Abstract
Proximal tubule (PT) cells are critical targets of acute ischemic injury. Elimination of the mitochondrial fusion protein mitofusin 2 (Mfn2) sensitizes PT cells to apoptosis in vitro. However, the role of PT Mfn2 in ischemic AKI in vivo is unknown. To test its role, we evaluated the effects of conditional KO of PT Mfn2 (cKO-PT-Mfn2) on animal survival after transient bilateral renal ischemia associated with severe AKI. Forty-eight hours after ischemia, 28% of control mice survived compared with 86% of cKO-PT-Mfn2 animals (P<0.001 versus control). Although no significant differences in histologic injury score, apoptosis, or necrosis were detected between genotypes, cKO-PT-Mfn2 kidneys exhibited a 3.5-fold increase in cell proliferation restricted to the intrarenal region with Mfn2 deletion. To identify the signals responsible for increased proliferation, primary PT cells with Mfn2 deficiency were subjected to stress by ATP depletion in vitro. Compared with normal Mfn2 expression, Mfn2 deficiency significantly increased PT cell proliferation and persistently activated extracellular signal–regulated kinase 1/2 (ERK1/2) during recovery from stress. Furthermore, stress and Mfn2 deficiency decreased the interaction between Mfn2 and Ras detected by immunoprecipitation, and purified Mfn2 dose-dependently decreased Ras activity in a cell-free assay. Ischemia in vivo also reduced the Mfn2-RAS interaction and increased both RAS and p-ERK1/2 activity in the renal cortical homogenates of cKO-PT-Mfn2 mice. Our results suggest that, in contrast to its proapoptotic effects in vitro, selective PT Mfn2 deficiency accelerates recovery of renal function and enhances animal survival after ischemic AKI in vivo, partly by increasing Ras-ERK–mediated cell proliferation.
Keywords: acute renal failure, apoptosis, mitochondria, cell signaling
Efforts to modify the untoward consequences of renal ischemia have primarily focused on limiting the initial degree of cell and tissue injury that contribute to AKI. However, because the timing of the ischemic insult is often unknown in human AKI, therapeutic efforts that accelerate epithelial cell proliferation are highly practical for promoting organ recovery. During ischemic organ failure, epithelial cell mitochondria in the proximal tubule (PT) undergo dramatic morphologic changes.1–4 Select proteins induce mitochondria to undergo fission or fusion, resulting in short or elongated mitochondria, respectively. In fact, organelle shape is a life-and-death matter because mitochondrial morphology mediates its susceptibility to outer membrane injury by BCL2 family proteins that cause apoptosis.1,3,4
In the kidney, mitofusins 1 and 2 (Mfn1 and 2), both located on the outer mitochondrial membrane, have emerged as key determinants of mitochondrial fusion and organelle function.4,5 These proteins optimize oxidative metabolism by ensuring dynamic mitochondrial remodeling,5 facilitate mitochondrial division during mitosis,6,7 and are required for proper mitochondrial quality control and recycling by mitophagy.8 In neurons and epithelial cells, mitochondrial fission and fusion are critical for maintaining polarity by promoting mitochondrial transport to the appropriate intracellular location.7,9 In addition to their role in controlling organelle shape and size, mitofusins control calcium flux between endoplasmic reticulum and mitochondria.10 Recently, select proteins that regulate mitochondrial morphology have also been shown to exert potent effect on cell proliferation,11–13 a process that is critical for organ repair and recovery after an ischemic insult. After renal ischemia, extracellular regulated kinases (ERK) have been implicated in promoting cell proliferation and repair.14–16
Although in vitro studies, including our own, provide a strong link between mitofusins and PT cell apoptosis,1,4 the hypothesis that mitofusins regulate ischemic AKI in vivo has not been directly tested. On one hand, selective deletion of Mfn2 in the PT might aggravate ischemic AKI by increasing renal cell apoptosis.4 However, Mfn2 (previously known as hyperplasia suppressor gene or HSG) could also exert potent “off target” effects on cell proliferation through p21Ras that are independent of mitochondrial dynamics.11,12 If Mfn2 suppressed proliferation, a major contributor to renal recovery after stress,14,16 then reducing Mfn2 in the PT might accelerate organ recovery. To assess the role of cell type–specific Mfn2 in mediating organ dysfunction after ischemia, we conditionally manipulated Mfn2 expression in the PT epithelial cell, a primary target of ischemic stress.1,4,17 We determined that PT Mfn2 deficiency markedly increases animal survival after transient renal ischemia. We suggest that this protection is partly mediated by enhanced proliferation of PT epithelial cells after stress. Using complementary in vivo and in vitro studies, we identified that selective Mfn2 deficiency in the PT promotes proliferation by activating Ras and ERK1/2 signaling pathways. This study identifies a mechanistic approach for increasing the proliferation of surviving PT cells after a severe, potentially lethal ischemic event.
Results
Establishment of a Mouse Model with Inducible Deletion of Mfn2 Expression Specifically in Renal PT Cells
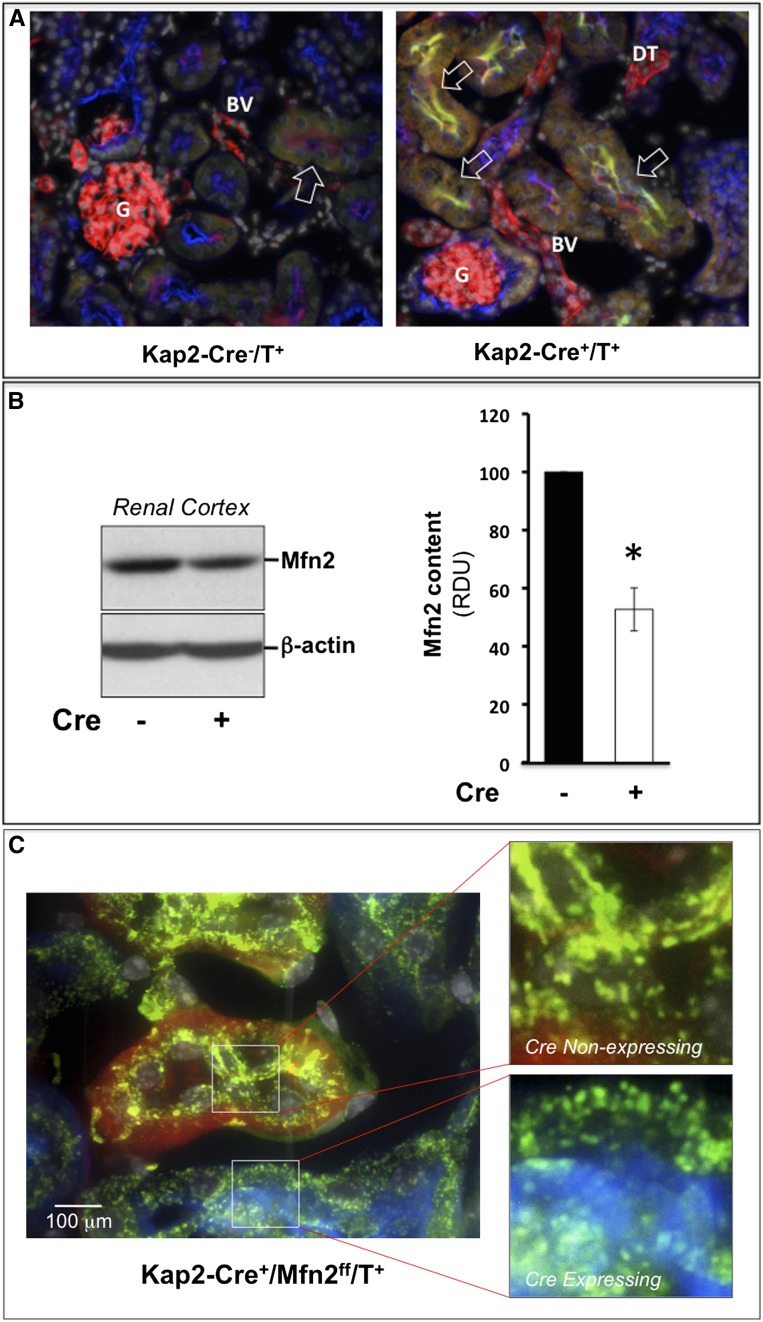
Triple-crossing was used to assess the efficacy of Kap2-Cre–driven PT Mfn2 knockout (KO) and yielded Kap2-Cre+/Mfn2ff/tomato and Kap2-Cre-/Mfn2ff/tomato littermates (genotyping data not shown) under the control of a testosterone-inducible, PT-specific KAP2 promoter.18 In male Kap2-Cre+/Mfn2f/f/tomato mice, endogenous testosterone failed to activate KAP2-Cre in the PT or cause conditional KO of Mfn2 (data not shown). Within 2 weeks of exogenous testosterone administration, mean serum testosterone levels increased by >14.5-fold in both male and female mice (53±62–772±240 ng/dl). In Kap2-Cre+/Mfn2f/f/tomato mice, testosterone treatment resulted in selective elimination of PT tomato red expression; green fluorescent protein (GFP) appeared and Mfn2 was selectively eliminated (Figure 1A, right versus left panel). In contrast, both glomeruli and blood vessels continued to appear red in testosterone-treated mice. Immunoblot showed that testosterone treatment reduced Mfn2 content by about 50% (P<0.05) as compared with renal cortical homogenates from animals lacking Kap2Cre (Figure 1B). The reduction in Mfn2 content in these tissue extracts reflects the fact that the PT epithelial cell represents only one of the cell types present in renal cortex and that Kap2Cre expression is incomplete. Because Mfn2 is critical for maintaining mitochondrial fusion in murine kidney cells,1,4 site-specific Mfn2 KO should change organelle shape from elongated to short or “punctate” in Kap2Cre positive PT epithelial cells. In fact, mitochondria did undergo a marked shape change in Kap2Cre-expressing PT cells. Specifically, Kap2Cre-positive tubules that express Cre (i.e., GFP-positive tubules) exhibited punctate (Figure 1C, upper inset) versus elongated mitochondria (lower panel) in adjacent PTs that contained red fluorescent protein and failed to express Kap2Cre.
Figure 1.
Exogenous testosterone treatment reduces proximal tubule Mfn2 expression and alters mitochondrial morphology of Kap2-Cre+/Mfn2f/f/tomato and Kap2-Cre-/Mfn2f/f/tomato littermates. (A) Effect of PT-specific Kap2-Cre expression in the renal cortices of tomato red mice treated with testosterone (T); in the absence of Kap2-Cre (left-hand panel), tomato red fluorescence appears in PTs (PT), glomeruli (G), and blood vessels (BV); in the presence of Kap2-Cre (right panel), many PTs suppress tomato red and express GFP; glomeruli and distal tubules (DT) remain red. Red=tomato protein; green=Mfn2; yellow=PT-specific lectin. (B) Immunoblot of steady-state Mfn2 protein content in homogenized renal cortices harvested from tomato red mice either with (Cre+) or without (Cre−) Cre expression in the presence of testosterone (T; left panel); densitometric analysis of three separate experiments (right panel). *P<0.05; n=3. RDU, relative density units; error bars represent mean±SEM. (C) Mitochondrial morphology after testosterone exposure in adjacent cortical PTs of a Kap2-Cre+/Mfn2f/f/tomato mouse after induction of Cre expression with testosterone; Mfn2-replete tubule with persistent tomato red expression and elongated mitochondria (upper inset); punctate mitochondria within a GFP fluorescent tubule (blue; lower inset); mitochondria are stained for F1F0 ATPase, an intrinsic outer mitochondrial membrane protein (green) at ×400 magnification.
Effect of Conditional Knockout-PT Mfn2 on Animal Survival, Renal Function, and Histologic Injury
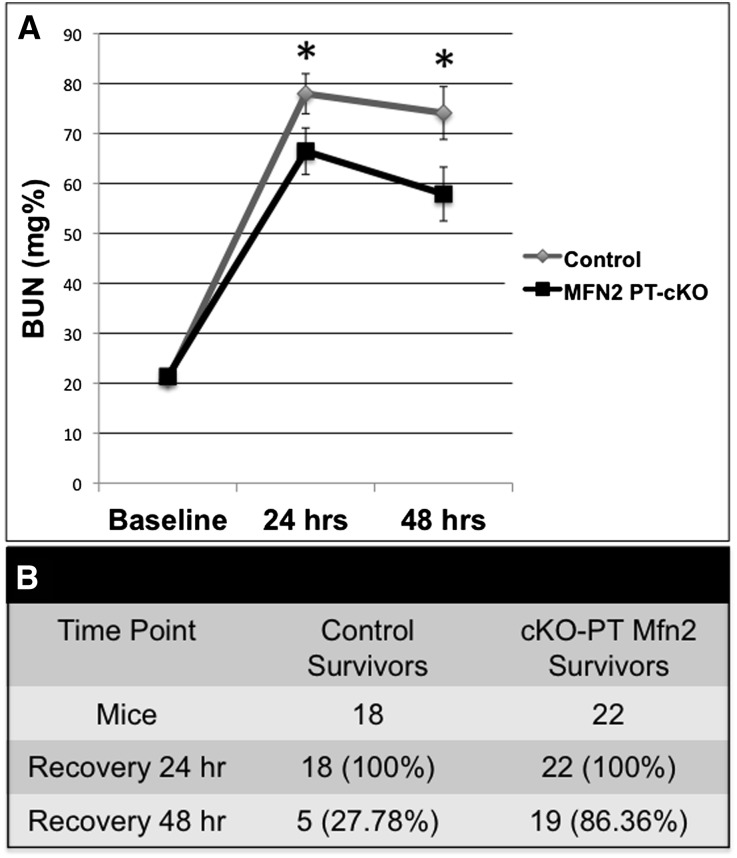
Once the positive effect of exogenous testosterone on Kap2Cre expression was determined in the tomato red mouse, Kap2-Cre and Mfn2ff mice were crossed and littermates were used for all additional experiments. Twenty-four and forty-eight hours after 28 minutes of bilateral renal pedicle clamping, renal function was assessed in surviving Kap2-Cre/Mfn2ff mice with conditional KO of PT (cKO-PT) Mfn2. The mean serum BUN level (a reliable estimate of renal function in this ischemia model that parallels measures of serum creatinine in this model19–21) was significantly lower in mice with cKO-PT Mfn2 (P<0.04) at both time points (Figure 2A). Importantly, BUN was measured in only 9 of 18 of the surviving control animals, likely underestimating the severity of AKI in this group. In fact, <30% of the surviving control and >85% of cKO-PT Mfn2 mice survived more than 48 hours after renal ischemia (Figure 2B). To quantify the severity of ischemic injury, randomly selected tissue sections were blindly examined 24 hours after ischemia. After ischemia, tissues sections harvested from both control and cKO-PT Mfn2 exhibited marked changes in the degree of tubular injury, vascular congestion, and inflammation (Table 1). However, no statistically significant difference in the severity of necrosis or apoptosis between groups was noted in postischemic tissue at the time point of maximal renal dysfunction, suggesting that tubular recovery, potentially mediated by PT cell regeneration, was more robust in cKO-PT Mfn2 mice.
Figure 2.
Conditional KO of PT Mfn2 (cKO-PT Mfn2) improves postischemic renal function and animal survival. (A) Serial BUN levels 24 and 48 hours after ischemia in control versus cKO-PT Mfn2 littermate mice. The significant difference in BUN observed between Kap2-MFN2 cKO and control animals was compared using repeated-measures ANOVA with the last BUN observation carried forward for nonsurviving animals. *P<0.05; n=18 or 22; error bars represent mean±SEM. (B) Animal survival at each time point after ischemia. *P<0.01 by chi-squared analysis.
Table 1.
Histologic score after renal ischemia
| Variable | Control | MFN2 PT-cKO | P Value |
|---|---|---|---|
| Simplification | 2.2±0.38 | 2.8±0.25 | >0.05 |
| Obstruction | 2.0±0.45 | 2.5±0.5 | >0.05 |
| Congestion | 1.6±0.68 | 2±0.58 | >0.05 |
| Necrosis | 0.4±0.25 | 1±0.58 | >0.05 |
| Apoptosis | 2.0±0.44 | 2.3±0.48 | >0.05 |
| Inflammation | 2.0±0.63 | 2.5±0.5 | >0.05 |
| Total Injury Score | 10.2±2.7 | 13±2.6 | >0.05 |
Unless otherwise noted, values are expressed as the mean±SD.
Effect of cKO-PT Mfn2 on Postischemic Proximal Tubule Proliferation
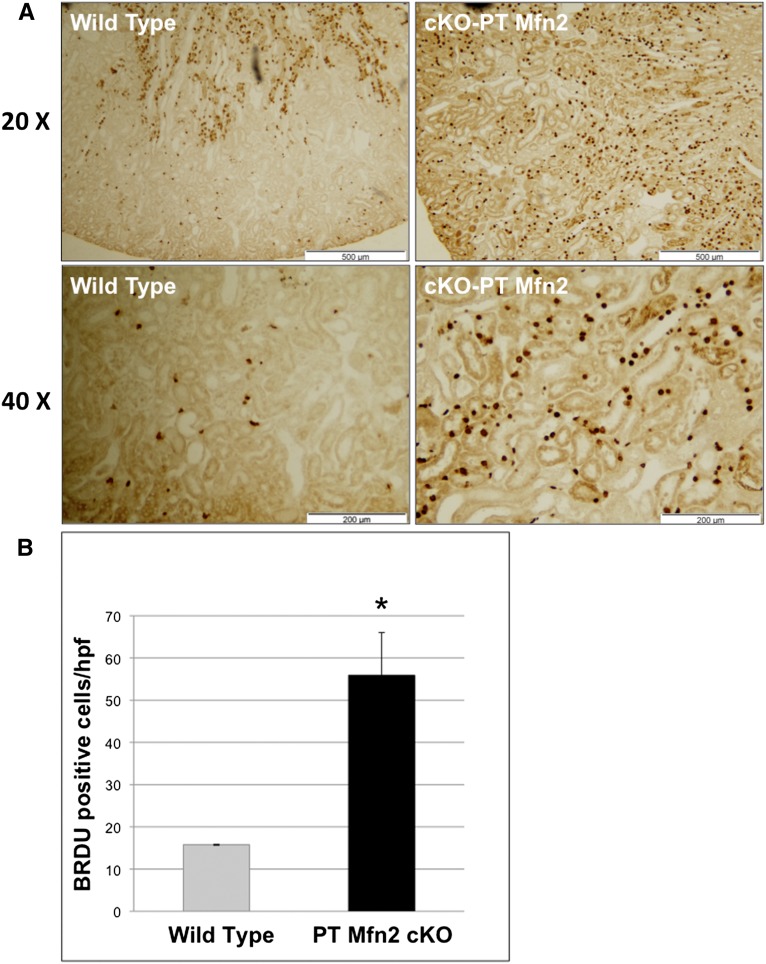
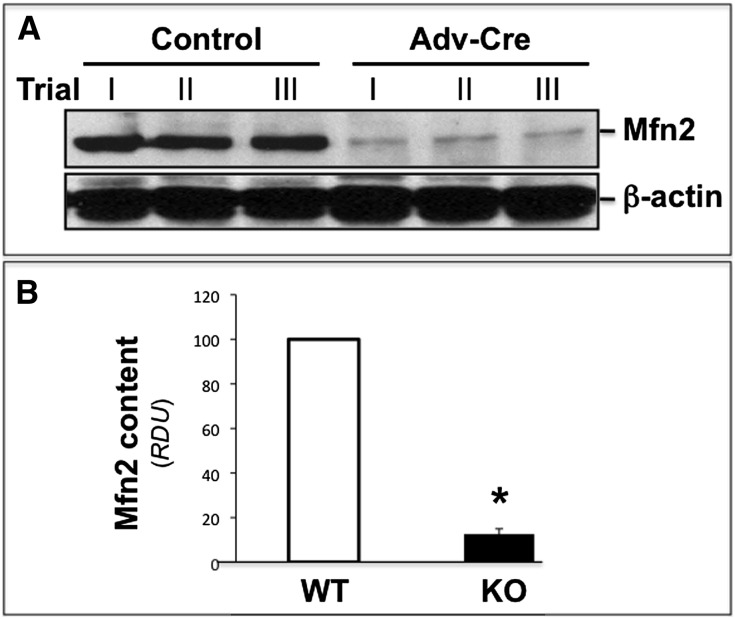
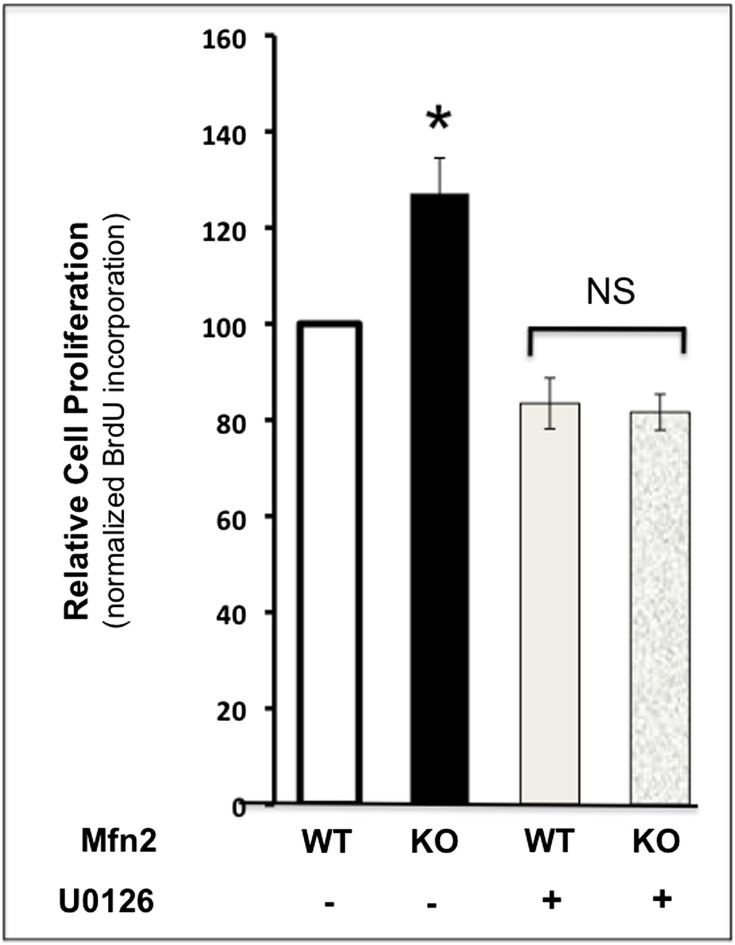
Compared with control, bromodeoxyuridine (BrdU) incorporation, a marker of cell proliferation, was dramatically increased in the renal cortices of cKO-PT Mfn2 mice (Figure 3A). At higher magnification, selective BrdU incorporation in the PT, the structure lacking Mfn2, was clearly evident (Figure 3A, lower panels). Only about 15% of control PTs incorporated BrdU after ischemia. In contrast, >50% of PTs stained with BrdU in postischemic Mfn2 KO kidneys (P<0.05) (Figure 3B). The amount of BrdU staining in the pre- or postischemic renal medulla did not significantly differ between these two groups (0.1 versus 0.15 cells/high-power field in control and cKO-PT Mfn2, respectively). Commensurate with similar baseline rates of cell proliferation, no difference in renal size was observed between control and cKO-PT mice (data not shown). In primary PT cells harvested from Mfn2f/f mice, exposure to adenovirus containing Cre resulted in an 80%–90% reduction in steady-state Mfn2 content in vitro (Figure 4, A and B). Twenty-four hours after ATP depletion, Mfn2 KO (Cre plus) cells exhibited significantly higher proliferation rates, as evidenced by greater BrdU incorporation compared with control exposed to empty AdV (Cre minus; P<0.05). The addition of an ERK1/2 inhibitor (U0126, 20 μM) completely prevented the increase in proliferation associated with Mfn2 deficiency (Figure 5). In the absence of ischemic stress, cell proliferation did not differ between Mfn2-replete and -deplete proximal tubule cells (data not shown).
Figure 3.
cKO-PT Mfn2 increases cell proliferation after ischemia in vivo. (A) Low-power (×20) view of BrdU incorporation in renal cortical tissue harvested from control versus cKO-PT Mfn2 mice 24 hours after ischemia (upper panels); higher power (×40) view of BrdU incorporation (lower panels). (B) Proliferation score in BrdU-positive cells in the renal cortices of wild-type versus cKO-PT Mfn2 mice; error bars represent mean±SEM. *P<0.05; n=3 control; n=5 cKO-PT Mfn2.
Figure 4.
Cre adenovirus reduces proximal tubule Mfn2 expression in primary cells. (A) Steady-state Mfn2 content in lysates obtained from primary cultured proximal tubule cells of Mfn2 floxed mice exposed to empty adenovirus (control) or adenovirus containing Cre (AdV-Cre; upper panel); β-actin loading control (lower panel). (B) Densitometric analysis of Mfn2 content before and after Mfn2 KO in vitro in three separate studies performed as described; error bars represent mean±SEM. *P<0.05 versus wild type. WT, wild type.
Figure 5.
ERK1/2 inhibition antagonizes the protective effect of Mfn2 deficiency on cell proliferation after ATP depletion in vitro. Proliferation measured by BrdU incorporation into primary renal cells after transient ATP depletion in the absence (minus) versus presence (plus) of 20 μM U0126, an ERK1/2 inhibitor. n=4; error bars represent mean±SEM. *P<0.05 KO versus wild type. NS, nonsignificant; WT, wild type.
Mfn2 Knockout Regulates Proximal Tubule Cell Proliferation via an ERK1/2-Dependent Pathway
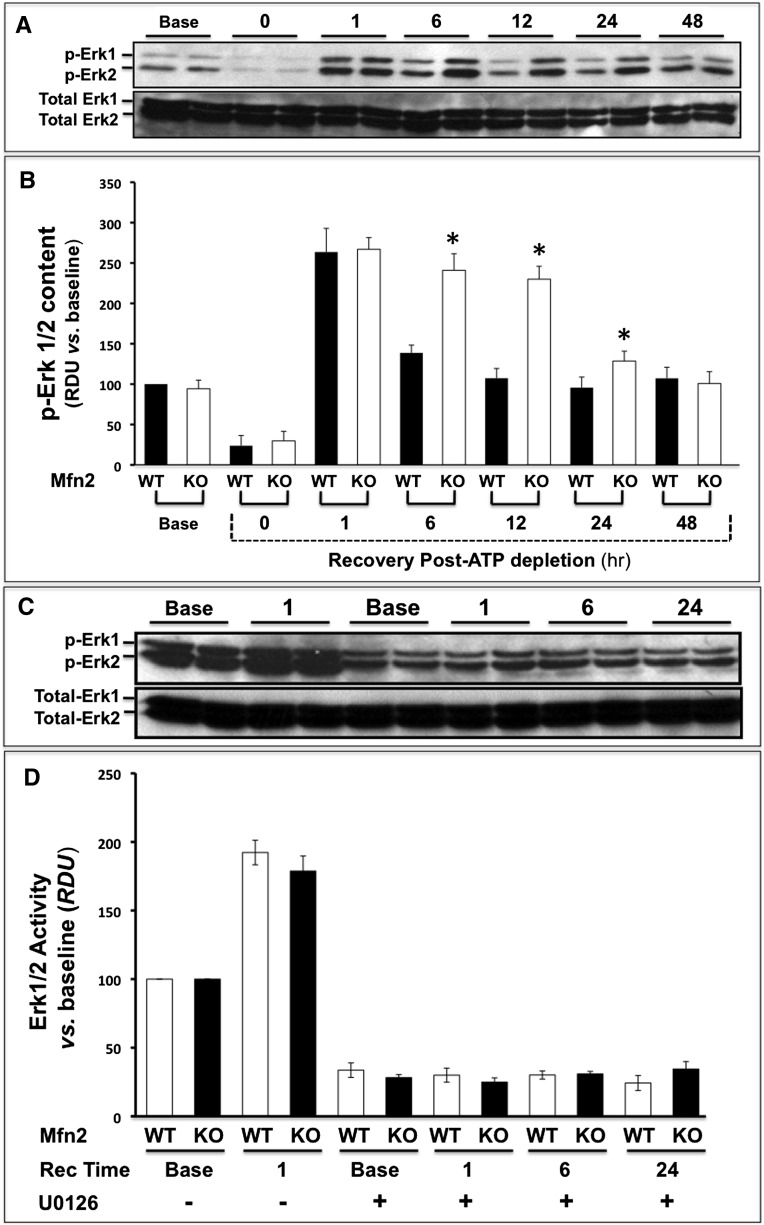
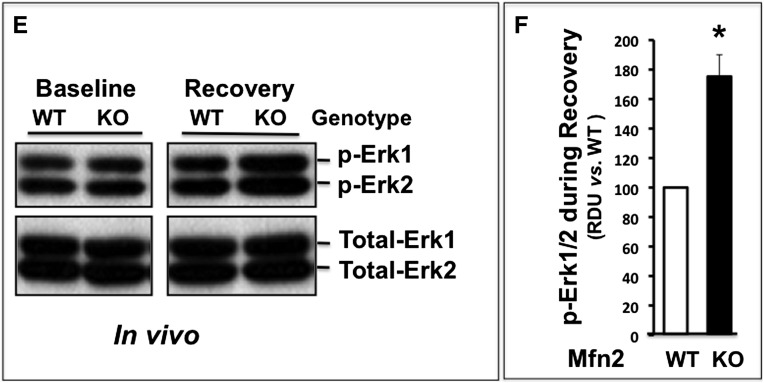
Compared with baseline, ATP depletion inactivates extracellular regulated kinase (phospho-ERK1/2) in both control and Mfn2 KO primary cells (Figure 6, A and B). In both groups, ERK1/2 was markedly activated above baseline within 1-hour recovery from stress. However, kinase activation was significantly greater in Mfn2 KO cells at 6, 12, and 24 hours after injury (P<0.05 at each time point). In contrast to the marked changes in p-ERK1/2 associated with ATP depletion and recovery, total ERK1/2 content remained constant at all time points (Figure 6, lower panels). The presence of U0126 (20 μM) similarly decreased the basal rate of proliferation both in wild-type and Mfn2-deficient renal cells and completely prevented the increase in proliferation associated with ATP depletion in both groups (Figure 6, C and D). Although ERK1/2 activity was almost identical before ischemic stress (i.e., at baseline), kinase activation was 80% greater than control in renal cortical homogenates harvested from cKO-PT Mfn2 mice after ischemia in vivo (Figure 6, E and F).
Figure 6.
Mfn2 deficiency-mediated enhances ERK1/2 activity after ATP depletion or renal ischemia. (A) Immunoblot analysis of phospho- and total ERK1/2 content in lysates at baseline (Base), immediately after (0 Recovery) and at 1- to 48-hour recovery. (B) Densitometric analysis of phospho- and total ERK1/2 content (ERK activity) in lysates harvested from control (wild type [WT]; black bars) versus Mfn2 KO (KO; white bars) cells before (base) and at 0- to 48-hour recovery after ATP depletion; error bars represent mean±SEM. n=3 separate studies. *P<0.05; n=3–4. (C) Immunoblot analysis of phospho- and total ERK1/2 content before and after ATP depletion in the presence versus absence of 20 μM U0126. (D) Densitometric analysis of three separate studies performed as described in part C; no significant differences between WT and KO were detected at any time point; error bars represent mean±SEM. (E) Immunoblot of phospho- and total ERK1/2 content in homogenates of renal cortex harvested from control or Mfn2 KO mice before and after renal ischemia. (F) Densitometric analysis of three separate studies performed as described in part E; error bars represent mean±SEM. *P<0.05 versus wild type.
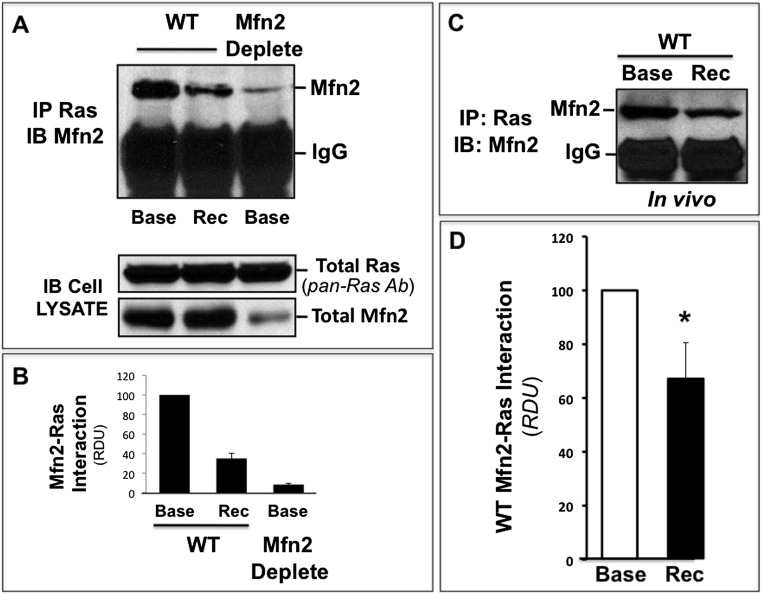
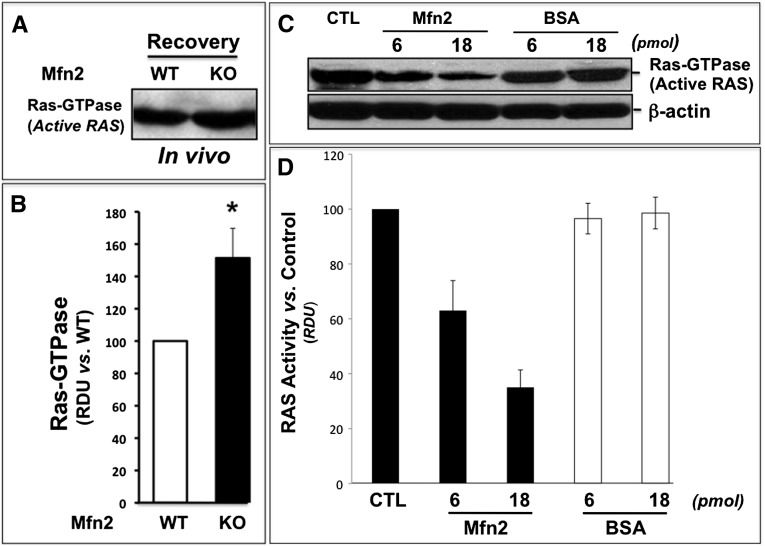
Interestingly, marked interaction between Mfn2 and Ras, an upstream ERK activator, was detected in immunoprecipitates harvested at baseline from nonstressed, Mfn2-replete primary PT cells (Figure 7A, left lane, upper panel). In contrast, decreased Mfn2-Ras interaction was detected during recovery from ATP depletion and in nonstressed renal cells with Mfn2 deficiency (Figure 7, A and B, middle and right lanes, respectively, in upper panel). In Mfn2 KO cells, decreased Mfn2-Ras interaction is clearly caused by a marked decrement in Mfn2 (Figure 7A, bottom panel) because equivalent amounts of Ras were detected in each sample. Similar to the effects of ATP depletion in vitro, Mfn2-RAS interaction also significantly decreased by about 35% in the renal cortices of mice subjected to ischemia in vivo (Figure 7, C and D). Similar molecular mass of Ras (22 kD) and immunoglobulin light chain (30 kD) precluded reciprocal immunoprecipitation studies. Commensurate with decrement in Mfn2 and Ras interaction during stress, renal cortical Ras activity significantly increased by 50% after ischemia in vivo (Figure 8, A and B). Finally, picomolar amounts of purified human MFN2 markedly decreased Ras activity in a dose-dependent manner by as much as 80% in a cell-free assay (Figure 8, C and D).In contrast, identical concentrations of BSA did not alter Ras activity.
Figure 7.
ATP depletion or renal ischemia reduce Mfn2-Ras interaction. (A) Immunoprecipitates obtained from 600 μg total protein harvested from lysates of Mfn2-replete (wild-type [WT]) cells were probed for total Mfn2 content at baseline (Base; lane 1), 1 hour after ATP depletion (recovery [Rec] 1 hour, lane 2) using a pan-Ras antibody (total Ras; upper panel) in primary renal cells with Mfn2 KO (Mfn2 deplete, lane 3). Cell lysates probed for Ras/Mfn2 content (lower panel). (B) Densitometric analysis of three separate immunoprecipitation studies performed as described in part A. (C) Immunoprecipitates obtained from renal cortical homogenates of control mice before or after ischemia using a pan-Ras antibody; precipitates were probed for both Mfn2 and total Ras content. IB, immunoblotting; IM, immunoprecipitation. (D) Densitometric analysis of three separate studies performed as described in part C. *P<0.05 recovery (Rec) versus baseline (Base).
Figure 8.
Renal ischemia and cKO-PT Mfn2 enhance, whereas purified Mfn2 decreases Ras activity. (A) Equivalent amounts of human MFN2 (molecular mass, 82 kD) or BSA (molecular mass, 66 kD) were added to a cell-free Ras activity assay as described in the Concise Methods. Representative immunoblot (upper panel) showing Ras GTPase activity (Ras activity) under control conditions (CTL) and after the addition of 6- or 18-pmol/L human MFN2 or BSA (upper panel); β-actin loading control (lower panel). (B) Densitometric analysis of three separate studies; error bars represent mean±SEM. *P<0.05 Mfn2 versus BSA; n=3. (C) Effect of renal ischemia on Ras activity (Ras-GTPase); renal cortical homogenates were analyzed after 28 minutes of ischemia with 6-hour recovery. (D) Densitometric analysis of three separate studies performed as described in part A; error bars represent mean±SEM. *P<0.05 control (wild type [WT]) versus KO mice.
Discussion
Although direct evidence is sparse, the PT has been presumed to be a critical mediator of acute ischemic organ failure for decades.22–24 In this study, we altered the expression of a single protein in the PT epithelial cell to assess (1) its role in mediating ischemic organ failure in vivo and (2) the potential effects of Mfn2 on PT epithelial cell death and organ recovery after ischemic stress. Because Mfn2 KO exerts potent untoward effects on renal cell survival after in vitro stress caused by exposure to metabolic inhibitors,1,4 we initially predicted that conditional KO of PT Mfn2 (cKO-PT Mfn2) would enhance tubular injury, exacerbate organ failure, and decrease animal survival. Unexpectedly, cKO-PT Mfn2 dramatically increased animal survival after a standardized ischemic insult that caused severe AKI and death. In fact, cKO-PT Mfn2 also decreased the severity of renal failure. The modest 15%–20% higher BUN in control probably underestimates a larger decrease in GFR on days 1 and 2 after ischemia because 50% of control mice (9 of 18) died within 24 hours and >70% (13 of 18) died within 48 hours after stress, precluding inclusion of their BUN measurements. Although the severity of tubular injury (simplification or congestion), obstruction, and tissue inflammation did not significantly differ in cKO-PT Mfn2 mice (Table 1), the degree of histologic evaluation poorly reflects organ function after ischemia.25 Similarly, no differences in the degree of cell apoptosis or necrosis were detected 24 hours after ischemia in control versus cKO-PT Mfn2 mice, the period of maximal organ dysfunction.19–21
The absence of marked changes in the degree of cell death associated with PT Mfn2 deficiency is unexpected. In renal and nonrenal cell types, filamentous mitochondria are relatively resistant to apoptosis compared with fragmented ones.1,2,4,26 This sensitivity to apoptosis is partly due to their increased susceptibility to Bax-mediated mitochondrial injury.1,4 Renal cell apoptosis, especially involving the epithelial cells of the PT, exacerbates ischemic AKI,4,19–21 strongly suggesting that mitofusin deletion (and the accompanying mitochondrial shortening) would promote organ failure. This hypothesis is consistent with the observation that pharmacologic treatment of mice with an inhibitor of the mitochondrial fission protein Drp-1, improved organ function after renal ischemia in mice.2 Similar to the present observations, however, major discrepancies between the untoward in vitro and favorable in vivo consequences of Mfn2 deficiency have been reported. In isolated cardiac myocytes, Mfn2 deficiency increased apoptotic cell death but enhanced myocardial performance after ischemia in vivo.26 This discrepancy could be due to the fact that apoptosis minimizes inflammation or that rapid clearance of severely damaged cells facilitates subsequent repair. Alternatively, the beneficial effect of Mfn2 deficiency after ischemic stress could overshadow its potential for promoting apoptosis.
Ischemic renal injury results in the loss of epithelial cells and cell growth arrest in surviving ones. Subsequent activation, proliferation, and redifferentiation of surviving cells repopulate the tubule, a critical hallmark of renal recovery after ischemia.16,27,28 Compared with control, BrdU incorporation increased 3-fold in the renal cortex of Mfn2 conditional KO mice (Figure 3A). Furthermore, Mfn2 deficiency increased proliferation exclusively in the PT, the only structure targeted for Mfn2 knockout (Figure 1, A and C). In contrast, cell proliferation in the renal medulla did not differ between groups before or after renal ischemia.
Although the source of proliferating cells after a renal insult is debated, recent evidence suggests that surviving, resident PT epithelial cells are more important for replacing epithelial cells lost during injury and organ recovery than stem cells derived from the bone marrow or the kidney itself.29–33 Once attached to the tubular basement membrane, immature cells redifferentiate, regain mature surface markers such as vimentin, and then contribute to vectoral solute transport, a hallmark of normal epithelial cell and organ function.32,34 In our study, primary proximal tubule cells with Mfn2 KO exhibited significantly higher rates of proliferation (BrdU incorporation) only 24 hours after metabolic stress in vitro (Figure 4C), a time point that matches our in vivo observations (Figure 3, A and B). Although the in vitro proliferative response was less robust than that detected in vivo, longer periods of cell growth (e.g., up to 9 days) enhance the effect of Mfn2 deficiency on cell proliferation.11,12
Recent investigations provide mechanistic clues for Mfn2-mediated PT cell proliferation. Previously referred to as hyperplasia suppressor gene, Mfn2 has multiple mechanism for regulating cell proliferation, including a Ras-like motif35; an amino terminal domain that binds active Ras12; and a carboxy terminal domain that binds RAF-1, a kinase downstream of Ras.12 In fact, Mfn2 has been linked to Ras-mediated proliferation in glomerular mesangial cells.13 In the present study, substantial evidence implicates RAS signaling as central to the effect of Mfn2 deficiency on renal epithelial cell growth. First, ATP depletion (Figure 6, C and D) activates RAS. Second, Mfn2 deficiency increases RAS activity after ischemia in vivo (Figure 8, A and B). Third, ischemic stress decreases the interaction between Mfn2 and active RAS in a manner predicted to increase free RAS (Figure 7, A–D). Finally, purified Mfn2 decreases RAS activity in a dose-dependent manner in a cell-free assay (Figure 8, C and D).
We suggest the decrement in Mfn2-Ras interaction caused by selective MFN2 deficiency increases free Ras and ERK1/2 activation, resulting in postischemic PT cell proliferation with improved animal survival. Because no difference in the rate of cell proliferation was detected in the absence of ischemic stress, we hypothesize that the antiproliferative effect of Mfn2 is evident only in the presence of signals that activate Ras/ERK. The observation that Ras and ERK are activated by renal ischemia36 in response to multiple growth factors, including hepatocyte growth factor,37 EGF,14 PDGF,38 TGF-β,31 and IGF,39 support this contention. Importantly, an established ERK inhibitor completely prevented the increase in ERK activation (Figure 6, C and D) and cell proliferation (Figure 5) caused by Mfn2 deficiency. These observations indicate that ERK is a relevant proliferative signal in our ischemia model. Interestingly, Mfn2-deficient proximal tubule cells exhibited persistent ERK1/2 activation 6–24 hours after ischemic stress (Figure 6, A and B, E and F), the same time frame in which marked epithelial cell proliferation was detected both in vitro (Figure 5) and in vivo (Figure 3, A and B). In fact, the stimulatory effect of Mfn2 deficiency on PT cell proliferation may be even greater because other investigators have shown proliferation peaks between 48 and 72 hours after renal ischemia.4
ERK activation is required for cell proliferation.40 In the kidney, the preponderance of evidence suggests that ERK 1/2 activation protects against oxidant, hypoxic, and ATP depletion–mediated injury of renal epithelial cells in vitro41,42 as well as oxidant and ischemia-reperfusion injury in vivo.16,43 Although GFR was not measured, biochemical ERK inhibition promoted the retention of focal adhesions and preserved the actin cytoskeletal network in a single study of renal ischemia.44 In contrast to ischemia/reperfusion models, ERK inhibition (versus activation) protects against cis-platinum–induced AKI, partly by its effect on apoptosis inhibition45 and partly by modulating renal epithelial cell cilia.46 However, few studies (including the present report) have used molecular techniques to selectively manipulate individual ERKs.
Presumably, sequestration/inactivation of Ras (and perhaps Raf) by Mfn2 limits PT cell division after stress by interfering with ERK1/2. On the basis of our current observations, we suggest that disrupting Mfn2-Ras interaction is a promising mechanism for promoting renal epithelial cell growth and organ function, as well as for enhancing survival after severe ischemia. Despite the potential for Mfn2 depletion to aggravate apoptosis in vitro,1,4,26 we suggest that Mfn2 deficiency dramatically protects against renal ischemia in vivo, partly by accelerating PT cell proliferation.
CONCISE METHODS
Chemicals
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated.
Animals
Because embryonic Mfn2 KO is lethal, mitofusin 2 floxed animals (Mfn2f/f) were generated by Dr. Chan’s laboratory.47 Conditional PT Mfn2 KO was created by breeding Mfn2f/f animals with animals expressing Cre recombinase under the control of the Kap2 promoter (Kap2-Cre, B6.Cg-Tg(Kap-cre)29066/2Sig/J18), resulting in Kap2-Cre+/Mfn2f/f mice. After additional crossing with the tomato red mouse, Kap2-Cre+/Mfn2ff/tomato and Kap2-Cre-/Mfn2ff/tomato littermates were studied. The Kap2 promoter requires the addition of testosterone to active and express Cre (Dr. Curt Sigmund, University of Iowa, personal communication). Silastic tubes (Φ=1.5 mm; Dow Corning, Midland, MI) were filled with 1 mg testosterone propionate (MP Biomedicals, Solon, OH) and sealed with cyanoacrylate glue before subdermal implantation in the right flank of both Kap2-Cre+/Mfn2f/f/tomato and Kap2-Cre-/Mfn2f/f/tomato littermates for 2 weeks. The effect of this maneuver on serum testosterone levels was assessed using liquid chromatography/mass spectometry to measure total testosterone (Hormone Assay Laboratory, Boston University School of Medicine, Boston, MA). In all subsequent renal ischemia studies, histology, renal function, and biochemical measures were assessed in Kap2Cre plus/MFN2ff mice crossed with Kap2Cre minus/MFN2ff animals. The resulting MFN2ff mice were each treated with exogenous testosterone. To minimize the potential effects of background genetic differences, littermates were subjected to genotyping and then divided into two experimental groups: Kap2 Cre positive or Kap2 Cre negative.
Renal Ischemia In Vivo
Six-week-old mice weighing 22–26 g were used to perform ischemia/reperfusion studies under standardized conditions as described elsewhere.19 After intramuscular injection of buprenorphine (0.075 mg/kg body wt) and intraperitoneal injection of tribromoethanol (250 mg/kg body wt), both kidneys were exposed by a midline incision. Sham-operated mice received identical surgical procedures with the exception that clamps were not applied. For PT-specific Mfn2 KO studies, bilateral renal pedicle occlusion was performed for 28 minutes to assess renal function, survival, histology, BrdU incorporation, apoptosis, and necrosis. After clamp removal, restoration of blood flow was visually confirmed for 1 minute. Renal histology was examined in stained, 5-μm tissue sections (described below). Tubular injury score was assessed in kidney tissue sections fixed with 10% formaldehyde, embedded in paraffin, and cut into 8-μm sections before staining with hematoxylin-eosin reagent. Total injury scores were calculated by a single blinded observer from three randomly selected tissue sections using a 0–3 semi-quantitative score (0=normal structure and 3=severe injury) of brush border simplification, intratubular obstruction, vascular congestion, inflammation, and both apoptosis and necrosis as previously reported.20
Primary PT Cell Culture
Three-week-old male mice were euthanized by overdose with inhaled CO2 using established protocols. Immediately after euthanasia, the kidneys were removed and maintained on ice. The cortex was collected, minced, and then digested in a solution with collagenase for 60 minutes at 37°C. The collagenase was neutralized by calf serum and the digested cortex was washed twice with buffered F12 medium. Mouse cells were maintained in a 50/50 (vol/vol) mixture of DMEM-Ham F12 medium containing insulin (5 mg/L), hydrocortisone (50 nM), and apotransferrin (500 μg/L) and were stored at 37°C for 5–7 days in a 5% CO2 incubator.
Metabolic Stress
ATP depletion, an established model of renal ischemia,48–52 was induced by exposing cells to glucose-free DMEM that contained adenosine, 1 mM; allopurinol, 0.2 mM; and rotenone, 10 mM (a complex I inhibitor), at 37°C in the presence of 5% CO2 as previously reported.52 Cells were rinsed three times with glucose-free DMEM followed by DMEM containing the preceding metabolic inhibitors for 60–90 minutes at 37°C with 5% CO2. Cell recovery was initiated by replacing the medium with DMEM containing adenosine, 1 mM; allopurinol, 0.2 mM; and heptanoic acid, 1 mM (a substrate that bypasses complex I inhibition). These maneuvers result in a 90% reduction in cell ATP content during the period of metabolic stress with prompt recovery of ATP content during recovery.52
In Vitro Mfn2 KO
An adenovirus expressing Cre recombinase was used to transduce Mfn2f/f primary PT cells. The cells were infected with adenovirus (multiplicity of infection, 10) overnight and the medium was replaced with complete primary culture media for 48 hours before the experiments. Recombination of the loxP sites by Cre recombinase deleted the floxed Mfn2 gene, creating Mfn2-deficient cells in culture. Adenovirus without Cre was used as control.
Antibodies and Immunoprecipitation
Mfn2 (XX-1) and Pan-Ras antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). p-ERK1/2, total ERK1/2, and β-actin antibodies were purchased from Cell Signaling Technology (Danvers, MA). The appropriate secondary antibody conjugated to AlexFluor-488 (Invitrogen) was used for immunofluorescence, and horseradish peroxidase (Bio-Rad) was used for immunoblot analysis.
Immunoprecipitation was performed as recently described by our laboratory.53 In brief, 7.5×106 cells were harvested in buffer containing NaCl, 150 mM; Tris HCl, 10 mM; EDTA, 5 mM; EGTA, 1 mM; 1% Triton X-100; and 0.5% NP-40, with protease inhibitors at a pH of 7.4. Lysates were centrifuged at 10,000 g for 10 minutes at 4°C. Samples containing 400 µg total protein were incubated overnight at 4°C with 2 µg of antibody bound to protein A/G beads. The immune complex was then precipitated and an immunoblot analysis performed to verify the identity of the antigen. Equal amounts of supernatant served as an input control.
Immunoblot Analysis
Cells were lysed in NP-40 lysis buffer containing 150 mM sodium chloride; 50 mM Tris at a pH of 7.5; 1 mM EDTA; 1% nonidet-P-40; and protease inhibitors aprotinin (10 µg/ml), leupeptin (2.5 µg/ml), and 1 mM phenylmethylsulfonyl fluoride. Aliquots of lysates containing 20–30 µg/lane of protein were separated on 8%–12% Tris-glycine polyacrylamide gels. Antigens were detected with enhanced chemiluminescence reagents (Amersham, Piscataway, NJ) using specific antibodies.
Tissue Staining
For immunofluorescent staining of tissue, sections were washed three times for 5 minutes each in PBS, followed by 5 minutes in PBS containing 0.1% SDS. After three additional washes, tissue sections were blocked for 1 hour in PBS containing 5% normal goat serum and 0.3% Triton X-100. Following three washes, sections were incubated for 90 minutes with antibodies against F1F0 ATPase (2 μg/ml) in PBS with 1% BSA and 0.3% Triton X-100. After three PBS washes, sections were incubated in AlexaFluor 488 (1:250 dilution) and AlexaFluor 594 (1:500 dilution) conjugated secondary antibodies (1% BSA, 0.3% Triton X-100 in PBS) for 1 hour, washed three final times in PBS, mounted with Gelvatol with or without Hoechst, and examined by confocal microscopy (PerkinElmer Ultraview Scanning Disk Microscope, Wellesley, MA). Slides were also imaged by phase contrast and wide-field epifluorescence microscopy followed by deconvolution using ImageJ software.
For immunohistochemical tissue staining, sections were deparaffinized, heated in 10 mM sodium citrate (pH, 5.5) for 1 hour in a pressure cooker, and stained with anti-BrdU (1:160 dilution) following the ABC elite kit directions (Vector Laboratories, Burlingame, CA). Sections were developed with Immpact DAB (Vector Laboratories).
Cell Proliferation Assay In Vivo and In Vitro
BrdU, a synthetic thymidine analogue, can be incorporated into newly synthesized DNA, providing a test of DNA replication, as an indirect measure of cell proliferation. The assay was performed in vivo by giving the mice a single intraperitoneal injection of 1 mg BrdU in 200 μl of sterile PBS before ischemia. BrdU was detected in kidney sections as described earlier. The assay was performed in vitro as described in the product manual from EMD Millipore (Billerica, MA). BrdU incorporation was detected by addition of peroxidase substrate. Spectrophotometric detection was performed at a wavelength of 450 nm.
Ras Activity Assay
A Ras Activation Assay kit (Cell BioLabs, Inc., San Diego, CA) was used to measure enzyme activity. Mfn2 KO PT cells were stressed with 70 minutes of ATP depletion, followed by 1-hour recovery or renal cortical homogenates after 28-min ischemia with 6-hour recovery. The protein was extracted using the manufacturer’s lysis buffer and divided into five aliquots (600 μg each). Purified recombinant human MFN2 protein (OriGene, Rockville, MD) or human serum albumin (Sigma-Aldrich) was added to the protein lysis and incubated on ice for 90 minutes. Ras activity was measured using the Ras-binding domain of Raf-1 to pull down active Ras according to the manufacturer’s protocol. Following separation by SDS PAGE, proteins were transferred to membranes that were probed with an anti–pan-Ras antibody.
BUN Assay
Mouse whole blood was collected via the tail vein before and after ischemia and from the heart at the time of euthanasia. Samples were spun in a microcentrifuge at 4000 rpm for 30 minutes and serum was collected. BUN was measured using a QuantiChrom BUN assay kit (Bioassay Systems, Hayward, CA) following the manufacturer’s protocol. Estimates of GFR using serum BUN or Cr are almost identical in this ischemia AKI model.20,21
Statistical Analyses
Data were analyzed using Excel (Microsoft, Redmond, WA). Significant differences between groups were determined by ANOVA using repeated measures adjusted by Scheffe test, with P values <0.05 considered to represent statistically significant differences.
Disclosures
None.
Acknowledgments
National Institutes of Health (NIH) grant DK-53387 (S.C.B.), NIH-DK-090558 (R.G.B.), and NIH-DK090143 (A.H.) each supported this research. M.L. is an Evans Research Fellow supported by the Evans Medical Foundation, Boston University Medical Center.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Brooks C, Cho SG, Wang CY, Yang T, Dong Z: Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis. Am J Physiol Cell Physiol 300: C447–C455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanhooren V, Liu XE, Desmyter L, Fan YD, Vanwalleghem L, Van Molle W, Dewaele S, Praet M, Contreras R, Libert C, Chen C: Over-expression of heat shock protein 70 in mice is associated with growth retardation, tumor formation, and early death. Rejuvenation Res 11: 1013–1020, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie Z-J, Dong Z: Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci U S A 104: 11649–11654, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gall JM, Wang Z, Liesa M, Molina A, Havasi A, Schwartz JH, Shirihai O, Borkan SC, Bonegio RG: Role of mitofusin 2 in the renal stress response. PLoS ONE 7: e31074, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks C, Wei Q, Cho SG, Dong Z: Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K: Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282: 11521–11529, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K: Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol 11: 958–966, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Twig G, Elorza A, Molina AJA, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS: Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC: Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141: 280–289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sebastián D, Hernández-Alvarez MI, Segalés J, Sorianello E, Muñoz JP, Sala D, Waget A, Liesa M, Paz JC, Gopalacharyulu P, Orešič M, Pich S, Burcelin R, Palacín M, Zorzano A: Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci U S A 109: 5523–5528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D, Li P, Qiu X, Wen S, Xiao RP, Tang J: Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol 6: 872–883, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Chen KH, Dasgupta A, Ding J, Indig FE, Ghosh P, Longo DL: Role of mitofusin 2 (Mfn2) in controlling cellular proliferation. FASEB J 28: 382–394, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan-Xin T, Tian-Lei C, Ben W, Wei-Hua W, Ping F: Effect of mitofusin 2 overexpression on the proliferation and apoptosis of high-glucose-induced rat glomerular mesangial cells. J Nephrol 25: 1023–1030, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Chen JK, Harris RC: Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int 82: 45–52, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon DS, Kwon CH, Kim JH, Woo JS, Jung JS, Kim YK: Signal transduction of MEK/ERK and PI3K/Akt activation by hypoxia/reoxygenation in renal epithelial cells. Eur J Cell Biol 85: 1189–1199, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Jang HS, Han SJ, Kim JI, Lee S, Lipschutz JH, Park KM: Activation of ERK accelerates repair of renal tubular epithelial cells, whereas it inhibits progression of fibrosis following ischemia/reperfusion injury. Biochim Biophys Acta 1832: 1998–2008, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Havasi A, Gall JM, Mao H, Schwartz JH, Borkan SC: Beta-catenin promotes survival of renal epithelial cells by inhibiting Bax. J Am Soc Nephrol 20: 1919–1928, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Zhou X, Davis DR, Xu D, Sigmund CD: An androgen-inducible proximal tubule-specific Cre recombinase transgenic model. Am J Physiol Renal Physiol 294: F1481–F1486, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueki S, Takagi J, Saito Y: Dual functions of transglutaminase in novel cell adhesion. J Cell Sci 109: 2727–2735, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Havasi A, Gall J, Bonegio R, Li Z, Mao H, Schwartz JH, Borkan SC: GSK3beta promotes apoptosis after renal ischemic injury. J Am Soc Nephrol 21: 284–294, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Gall JM, Bonegio RG, Havasi A, Hunt CR, Sherman MY, Schwartz JH, Borkan SC: Induction of heat shock protein 70 inhibits ischemic renal injury. Kidney Int 79: 861–870, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin DM, Jeon JH, Kim CW, Cho SY, Kwon JC, Lee HJ, Choi KH, Park SC, Kim IG: Cell type-specific activation of intracellular transglutaminase 2 by oxidative stress or ultraviolet irradiation: implications of transglutaminase 2 in age-related cataractogenesis. J Biol Chem 279: 15032–15039, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Padanilam BJ: Cell death induced by acute renal injury: A perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol 284: F608–F627, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Venkatachalam MA, Bernard DB, Donohoe JF, Levinsky NG: Ischemic damage and repair in the rat proximal tubule: Differences among the S1, S2, and S3 segments. Kidney Int 14: 31–49, 1978 [DOI] [PubMed] [Google Scholar]
- 25.Rosen S, Heyman SN: Difficulties in understanding human “acute tubular necrosis”: Limited data and flawed animal models. Kidney Int 60: 1220–1224, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O’Shea KM, Riley DD, Lugus JJ, Colucci WS, Lederer WJ, Stanley WC, Walsh K: Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol 31: 1309–1328, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devarajan P: Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Havasi A, Haegele JA, Gall JM, Blackmon S, Ichimura T, Bonegio RG, Panchenko MV: Histone acetyl transferase (HAT) HBO1 and JADE1 in epithelial cell regeneration. Am J Pathol 182: 152–162, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV: Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115: 1743–1755, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Racusen LC, Fivush BA, Li YL, Slatnik I, Solez K: Dissociation of tubular cell detachment and tubular cell death in clinical and experimental “acute tubular necrosis”. Lab Invest 64: 546–556, 1991 [PubMed] [Google Scholar]
- 31.Wen X, Murugan R, Peng Z, Kellum JA: Pathophysiology of acute kidney injury: a new perspective. Contrib Nephrol 165: 39–45, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Lee PT, Lin HH, Jiang ST, Lu PJ, Chou KJ, Fang HC, Chiou YY, Tang MJ: Mouse kidney progenitor cells accelerate renal regeneration and prolong survival after ischemic injury. Stem Cells 28: 573–584, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Geng H, Lan R, Wang G, Siddiqi AR, Naski MC, Brooks AI, Barnes JL, Saikumar P, Weinberg JM, Venkatachalam MA: Inhibition of autoregulated TGFbeta signaling simultaneously enhances proliferation and differentiation of kidney epithelium and promotes repair following renal ischemia. Am J Pathol 174: 1291–1308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hertig A, Verine J, Mougenot B, Jouanneau C, Ouali N, Sebe P, Glotz D, Ancel PY, Rondeau E, Xu-Dubois YC: Risk factors for early epithelial to mesenchymal transition in renal grafts. Am J Transplant 6: 2937–2946, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Chen CC, Liu ZM, Wang HH, He W, Wang Y, Wu WD: Effects of ulinastatin on renal ischemia-reperfusion injury in rats. Acta Pharmacol Sin 25: 1334–1340, 2004 [PubMed] [Google Scholar]
- 36.Pombo CM, Bonventre JV, Avruch J, Woodgett JR, Kyriakis JM, Force T: The stress-activated protein kinases are major c-Jun amino-terminal kinases activated by ischemia and reperfusion. J Biol Chem 269: 26546–26551, 1994 [PubMed] [Google Scholar]
- 37.Fiaschi-Taesch NM, Santos S, Reddy V, Van Why SK, Philbrick WF, Ortega A, Esbrit P, Orloff JJ, Garcia-Ocaña A: Prevention of acute ischemic renal failure by targeted delivery of growth factors to the proximal tubule in transgenic mice: The efficacy of parathyroid hormone-related protein and hepatocyte growth factor. J Am Soc Nephrol 15: 112–125, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Watton SJ, Downward J: Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr Biol 9: 433–436, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Takeda R, Nishimatsu H, Suzuki E, Satonaka H, Nagata D, Oba S, Sata M, Takahashi M, Yamamoto Y, Terauchi Y, Kadowaki T, Kangawa K, Kitamura T, Nagai R, Hirata Y: Ghrelin improves renal function in mice with ischemic acute renal failure. J Am Soc Nephrol 17: 113–121, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Johnson GL, Lapadat R: Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298: 1911–1912, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Arany I, Megyesi JK, Kaneto H, Tanaka S, Safirstein RL: Activation of ERK or inhibition of JNK ameliorates H(2)O(2) cytotoxicity in mouse renal proximal tubule cells. Kidney Int 65: 1231–1239, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Arany I, Megyesi JK, Nelkin BD, Safirstein RL: STAT3 attenuates EGFR-mediated ERK activation and cell survival during oxidant stress in mouse proximal tubular cells. Kidney Int 70: 669–674, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Ishizuka S, Yano T, Hagiwara K, Sone M, Nihei H, Ozasa H, Horikawa S: Extracellular signal-regulated kinase mediates renal regeneration in rats with myoglobinuric acute renal injury. Biochem Biophys Res Commun 254: 88–92, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Alderliesten M, de Graauw M, Oldenampsen J, Qin Y, Pont C, van Buren L, van de Water B: Extracellular signal-regulated kinase activation during renal ischemia/reperfusion mediates focal adhesion dissolution and renal injury. Am J Pathol 171: 452–462, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim YK, Kim HJ, Kwon CH, Kim JH, Woo JS, Jung JS, Kim JM: Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J Appl Toxicol 25: 374–382, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Wei Q, Dong G, Dong Z: ERK-mediated suppression of cilia in cisplatin-induced tubular cell apoptosis and acute kidney injury. Biochim Biophys Acta 1832: 1582–1590, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC: Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160: 189–200, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruchalski K, Mao H, Singh SK, Wang Y, Mosser DD, Li F, Schwartz JH, Borkan SC: HSP72 inhibits apoptosis-inducing factor release in ATP-depleted renal epithelial cells. Am J Physiol Cell Physiol 285: C1483–C1493, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Li F, Mao HP, Ruchalski KL, Wang YH, Choy W, Schwartz JH, Borkan SC: Heat stress prevents mitochondrial injury in ATP-depleted renal epithelial cells. Am J Physiol Cell Physiol 283: C917–C926, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Borkan SC, Wang YH, Lam KT, Brecher P, Schwartz JH: Hepatic alpha 2 mu-globulin: A potential metabolic role in the rat proximal tubule. Am J Physiol 271: F527–F538, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Ruchalski K, Mao H, Li Z, Wang Z, Gillers S, Wang Y, Mosser DD, Gabai V, Schwartz JH, Borkan SC: Distinct hsp70 domains mediate apoptosis-inducing factor release and nuclear accumulation. J Biol Chem 281: 7873–7880, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Doctor RB, Bacallao R, Mandel LJ: Method for recovering ATP content and mitochondrial function after chemical anoxia in renal cell cultures. Am J Physiol 266: C1803–C1811, 1994 [DOI] [PubMed] [Google Scholar]
- 53.Havasi A, Wang Z, Gall JM, Spaderna M, Suri V, Canlas E, Martin JL, Schwartz JH, Borkan SC: Hsp27 inhibits sublethal, Src-mediated renal epithelial cell injury. Am J Physiol Renal Physiol 297: F760–F768, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]