Abstract
The generation of reactive oxygen species (ROS), particularly superoxide, by damaged or dysfunctional mitochondria has been postulated to be an initiating event in the development of diabetes complications. The glomerulus is a primary site of diabetic injury, and podocyte injury is a classic hallmark of diabetic glomerular lesions. In streptozotocin-induced type 1 diabetes, podocyte-specific EGF receptor (EGFR) knockout mice (EGFRpodKO) and their wild-type (WT) littermates had similar levels of hyperglycemia and polyuria, but EGFRpodKO mice had significantly less albuminuria and less podocyte loss compared with WT diabetic mice. Furthermore, EGFRpodKO diabetic mice had less TGF-β1 expression, Smad2/3 phosphorylation, and glomerular fibronectin deposition. Immunoblotting of isolated glomerular lysates revealed that the upregulation of cleaved caspase 3 and downregulation of Bcl2 in WT diabetic mice were attenuated in EGFRpodKO diabetic mice. Administration of the SOD mimetic mito-tempol or the NADPH oxidase inhibitor apocynin attenuated the upregulation of p-c-Src, p-EGFR, p-ERK1/2, p-Smad2/3, and TGF-β1 expression and prevented the alteration of cleaved caspase 3 and Bcl2 expression in glomeruli of WT diabetic mice. High-glucose treatment of cultured mouse podocytes induced similar alterations in the production of ROS; phosphorylation of c-Src, EGFR, and Smad2/3; and expression of TGF-β1, cleaved caspase 3, and Bcl2. These alterations were inhibited by treatment with mito-tempol or apocynin or by inhibiting EGFR expression or activity. Thus, results of our studies utilizing mice with podocyte-specific EGFR deletion demonstrate that EGFR activation has a major role in activating pathways that mediate podocyte injury and loss in diabetic nephropathy.
Keywords: apoptosis, chronic diabetic complications, NADPH oxidase, TGF-β, podocyte
The incidence of diabetic nephropathy continues to increase worldwide in association with the epidemic rise in diabetes. In the United States, diabetic kidney disease is now the major cause of end stage kidney disease1 and increases the rate of other complications, such as cardiovascular disease.2 Twenty to forty percent of all patients with diabetes will develop some form of kidney disease during their life.3
Podocytes are highly specialized cells characterized by formation of foot processes that are interconnected by the slit diaphragm, which is a critical component of the glomerular filtration barrier. In both experimental animals4 and patients with type 2 diabetes, a reduction in the number of podocytes per glomerulus is associated with broadening of podocyte foot processes and is thought to contribute to the progression of diabetic nephropathy.5 Moreover, it is now widely recognized that proteinuria, specifically microalbuminuria, is one of the earliest clinically identifiable markers of diabetes-induced renal damage, and appearance of proteinuria indicates a compromised glomerular filtration barrier. Recent studies suggested that reactive oxygen species (ROS) production, mediated primarily by NADPH oxidases of the Nox family, mediates podocyte injury, including podocyte apoptosis and detachment from the glomerular basement membrane.6,7
The EGF receptor (EGFR) is a member of the ErbB family of receptor tyrosine kinases that consist of an extracellular ligand-binding domain, a single membrane-spanning region, a homologic cytoplasmic protein tyrosine kinase domain, and a C-terminal tail with multiple phosphorylation sites. Ligand binding to EGFR leads to activation of the intrinsic kinase domain and subsequent phosphorylation on specific tyrosine residues within the cytoplasmic tail, including Y1068 and Y1173. In addition, EGFR may be activated by nonligand-associated intracellular Src family kinases, indicated by phosphorylation at Y845. EGFR is widely expressed in the mammalian kidney, including the glomeruli, proximal tubules, and cortical and medullary collecting ducts.8–10 There is increasing evidence that EGFR is an important mediator of cell fate decisions, such as proliferation, cell lineage determination and differentiation, migration and even cell death.11,12 Aberrant EGF receptor signaling pathway activations is associated with tumorogenesis,12,13 and we and other investigators have demonstrated that the EGFR activation is a pivotal mediator for renal fibrosis and may interact with TGF-β signaling.14–17
In this study, we investigated the role of podocyte EGFR in the development of diabetic nephropathy by using podocyte-specific EGFR deletion mice and determined that hyperglycemia-induced ROS production activates the Src kinase and thereby induces EGFR activation-dependent phosphorylation of ERK, activation of the TGF-β–Smad2/3 signaling pathway, downregulation of Bcl2, and upregulation of cleaved caspase 3 and leads to a reduction of the podocyte number per glomerulus and development of albuminuria, the hallmarks of early diabetic nephropathy.
Results
Podocyte EGFR Deletion Attenuated Albuminuria and Podocyte Loss Induced by Hyperglycemia
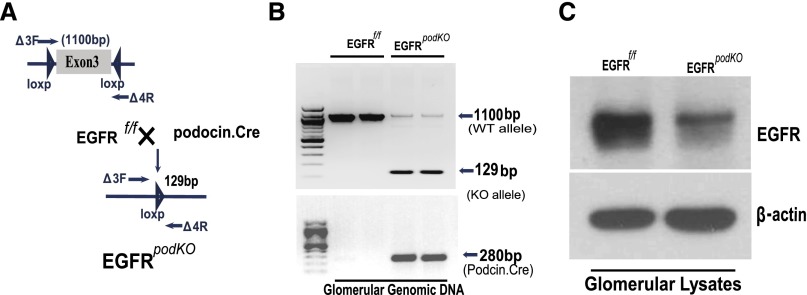
Activation of EGFR in mammalian kidney cells including podocytes is an important step in the regulation of cell survival, migration, proliferation, and differentiation.10,11,18,19 Recent studies by us and others indicate that administration of EGFR tyrosine kinase inhibitors can slow progression of diabetic nephropathy in experimental animals17,20 To study the potential role of EGFR expressed in diabetic podocytes, we developed a podocyte-specific EGFR knockout mouse (EGFRpodKO) by crossing EGFRflox/flox mice21 with Podocin.Cre mice22 (Figure 1A). Effective cleavage of the EGFR floxed gene was verified by PCR analysis of isolated glomerular genomic DNA, with excision of exon 3 of the EGFR encoding gene (Figure 1B). Effective deletion of EGFR protein expression in podocytes was confirmed by immunoblotting analysis of isolated glomerular lysates (Figure 1C). Both EGFRpodKO and wild-type (WT) mice were made diabetic by the streptozotocin injection 1 week after unilateral nephrectomy. The two groups of mice developed comparable levels of hyperglycemia within 6 days, which persisted through the course of the study.
Figure 1.
Development of podocyte-specific EGFR deletion mice. (A) Schematic for the generation of EGFRpodKO mice by crossing Podocin.Cre mice with EGFRflox/flox mice. (B) EGFR deletion of exon 3 and Cre expression are verified by PCR using glomerular genomic DNA as the template. (C) EGFR attenuation in podocytes is confirmed by immunoblotting of isolated glomerular lysates. Data are representative of three separate experiments (n=5–7 mice/group).
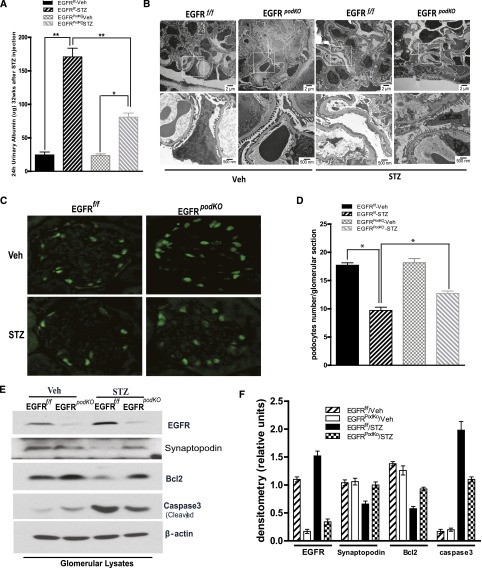
Podocytes are essential for the integrity of the glomerular filtration barrier, and proteinuria is an indication of a compromised glomerular filtration barrier. The diabetic EGFRpodKO mice developed significantly less albuminuria compared with the WT mice (urinary albumin/creatinine ratio WT versus EGFRpodKO: 170.80±12.93 versus 80.8±6.14 µg/mg; P<0.001; n=5–7 mice/group) (Figure 2A). Electron microscopy revealed more severe segmental podocyte foot process effacement in the WT mice (Figure 2B).
Figure 2.
Proteinuria and podocyte loss are attenuated in mice with selective deletion of EGFR in podocytes. (A) EGFRpodKO mice aged 9–10 weeks and their control littermates are subjected to five consecutive STZ injections. Mouse urine is collected for 24 hours and creatinine and albumin are measured at 32 weeks after STZ injection (n=5–7 per group). (B) Representative micrographs of electron microscopy from different groups of mouse kidney (n=3–5 per group). (C) Representative kidney sections are stained with the antibody against WT1. (D) Podocyte numbers are counted randomly in 15 glomeruli/section (n=5–7 per group). (E) Immunoblotting of isolated glomeruli lysates from EGFRpodKO mice and their control littermates 32 weeks after STZ injection are analyzed with the indicated antibodies (n=5–7 mice/group). (F) Densitometry of the data in E. Data are presented as the mean±SEM. *P<0.05; **P<0.001. Veh, vehicle; STZ, streptozocin.
Podocyte detachment from the glomerular basement membrane is proposed to be an early feature of diabetic kidney injury and may predict the progression of diabetic nephropathy, but the precise mechanisms of podocyte loss under diabetic conditions have not yet been completely elucidated. We observed significantly more podocytes loss in WT diabetic mice compared with the EGFRpodKO diabetic mice (podocyte number/glomerulus: WT versus EGFRpodKO: 9.71±0.56 versus 12.71±0.42, P<0.01, n=5–7 mice/group) (Figure 2, C and D). Further analysis of expression of a differentiated podocyte cell marker protein, synaptopodin,23 in isolated glomerular lysates indicated much less synaptopodin expression in WT mouse glomeruli (Figure 2, E and F). To determine the mechanism of podocyte loss, we isolated glomeruli and analyzed the expression levels of Bcl2, an antiapoptotic protein,24 and cleaved caspase3, a proapoptotic protein.25 We found that the expression of Bcl2 was downregulated and the expression of cleaved caspase3 was upregulated in WT diabetic glomeruli, and these alterations were significantly less in EGFRpodKO mice (Figure 2, E and F).
EGFR Deletion in Podocytes Attenuated the Increased TGF-β Signaling in Diabetic Kidney
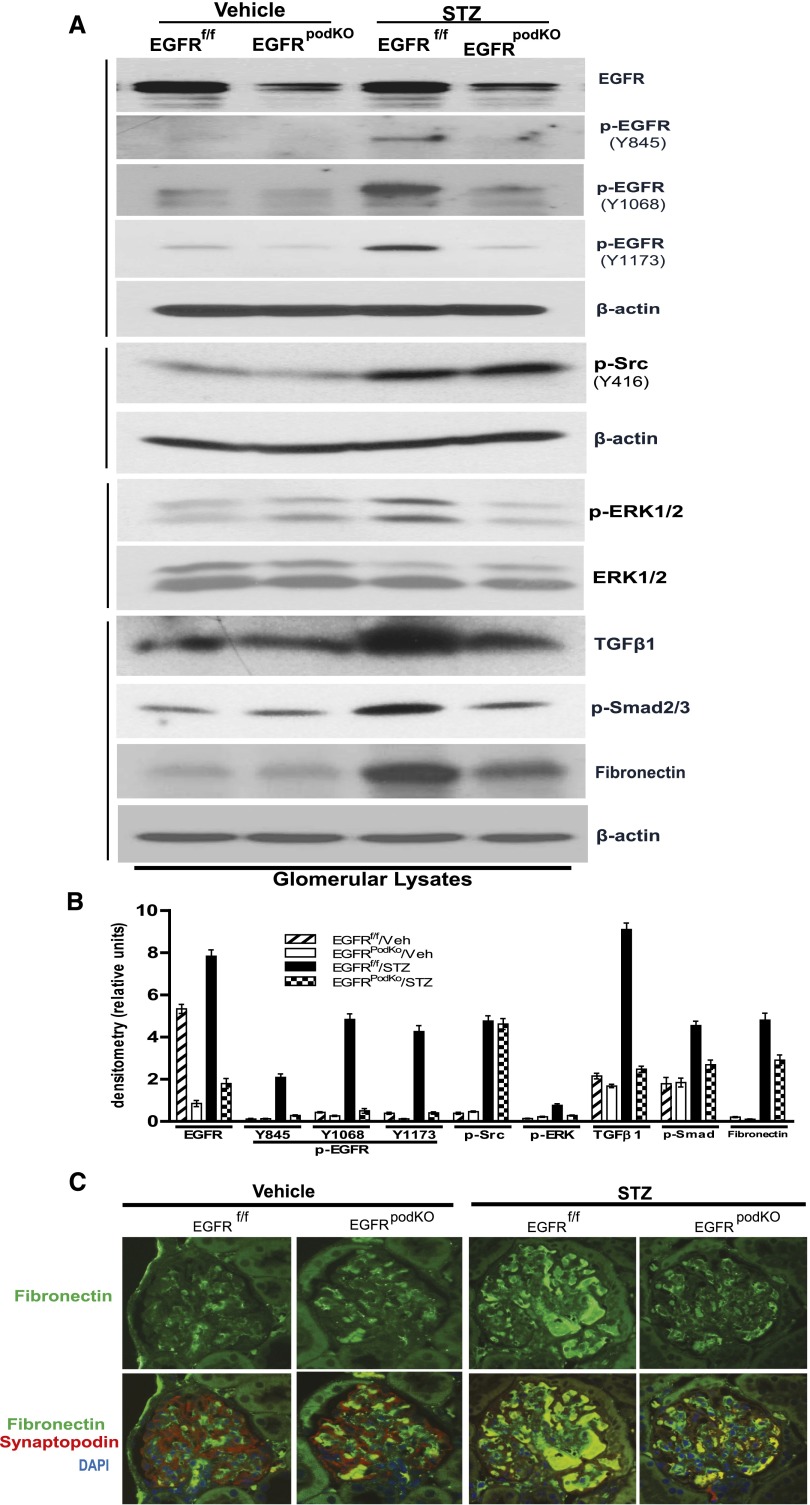
Activation of the TGF-β signaling pathway plays a major role in the progression of diabetic nephropathy through induction of extracellular matrix accumulation by enhancing synthesis of collagen, fibronectin, and laminin, as well as by inhibiting matrix metalloproteinase-mediated extracellular matrix degradation.26 Our previous study found that in response to chronic angiotensin II infusion in renal proximal tubule epithelial cells, TGF-β–Smad2/3 signaling pathway was activated through a c-Src kinase–mediated EGFR-ERK signaling pathway activation.15 To determine whether the TGF-β signaling pathway was activated in diabetic podocytes through an EGFR-dependent mechanism, we examined and found increased phosphorylation levels of EGFR at three different sites (Y845, Y1068 and Y1173) as well as increased phosphorylation of c-Src, ERK1/2 as well as Smad2/3. The expression levels of TGF-β and fibronectin were upregulated in the glomeruli isolated from WT diabetic mice. There was marked inhibition of phosphorylation of EGFR at all three tyrosine residues, ERK and Smad2/3 phosphorylation, and TGF-β and fibronectin expression in EGFRpodKO diabetic mice. However, c-Src phosphorylation was unchanged (Figure 3, A and B). Immunofluorescence of the mouse kidney sections with antibodies against fibronectin confirmed that upregulated fibronectin in glomeruli was markedly blunted in EGFRpodKO diabetic mice (Figure 3C).
Figure 3.
Deletion of EGFR in podocytes attenuates ERK activation, TGFβ-Smad2/3 signaling activation, and fibronectin expression, but not Src phosphorylation, in diabetic kidney glomeruli. (A) Immunoblotting of isolated glomeruli lysates from EGFRpodKO mice and their control littermates 32 weeks after STZ injection are analyzed with indicated antibodies. (B) Densitometry of the data in A. (C) Immunoreactivity in diabetic EGFRpodKO mice and littermate controls with specific antibodies against fibronectin (green), podocyte marker synaptopodin (red), and DAPI (blue) (n=5–7 mice/group). Veh, vehicle; STZ, streptozocin.
ROS-Mediated Podocyte c-Src and EGFR/ERK Phosphorylation
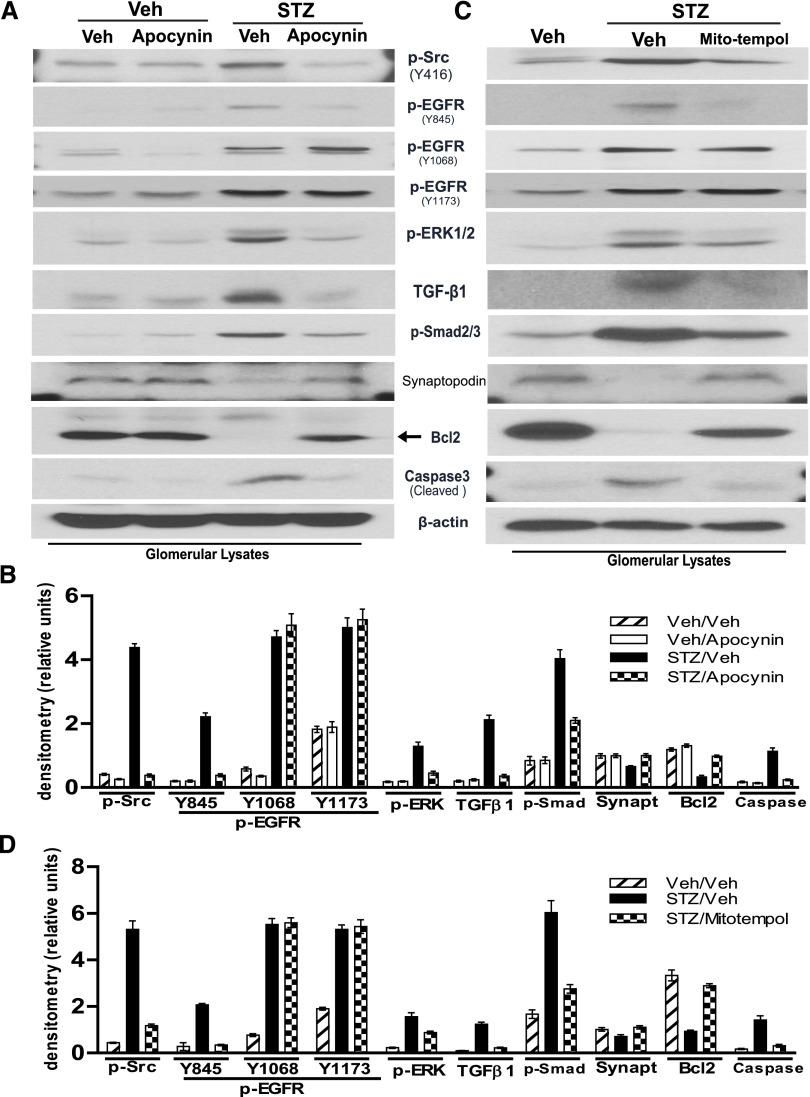
Elevated levels of ROS due to either increased production or decreased antioxidant function in the kidney are implicated in the initiation and progression of diabetic nephropathy.1 Our recent studies demonstrated that NADPH oxidase–derived ROS production–mediated c-Src kinase activation was an upstream mediator of EGFR activation in renal proximal tubular epithelial cells in response to chronic angiotensin II treatment.15 A recent study also indicated that inhibition of c-Src kinase prevented progression of diabetic nephropathy.27 To examine whether NADPH oxidase–dependent ROS production induced c-Src kinase–dependent activation of the EGFR-ERK signaling pathway and TGF-β-Smad2/3 signaling pathway in podocytes, WT diabetic mice were administered a mitochondria-targeted ROS scavenger, mito-tempol (10 mg/kg per day intraperitoneally), or a cell-permeable NADPH-oxidase inhibitor, apocynin (5mg/kg per day intraperitoneally), for 3 weeks with the first dose being given 2 days after initiation of diabetes. Both mito-tempol and apocynin treatments inhibited phosphorylation of c-Src, EGFR (Y845), ERK1/2, and Smad2/3; prevented upregulation of TGF-β expression; and prevented the alterations of synaptopodin, Bcl2, and cleaved caspase3 expression in diabetic mouse glomeruli. However, upregulated EGFR phosphorylation at both tyrosine 1068 (Y1068) and tyrosine 1173 was not affected by mito-tempol or apocynin administration (Figure 4).
Figure 4.
Inhibition of ROS production blunts the signaling pathway alterations in WT diabetic mouse glomeruli. WT 129svj mice aged 9–10 weeks are subjected to five consecutive STZ injections. (A and C) Apocynin (A) or mito-tempol (C) is administered by intraperitoneal injections starting 2 days after STZ injection for 3 weeks. Immunoblotting of isolated glomeruli lysates from control, diabetic mice with or without treatment is performed by using indicated antibodies. (B and D) Densitometry of the data in A and C, respectively (n=5–7 mice/group). Veh, vehicle; STZ, streptozocin.
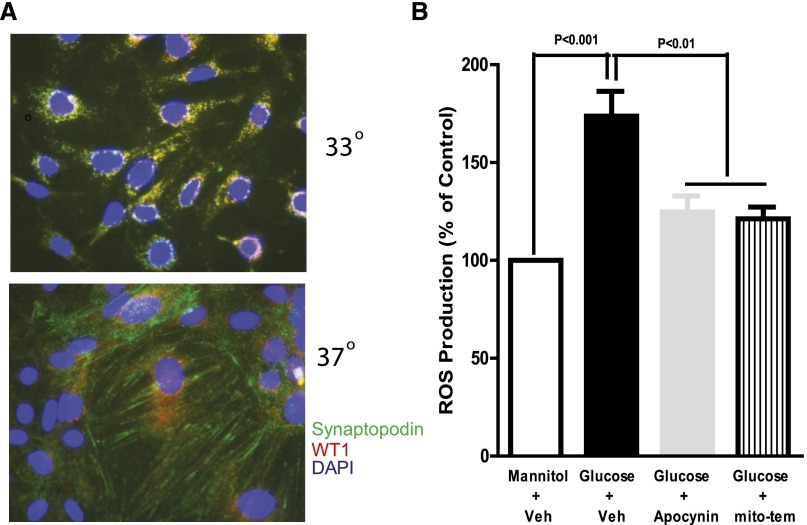
To further confirm the role of ROS in activation of the EGFR-ERK pathway and TGF-β–Smad2/3 signaling pathway in podocytes, we utilized a conditionally immortalized mouse podocyte line. The cells were maintained at 33° in culture medium with IFNγ and then differentiated at 37°C in culture medium without IFNγ for 10 days, as previously described23 (Figure 5A). When the differentiated cultured podocytes were exposed to high glucose (25 mM) after 24 hours of quiescence, ROS production increased significantly within 4 hours, and treatment of the cells with either apocynin or mito-tempol markedly blunted the increased ROS (Figure 5B).
Figure 5.
Apocynin or mito-tempol inhibits ROS production in response to high-glucose treatment in cultured podocytes. (A) Synaptopodin staining is performed to verify the differentiation of podocyte cells. (B) High glucose (25 mM) increases ROS production, measured by fluorescence intensity of DCFH, which is inhibited by the NADPH oxidase inhibitor apocynin or mito-tempol in differentiated podocytes. DCFH, 2′,7′-dichlorodihydrofluorescin; Veh, vehicle; mito-tem, mito-tempol.
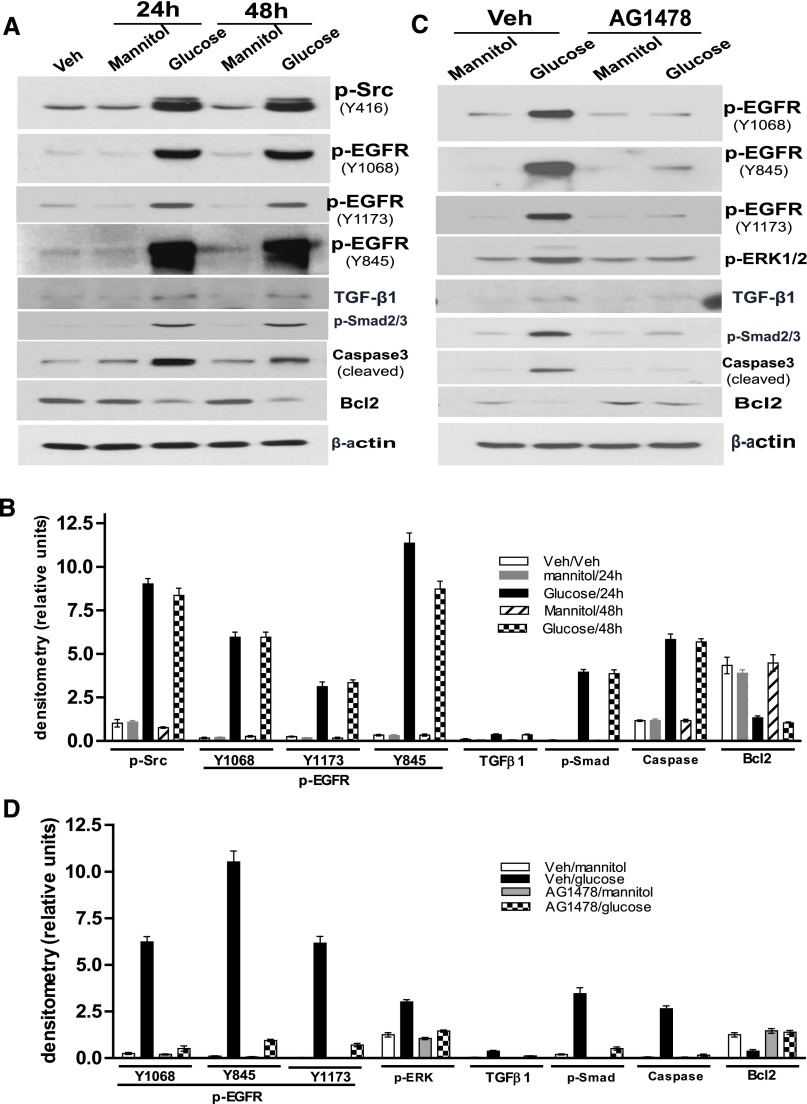
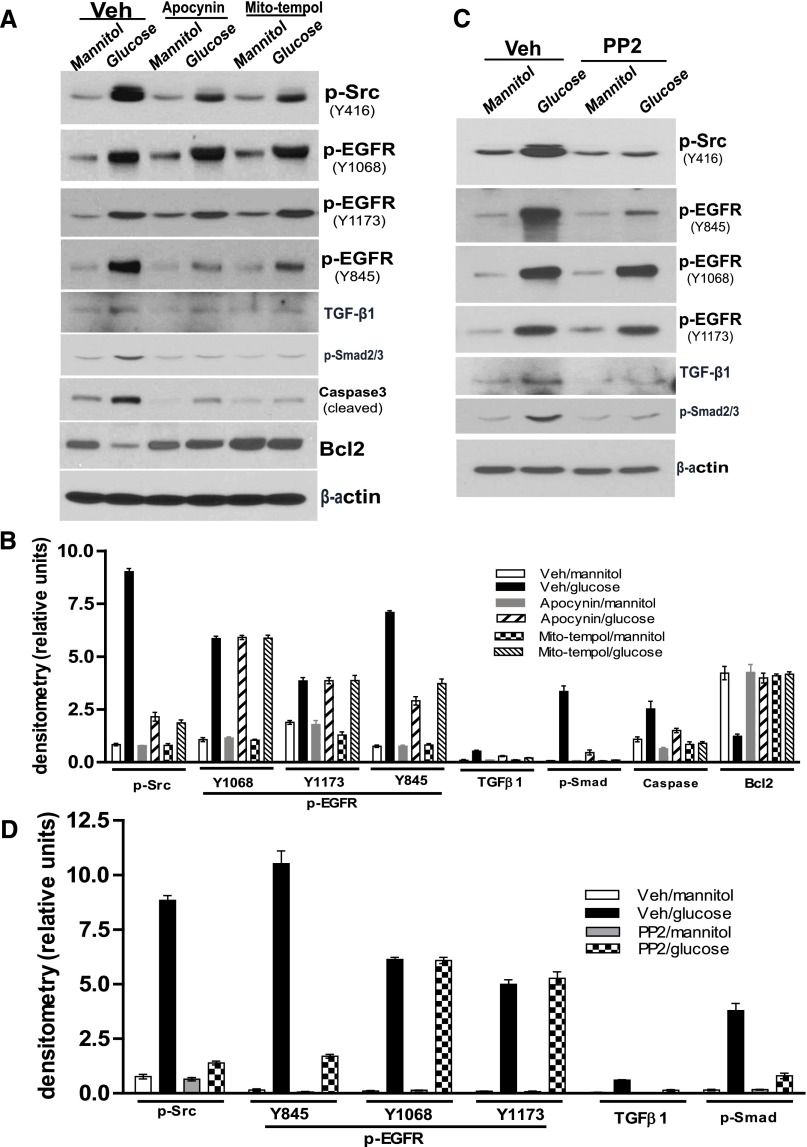
High-glucose treatment of the differentiated podocytes for 24 or 48 hours markedly increased phosphorylation of c-Src at Y416 and EGFR at Y1068, Y1173, and Y845, and also increased TGF-β, phosphor-Smad2/3, and cleaved caspase3 expression and decreased Bcl2 expression (Figure 6, A and B). Pretreatment of the differentiated podocytes with the EGFR tyrosine kinase inhibitor, AG1478, not only blocked EGFR to ERK and TGF-β–Smad2/3 signals but also prevented altered expression of cleaved caspase3 and Bcl2 (Figure 6, C and D). Apocynin or mito-tempol pretreatment of the differentiated podocytes markedly inhibited c-Src and EGFR tyrosine 845 (Y845) phosphorylation and activation of TGF-β–Smad2/3 signaling, but not EGFR phosphorylation at tyrosine 1068 (Y1068) or tyrosine 1173 (Y1173). In addition, both elevation of cleaved caspase3 and reduction of Bcl2 expression in response to high-glucose treatment were prevented by either apocynin or mito-tempol treatment (Figure 7, A and B). To confirm that c-Src kinase phosphorylation is upstream of EGFR phosphorylation and its downstream signaling, we determined that the c-Src kinase inhibitor, PP2, inhibited EGFR tyrosine 845 (Y845) phosphorylation-mediated TGF-β–Smad2/3 signaling pathway activation in the differentiated podocytes in response to high-glucose treatment, but not EGFR phosphorylation at tyrosine 1068 (Y1068) and tyrosine 1173 (Y1173) (Figure 7, C and D).
Figure 6.
Blocking EGFR phosphorylation inhibits the effects of high-glucose treatment on cultured podocytes. (A) With exposure of the differentiated podocytes to high glucose (25 mM) for either 24 or 48 hours, phosphorylation of Src at Y416; EGFR at Y845, Y1173, and Y1068; and Smad2/3 is increased. TGFβ and cleaved caspase3 expression is increased, but Bcl2 expression is inhibited. (B) Densitometry of the data in A. (C) In cultured differentiated podocytes, AG1478 treatment inhibits high-glucose–induced phosphorylation of EGFR and ERK, activation of TGFβ-Smad2/3 signaling, and expression alterations of cleaved caspase3 and Bcl2. (D) Densitometry of the data in C. Results from three separate experiments are shown. Veh, vehicle.
Figure 7.
Blocking ROS production or c-Src kinase activity inhibits the effects of high-glucose treatment on cultured podocytes. (A) Pretreatment of the differentiated podocytes with either apocynin or mito-tempol inhibited phosphorylations of c-Src at Y416 and EGFR at Y845, damped TGFb-Smad2/3 signaling activation and blunted the alterations of cleaved caspase3 and Bcl2 expression in response to high glucose treatment. (B) Densitometry of the data in A. (C) Pretreatment of the differentiated podocytes with PP2 not only inhibited c-Src phosphorylation, but also restricted phosphorylation of EGFR and activation of TGFβ-Smad2/3 signaling pathway in response to high glucose treatment. (D) Densitometry of the data in C. Representative data from three separate experiments are shown. Veh, vehicle.
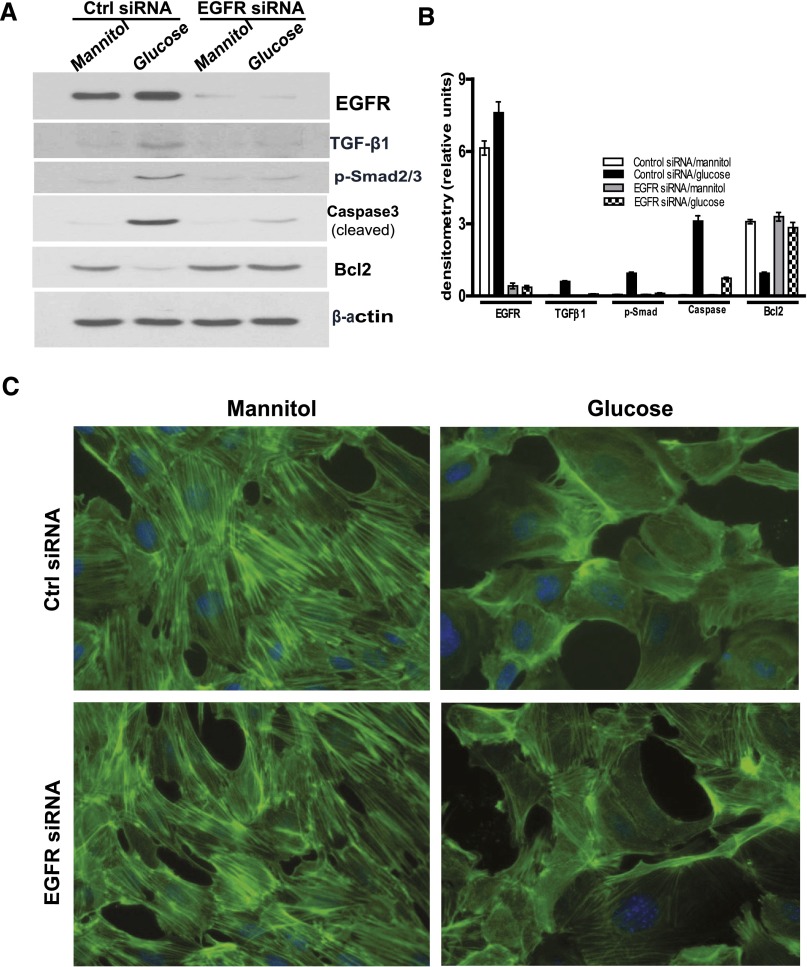
Knocking Down EGFR-Inhibited TGF-β–Smad2/3 Signaling and Prevented Alterations of Bcl2 and Cleaved Caspase3 Expression in Podocytes in Response to High-Glucose Treatment
To further investigate whether EGFR activation was essential for activation of the TGF-β–Smad2/3 signaling pathway in podocytes in response to high-glucose treatment, we knocked down EGFR protein expression by transfection of specific mouse small interfering RNA (siRNA) sequences in the cultured immortal mouse podocytes and found that downregulation of EGFR expression markedly attenuated TGF-β–Smad2/3 activation and alteration of cleaved caspase3 and Bcl2 expression in response to high-glucose exposure (Figure 8, A and B). For further investigation of cell morphologic changes in response to high-glucose treatment, the differentiated podocytes were stained with fluorescence-labeled phalloidin, which binds polymerized f-actin, after mannitol or glucose treatment. There was a striking reduction in actin stress fiber formation when the cells were exposed to high glucose. Knockdown of EGFR expression by siRNA limited the reduction in actin stress fiber formation in response to high-glucose treatment (Figure 8C).
Figure 8.
Knocking down EGFR expression inhibits the effects of high-glucose treatment on cultured podocytes. (A) In cultured differentiated podocytes, specific EGFR siRNA knocks down expression of EGFR-inhibited high-glucose–induced TGFβ-Smad2/3 signaling activation and alterations of expression of cleaved caspase3 and Bcl2. (B) Densitometry of the data in A. (C) Representative images of phalloidin staining patterns observed in control or EGFR siRNA transfected differentiated podocytes in response to mannitol or glucose treatment. Representative data from three separate experiments are shown. Ctrl, control.
Discussion
This study provided evidence for the first time that podocyte-specific deletion of EGFR in mice attenuated albuminuria and podocyte loss in the diabetic kidney. In an attempt to determine the underlying mechanism, we found that EGFR deletion in podocytes inhibited TGF-β upregulation and activation of Smad2/3 in glomeruli of diabetic mice. Administration of a mitochondrial-targeted antioxidant (mito-tempol) or a cell-permeable NADPH-oxidase inhibitor (apocynin) to the WT diabetic mice inhibited c-Src phosphorylation at Y416 (which is known to activate Src kinase28,29), inhibited EGFR at Y845 (a c-Src kinase-mediated phosphorylation site14,30), inhibited the TGF-β–Smad signaling pathway, and prevented proapoptotic mediators both in glomeruli from diabetic mice and in cultured podocytes exposed to hyperglycemia. Therefore, our results suggest that ROS-mediated activation of EGFR by c-Src kinase is a key mechanism of podocyte dysfunction and loss during the development of diabetic nephropathy.
In isolated diabetic mouse glomeruli from WT diabetic mice, inhibition of NADPH oxidase by apocynin or mito-tempol reduced EGFR phosphorylation at tyrosine 845 (Y845), but did not affect EGFR phosphorylation at tyrosine 1068 (Y1068) and at tyrosine 1173 (Y1173), which are well known autophosphorylation sites mediated by EGFR ligands.31 In vitro, mito-tempol or apocynin treatment of the differentiated podocytes not only blocked ROS production, but also inhibited c-Src kinase activation and phosphorylation of EGFR at tyrosine 845 (Y845) without affecting EGFR phosphorylation at tyrosine 1068 (Y1068) and at tyrosine 1173 (Y1173).
There is increasing evidence that continuous and aberrant EGFR activation is related to renal fibrogenesis.15,16,32,33 In this regard, we recently reported that in a model of accelerated diabetic nephropathy (endothelial nitric oxide synthase–streptozotocin mice), administration of the EGFR tyrosine kinase inhibitor, erlotinib, markedly reduced albuminuria and glomerulosclerosis.20 Similarly, studies in diabetic rats reported that a different EGFR tyrosine kinase inhibitor, PKI 166, attenuated early glomerular enlargement and preserved podocytes.17 Deletion of podocyte EGFR did not reduce c-Src kinase activation in diabetic glomeruli. By contrast, inhibition of ROS production in vivo or in vitro inhibited not only c-Src activation but also phosphorylation of EGFR at Y845, without decreasing EGFR phosphorylation at Y1068 and Y1173, suggesting that EGFR may be activated by both ligand-mediated and ligand-independent pathways in diabetes. In this regard, we recently reported increased expression of the EGFR ligand, HB-EGF, in diabetes that was mediated by endothelial dysfunction, and endothelial-specific HB-EGF deletion reduced progression of diabetic nephropathy.34
Previous studies by us and others indicated that activation of EGFR contributes to recovery from AKI35–37 by accelerating tissue regeneration but is detrimental in response to chronic kidney insults by increasing profibrotic pathways. We suggest that mild or moderate AKI induces acute and self-limited EGFR activation to promote cell repair and proliferation, whereas more severe AKI or a continuous chronic kidney injury will induce continued EGFR activation, which in turn will activate TGF-β and other signaling pathways mediating progressive renal fibrosis.
Increased ROS production has been posited to be a mediator of the injury associated with diabetes in various target organ systems, including the kidney.38,39 There are a number of potential sources for increased ROS production in the diabetic kidney, including NADPH oxidase, advanced glycation end products, defects in the polyol pathway, uncoupled nitric oxide synthase, and alterations in the mitochondrial respiratory chain via oxidative phosphorylation.40 It was proposed that overproduction of superoxide by mitochondria provides a unifying explanation for many of the pathophysiologic mechanisms underlying diabetic complications,41 although recent studies questioned this hypothesis.42 However, it was noteworthy that in the current studies, hyperglycemia-induced ROS production in cultured podocytes was inhibited both by an NADPH oxidase inhibitor, apocynin, and a mitochondria-targeted SOD, mito-tempol.
In summary, we found that in isolated glomerular lysates from streptozotocin injection induced type 1 diabetic WT or EGFRpodKO mice or cultured immortal podocytes after exposure to high-glucose medium, increased glomerular ROS production, subsequent Src kinase activation was upstream of EGFR activation in podocytes, and the activated EGFR induced TGF-β–Smad2/3 signaling and proapoptic signaling, which led to reduction of podocyte number by apoptosis. In light of recent studies indicating that detrimental effects of TGF-β in diabetic nephropathy may be mediated by induction of mitochondrial Nox4 and increased ROS production via activation of TGF-β receptor dependent–Smad2/3 signaling,43,44 we propose that there may exist a vicious cycle, with high glucose directly activating NADPH oxidase–dependent ROS production and subsequent Src kinase–mediated EGFR signaling leading to TGF-β–Smad2/3 activation, which may in turn further activate mitochondrial NADPH oxidase to increase ROS production and Src kinase activity, thereby enhancing TGF-β–mediated podocyte injury.
Concise Methods
Reagents and Antibodies
Antibodies against EGFR, p-EGFR (Y1173, Y1068, and Y845), p-SRC (Y416), Bcl2, cleaved Caspase3, TGF-β1, and p-Smad2/3 were purchased from Cell Signaling Technology (Beverly, MA). Antibodies against WT1, p-ERK1/2, total ERK, and all secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Alexa Fluor 488– or Alexa Fluor 594–conjugated secondary antibodies were from Life Technologies (Grand Island, NY). The specific EGFR tyrosine kinase inhibitor AG1478 and the inhibitor of Src tyrosine kinase PP2 were from EMD Millipore (Billerica, MA). Antibodies against fibronectin, streptozocin, 2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl) triphenylphosphonium chloride (mito-tempol), 4′-hydroxy-3′-methoxyacetophenone (apocynin), 2′,7′-dichlorodihydrofluorescin diacetate, and all other reagents were from Sigma-Aldrich (St. Louis, MO).
Generation of EGFRpodKO Null Mice
The Vanderbilt University Institutional Animal Care and Use Committee approved all experiments and all experiments were conducted according to National Institutes of Health guidelines. EGFRflox/flox mice, which were generated as we previously described,15 were back-crossed onto a 129 Svj background for 10 generations followed by crossing with transgenic mice carrying Cre recombinase under the control of the podocin promoter (Pod-Cre),22 which were also back-crossed onto the 129 Svj background for 10 generations. Age-matched EGFRflox/flox littermates lacking the pod-Cre transgene were used as WT controls for Pod-Cre(+) EGFRflox/flox (EGFRpodKO) mice lacking the EGFR in podocytes. Mice were genotyped by PCR with the following primers: 5′-CTTTGGAGAACCTGCAGATC-3′ and 5′-CTGCTACTGGCTCAAGTTTC-3′ for verification of the EGFR gene floxed mice, 5′-ACACTAGCACTGACTGCTGG-3′ and 5′-CTGCTACTGGCTCAAGTTTC-3′ for verification of the EGFR WT mice, and 5′- GCATAACCAGTGAAACAGCATTGCTG-3′ and 5′-GGACATGTT CAGGGATCGCCAGGCG-3′ for verification of pod-Cre expression.
Induction of Diabetes in EGFRpodKO and WT Mice
Ten-week-old male EGFRpodKO and their WT mice or WT 129 Svj mice were injected daily with streptozocin (prepared freshly in 0.1 mol/L citrate buffer, pH 4.5, and given at a dose of 50 mg/kg body weight intraperitoneally) or vehicle alone for 5 consecutive days to induce type 1 diabetes. One week before streptozocin injection, unilateral nephrectomy surgery was performed in those mice that were used to analyze urine creatinine and albumin. Blood glucose was measured using the OneTouch Basic Blood Glucose Monitoring System (LifeScan, Milpitas, CA) on blood samples obtained via the saphenous vein after 5 hours of food deprivation. Mice were euthanized at indicated time points.
Measurement of Urine Creatinine and Albumin
We collected 24-hour urine samples from uninephrectomized EGFRpodKO and WT mice 32 weeks after either streptozocin or vehicle injection. Urinary albumin levels were detected by a murine microalbuminuria ELISA kit (AlbuwellM) and the urinary creatinine was measured by using a microplate assay kit (Creatinine Companion; Exocell, Philadelphia, PA). All measurements were performed in triplicate and the ratio of urinary albumin (in micrograms per milliliter) to creatinine (in milligrams per milliliter) was calculated and expressed in micrograms per milligram. Results are expressed as the mean±SEM.
Isolation of Glomeruli by Using Dynabeads M-450
Three weeks after induction of hyperglycemia by streptozocin injection, diabetic and control mouse glomeruli were isolated as previously described with minor modifications .45 Briefly, mice were anesthetized by an intraperitoneal injection of Nembutal Sodium solution (50 mg/kg) and perfused with 4×107 Dynabeads (0.25 ml of commercial Dynabeads M-450 Tosylactivated beads) diluted in 20 ml of PBS through the heart. The kidneys were removed, minced, and digested in collagenase (1 mg/ml collagenase A, 100 U/ml deoxyribonuclease I in HEPES-buffered salt solution) at 37°C for 30 minutes with gentle agitation. The collagenase-digested tissue was gently pressed through a 100-µm cell strainer using a flattened pestle, and the cell strainer was then washed with 5 ml of HEPES-buffered salt solution. The filtered cells were passed through a new cell strainer without pressing and the cell strainer was washed with 5 ml of HEPES-buffered salt solution. The cell suspension was then centrifuged at 200×g for 5 minutes. The supernatant was discarded and the cell pellet was resuspended in 2 ml of HEPES-buffered salt solution. Finally, glomeruli containing Dynabeads were gathered by a magnetic particle concentrator and washed three times with ice-cold HEPES-buffered salt solution. The isolated glomeruli were lysed in RIPA buffer followed by immunoblotting analysis.
Cell Culture
Immortalized mouse podocyte cells, from Dr. Peter Mundel (Harvard Medical School), were cultured as previously described.23 Briefly, cells were maintained at 33°C in RPMI 1640 medium containing 100 U/ml IFNγ and 10% FBS and induced to differentiate by shifting them to 37°C and culturing in RPMI 1640 medium containing 10% FBS without IFNγ for 10 days. The differentiated podocytes were made quiescent in RPMI 1640 medium containing 5.5 mM of glucose and 1% FBS for 24 hours followed by treatment with 25 mM mannitol or glucose with or without mito-tempol (300 μM) or apocynin (500 μM), AG1478 (1 μM), and PP2 (5 μM) for 24 or 48 hours.
Immunoblotting
Immunoblotting procedures were performed as previously described with minor modifications.15 Briefly, for in vitro experiments, differentiated podocytes were cultured for 24 or 48 hours in quiescent medium containing 25 mM of mannitol or glucose with or without mito-tempol, apocynin, or AG1478 followed by harvesting in RIPA buffer. For in vivo experiments, isolated EGFRpodKO and/or WT mouse glomeruli were lysed in RIPA buffer and equal amounts of protein lysate were loaded directly onto 7.5%–15% SDS-PAGE gels, transferred onto Immobilon-P transfer membranes (EMD Millipore, Bedford, MA), and probed with the indicated primary antibody. The primary antibodies were detected with peroxidase-labeled goat anti-rabbit IgG or goat anti-mouse IgG and were exposed on film by using enhanced chemiluminescence (Amersham Biosciences, Ltd., Buckinghamshire, UK).
Transfection of EGFR siRNA
Mouse EGFR ON-TARGETplus SMARTpool siRNA (L-040411-00-0005) or negative control siRNA (Thermo Fisher Scientific, Lafayette, CO) were transfected into differentiated mouse podocytes by the Lipofectamine method (Invitrogen, Carlsbad, CA) as previously described.14 Three days after transfection, the cells were made quiescent in RPMI 1640 medium containing 5.5 mM of glucose and 1% FBS for 24 hours followed by treatment with 25 mM of mannitol or glucose for 24 hours. Cells were harvested in RIPA buffer and the lysates were analyzed by immunoblotting or stained by phalloidin-FITC.
Measurement of Intracellular ROS Generation
Differentiated podocytes were cultured in 24-well plates and made quiescent in RPMI 1640 medium (containing 5.5 mM of glucose) containing 1% FBS for 24 hours, followed by washing once with HEPES-buffered salt solution (pH=7.4) (0.5 ml/well) containing 25 mM HEPES, 120 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 25 mM NaHCO3, and 5.5 mM glucose. The cells were then left untreated or treated with mito-tempol (300 μM) or apocynin (500 μM) for 30 minutes before the addition of 25 mM glucose or 25 mM mannitol and 2′,7′-dichlorodihydrofluorescin diacetate (100 μM) for 4 hours. The fluorescence intensity was measured using a fluorescence multiwell plate reader with excitation and emission wavelengths of 485 nm and 530 nm, respectively.
Immunofluorescence Analyses
For mouse kidney tissues, immunofluorescence was performed on paraffin-embedded tissues fixed by 4% paraformaldehyde, using standard techniques as previously described.15 Five-micrometer kidney sections were deparaffinized, rehydrated, subjected to antigen retrieval, and then incubated with rabbit primary antibodies (WT1, p-Smad2/3, and fibronectin) and mouse primary antibodies (synaptopodin) in 5% goat serum in PBS for 1 hour followed by incubation with Alexa Fluor 594– or Alexa Fluor 488–conjugated secondary antibodies for 1 hour. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). For cultured differentiated podocytes, the cells were fixed with 4% paraformaldehyde/PBS, permeabilized with 0.1% Triton X-100/PBS, and washed three times with PBS. After incubation with mouse anti-synaptopodin antibody (1:500) for 1 hour at room temperature. Cells were washed three times with PBS and then incubated with Alexa Fluor 488–conjugated donkey anti-mouse antibody for 1 hour. Nuclei were counterstained with DAPI. Cells were treated with 25 mM of mannitol or glucose for 24 hours after 24 hours of quiescence as described above. The differentiated podocytes were incubated with phalloidin/FITC (50 μg/ml) at room temperature for 40 minutes. The cells were washed once and counterstained by DAPI followed by three times of PBS washing before being covered with coverslips. Images were captured by using an Axioplan2 fluorescence microscope and an AxioCam HRc digital camera (Carl Zeiss).
Statistical Analyses
Data are presented as the mean±SEM for at least three separate experiments (each in triplicate). An unpaired t test was used for statistical analyses. ANOVA and Bonferroni t tests were used for multiple-group comparisons. A P value <0.05 compared with the control was considered statistically significant.
Disclosures
None.
Acknowledgments
This work was supported by funds from the Department of Veterans Affairs and the National Institutes of Health (grants DK51265, DK62794, and DK95785 to R.C.H. and DK83575 to J.-K.C.).
Some of the data in this article were presented as an oral presentation during a free communication session at the 2013 American Society of Nephrology Annual Meeting, held November 5–10, 2013, in Atlanta, Georgia.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Stanton RC: Oxidative stress and diabetic kidney disease. Curr Diab Rep 11: 330–336, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Stamler J, Vaccaro O, Neaton JD, Wentworth D: Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 16: 434–444, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Caramori ML, Mauer M: Diabetes and nephropathy. Curr Opin Nephrol Hypertens 12: 273–282, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Reidy K, Susztak K: Epithelial-mesenchymal transition and podocyte loss in diabetic kidney disease. Am J Kidney Dis 54: 590–593, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, Abboud HE: Mechanisms of podocyte injury in diabetes: Role of cytochrome P450 and NADPH oxidases. Diabetes 58: 1201–1211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Susztak K, Raff AC, Schiffer M, Böttinger EP: Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 8.Harris DC, Chan L, Schrier RW: Remnant kidney hypermetabolism and progression of chronic renal failure. Am J Physiol 254: F267–F276, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Harris RC: Response of rat inner medullary collecting duct to epidermal growth factor. Am J Physiol 256: F1117–F1124, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Breyer MD, Redha R, Breyer JA: Segmental distribution of epidermal growth factor binding sites in rabbit nephron. Am J Physiol 259: F553–F558, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Zeng F, Singh AB, Harris RC: The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp Cell Res 315: 602–610, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pines G, Köstler WJ, Yarden Y: Oncogenic mutant forms of EGFR: Lessons in signal transduction and targets for cancer therapy. FEBS Lett 584: 2699–2706, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi K, Ito F: EGF receptor in relation to tumor development: Molecular basis of responsiveness of cancer cells to EGFR-targeting tyrosine kinase inhibitors. FEBS J 277: 316–326, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Chen JK, Harris RC: Angiotensin II induces epithelial-to-mesenchymal transition in renal epithelial cells through reactive oxygen species/Src/caveolin-mediated activation of an epidermal growth factor receptor-extracellular signal-regulated kinase signaling pathway. Mol Cell Biol 32: 981–991, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC: EGFR signaling promotes TGFβ-dependent renal fibrosis. J Am Soc Nephrol 23: 215–224, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang J, Liu N, Tolbert E, Ponnusamy M, Ma L, Gong R, Bayliss G, Yan H, Zhuang S: Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am J Pathol 183: 160–172, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Advani A, Wiggins KJ, Cox AJ, Zhang Y, Gilbert RE, Kelly DJ: Inhibition of the epidermal growth factor receptor preserves podocytes and attenuates albuminuria in experimental diabetic nephropathy. Nephrology (Carlton) 16: 573–581, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Coaxum SD, Garnovskaya MN, Gooz M, Baldys A, Raymond JR: Epidermal growth factor activates Na(+/)H(+) exchanger in podocytes through a mechanism that involves Janus kinase and calmodulin. Biochim Biophys Acta 1793: 1174–1181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bollée G, Flamant M, Schordan S, Fligny C, Rumpel E, Milon M, Schordan E, Sabaa N, Vandermeersch S, Galaup A, Rodenas A, Casal I, Sunnarborg SW, Salant DJ, Kopp JB, Threadgill DW, Quaggin SE, Dussaule JC, Germain S, Mesnard L, Endlich K, Boucheix C, Belenfant X, Callard P, Endlich N, Tharaux PL: Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med 17: 1242–1250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang MZ, Wang Y, Paueksakon P, Harris RC: Epidermal growth factor receptor inhibition slows progression of diabetic nephropathy in association with a decrease in endoplasmic reticulum stress and an increase in autophagy. Diabetes 63: 2063–2072, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee TC, Threadgill DW: Generation and validation of mice carrying a conditional allele of the epidermal growth factor receptor. Genesis 47: 85–92, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Hockenbery DM, Zutter M, Hickey W, Nahm M, Korsmeyer SJ: BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A 88: 6961–6965, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, Munday NA, Raju SM, Smulson ME, Yamin TT, Yu VL, Miller DK: Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376: 37–43, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Gruden G, Perin PC, Camussi G: Insight on the pathogenesis of diabetic nephropathy from the study of podocyte and mesangial cell biology. Curr Diabetes Rev 1: 27–40, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Taniguchi K, Xia L, Goldberg HJ, Lee KW, Shah A, Stavar L, Masson EA, Momen A, Shikatani EA, John R, Husain M, Fantus IG: Inhibition of Src kinase blocks high glucose-induced EGFR transactivation and collagen synthesis in mesangial cells and prevents diabetic nephropathy in mice. Diabetes 62: 3874–3886, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kmiecik TE, Shalloway D: Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell 49: 65–73, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Piwnica-Worms H, Saunders KB, Roberts TM, Smith AE, Cheng SH: Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell 49: 75–82, 1987 [DOI] [PubMed] [Google Scholar]
- 30.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ: c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem 274: 8335–8343, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Sorkin A, Waters C, Overholser KA, Carpenter G: Multiple autophosphorylation site mutations of the epidermal growth factor receptor. Analysis of kinase activity and endocytosis. J Biol Chem 266: 8355–8362, 1991 [PubMed] [Google Scholar]
- 32.Chung H, Ramachandran R, Hollenberg MD, Muruve DA: Proteinase-activated receptor-2 transactivation of epidermal growth factor receptor and transforming growth factor-β receptor signaling pathways contributes to renal fibrosis. J Biol Chem 288: 37319–37331, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, Bayliss G, Dworkin LD, Yan H, Zhuang S: Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol 23: 854–867, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyazawa T, Zeng F, Wang S, Fan X, Cheng H, Yang H, Bian A, Fogo AB, Harris RC: Low nitric oxide bioavailability upregulates renal heparin binding EGF-like growth factor expression. Kidney Int 84: 1176–1188, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin JJ, Cybulsky AV, Goodyer PR, Fine RN, Kaskel FJ: Insulin-like growth factor-1 enhances epidermal growth factor receptor activation and renal tubular cell regeneration in postischemic acute renal failure. J Lab Clin Med 125: 724–733, 1995 [PubMed] [Google Scholar]
- 36.Wang Z, Chen JK, Wang SW, Moeckel G, Harris RC: Importance of functional EGF receptors in recovery from acute nephrotoxic injury. J Am Soc Nephrol 14: 3147–3154, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Chen JK, Harris RC: Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int 82: 45–52, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Forbes JM, Coughlan MT, Cooper ME: Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57: 1446–1454, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Kashihara N, Haruna Y, Kondeti VK, Kanwar YS: Oxidative stress in diabetic nephropathy. Curr Med Chem 17: 4256–4269, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brownlee M: The pathobiology of diabetic complications: A unifying mechanism. Diabetes 54: 1615–1625, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, Andreyev A, Quach T, Ly S, Shekhtman G, Nguyen W, Chepetan A, Le TP, Wang L, Xu M, Paik KP, Fogo A, Viollet B, Murphy A, Brosius F, Naviaux RK, Sharma K: AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest 123: 4888–4899, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stieger N, Worthmann K, Teng B, Engeli S, Das AM, Haller H, Schiffer M: Impact of high glucose and transforming growth factor-β on bioenergetic profiles in podocytes. Metabolism 61: 1073–1086, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Das R, Xu S, Quan X, Nguyen TT, Kong ID, Chung CH, Lee EY, Cha SK, Park KS: Upregulation of mitochondrial Nox4 mediates TGF-β-induced apoptosis in cultured mouse podocytes. Am J Physiol Renal Physiol 306: F155–F167, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]