striking reduction in the density of peritubular capillaries. Consequently, elimination of tubular Vegfa caused pronounced polycythemia because of increased renal erythropoietin (Epo) production. Reducing hematocrit to normal levels in tubular Vegfa–deficient mice resulted in a markedly augmented renal Epo production, comparable with that observed in anemic wild-type mice. Here, we show that tubulovascular cross-talk by Vegfa is essential for maintenance of peritubular capillary networks in kidney. Disruption of this communication leads to increased renal Epo production and resulting polycythemia, presumably to counterbalance microvascular losses.
Keywords: peritubular capillary rarefaction, erythropoietin, polycythemia, vascular, endothelial growth factor inhibition, kidney
The kidney is a highly organized structure containing complex vascular networks, which are critical for its function.1 Blood from the primary renal arterial system is filtered across glomerular capillary loops before entering efferent arterioles. Peritubular capillaries run off the efferent arterioles and are situated in close contact with the renal tubules, whereas juxtamedullary vessels branching off the efferent arterioles form the vasa recta.1 The unique organization of the renal vasculature leads to substantial differences in oxygen tension, with partial pressures of oxygen varying from between 30 and 50 mmHg in the cortex (CTX) to below approximately 10–20 mmHg in the medulla.2,3 Importantly, insufficient delivery of oxygen to the kidney in relation to its oxygen consumption promotes or further augments hypoxia. Such changes in renal oxygen tension are sensed by renal erythropoietin (Epo)-producing cells (REPs), which respond by amending their Epo production to enhance erythropoiesis and hence, the oxygen-carrying capacity of the blood.4–6 As in any organ, oxygenation is intimately linked to the generation and maintenance of a broad vascular network. However, it is currently not known how postglomerular vascular networks develop and are maintained. Vascular endothelial growth factor A (VEGFA) plays a critical role in blood vessel development by promoting vasculogenesis and angiogenesis. In addition, VEGFA and its receptors (VEGFR) remain expressed in a subset of cells throughout adulthood, where the system exerts key functions, often related to maintenance of specialized vascular beds.7–10
Collectively, our understanding of the extraglomerular VEGFA system within the kidney and its potential role in development and maintenance of the renal microcirculation is incomplete. Using a range of targeted transgenic strategies, we examined the physiologic role of extraglomerular Vegfa in kidney. Vegfa expression was found in renal tubular epithelial cells, with the highest expression in the thick ascending limb (TAL), presumably allowing tubulovascular cross-talk to its receptor (Kdr/Vegfr2) located almost exclusively on peritubular capillary endothelial cells in the postglomerular vasculature. Excision of Vegfa from renal tubules was used to examine its role in the maintenance of the peritubular microvasculature. Loss of tubular Vegfa resulted in a substantial reduction in peritubular capillary density and promoted marked polycythemia. These findings are relevant for understanding how the renal microvasculature is maintained and stress the important physiologic role of tubular Vegfa in mediating cross-talk between the tubular system and the vasculature in kidney.
Results
Segmental Expression of Vegfa and Its Receptors along the Nephron and in Renal Microvasculature
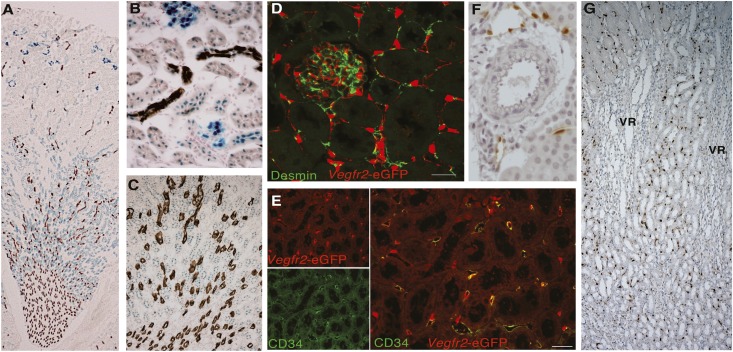
Intrarenal localization of Vegfa was evaluated in animals that express β-galactosidase (LacZ) in the 3′ untranslated region of the Vegfa gene.11 High expression of Vegfa in podocytes was noted as reported previously (Vegfa-LacZ in blue in Figure 1).12 In 6- to 14-week-old mice, the tubular expression of Vegfa localized most abundantly to the TAL, with lesser expression in proximal and distal cortical tubules (Figure 1, A–C). Aquaporin 2 was used as a collecting system marker and stained in brown (Figure 1, A–C). Changes in tubular Vegfa expression were noted during development, with the highest expression in the medulla (Supplemental Figure 1). Transgenic mice with enhanced green fluorescent protein (eGFP) inserted into exon 1 of the Vegfr2 gene were used to evaluate the intrarenal distribution of the receptor.13 Here, Vegfr2 gene regulatory elements drive eGFP expression. The eGFP signal localizes predominantly to the nuclei and cytoplasmic domains of Vegfr2-expressing cells. Overall, Vegfr2 expression showed a restricted pattern, localizing to endothelial cells in the peritubular capillaries, in addition to expression in glomerular capillary loops, which is in line with previous observations (Figure 1, D–G).14,15 Membranous markers of fibroblasts and endothelial cells were used to confirm the cellular localization of Vegfr2 to endothelial cell populations. Throughout the kidney, Vegfr2-driven eGFP expression did not localize with cells expressing desmin, a marker of mesangial cells and fibroblasts in kidney (Figure 1D).16 However, Vegfr2-driven eGFP signal localized to peritubular capillary cells expressing the membranous endothelial marker, CD34 (Figure 1E), as well as glomerular capillary loops (Supplemental Figure 2A). Expression of the Vegfr2 receptor was absent from arteries, arterioles, and vasa recta capillary bundles of the outer medulla (OM) (Figure 1, F and G), whereas some expression was noted in larger veins (Supplemental Figure 2, B and C).
Figure 1.
Localization and characterization of the intrarenal Vegfa system. (A–C) Vegfa-driven LacZ expression (blue) coupled with immunostaining for Aquaporin 2 (brown) in (A) whole kidney, (B) higher magnification of CTX, and (C) OM. Original magnification, ×25 in A; ×400 in B; ×200 in C. (D–F) Representative confocal images of kidney sections immunostained for Vegfr2-driven eGFP expression (red) and costained in green with (D) desmin, a marker of fibroblast and mesangial cells, or (E) the endothelial cell marker CD34. Scale bar, 23.71 μm. (F) Cross-section of the XY plane of kidney showing absence of Vegfr2-driven eGFP expression in the renal macrovasculature. (G) Cross-section of the XZ plane of kidney showing localization of Vegfr2-driven eGFP expression to peritubular capillaries but not vasa recta capillary bundles of the OM (VR). Original magnification, ×100.
Tubular Vegfa Deletion Produces a Smaller but Histologically Normal Kidney
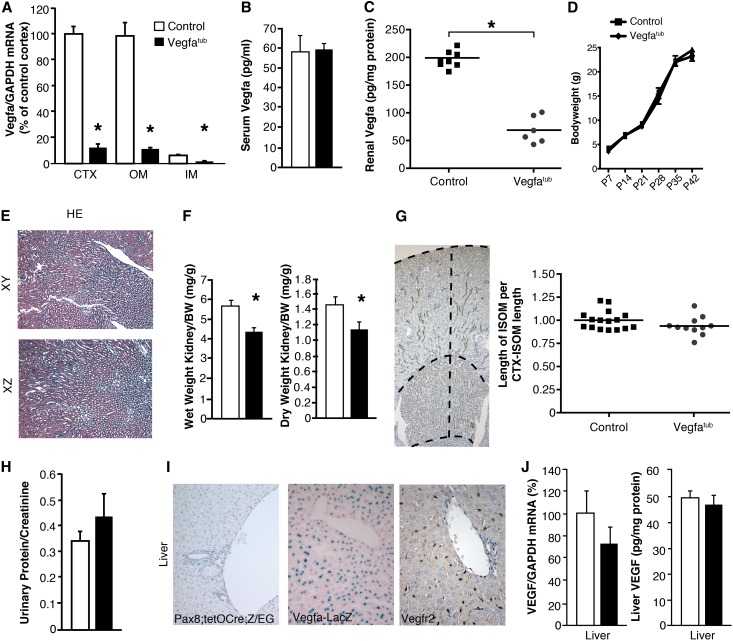
To evaluate the role of tubular Vegfa in microvascular development, we bred mice homozygous for two floxed Vegfa alleles17 to bitransgenic mice carrying Pax8-reverse tetracycline-controlled transactivator (rtTA)18 and tetracycline-responsive promoter element placed in front of CRE recombinase (Tet-O-Cre).19 Subsequent crosses and induction with doxycycline, generated mice with selective deletion of the Vegfa gene from renal tubules (Vegfatub). Vegfatub mice receiving doxycycline from conception had an approximately 60% reduction in genomic Vegfa in kidney CTX and OM (Supplemental Figure 3), whereas renal Vegfa mRNA expression was reduced substantially farther (approximately 85%) (Figure 2A). No change was observed in serum Vegfa levels, whereas a marked reduction was found in renal Vegfa protein abundance (Figure 2, B and C). Deletion of Vegfa in tubules did not affect body weight (Figure 2D), and the kidneys seemed normal histologically by light microscopy (Figure 2E). However, the kidney weight to body weight ratio decreased. This difference is particularly visible in Vegfatub mice after 8 weeks (Figure 2F). The length of the inner stripe of OM (ISOM), which contains the majority of vasa recta bundles, was measured as a ratio to the length between the CTX and the end of the ISOM. Here, no changes in the relative length of the ISOM were observed between the groups, which is in line with absent expression of Vegfr2 in the vasa recta bundles (Figure 2G). In addition, no change in urinary protein excretion was found (Figure 2H). Although Cre recombinase activity has been reported in periportal hepatocytes of transgenic Pax8-rtTA;Tet-O-Cre mice18 and both Vegfa and its Vegfr2 receptor are expressed in liver, we did not detect any hepatic Cre activity using reporter Z/EG mice (Figure 2I) or changes in hepatic Vegfa mRNA expression or Vegfa protein abundance (Figure 2J).
Figure 2.
Tubular Vegfa deletion produces a small but histologically normal kidney. (A) Renal Vegfa mRNA expression after early tubular Vegfa excision by doxycycline in different kidney zones: CTX, OM, and inner medulla (IM; n=7–11 per group). (B and C) Serum (n=5–7 per group) and renal Vegfa (n=6–8 per group) concentrations in control and Vegfatub mice. (D) Body weight measured from postnatal days 7–42 (n=6–11 per group). (E) Hematoxylin and eosin (HE) stain of kidney from Vegfatub mice. Original magnification, ×100. (F) Kidney weight to body weight ratio in 8-week-old mice (n=5–8 per group). (G) Nkcc2 staining was used to evaluate the length of ISOM as a ratio of the length from CTX to the bottom of ISOM (CTX-ISOM; n=8–9 per group). Original magnification, ×50. (H) Urinary protein-to-creatinine ratio in 8-week-old Vegfatub mice and controls (n=6 per group). (I) Excision efficiency of Pax8 in liver (no excision detected). Expression of Vegfa and Vegfr2 in liver. Original magnification, ×400. (J) Hepatic Vegfa mRNA (n=13 per group) and protein abundance (n=6–8 per group) in Vegfatub mice and littermate controls. Data are presented as means±SEMs. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P<0.05 relative to control animals.
Specific Deletion of Vegfa from Tubular Epithelia Impairs Development of Peritubular Capillary Networks
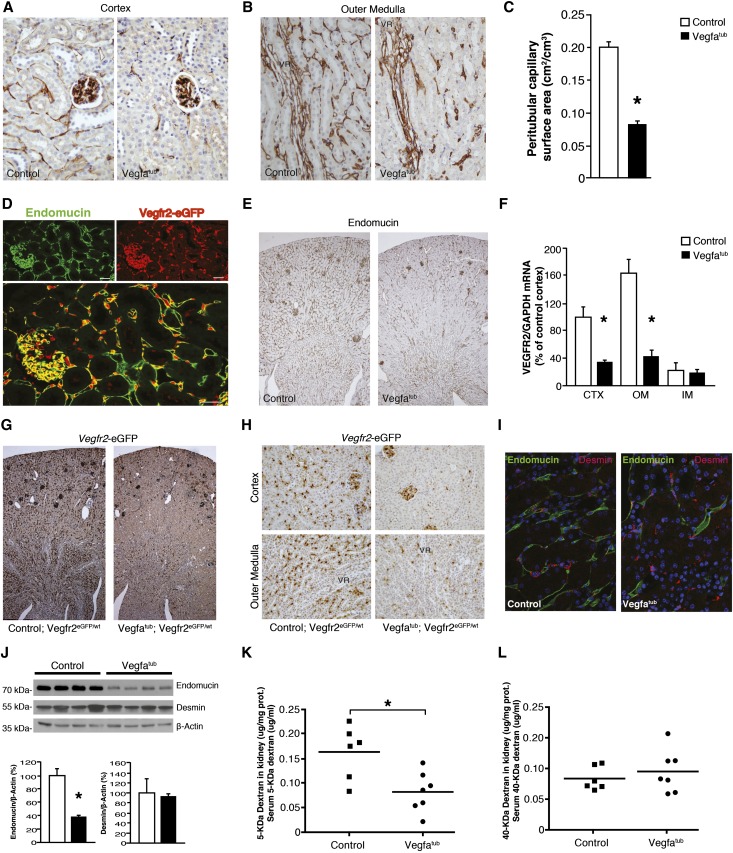
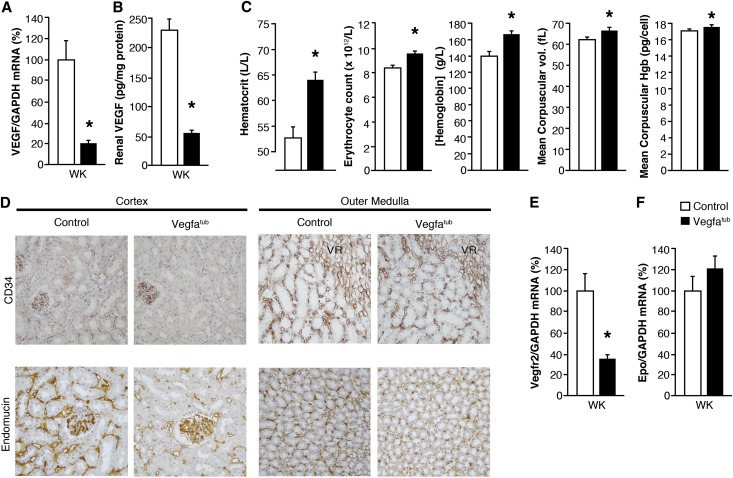
CD34 was used as a marker of endothelial cells within kidney. As shown in Figure 1E and Supplemental Figure 2A, CD34 localized to the peritubular capillaries, which also express eGFP driven by the Vegfr2 gene. Absence of tubular Vegfa led to a marked capillary rarefaction in CTX and OM (Figure 3, A and B). To quantitate the capillary loss, morphometric analysis was performed on cortical peritubular capillaries (Figure 3C). Here, a marked reduction was found in the peritubular capillary surface area of Vegfatub mice (0.085±0.003 versus 0.202±0.0009 cm2/cm3, P<0.05). In the kidney, endomucin localizes to the same peritubular capillaries as Vegfr2 but also seems expressed in all veins, while being absent from arteries and arterioles (Figure 3D). Thus, endomucin is a suitable alternative endothelial marker, and we used it to confirm the effect of Vegfa depletion on peritubular microvasculature. Again, a pronounced decrease in endomucin staining was observed in Vegfatub mice, consistent with capillary rarefraction (Figure 3E). In addition, deletion of Vegfa from tubules caused a selective decrease in the abundance of vascular endothelial cell markers, most notably in Vegfr2 and Tie2 but not the Tie2 ligands (Figure 3F, Supplemental Figure 4, A–F). To evaluate if the decrease in Vegfr2 resulted from downregulation of mRNA abundance or loss of peritubular capillary endothelial cells, mice expressing eGFP driven by the Vegfr2 gene (Vegfr2eGFP/wt) were crossed with Vegfatub mice to generate Vegfatub;Vegfr2eGFP/wt mice. Tubular Vegfa gene excision at embryonic day 0 led to a striking loss of Vegfr2-eGFP–positive cells throughout the kidney (Figure 3, G and H). Desmin is expressed by fibroblasts, which are found in close association with peritubular capillaries (Figure 1D, Supplemental Figure 4G). Reducing tubular Vegfa decreases capillary density without affecting the number of desmin-positive cells (Figure 3I). A marked reduction in endomucin was confirmed by Western blotting, whereas desmin remained unchanged (Figure 3J). To evaluate changes in permeability and/or perfusion of the kidney, animals were injected with 5 and 40 kD fluorescently labeled dextrans (Figure 3, K and L). A significant reduction in the amount of 5 kD dextran extravasated from blood into the kidney was found, suggesting that vessel permeability in the kidney may be altered.
Figure 3.
Deletion of tubular Vegfa promotes capillary rarefaction of postglomerular vessels. (A) Representative images of CD34 expression in cortical vessels of Vegfatub mice and littermate controls (n=5–6 per group). Original magnification, ×400. (B) CD34 expression in OM vessels in Vegfatub mice and controls (n=5–6 per group). Original magnification, ×400. (C) Morphometric analysis of surface area of CD34-positive peritubular capillaries in kidney CTX from Vegfatub mice and littermate controls (n=5–6 per group). (D) Localization of Vegfr2-driven eGFP expression (red) and endomucin (green) in kidney CTX. Scale bar, 23.71 μm. (E) Representative images of endomucin expression in controls and Vegfatub (n>6 per group). Original magnification, ×50. (F) Vegfr2 mRNA expression in different kidney zones after early tubular Vegfa excision (n=7–11 per group). (G) Vegfr2-driven eGFP expression in controls and Vegfatub mice. Original magnification, ×50. (H) Higher-magnification (×400) representative images of Vegfr2-driven eGFP expression in kidney CTX and OM from animals lacking tubular Vegfa and controls. (I) Colocalization of desmin (red) and endomucin (green) in kidney OM between groups and nuclear stain 4',6-diamidino-2-phenylindole (DAPI; blue). Original magnification, ×400. (J) Immunoblots and corresponding densiometry of whole-kidney lysates from controls and Vegfatub against endomucin and desmin corrected for β-actin (n=4–5 per group); (K) 5 and (L) 40 kD dextran permeability in kidney corrected for serum concentration (n=6–7 per group). Data are presented as means±SEMs. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; VR, vasa recta capillary bundles of the OM. *P<0.05 relative to control animals.
Increased Renal EPO Production and Polycythemia in Mice Lacking Vegfa in Tubular Epithelial Cells
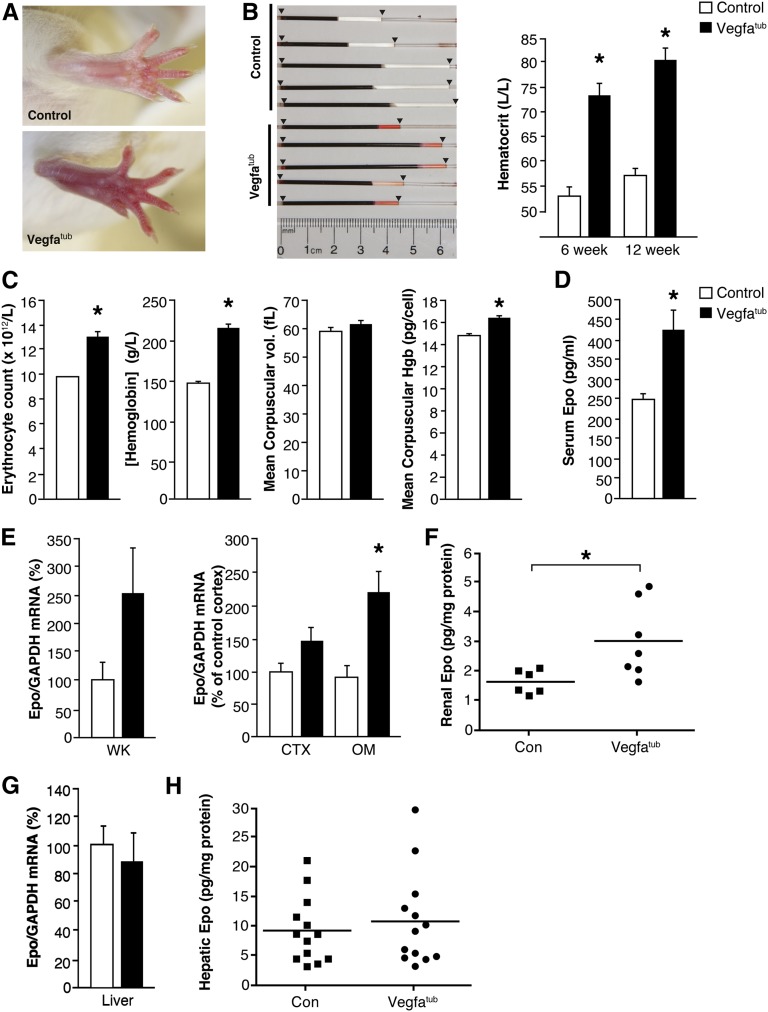
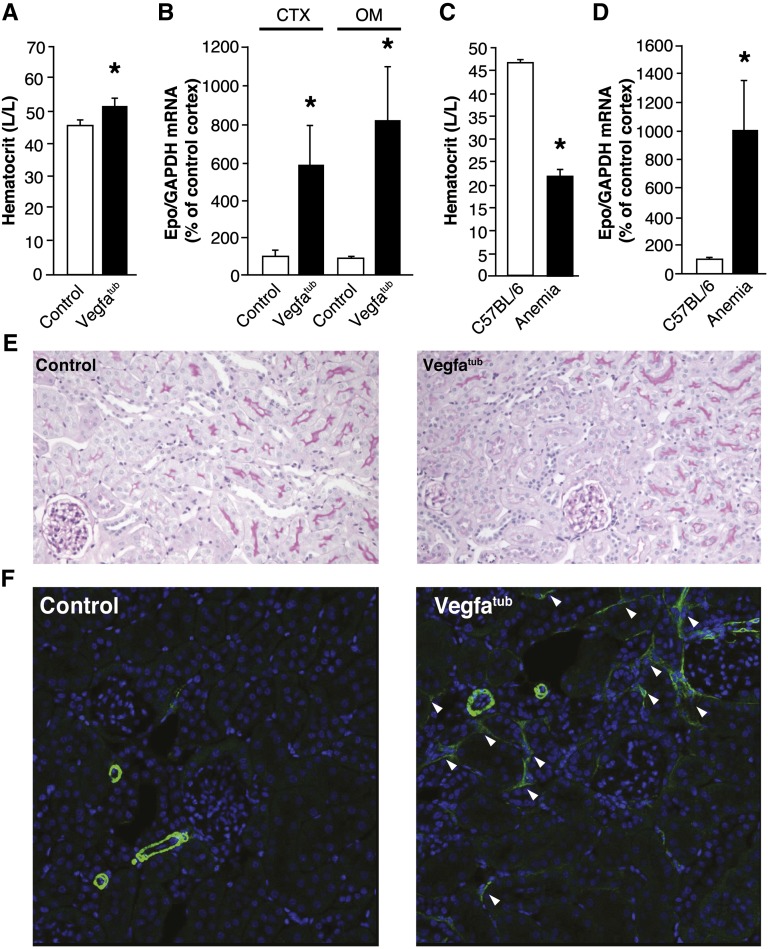
Embryonic tubular Vegfa deletion causes a reddish/pink coloration of extremities (Figure 4A), which was caused by pronounced polycythemia reflected as a marked increase in hematocrit (Figure 4B) because of increased erythrocyte counts (Figure 4C). Moreover, erythrocyte hemoglobin content was also elevated (Figure 4C). The polycythemia observed in Vegfatub mice is associated with increased circulating levels of Epo (Figure 4D) resulting from an augmented mRNA (Figure 4E) and protein expression of intrarenal Epo. By contrast, Epo abundance in liver is unaffected in Vegfatub mice (Figure 4, G and H).
Figure 4.
Deletion of tubular Vegfa promotes marked polycythemia. (A) Erythema due to polycythemia is evident in tubular Vegfa-deficient mice. (B) Hematocrit determined in controls and Vegfatub mice at 6 (n=6–9 per group) and 12 weeks (n=5 per group). (C) Erythrocyte count, hemoglobin concentration, mean corpuscular volume, and mean corpuscular hemoglobin between groups (n=6 per group). (D) Serum Epo concentration (n=6–7 per group). (E) Renal Epo mRNA expression in whole kidney (WK; n=5–7 per group) as well as CTX and OM of controls and Vegfatub mice (n=8–11 per group). (F) Renal Epo content (n=6–7 per group). G) Hepatic Epo mRNA abundance (n=9–13 per group) and (H) hepatic Epo protein content (n=13 per group). Data are presented as means±SEMs. Con, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Hgb, hemoglobin. *P<0.05 relative to control animals.
Normalizing Hematocrit in Vegfatub Mice Markedly Augments Epo Production
The marked polycythemia may reflect a lower than normal delivery of oxygen to the REPs because of loss of blood vessels. If this prediction is correct, further augmentation in renal Epo production is expected if the hematocrit is lowered to normal physiologic levels in Vegfatub mice. To test this hypothesis, blood was withdrawn from polycythemic Vegfatub mice until the hematocrits were reduced toward wild-type levels (52.6±1.8 in Vegfatub versus 46.4±1.1 in controls in percent hematocrit) (Figure 5A). In Vegfatub mice with normalized hematocrit, renal Epo mRNA expression was markedly increased by 600% and 800% in the CTX and OM, respectively (Figure 5B), consistent with a shift in oxygen delivery to the REPs in Vegfatub mice. To compare this dramatic change in Epo expression, we performed a similar experiment in wild-type mice. Here, induction of nonhemolytic anemia was achieved, which was evidenced by a marked reduction in hematocrit (21.9±1.2% versus 47.0±0.1%) (Figure 5C). Correspondingly, nonhemolytic anemia promoted a pronounced increase in the renal Epo expression (Figure 5D), similar to Vegfatub mice with normalized hematocrit. The similar augmentation of Epo production in these two models of anemia and normalization of polycythemia supports the notion that the kidney in Vegfatub mice with physiologic hematocrit concentrations receives less oxygenated blood, almost equivalent to what is observed in anemic mice. Histologically, tubular structures and glomeruli appeared unperturbed by the normalization of hematocrit in Vegfatub mice (Figure 5E). However, accumulation of α-smooth muscle actin (α-SMA) -positive cells was detected in Vegfatub mice. Immunohistochemically, α-SMA shows an expression pattern limited to vascular smooth muscle cells in larger vessels and the descending vasa recta in adult wild-type kidney (Supplemental Figure 5, A and B). Conversely, in kidneys from Vegfatub mice with normalized hematocrits, we observed patches of stellate-shaped α-SMA–positive cells in the peritubular cavities (Figure 5F), which were not observed in control animals (Figure 5F) or Vegfatub mice, without any reductions in hematocrit (data not shown). Overall, kidney injury markers were not changed significantly between the groups, although several of the Vegfatub mice had large increases in the expression of these factors (Supplemental Figure 5C).
Figure 5.
Normalizing hematocrit in Vegfatub mice augments renal Epo production. (A) Hematocrit in control littermates and Vegfatub mice (n=8 per group). (B) Renal Epo mRNA expression in controls and Vegfatub mice after normalization of hematocrit (n=8 per group). (C) Hematocrit in C57BL/6 controls and animals subjected to nonhemolytic anemia. (D) Renal Epo mRNA abundance in control and anemic C57BL/6 mice. (E) Periodic acid–Schiff staining of kidney (n=8 per group). Original magnification, ×200. F) Immunohistochemical staining of α-SMA (green) and the nuclear stain 4',6-diamidino-2-phenylindole (DAPI; blue) in kidney of control animals and Vegfatub mice with normalized hematocrit (n=6 per group). Arrowheads mark stellate-shaped α-SMA–positive cells in the peritubular cavities. Original magnification, ×400. Data are presented as means±SEMs. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P<0.05 relative to control animals.
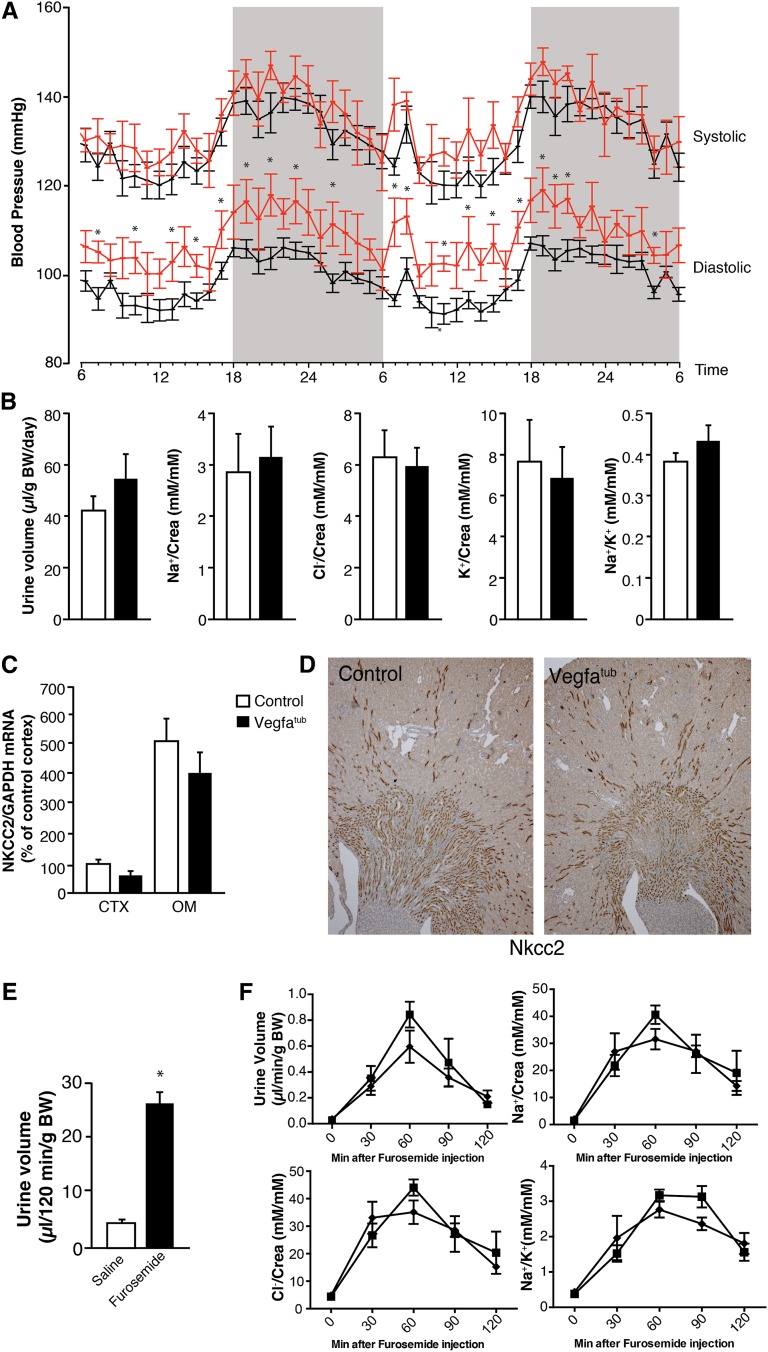
Effect of Tubular Vegfa Elimination on BP and Electrolyte Excretion
To investigate the influence of embryonic tubular Vegfa deletion on BP, mice were implanted with radiotelemetry transmitters. No changes were observed in systolic BP between the groups, although Vegfatub displayed mildly increased diastolic BP when maintained on a normal diet (Figure 6A). No differences in pressure were observed between the groups when dietary NaCl was altered or after chronic infusion of angiotensin II (Supplemental Figure 6). Urinary water and solute excretions were evaluated in animals placed in metabolic cages. No overt polyuria was observed in Vegfatub mice, which is in line with preserved vasa recta function (Figure 6B). The urinary excretion of electrolytes normalized to urinary creatinine also remained unchanged (Figure 6B). Because Vegfa seems to be expressed most abundantly in the TAL, expression of Nkcc2 was evaluated. Renal Nkcc2 mRNA expression remained unchanged (Figure 6C), and no marked changes in Nkcc2 abundance were observed by immunohistochemistry (Figure 6D). Injection of furosemide elicited a marked diuresis in mice (Figure 6E); however, changes in urinary volume and electrolyte excretions after furosemide administration were similar between groups (Figure 6F).
Figure 6.
Effects of early deletion of tubular Vegfa on BP and renal electrolyte handling. (A) Representative BP trace showing mildly increased diastolic BP and no change in systolic BP (n=6–8 per group). (B) Twenty-four–hour urinary volume, urinary electrolyte excretion corrected for creatinine, and Na+/K+ ratio in Vegfatub mice and littermate controls (n=8–9 per group). (C) Nkcc2 mRNA (n=8–11 per group) and (D) Nkcc2 protein expression (n>6 per group). Original magnification, ×50. (E) Furosemide response in mice (n=4 per group). (F) Changes in urinary volume, electrolyte excretion, and Na+/K+ ratio in controls (▪) and Vegfatub mice (♦; n=6 per group) after furosemide administration. Data are presented as means±SEMs. BW, body weight; Crea, creatinine; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P<0.05 relative to control animals.
Late-Onset Vegfa Gene Deletion Results in Less Severe Capillary Rarefaction and Polycythemia
Animals were administered doxycycline from postnatal day 21 to 42 to induce tubular Vegfa deletion. Late-onset Vegfa gene deletion lead to a reduction in renal Vegfa gene expression (Figure 7A) and Vegfa protein abundance (Figure 7B) comparable with embryonically induced Vegfatub mice. There was an increase in hematocrit and related hematologic parameters (Figure 7C), but these findings were not as pronounced as those in Vegfatub mice undergoing early induction. CD34 and endomucin expression were both reduced after late-onset Vegfa deletion (Figure 7D). Similarly, Vegfr2 expression was again downregulated in kidney (Figure 7E), whereas there were no detectable changes in renal Epo expression (Figure 7F).
Figure 7.
Effects of late induction of tubular Vegfa excision. (A) Renal Vegfa mRNA expression (n=4–5 per group) and (B) Vegfa concentrations in whole kidney (WK) after late tubular Vegfa excision by doxycycline in control and Vegfatub mice (n=4–5 per group). (C) Hematocrit, erythrocyte count, hemoglobin concentration, mean corpuscular volume, and mean corpuscular hemoglobin (Hgb) between groups (n=4 per group). (D) Representative images of CD34 and endomucin expression in the CTX and OM of controls and Vegfatub mice (n=4–5 per group). Original magnification, ×400. (E) Vegfr2 expression in Vegfatub animals with late induction of Vegfa excision (n=4–5 per group). (F) Epo expression in kidney (n=4–5 per group). Data are presented as means±SEMs. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; VR, vasa recta capillary bundles of the OM. *P<0.05 relative to control animals.
Discussion
VEGFA plays a key role in a number of physiologic and pathophysiologic processes. Here, we explored the physiologic role of tubular Vegfa in kidney and showed its critical requirement for maintenance of peritubular microvasculature. Our study is the first to identify a direct causal relationship between tubular Vegfa and peritubular microvascular networks. Specifically, genetic ablation of tubular Vegfa results in selective dropout of peritubular microvessels. This pronounced peritubular capillary rarefaction is associated with a marked increase in intrarenal Epo production, stimulating erythropoiesis.
Elimination of tubular Vegfa led to a marked reduction in renal Vegfa expression, suggesting that Vegfa production by the renal tubules contributes substantially to total renal Vegfa production. This observation is well in line with the observed localization of Vegfa throughout the tubular system, which is outlined in Figure 1, A–C. Using the Vegfa-driven LacZ reporter, expression of Vegfa did appear highest in the glomerular podocytes, but because they occupy a relatively low portion of the total kidney volume, it explains the large reduction in total renal Vegfa after tubular Vegfa elimination. Importantly, no changes were observed in the systemic concentration of Vegfa in Vegfatub mice, suggesting that Vegfa produced by the renal tubules does not contribute substantially to circulating Vegfa levels.
The uniquely situated peritubular REPs in the corticomedullary boundary region respond to reductions in renal oxygen tension by altering their production of Epo.6,20 Most REPs do not produce Epo under normal conditions, although they retain the ability to do so, which can shift dramatically when changes in oxygen delivery occur.6 During chronic anemia, Epo-producing REPs can be found broadly distributed throughout the interstitium of the CTX and OM.6 Elevated Epo production increases hematocrit and consequently, the oxygen-carrying capacity of the blood. On the basis of the peritubular localization of the REPs, it has been hypothesized that reduced oxygen delivery caused by changes in peritubular capillary density is likely to increase Epo production. Observations made in this study support this notion. Here, a selective reduction in peritubular capillary endothelial cells results in pronounced polycythemia in Vegfatub mice. Despite the severe polycythemia, the increase in renal and systemic Epo levels remains lower than that observed in anemic mice, which may relate to the animals reaching a new steady state. In fact, increasing hematocrit by artificially elevating systemic Epo concentrations normally results in downregulation of Epo expression within the kidney.6,21 Thus, the absence of such reductions in renal Epo abundance in Vegfatub mice exhibiting marked polycythemia further suggests that the secretion of Epo is inappropriately high and may result from the capillary rarefaction. Moreover, when hematocrit was lowered close to physiologic control levels in Vegfatub mice, the renal Epo response was similar to what is observed in anemic wild-type mice. Collectively, these data support the notion that oxygen tension at the REPs is reduced in Vegfatub mice. However, detailed physiologic measurements of renal tissue pO2 in kidneys of Vegfatub mice are necessary to definitively show whether it, in fact, occurs in our transgenic model.
As outlined by Boor and Floege,22 most studies directed at understanding the process of renal fibrosis use the term myofibroblast for a renal interstitial cell, which is positive for the marker α-SMA. Thus, α-SMA remains the best established marker of myofibroblasts in the kidney.22,23 Although the origin of renal myofibroblasts has been debated,23 the presence of α-SMA–positive cells in kidney can lead to scarring. In this study, we detected α-SMA–positive cells in the peritubular cavities, which is suggestive of myofibroblast accumulation, but it occurred only when the hematocrit was lowered in Vegfatub mice. The observed appearance of myofibroblasts only in this condition implies that scarring could ensue and reveal an important protective role for the elevated hematocrit in Vegfatub mice. Myofibroblast formation in kidney may depend on environmental cues, such as the presence of inflammatory molecules.5 Alternatively, normalization of hematocrit in Vegfatub mice could be envisioned to promote enhanced stabilization of hypoxia-inducible factors and augment the expression of their target genes, such as platelet-derived growth factor,24 which has previously been shown to be important for myofibroblast formation.25 Whether the myofibroblasts are derived from REPs or other cell types remains to be determined.5,23
Systemic Vegf inhibition in adult mice causes capillary regression, reduces capillary fenestration, and diminishes Vegfr2 expression in various organs.8 Similar effects are observed in the kidney of Vegfatub mice and most obvious after early induction of tubular Vegfa excision. Mice with doxycycline-induced Vegfa excision from postnatal day 21 to 42 develop the same reductions in tubular Vegfa but display less pronounced capillary rarefaction, likely because of the later onset and therefore, less compromised endothelial cell health. The extent to which this phenomena occurs during VEGFA inhibition in patients remains undetermined. However, it is clear from studies directed at evaluating capillary rarefaction in other vascular beds that dramatic decreases in capillary density occur after VEGFA inhibition in both the buccal mucosa in patients receiving bevacizumab26 and mice receiving various forms of VEGFA and VEGFR inhibitors.8 Whether the observed renal damage in a subset of patients treated with antiangiogenics is accelerated by VEGFA inhibitor–dependent reductions in renal peritubular microvasculature has not been addressed.
In conclusion, tubular Vegfa is necessary and critical for maintenance of the peritubular microvasculature by directing tubulovascular cross-talk with the Vegfr2-expressing endothelial cells in kidney (i.e., those comprising the peritubular capillaries). This interaction seems to be a prerequisite for the REPs to adequately sense systemic circulating oxygen content and amend their production of Epo accordingly. Thus, our findings delineate an important physiologic role of the tubular Vegfa system. A delicate balance of tubular Vegfa likely exists, because overexpression of tubular Vegfa results in renal fibrosis and cysts.27 It is interesting to speculate that targeted activation of the tubular VEGFA system may provide a novel treatment for patients suffering from a variety of acute and chronic kidney diseases characterized by peritubular capillary rarefaction.
Consice Methods
Animal Models
Mice expressing β-galactosidase (LacZ) downstream of an internal ribosome entry site in the 3′ untranslated region of the Vegfa gene and mice expressing eGFP in exon 1 of Vegfr2 gene (Vegfr2eGFP/wt) have been described previously.12,13 Similarly, mice with a floxed Vegfa allele have been described elsewhere.17 B6.Cg-Tg(Pax8-rtTA2S*M2)1Koes/J expressing the rtTA under regulation of the murine Pax8 promoter (Pax8-rtTA) has been validated previously18 and was obtained from The Jackson Laboratory (Bar Harbor, ME). Mice expressing the Tet-O-Cre have been described previously.19 Intercrossing these mice generated a Vegfaflox/flox;Pax8-rtTA;Tet-O-Cre strain that excises Vegfa specifically in renal tubules on doxycycline administration (referred to as Vegfatub). Control animals expressed the same transgenes but were Tet-O-Cre–negative. Moreover, to evaluate expression of Vegfr2 in kidney after Vegfa deletion, Vegfr2eGFP/wt mice were crossed heterozygously to generate Vegfatub;Vegfr2eGFP/wt mice. LacZ/eGFP reporter mice that express eGFP upon Cre-mediated excision (Z/EG)28 were crossed with Pax8-rtTA;Tet-O-Cre mice to evaluate excision efficiency. Genotyping primers are listed in Supplemental Figure 6. To promote excision of the Vegfa gene, animals were administered doxycycline in drinking water (0.4 g doxycycline per 100 ml solution containing 5% sucrose; Sigma-Aldrich, Oakville, ON, Canada) either from conception to weaning at postnatal day 21 or from postnatal day 21 to 42 when noted.
Animal Experimental Protocols
Metabolic Cage Experiments
Mice were individually housed in metabolic cages, where they were maintained on a standard rodent diet (2918 diet; Harlan Teklad Global, Mississauga, ON, Canada) with free access to food and water; 24-hour urine was collected for analysis.
Furosemide Response
Furosemide (7.5 mg/kg body wt dissolved in 2.5% DMSO in physiologic saline; Sigma-Aldrich) was injected intraperitoneally in control and Vegfatub mice. Total injection volume was 750 μl per mouse to stimulate diuresis. Urine was collected every 30 minutes postinjection for 120 minutes.
Vascular Permeability Assay
Dextran permeability was determined in Vegfatub and control littermates. Animals were injected intravenously through the tail vein with 0.5 mg 40 kD dextran labeled with rhodamine (Invitrogen, Burlington, Canada) and 0.25 mg 5 kD dextran labeled with FITC (Invitrogen). Mice were coadministered 40 units Heparin (Sigma-Aldrich) in a total injection volume of 200 μl PBS; 20 minutes after injection, mice were anesthetized with isoflurane (3%, 1 L/min; Aerrane, Baxter, Canada), and 30 minutes after injection, blood was withdrawn by perforating the orbital vessels. Immediately thereafter, mice were perfused with PBS through the left ventricle. Tissue was collected, homogenized, and lysed in PBS with 1% NP40. Concentrations of dextran-coupled dyes were determined in kidney lysates and serum using a fluorometer (Infinite 200 PRO; Tecan Group AG).
Hematocrit Reduction
To reduce hematocrit in Vegfatub mice and induce nonhemolytic anemia in C57BL/6 mice, daily blood collections were done until the desired hematocrit was reached. Mice received subcutaneous injections of an equivalent volume of saline to offset volume loss. Animals were euthanized 1 day after the last blood collection.
Telemetry
BPs were measured in conscious, unrestrained 8- to 12-week-old male Vegfatub and control (n=6–8) mice using radiotelemetry as described previously.29 Arterial BP was collected, stored, and analyzed using Dataquest A.R.T. software (version 4.0; Data Sciences International, Saint Paul, MN). BPs were measured beginning 7 days after the catheter implantation when the mice had re-established normal circadian rhythms.30 Telemetry data were collected continuously with sampling every 5 minutes for 10-second intervals. Baseline measurements were recorded for 7 consecutive days while mice ingested a normal diet (0.4% NaCl). On day 8, mice ingested a low-salt (<0.002% NaCl; Harlan Teklad, Indianapolis, IN) diet for 1 week. Mice were returned to normal sodium diet for at least 3 days before subjecting them to high dietary NaCl (6% NaCl; Harlan Teklad) for 1 week. Subsequently, while on a normal salt diet, an osmotic minipump (Alzet, Cupertino, CA) infusing angiotensin II (A9525; Sigma-Aldrich) at a rate of 1000 ng/kg per minute was implanted subcutaneously, and BP measurements were performed. Telemetry devices and osmotic minipumps were implanted under deep isoflurane anesthesia.
Experimental procedures performed at the Toronto Center for Phenogenomics were approved by the Animal Care Committee and conducted in accordance with guidelines established by the Canadian Council on Animal Care. Similarly, experimental protocols performed at Durham Veterans Affairs Medical Center were approved by the Duke University and Durham Veterans Affairs Medical Center Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Real-Time PCR
When noted, kidneys were divided by gross dissection into upper CTX, OM, and inner medulla. RNA was extracted using TRIzol Total RNA Isolation Reagent (Invitrogen) and processed into cDNA. The cDNA was mixed with iTaq SYBR Green Supermix (Bio-Rad, Hercules, CA) and gene-specific primers (listed in Supplemental Figure 6). Samples were read using CFX96 and CFX384 real-time detection systems (Bio-Rad).
ELISA
Tissue was lysed in buffer of 1% Nonidet P40, 0.5% sodium deoxycholic acid, and 1% SDS in PBS and spun down at 13,000 rpm for 10 minutes, and the resulting supernatant was further processed for ELISA. Kits for detection of mouse Vegfa (MMV00) and EPO (MEP00B) were obtained from R&D Systems (Minneapolis, MN) and used according to the manufacturer’s instructions. Samples were measured on a µQuant Microplate Spectrophotometer (BioTek Instruments Inc., Winooski, VT).
Determination of Blood and Urinary Parameters
Urinary protein was measured using Bradford assay (500–0006; Bio-Rad). Urinary creatinine was determined using the method of Jaffe (KGE005; R&D Systems). Urinary electrolytes was determined by ion chromatography using an 800-series modular Metrohm IC System (Metrohm Nordic, Glostrup, Denmark) customized for simultaneous analysis of cations using a Metrosep C4 150 Column and anions using a Metrosep A SUPP 5–150 Column. Complete blood counts were determined using a Hemavet 950FS Hematology Analyzer (Drew Scientific Inc., Dallas, TX). Alternatively, hematocrit was determined using heparin-coated 75-mm microhematocrit tubes in a Readacrit centrifuge (Clay Adams, Parsippany, NJ).
Immunohistochemistry and Western Blotting
Tissues were fixed in 4% paraformaldehyde in PBS and either (1) dehydrated and embedded in paraffin or (2) placed in 30% sucrose overnight and subsequently snap-frozen. Sections were stained with hematoxylin and eosin stain for histologic evaluation. Immunohistochemistry and immunoblotting were performed essentially as described previously,31 with minor modifications.
The anti-CD34 signal was amplified using the ImmPRESS Polymerized Reporter Enzyme Staining System (Vector Laboratories) for light microscopy and the Catalyzed Signal Amplification System (DAKO) in conjunction with Alexa488 streptoavidin-coupled secondary antibodies for visualization using confocal microscopy. Lysates used for Western blotting were extracted in buffer of 1% Nonidet P40, 0.5% sodium deoxycholic acid, and 1% SDS in PBS as indicated above containing protease inhibitor and phosphatase inhibitor 2 cocktails (Sigma-Aldrich). Antibodies used are listed in Supplemental Figure 7.
Morphometric Analyses of Peritubular Capillary Surface Area
The surface areas of peritubular capillaries per volume of tissue were estimated in the renal CTX, where the orientation of the capillaries is assumed to be isotropic. Intersections of the capillary surface with a Merz grid were counted using FIJI image processing software32 on 14–17 images from each animal (n=5–6 per group) obtained at ×400 original magnification. The capillary surface area per volume was estimated using the stereological formula: 2×(number of intercepts between capillary surface and test line)/(length of test line) as described previously.33
Statistical Analyses
Values are presented as means±SEMs; n values refer to sample sizes per experimental group and are listed for each individual experiment in the figures. Comparisons between two groups were made using an unpaired t test (P<0.05 is considered statistically significant).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Tomokazu Souma for providing critical comments and helpful suggestions on the manuscript. The authors thank Kendraprasad Harpal and Inger Merete S. Paulsen for valuable experimental help and expert histological expertise. We also thank Janet Rossant and Andras Nagy for providing Vegfr2-eGFP and Vegfa-LacZ reporter mice. Kirsten Madsen and Anne Tinning are thanked for helpful advice on CD34 staining protocols.
H.D. is supported by the Danish Medical Research Council, the Novo Nordisk Foundation, the Carlsberg Foundation, and the A.P. Møller Foundation. M.A.S. is funded by Career Development Award IK2BX002240 from the Department of Veterans Affairs, Biomedical Laboratory Research and Development Service. This work was funded by Canadian Institutes of Health Research Grants M0P62931 and M0P77756, E-Rare Joint Translational Call (JTC 2011) for European Research Projects on Rare Diseases, and Terry Fox Group Grant (CIHR #TFF-105268) awarded to S.E.Q. S.E.Q. holds the Charles Mayo Chair of Medicine at the Feinberg School of Medicine and a Finnish Distinguished Professorship at the Oulu Biocenter. The BP studies were undertaken with resources and facilities at Durham Veterans Affairs Medical Center that were supported by Duke O'Brien Center for Kidney Research supported by the National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health under Award Number P30-DK096493.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Tubular Vascular Endothelial Growth Factor-A, Erythropoietin, and Medullary Vessels: A Trio Linked by Hypoxia,” on pages 997–998.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014010060/-/DCSupplemental.
References
- 1.Kriz W: Renal medullary circulation: Morphological characteristics of vessels and their organization. Klin Wochenschr 60: 1063–1069, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Brezis M, Heyman SN, Dinour D, Epstein FH, Rosen S: Role of nitric oxide in renal medullary oxygenation. Studies in isolated and intact rat kidneys. J Clin Invest 88: 390–395, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Donnelly S: Why is erythropoietin made in the kidney? The kidney functions as a ‘critmeter’ to regulate the hematocrit. Adv Exp Med Biol 543: 73–87, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Souma T, Yamazaki S, Moriguchi T, Suzuki N, Hirano I, Pan X, Minegishi N, Abe M, Kiyomoto H, Ito S, Yamamoto M: Plasticity of renal erythropoietin-producing cells governs fibrosis. J Am Soc Nephrol 24: 1599–1616, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamazaki S, Souma T, Hirano I, Pan X, Minegishi N, Suzuki N, Yamamoto M: A mouse model of adult-onset anaemia due to erythropoietin deficiency. Nat Commun 4: 1950, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Marti HH, Risau W: Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci U S A 95: 15809–15814, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM: VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 290: H560–H576, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eremina V, Quaggin SE: The role of VEGF-A in glomerular development and function. Curr Opin Nephrol Hypertens 13: 9–15, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A: Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol 212: 307–322, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Miquerol L, Langille BL, Nagy A: Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development 127: 3941–3946, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Ema M, Takahashi S, Rossant J: Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood 107: 111–117, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Robert B, St John PL, Hyink DP, Abrahamson DR: Evidence that embryonic kidney cells expressing flk-1 are intrinsic, vasculogenic angioblasts. Am J Physiol 271: F744–F753, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Robert B, St John PL, Abrahamson DR: Direct visualization of renal vascular morphogenesis in Flk1 heterozygous mutant mice. Am J Physiol 275: F164–F172, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Maxwell PH, Ferguson DJ, Nicholls LG, Johnson MH, Ratcliffe PJ: The interstitial response to renal injury: Fibroblast-like cells show phenotypic changes and have reduced potential for erythropoietin gene expression. Kidney Int 52: 715–724, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N: VEGF is required for growth and survival in neonatal mice. Development 126: 1149–1159, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Traykova-Brauch M, Schönig K, Greiner O, Miloud T, Jauch A, Bode M, Felsher DW, Glick AB, Kwiatkowski DJ, Bujard H, Horst J, von Knebel Doeberitz M, Niggli FK, Kriz W, Gröne HJ, Koesters R: An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med 14: 979–984, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan X, Suzuki N, Hirano I, Yamazaki S, Minegishi N, Yamamoto M: Isolation and characterization of renal erythropoietin-producing cells from genetically produced anemia mice. PLoS ONE 6: e25839, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osborne WR, Ramesh N, Lau S, Clowes MM, Dale DC, Clowes AW: Gene therapy for long-term expression of erythropoietin in rats. Proc Natl Acad Sci U S A 92: 8055–8058, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boor P, Floege J: The renal (myo-)fibroblast: A heterogeneous group of cells. Nephrol Dial Transplant 27: 3027–3036, 2012 [DOI] [PubMed] [Google Scholar]
- 23.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R: Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theilig F, Enke AK, Scolari B, Polzin D, Bachmann S, Koesters R: Tubular deficiency of von Hippel-Lindau attenuates renal disease progression in anti-GBM glomerulonephritis. Am J Pathol 179: 2177–2188, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, Shen J, Chen YM, Wu KD, Tsai TJ, Duffield JS, Lin SL: Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int 80: 1170–1181, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Steeghs N, Rabelink TJ, op ’t Roodt J, Batman E, Cluitmans FH, Weijl NI, de Koning E, Gelderblom H: Reversibility of capillary density after discontinuation of bevacizumab treatment. Ann Oncol 21: 1100–1105, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Hakroush S, Moeller MJ, Theilig F, Kaissling B, Sijmonsma TP, Jugold M, Akeson AL, Traykova-Brauch M, Hosser H, Hähnel B, Gröne HJ, Koesters R, Kriz W: Effects of increased renal tubular vascular endothelial growth factor (VEGF) on fibrosis, cyst formation, and glomerular disease. Am J Pathol 175: 1883–1895, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, Costantini F, Whitsett J, Quaggin SE, Nagy A: Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res 33: e51, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM: Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A 103: 17985–17990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butz GM, Davisson RL: Long-term telemetric measurement of cardiovascular parameters in awake mice: A physiological genomics tool. Physiol Genomics 5: 89–97, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Dimke H, Flyvbjerg A, Bourgeois S, Thomsen K, Frøkiaer J, Houillier P, Nielsen S, Frische S: Acute growth hormone administration induces antidiuretic and antinatriuretic effects and increases phosphorylation of NKCC2. Am J Physiol Renal Physiol 292: F723–F735, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A: Fiji: An open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruz-Orive LM, Weibel ER: Recent stereological methods for cell biology: A brief survey. Am J Physiol 258: L148–L156, 1990 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.