Abstract
In CKD, phosphate retention arising from diminished GFR is a key early step in a pathologic cascade leading to hyperthyroidism, metabolic bone disease, vascular calcification, and cardiovascular mortality. Tenapanor, a minimally systemically available inhibitor of the intestinal sodium-hydrogen exchanger 3, is being evaluated in clinical trials for its potential to (1) lower gastrointestinal sodium absorption, (2) improve fluid overload-related symptoms, such as hypertension and proteinuria, in patients with CKD, and (3) reduce interdialytic weight gain and intradialytic hypotension in ESRD. Here, we report the effects of tenapanor on dietary phosphorous absorption. Oral administration of tenapanor or other intestinal sodium-hydrogen exchanger 3 inhibitors increased fecal phosphorus, decreased urine phosphorus excretion, and reduced [33P]orthophosphate uptake in rats. In a rat model of CKD and vascular calcification, tenapanor reduced sodium and phosphorus absorption and significantly decreased ectopic calcification, serum creatinine and serum phosphorus levels, circulating phosphaturic hormone fibroblast growth factor-23 levels, and heart mass. These results indicate that tenapanor is an effective inhibitor of dietary phosphorus absorption and suggest a new approach to phosphate management in renal disease and associated mineral disorders.
Keywords: intestine, coronary calcification, phosphate uptake, epithelial sodium, transport, hyperphosphatemia, hypertrophy
In CKD, the impaired renal elimination of phosphorus (P) is insufficient to keep pace with absorption of dietary P from the gastrointestinal tract. P load increases early in the course of CKD, although frank hyperphosphatemia is usually not evident until the disease is well advanced.1 Endocrine responses to rising P levels include increased fibroblast growth factor-23 (FGF-23) levels and secondary hyperparathyroidism, which contribute to metabolic bone disease, ectopic calcification, renal failure, and cardiovascular disease progression.2–4 Hyperphosphatemia alone or combined with high serum calcium (Ca) is associated with increased mortality in patients on dialysis.5,6
Restrictions of dietary P to offset the effects of declining kidney function are no longer endorsed by Kidney Disease Improving Global Outcomes, because a recent study showed that dietary P was positively coupled to protein content and nutritional status in patients with ESRD and that liberal dietary P would actually increase survival.7 Pharmacologic agents capable of selectively decreasing dietary P absorption, such as P binders, continue to be the first line of treatment of P disorders in those patients. Orally administered P binders prevent systemic absorption of dietary phosphate by converting it to an insoluble form that is eliminated in the feces.8 Unfortunately, these agents all have important limitations.9–11 All have a high pill burden, and efficacy is limited by poor compliance associated with inconvenient administration and a high prevalence of gastrointestinal side effects.8,11,12 Ca-based binders can lead to hypercalcemia and vascular calcification.9
An alternative strategy for limiting systemic uptake of dietary P involves the inhibition of gastrointestinal tract P transporters. The design of nonsystemically absorbed inhibitors offers the possibility of reducing pill burden and gastrointestinal side effects associated with the large excess of binder needed to capture phosphate ions stoichiometrically, while maintaining the low risk of toxicity associated with a nonsystemic agent. Although there is a passive component to P absorption, evidence exists of a sodium (Na)-dependent phosphate cotransporter (NaPi2b; SLC34a2) in the small intestine,13 with activity that is influenced by the dietary load of P and vitamin D status14–16 among other factors.
We recently described the protective effects of tenapanor (previously referred to as RDX5791 or AZD1722), a minimally systemic inhibitor of Na-hydrogen exchanger 3 (NHE3), against Na-driven cardiac and renal damage in rodents.17 In an ascending multiple dose phase 1 clinical trial, tenapanor increased fecal Na content and reduced urine Na content as predicted on the basis of a mechanism of action involving local inhibition of intestinal NHE3.18
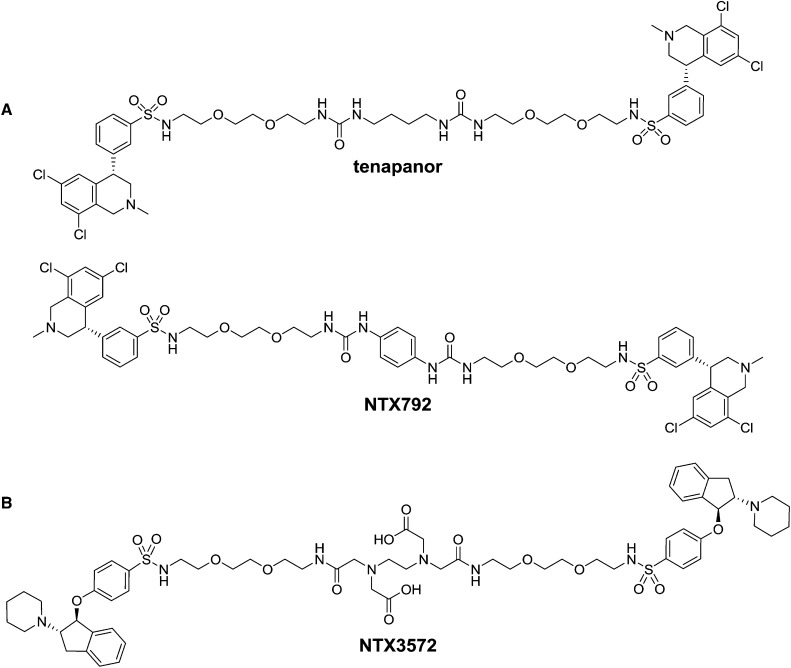
Novel minimally systemic NHE3 compounds have been developed19,20; among them are tenapanor and its close analog NTX792 (tetrahydroisoquinoline dimers) (Figure 1A) as well as NTX3572 (indane dimer) (Figure 1B). In this study, we show that these compounds, in addition to their inhibitory activity on Na uptake, decrease intestinal P uptake by a mechanism distinct from direct inhibition of phosphate transporters type 1 (PiT1; SLC20A1) and NaPi2b.
Figure 1.
Structures of NHE3 inhibitors with minimal systemic bioavailability. (A) Shown tetrahydroisoquinoline dimers are tenapanor [(S)-N,N′-(10,17-dioxo-3,6,21,24-tetraoxa-9,11,16,18-tetraazahexacosane-1,26-diyl)bis(3-((S)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)benzenesulfonamide) dihydrochloride] and NTX792 [(S)-N,N′-(2,2′-(2,2′-(2,2′-(1,4-phenylenebis(azanediyl))bis(oxomethylene)bis(azanediyl)bis(ethane-2,1-diyl))bis(oxy)bis(ethane-2,1-diyl))bis(oxy)bis(ethane-2,1-diyl))bis(3-((S)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)benzenesulfonamide) dihydrochloride]. (B) Shown indane dimer is NTX3572 [6-(carboxymethyl)-8-oxo-3-(2-oxo-2-(2-(2-(2-(4-((1S,2S)-2-(piperidin-1-yl)-2,3-dihydro-1H-inden-1-yloxy)phenylsulfonamido)ethoxy)ethoxy)ethylamino)ethyl)-17-(4-((1S,2S)-2-(piperidin-1-yl)-2,3-dihydro-1H-inden-1-yloxy)phenylsulfonamido)-12,15-dioxa-3,6,9-triazaheptadecan-1-oic acid].
We report that inhibition of intestinal sodium absorption by NHE3 in rats is accompanied by increased fecal P excretion and reduced urinary P excretion. Tenapanor markedly reduces ectopic calcification and protects renal function in a CKD rat model with vascular calcification. In aggregate, the data support a mechanism of action involving inhibition of dietary P uptake. These compounds may be useful clinically for the management of phosphate-related complications in renal disease.
Results
A Nonsystemic NHE3 Inhibitor Decreases Phosphate Absorption in Both Normal Renal Function and CKD Rats
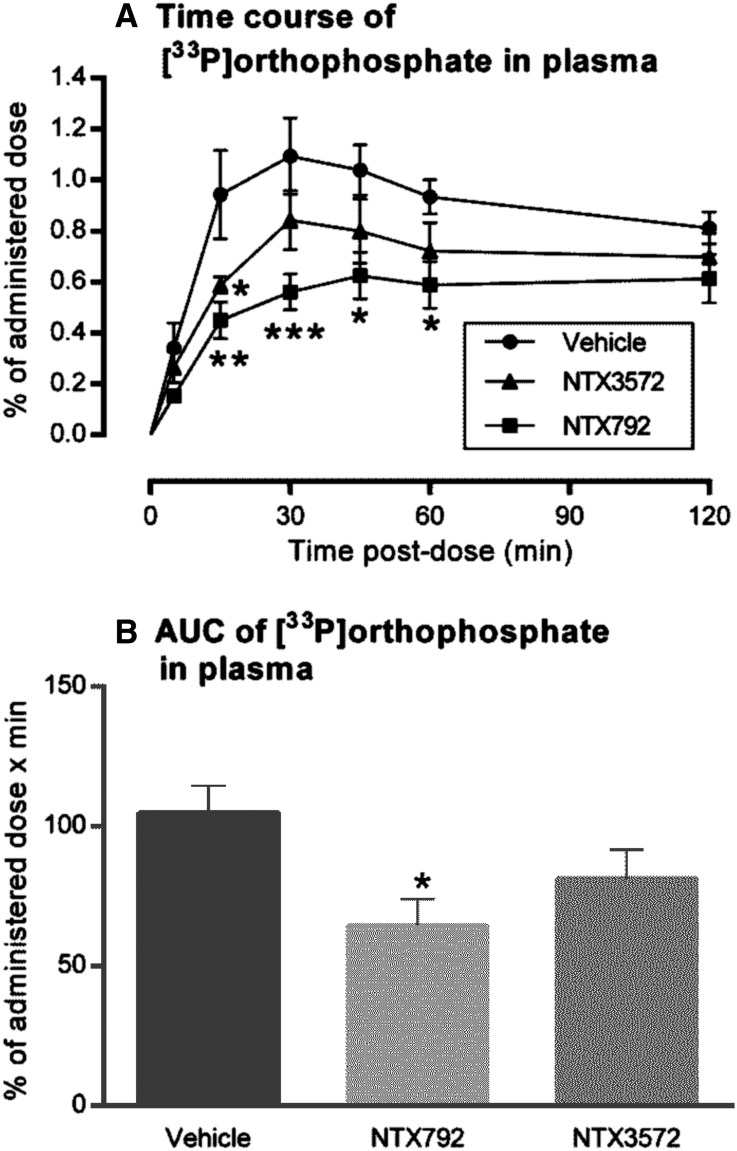
The effect of orally administered NHE3 inhibitors on absorption of phosphate from the gastrointestinal tract was evaluated in rats. Acute plasma levels of intragastrically administered [33P]orthophosphate were reduced in normal rats treated with the NHE3 inhibitor NTX792 compared with vehicle controls (Figure 2). NTX3572, an equipotent NHE3 inhibitor that shows lower persistence of inhibition in cultured cells (Table 1), also decreased [33P]orthophosphate uptake in rats but to a lesser extent (Figure 2).
Figure 2.
Attenuation of acute phosphate uptake with a gut-restricted NHE3 inhibitor. Oral coadministration of NTX792, NTX3572, or vehicle at 10 mg/kg to rats (n=4–5) along with a phosphate meal containing [33P]orthophosphate resulted in the plasma profiles indicated by (A) postdose and (B) corresponding area under curve (AUC) analysis. Data are represented by means±SEMs, and statistical significance from mean comparison with vehicle group is indicated by asterisks. *P≤0.05; **P≤0.01; ***P≤0.001.
Table 1.
Inhibition of select transporters in cell-based assays by test compounds
| Target | Tenapanor pIC50 | NTX792 pIC50 | NTX3572 pIC50 |
|---|---|---|---|
| Human NHE3, prompt | 8.3±0.3 | 8.6±0.2 | 8.0 |
| Rat NHE3, prompt | 8.0±0.3 | 8.4±0.4 | 8.9±0.1 |
| Rat NHE3, persistent | 8.4±0.3 | 8.1 | 6.2±0.1 |
| Human NaPi2b | ≤5.0 | n.a. | n.a. |
| Human PiT1 | ≤5.0 | n.a. | n.a. |
Inhibition response curves for compounds in each assay are expressed as pIC50. Results from several determinations are expressed as means±SDs. pIC50, negative log of the concentration that inhibits response by 50%; n.a., not measured.
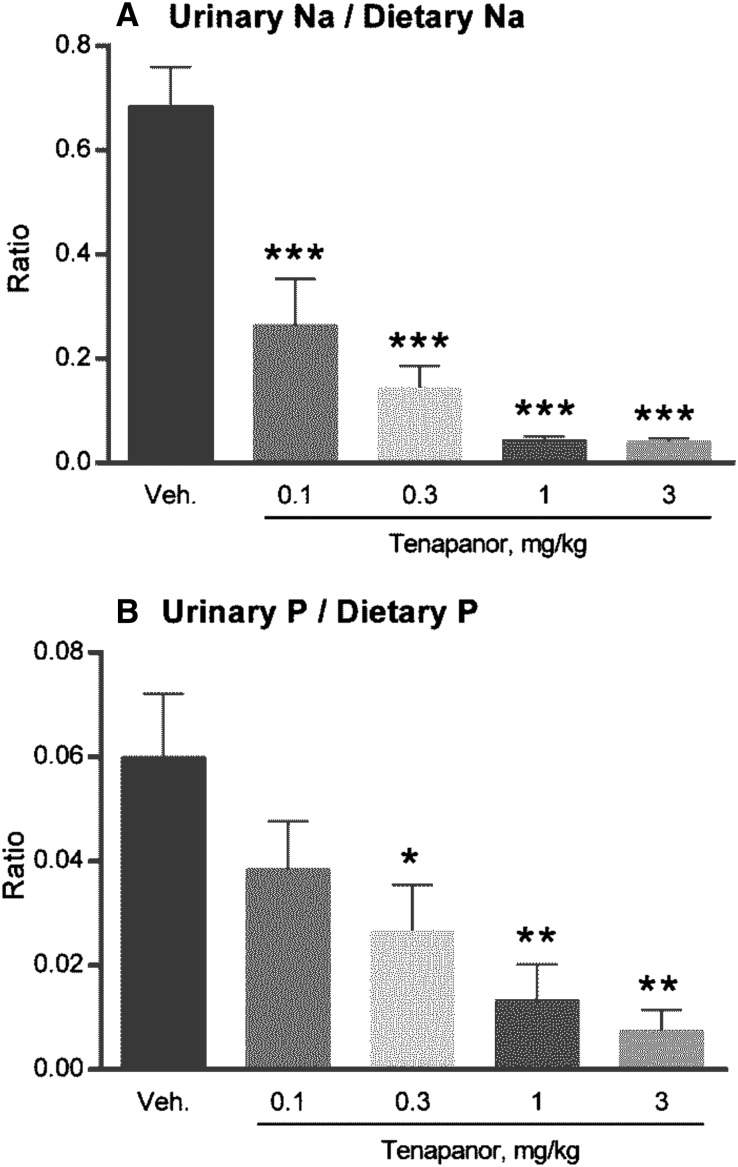
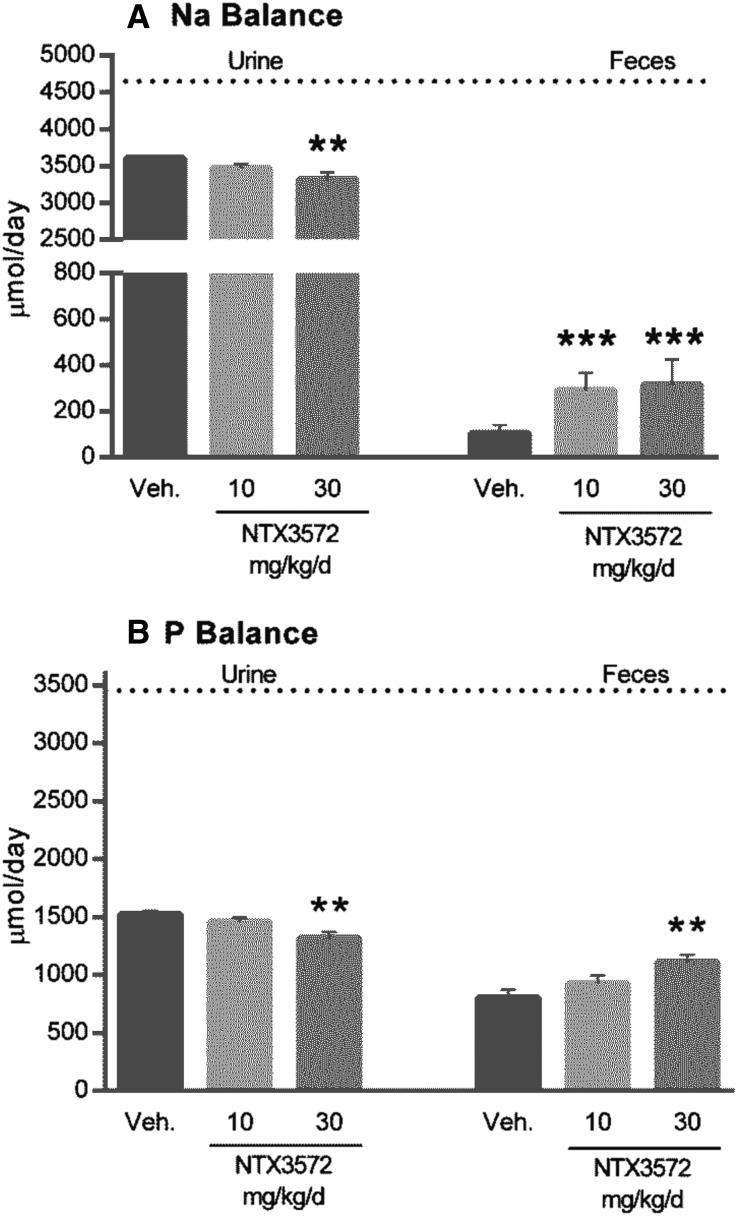
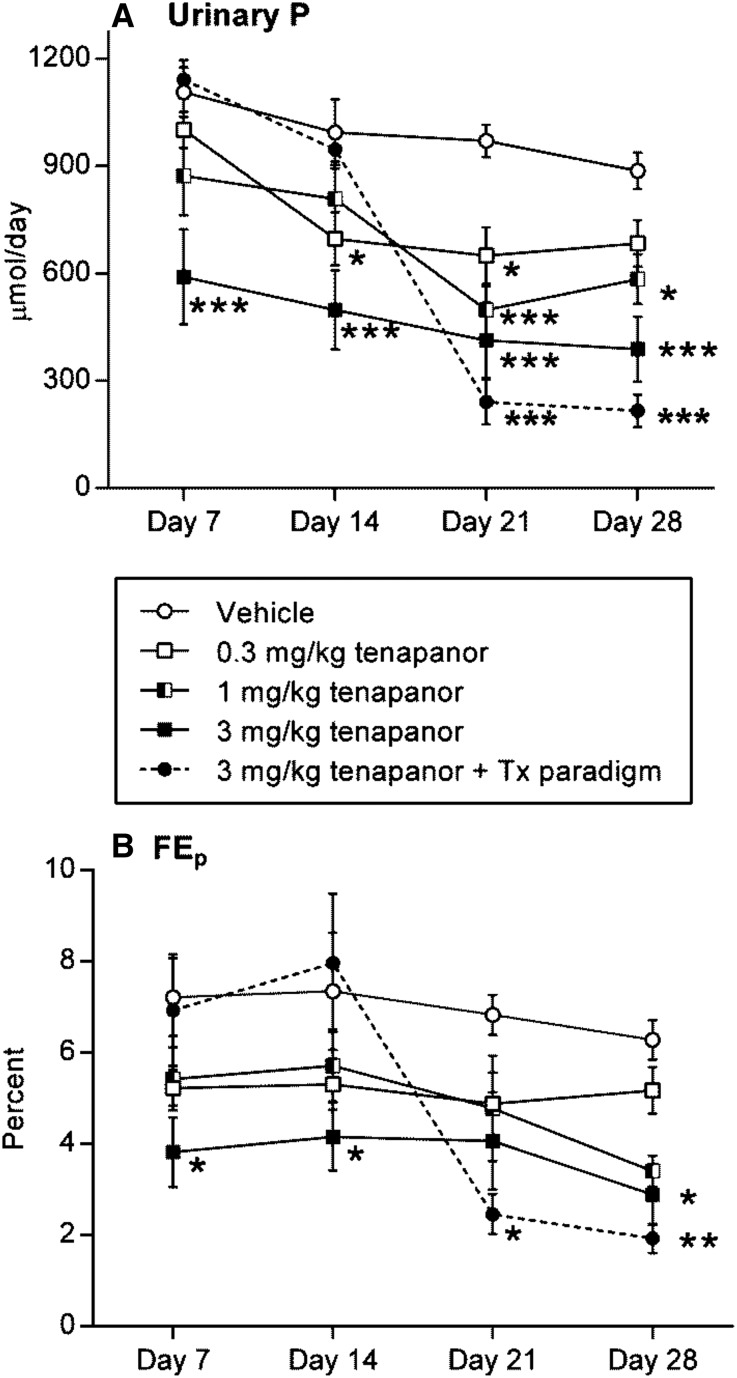
Urinary Na and P excretion was decreased on acute tenapanor administration (doses of 0.3, 1, and 3 mg/kg; P≤0.05, P≤0.01, and P≤0.01, respectively) (Figure 3B). Food intake was normal for all groups, except the 1 and 3 mg/kg tenapanor groups, each of which exhibited an average decrease of 15% in chow intake coinciding with onset of loose stools. The incidence of loose stools was shown to be linked to intestinal NHE3 inhibition diverting Na and water in the stool.17 Because liquid stools may lead to urine/feces crosscontamination, we performed an external P balance study with NTX3572, a compound selected for its lower-persistence NHE3 inhibition and reduced pharmacologic response compared with tenapanor and NTX792. NTX3572 (mixed in chow and fed to rats for 4 days) allowed for more precise fecal collection compared with NTX792 or tenapanor in rats, because the fecal form was not altered as dramatically. The average daily uptake and excretion of Na (Figure 4A) and P (Figure 4B) were determined for each rat from day 2 to 4. Fecal Na and P each increased and urinary Na and P each decreased in an NTX3572 dose-dependent manner. The 30-mg/kg per day dose reduced urinary Na, on average, by 304 μmol/d and increased fecal Na by 217 μmol/d compared with vehicle (Figure 4A). Similarly, urinary P decreased, on average, by 194 μmol/d, and fecal P increased by 323 μmol/d (Figure 4B). Urinary P was also measured in tenapanor-treated 5/6th nephrectomized (NPX) rats fed a high-salt diet for 28 days. Twenty-four–hour urinary phosphate excretion was consistently lower in tenapanor-treated NPX rats compared with untreated controls (Figure 5A). The fractional excretion of phosphate, estimated using creatinine clearance, was also decreased in rats treated with tenapanor compared with the vehicle group (Figure 5B).
Figure 3.
Tenapanor decreases urinary Na and P in rats relative to dietary intake. Male Sprague–Dawley rats (n=6) were orally administered vehicle (water) or tenapanor at the indicated doses by oral gavage just before the dark phase and then placed in metabolic cages for urine collection over a 16-hour period. Urine was analyzed for (A) Na and (B) P content. Mean comparisons between groups and vehicle group were analyzed by one-way ANOVA followed by Dunnett multiple comparison, and statistical significance is denoted by asterisks. *P≤0.05; **P≤0.01; ***P≤0.001.
Figure 4.
Increased fecal elimination of Na and P in rats treated with NTX3572 for 4 days. Rats (n=9) were fed a 0.6% bioavailable P diet for 4 days with or without NTX3572 mixed in the chow at the indicated doses. Daily urine and feces were collected, and the masses of excreted (A) Na and (B) P from each rat were determined and averaged from the last three 24-hour periods (days 2–4). The dotted lines indicate the daily average intake of Na or P of all rats: 4654 and 3455 μmol/d, respectively. Mean comparisons between groups and vehicle (Veh) group were analyzed by one-way ANOVA followed by Dunnett multiple comparison, and statistical significance is denoted by asterisks. **P≤0.01; ***P≤0.001.
Figure 5.
Tenapanor reduces urinary phosphate excretion in NPX rats. NPX rats fed a 4% NaCl diet were dosed with vehicle or tenapanor daily for 4 weeks. Weekly 24-hour urine collections were measured for phosphate using ion chromatography, and data are represented by (A) mass (micromoles per day) and (B) fractional excretion of phosphate (percentage). Data are represented by means±SEMs, and significance versus vehicle treatment determined by two-way ANOVA with Dunnett post hoc test is denoted by asterisks. Closed circle and dotted line, 3.0 mg/kg tenapanor interventional treatment paradigm (Tx paradigm); closed square, 3.0 mg/kg tenapanor prophylactic paradigm; open circle, vehicle; open square, 0.3 mg/kg tenapanor; split square, 1.0 mg/kg tenapanor. *P≤0.05; **P≤0.01; ***P≤0.001.
Tenapanor Reduces Vascular Calcification in CKD Rats
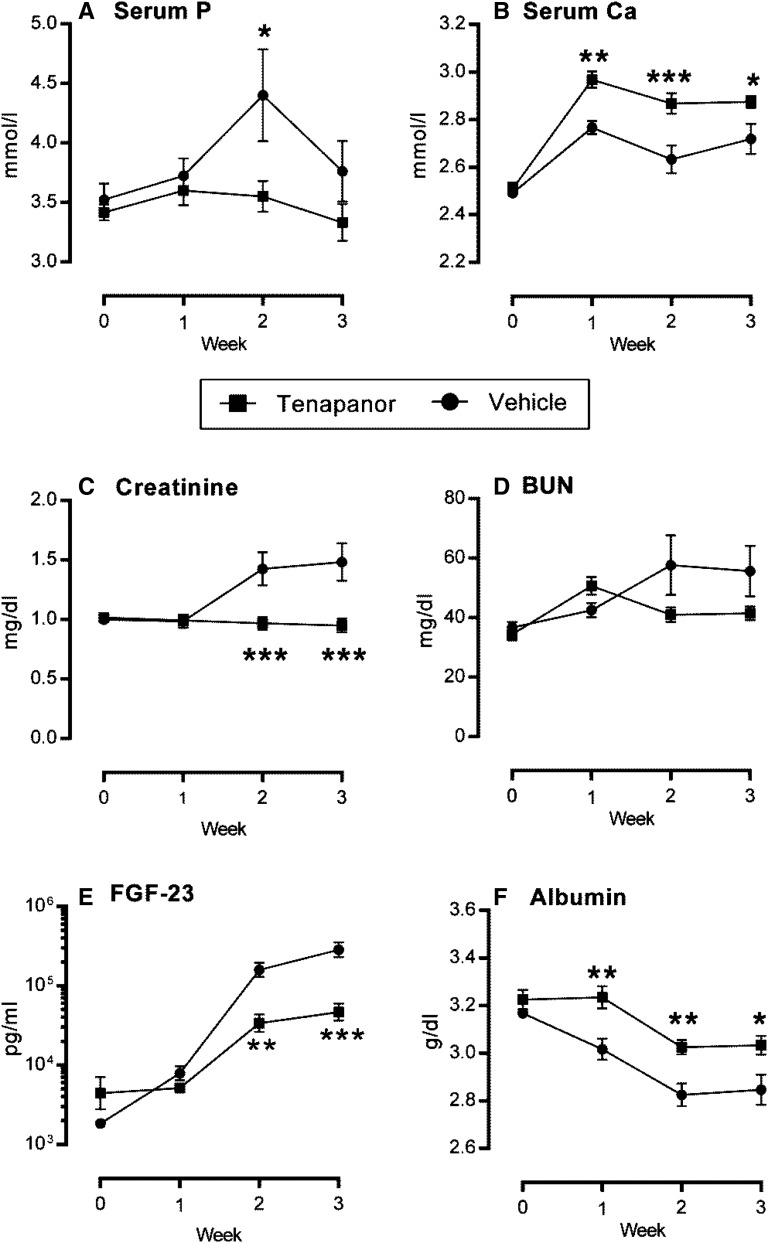
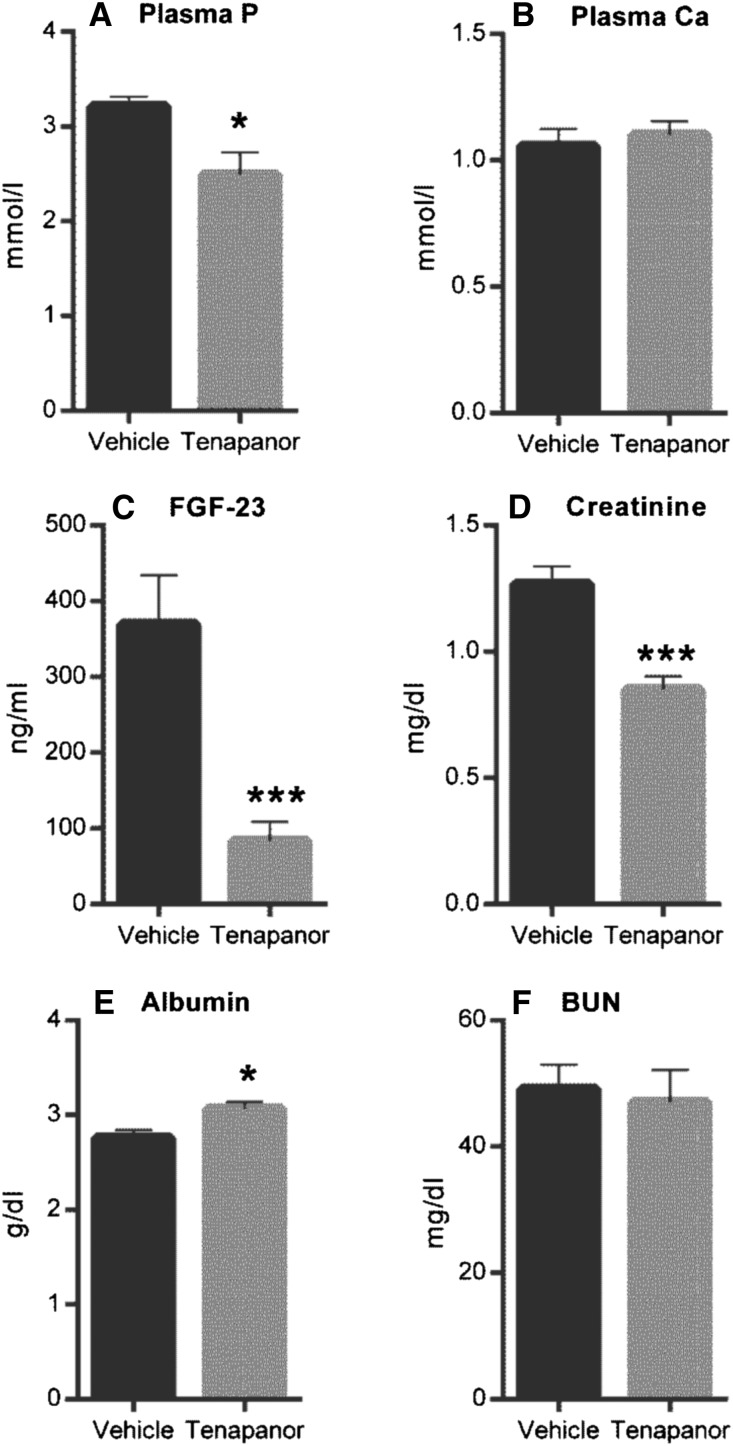
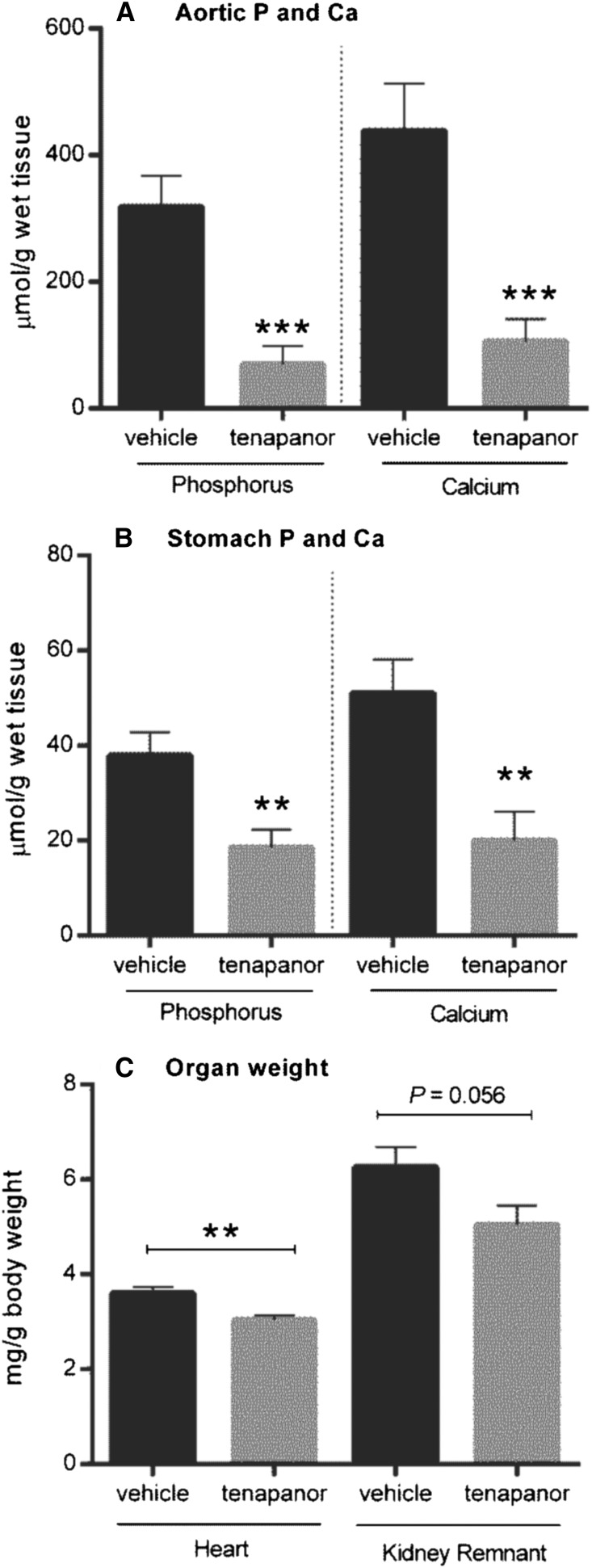
To investigate the potential use of tenapanor in the setting of renal failure, we used an NPX rat model of hyperphosphatemia with calcitriol administration and a synthetic P diet to enhance vascular and soft tissue calcification. In this model, ectopic vascular calcification was induced in the aorta and stomach over 28 days. Growth curves for both the vehicle and 5 mg/kg tenapanor groups were comparable after test diet initiation (Supplemental Figure 1A). There was no mortality over the course of the study in the tenapanor-treated group, whereas 3 of 12 animals in the vehicle group died or were euthanized on reaching a predetermined end point (Supplemental Figure 1B) (P=0.07). Treatment of NPX rats with tenapanor attenuated the decline in renal function compared with vehicle controls, which was shown by stabilization of creatinine and BUN over the course of the study (Figure 6, C and D). Serum creatinine did not increase over time and was lower in the tenapanor-treated group compared with the vehicle-treated group at weeks 2 and 3 (P≤0.001). Furthermore, the exaggerated hyperphosphatemia in this model was attenuated by tenapanor, which was evidenced by reduced serum P (Figure 6A) and lower concentration of serum FGF-23 at weeks 2 and 3 (Figure 6E). There was also a slight but significant increase in serum Ca in rats treated with tenapanor compared with vehicle controls (Figure 6B). After 3 weeks of treatment, serum bicarbonate was lower in the tenapanor-treated group compared with the vehicle group (22.8±0.7 versus 26.8±1.0 mEq/L; P≤0.01) but within the normal reference range. This observation is consistent with our previous finding that, at high doses, tenapanor (or NTX792, a close analog) can affect the acid–base balance in rats with renal insufficiency.17 Study termination occurred on day 27, which was 48 hours after the last calcitriol treatment. As expected in the absence of recent calcitriol treatment, circulating P and Ca were reduced in both groups at termination (compare Figure 6A with Figure 7A and compare Figure 6B with Figure 7B, respectively). Nonetheless, the final serum P levels were reduced in tenapanor-treated animals compared with vehicle controls (P≤0.05). Circulating FGF-23 levels were also significantly reduced compared with vehicle-treated controls (P≤0.001) as shown in Figure 7C. Creatinine levels of tenapanor-treated rats were lower than those of vehicle-treated rats, indicating protection from loss of renal function (Figure 7D) (P≤0.001). Analysis of tissues collected at the conclusion of the study showed that tenapanor greatly attenuated aortic (P≤0.001) and stomach (P≤0.01) mineralization (Figure 8, A and B, respectively) but did not significantly alter the jejunal brush border membrane expression of NHE3 or NaPi2b as determined by immunoblot analysis (Supplemental Figure 2). Tenapanor treatment was associated with a significant reduction in P and Ca content of the aortic arch and stomach compared with vehicle treatment, and an attenuation of renal (P=0.06) and cardiac (P≤0.01) hypertrophy was also apparent in the tenapanor-treated group compared with controls (Figure 8C). Taken together, these data show alleviation of ectopic calcification by tenapanor in this model of hyperphosphatemic uremia.
Figure 6.
Tenapanor improves uremic markers in the rat CKD disease model in life phase. Starting and weekly serum concentration measurements for (A) P, (B) Ca, (C) creatinine, (D) BUN, (E) FGF-23, and (F) albumin from vehicle- (closed circles) and 5 mg/kg per day tenapanor-treated (5 mg/kg per day; closed squares) rats. Data are represented by means±SEMs, and significance versus vehicle group determined by two-way ANOVA with Bonferroni post hoc test is denoted by asterisks. *P≤0.05; **P≤0.01; ***P≤0.001.
Figure 7.
Tenapanor improves uremic markers in the rat CKD model at the time of euthanasia. At euthanasia, blood was collected from rats fed chow with or with out tenapanor (5 mg/kg per day) for plasma from the remaining rats in the study and measured for (A) P, (B) Ca, (C) FGF-23, (D) creatinine, (E) albumin, and (F) BUN. Data are represented by means±SEMs, and mean comparison analysis was determined by t test with statistical significance denoted by asterisks. *P≤0.05; ***P≤0.001.
Figure 8.
Tenapanor improves ectopic vascular and soft tissue calcification as well as renal and heart hypertrophy in a rat CKD disease model. P and Ca contents were assessed in (A) the aortic arch and (B) the stomach from NPX rats after 28 days of calcitriol treatment fed chow with or without tenapanor (5 mg/kg per day). (C) Kidney remnant and heart weights as related to body weight were also measured for each rat. Data are represented by means±SEMs, and mean comparison analysis was determined by t test, with statistical significance denoted by asterisks. *P≤0.05; **P≤0.01; ***P≤0.001.
Discussion
These studies show that the nonsystemically absorbed NHE3 inhibitor tenapanor slows renal function decline, reduces hyperphosphatemia and circulating FGF-23, reduces vascular and soft tissue calcification, and reduces renal and cardiac hypertrophy in an animal model of CKD. Studies performed with this series of minimally systemic NHE3 inhibitors support the hypothesis that these effects are mediated by reducing P retention associated with reduced GFR by a mechanism involving a reduction of dietary P uptake in the gastrointestinal tract. Pharmacokinetic, autoradiography, and radiolabel recovery data on tenapanor17 strongly support the hypothesis of tenapanor exerting its effect locally in the gastrointestinal tract.
Support for the proposed mechanism of action is provided by the reduced absorption of intragastrically administered [33P]orthophosphate observed in normal rats treated with the NHE3 inhibitors NTX792 (a very close analog of tenapanor) and NTX3572. Additional support was provided by studies examining the effects of those NHE3 inhibitors on urinary and fecal P excretion. In normal rats, tenapanor administration reduced urinary P excretion; NTX3572 produced a dose-dependent reduction in urinary P excretion and a dose-dependent increase in fecal P excretion. Tenapanor also reduced urinary P excretion in the NPX rat. These unexpected effects cannot be explained by direct inhibition of known phosphate intestinal transporters, because tenapanor, when tested, had no measurable inhibition against NaPi2b or PiT1 (Table 1).
Currently available drugs for treating hyperphosphatemia (P binders) prevent systemic absorption of P in the gastrointestinal tract by converting phosphate to an insoluble form that is eliminated in the feces. Although it has been clearly shown that all of the available phosphate binders are effective in lowering serum phosphate,21 their efficacy is suboptimal. Clinical studies comparing the increase in fecal P and decrease in urinary P observed in patients treated with Renagel (crosslinked polyallylamine HCl salt) and Fosrenol (Lanthanum carbonate) with the theoretical P binding capacity of these agents suggest that only 25%–35% of the total binding capacity is used in vivo, estimated from (1) the increase in fecal P and decrease in urinary P from baseline or placebo observed on treatment22,23 and (2) the theoretical P binder maximum binding capacity (e.g., 5 and 7 mEq P/g binder for Renagel and Fosrenol, respectively). The limited efficacy may originate from endogenous anions competing for the phosphate binding sites and the rapid mucosal absorption of phosphate by diffusion or active transport through the gut epithelia. Large doses of these binders are required as a direct consequence of their limited efficiency. The vast majority of patients prescribed phosphate binders are noncompliant12 as a result of the number and size of tablets required (recommended doses for Renvela and Fosrenol are 0.8–1.6 g per meal22 and 0.5–1 g per meal, respectively),23 the three times daily dosing regimen, and the requirement for additional fluid taken with the bulky tablets in patients who were fluid restricted.
The pharmacologic activity of tenapanor was evaluated in the NPX rat, an animal model of CKD that features pronounced vascular calcification. Because of the repeated calcitriol injections, these rats do not show overt secondary hyperparathyroidism24 but do exhibit elevated serum P levels, elevated FGF-23 levels, and vascular and nonvascular tissue calcification. Tenapanor significantly reduced serum P and FGF-23, implying a normalizing effect on P balance (Figure 6). These observations are consistent with the phosphaturic role of FGF-23, which serves to reduce renal tubule phosphate reabsorption in the presence of increased dietary P absorption. A recent study showed that FGF-23 directly downregulates membrane expression of the renal NaPi2a (SLC34A1).25 Reduction of FGF-23 and concomitant reduction of the renal phosphate fractional excretion have previously been observed in clinical studies in patients with CKD treated with P binders.26,27
The reduced P intestinal intake mechanism posited for tenapanor is supported by the robust attenuation of ectopic deposition of Ca and phosphate (presumably as hydroxyapatite) in the aortic arch and stomach in tenapanor-treated rats (Figure 8). A study describing the klotho and NaPi2a double-knockout mouse line determined that these mice did not exhibit the vascular calcification found in the klotho single-knockout mice, despite having high levels of serum Ca and 1,25-dihydroxyvitamin D.28 The resulting hyperphosphaturia from the lack of renal NaPi2a transporter reduced the P burden in these mice, as demonstrated by the reduced serum P and circulating FGF-23, and it led Oshnishi et al.28 to the conclusion that whole-body P status could be a key factor in the pathogenesis of vascular calcification in patients with CKD. This result would be consistent with this study, because while circulating Ca was unaffected by tenapanor at study termination (Figure 7), it was elevated overall during the calcitriol dosing regimen included in the study, especially in the tenapanor-treated rats (Figure 6). Calcitriol is known to increase serum Ca but also further drives this rodent calcification model by enhancing dietary phosphate absorption.29 By reducing phosphate availability and the potential for calcification, the tenapanor-treated rats are presumably able to reach elevated levels of circulating soluble Ca in response to calcitriol compared with control rats, which was accompanied by a reduction in heart (16%; P≤0.01) and kidney (19%; P=0.06) hypertrophy noticeable above the survival bias (Figure 8). The reduction in cardiac hypertrophy could stem from the triple effect of (1) reduced intravascular volume caused by reduced intestinal Na intake, (2) attenuated vascular stiffening from ectopic calcification as a result of the reduced serum P, and (3) reduced FGF-23 levels secondary to reduced intestinal P uptake. Likewise, the reduction of renal hypertrophy and the serum creatinine levels in tenapanor-treated rats that do not rise over time (Figure 6) indicate a protection from further degradation of renal function, presumably from a reduced renal vascular hypertension and corresponding end organ damage caused by alleviation of arterial stiffening related to the calcification and overall reduced hypervolumia. FGF-23 increases very early in the course of CKD and is strongly associated with death and cardiovascular disease, including left ventricular hypertrophy and vascular calcification.2,30,31 Several studies have shown that reducing FGF-23 by means of treatment with P binders improves cardiovascular disease in patients who are normophosphatemic independent of serum P.12,26,27 Although the link between elevated FGF-23 levels and disease outcomes is becoming clearer, evidence of a direct role for FGF-23 in pathogenesis remains elusive. However, recently, a group has shown that FGF-23 enhances P-induced calcification in the aortas of CKD rats and Klotho-overexpressing vascular smooth muscle cells by promoting osteoblastic differentiation.32 Another group has presented results that suggest that elevated levels of FGF-23 contribute to heart hypertrophy through FGF-receptor mediated effects triggering a calcineurin signaling cascade vis-a-vis heart remodeling genes, independent of BP.33
Ultimately, despite normal serum phosphate levels, improved management of P and reduction of FGF-23 in patients with CKD may lead to decreased associated cardiovascular morbidity and mortality. P management continues to be the cornerstone treatment of mineral disorders in patients with renal failure. Despite recent progress in phosphate binder design (reviewed in refs. 9 and 11), the efficacy and patient compliance of P-lowering agents remain suboptimal. The selective inhibition of intestinal NHE3 by nonsystemically absorbed agents, like tenapanor, may offer improved efficacy over P binders on a dose-adjusted basis while preserving the safety advantages of nonabsorbed drugs. The mechanism of action of the effect of tenapanor on gastrointestinal P uptake remains under active investigation. Tenapanor is being investigated in the management of Na-mediated fluid overload in both patients with ESRD and CKD, and its clinical development for the treatment of hyperphosphatemia in patients with ESRD is in progress.
Concise Methods
Cell-Based Assay of NHE3 Activity (Prompt Inhibition)
Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH-sensitive dye method reported by Paradiso et al.34 Opossum kidney (OK) cells were obtained from the American Type Culture Collection (Manassas, VA) and propagated per their instructions. The rat NHE3 gene (GenBank accession no. M85300) or the human NHE3 gene (GenBank accession no. NM_004174.1) was introduced into OK cells through electroporation, and cells were seeded into 96-well plates and grown overnight. Medium was aspirated from the wells, and cells were washed two times with NaCl-Hepes buffer (100 mM NaCl, 50 mM Hepes, 10 mM glucose, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, pH 7.4) and then incubated for 30 minutes at room temperature with NH4Cl-Hepes buffer (20 mM NH4Cl, 80 mM NaCl, 50 mM Hepes, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, pH 7.4) containing 5 µM 2′,7′-Bis-(2-Carboxyethyl)-5-(and-6)-Carboxyfluorescein, Acetoxymethyl Ester (BCECF-AM). Cells were washed two times with ammonium-free, Na+-free Hepes of 100 mM choline, 50 mM Hepes, 10 mM glucose, 5 mM KCl, 2 mM CaCl2, and 1 mM MgCl2 (pH 7.4) and incubated in the same buffer for 10 minutes at room temperature to lower intracellular pH. NHE3-mediated recovery of neutral intracellular pH was initiated by addition of Na-Hepes buffer containing 0.4 μM ethyl isopropyl amiloride (a selective antagonist of NHE1 activity that does not inhibit NHE3) and 0–30 µM test compound, and the pH-sensitive changes in BCECF fluorescence (λex=505 nm and λem=538 nm) normalized to the pH-insensitive BCECF fluorescence (λex=439 nm and λem=538 nm) were monitored. Initial rates were plotted as the average of two or more replicates, and negative log of the concentration that inhibits response by 50% values were estimated using GraphPad Prism.
Cell-Based Assay of NHE3 Activity (Persistent Inhibition)
The ability of compounds to inhibit rat NHE3-mediated Na+-dependent H+ antiport after application and washout was measured using a modification of the pH-sensitive dye method described above. The rat NHE3 gene was introduced into OK cells through electroporation, and cells were seeded into 96-well plates and grown overnight. Medium was aspirated from the wells, and cells were washed two times with NaCl-Hepes buffer and then overlayed with NaCl-Hepes buffer containing 0–30 µM test compound. After a 60-minute incubation, the test drug containing buffer was aspirated from the cells, and cells were washed two times with NaCl-Hepes buffer without drug and then incubated for 30 minutes at room temperature with NH4Cl-Hepes buffer containing 5 µM BCECF-AM. Cells were washed two times with ammonium-free, Na+-free Hepes and incubated in the same buffer for 10 minutes at room temperature to lower intracellular pH. NHE3-mediated recovery of neutral intracellular pH was initiated (40 minutes after compound washout) by addition of Na-Hepes buffer containing 0.4 µM ethyl isopropyl amiloride, and the pH-sensitive changes in BCECF fluorescence were monitored.
Assay of PiT1 Inhibition
PiT1 (SLC20A1) is responsible for Na-dependent phosphate uptake into HEK293 cells.35 HEK293 cells were obtained from the American Type Culture Collection and propagated per their instructions. Cells were seeded into 96-well plates at 25,000 cells/well and cultured overnight. Medium was aspirated from the cultures, and the cells were washed one time with choline uptake buffer (14 mM Tris, 137 mM choline chloride, 5.4 mM KCl, 2.8 mM CaCl2, 1.2 mM MgSO4, 100 µM KH2PO4, 1 mg/ml BSA, pH 7.4). Cells were then overlayed with either choline uptake buffer or Na uptake buffer (14 mM Tris, 137 mM NaCl, 5.4 mM KCl, 2.8 mM CaCl2, 1.2 mM MgSO4, 100 µM KH2PO4, 1 mg/ml BSA, pH 7.4) containing 6–9 µCi/ml [33P]orthophosphate (PerkinElmer, Waltham, MA) and test compound. Each compound was tested at 12 concentrations ranging from 0.1 nM to 30 µM. Assays were run in duplicate, and compounds of interest were tested multiple times.
After incubation for 23 minutes at room temperature, assay mixtures were removed, and the cells were washed two times with ice-cold stop solution (137 mM NaCl, 14 mM Tris, pH 7.4). Cells were lysed by the addition of 20 μl 0.1% Tween 80 followed by 100 μl scintillation fluid and counted using a TopCount (PerkinElmer). The negative log of the concentration that inhibits response by 50% values of the test compounds were calculated using GraphPad Prism. Preliminary studies showed that, under these conditions, Na-dependent phosphate uptake was linear for at least 30 minutes and tolerated 0.6% (vol/vol) DMSO without deleterious effects.
Assay of NaPi2b
Assay of NaPi2b (SLC34a2) was performed as described above for PiT1; however, before the assay, HEK293 cells were transfected with expression clones of rat or human NaPi2b using Lipofectamine 2000, and a specific inhibitor of PiT1 was included in the Na-containing uptake buffer used during the assay. Expression clones for rat and human NaPi2b were obtained from Open Biosystems (catalog nos. MRN1768–9510282 and MHS1010–99823026, respectively; Thermo Fisher Scientific, Pittsburgh, PA). There are two putative splice variants of human NaPi2b designated as isoforms A and B (National Center for Biotechnology Information Reference Sequences NP_006415.2 and NP_001171470.1, respectively). The sequence of the open reading from in MHS1010–99823026 corresponds to isoform B; transfection with this construct was found to confer only very low levels of nonendogenous phosphate transport activity. The cDNA was, therefore, mutated to correspond with isoform A; transfection with this sequence conferred phosphate transport significantly over background. Thus, studies of the inhibition of human NaPi2b used isoform A exclusively.
In Vivo Studies with Rats
The experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Ardelyx, Inc. Sprague–Dawley rats were purchased from Charles River Laboratories (Hollister, CA). Animals were housed in microisolator cages kept in a temperature- (65°F to 75°F) and humidity-controlled (35%–55%) facility using a 12-hour light/dark cycle. Rats were fed a normal grain-based chow (2018 Teklad Global 18% Protein Rodent Diet) containing 0.65% P, 1% Ca, and 1.5 IU/g vitamin D3 purchased from Harlan-Teklad (Madison, WI) unless otherwise indicated and given water ad libitum. During an overnight period of fasting, the animals received water only.
Rat Acute Uptake Pharmacodynamic Studies
Acute uptake studies of [33P]orthophosphate were performed in 8-week-old male Sprague–Dawley rats purchased from Charles River Laboratories with jugular vein catheters surgically implanted by the vendor. After allowing for a postsurgical recovery period of 1 day, the rats were transferred to the Ardelyx, Inc. facility, where they were singly housed in microisolator cages and acclimated for at least 3 days before initiation of the study. To assess the effect of NHE3 inhibitors in acute dietary phosphate absorption, the appearance of radiolabeled phosphate in plasma was monitored in rats. After an overnight fast, rats (n=4–5) were administered intragastrically (10 ml/kg) a phosphate bolus containing [33P]orthophosphate (product NEZ080; PerkinElmer) with or without dispersed test article at the indicated dosage. This dosing solution contained 8 mM monobasic Na phosphate (1.25 µCi [33P]orthophosphate/µmol), 4 mM CaCl2, 0.4% (wt/vol) hydroxypropyl methocellulose, and 2% (wt/vol) dimethylsulfoxide prepared in water. Blood was sampled from conscious rats through implanted catheters after dosing at 5, 10, 15, 30, 45, 60, and 120 minutes postdose. From scintillation counting of the plasma, circulating labeled phosphate absorbed from the gastrointestinal tract was calculated. The relative amount of phosphate uptake from the administered dose to the total plasma volume was calculated using a body weight-based estimation of total circulating plasma36 and the scintillation counting of the initial dosing solution.
Urinary Electrolytes in Normal and Renal-insufficient Rats
Male Sprague–Dawley rats (7 weeks old) were orally dosed (5 ml/kg) with solutions containing 0.6, 0.2, 0.06, or 0.02 mg/ml tenapanor dissolved in deionized water. Groups of rats were dosed (n=6) with vehicle (deionized water) or tenapanor at the indicated dosage and placed in individual metabolic cages for overnight urine collection with free access to normal chow and water. Sixteen hours postdose, urine samples were collected, and the volume for each sample was determined. Feces was not collected from studies involving tenapanor, because samples could not be collected entirely or evenly from metabolic caging because of loss of fecal form as previously reported.17 Urine samples were centrifuged at 4,000 rpm, and the resulting supernatants (500 µl) were diluted and acidified with 6 N HCl acid (50 µl). Na chromatography analysis of urine was performed by injecting 10 µl each sample onto a Dionex ICS 3000 ion chromatograph system (Dionex; Thermo Fisher Scientific, Sunnyvale, CA). Cations were separated by an isocratic method using 25 mM methanesulfonic acid as the eluent on an IonPac CS12A 2 mm inner diameter×250 mm, 8-µm particle size cation exchange column (Dionex; Thermo Fisher Scientific). For phosphate analysis, sampling was performed with an AS autosampler (Dionex; Thermo Fisher Scientific) linked to a Dionex ICS-3000 ion chromatography system using an IonPac AS18 anion-exchange analytical column (2×250 mm, 7.5-µm particle size; Dionex; Thermo Fisher Scientific). Chromatography was performed using an isocratic method (35 mM KOH, 0.35 ml/min). The total mass of Na and phosphate urinated in the 16-hour collection period for each rat was calculated.
To examine the effect of tenapanor on urinary P in renal-compromised rats, a pharmacodynamics study was conducted at Plato BioPharma, Inc. (Westminster, CO). Male Sprague–Dawley rats (8–9 weeks old) were subjected to uninephrectomy 1 week before subtotal nephrectomy and weeklong recovery. After randomization by body weight, serum creatinine (>0.5 mg/dl), and BUN (>35 mg/dl), animals were fed a diet containing 4% NaCl or control chow (0.49% NaCl). Body weight, mortality, and morbidity were evaluated daily. Periodically, 24-hour urine samples were collected. Rats were dosed prophylactically with vehicle (water) or tenapanor (0.3, 1, or 3 mg/kg per day; n=12/dose group) through gavage incepting concomitantly with high-salt feeding. Dosing occurred daily just before the feeding period. After 2 weeks on study, 24 rats initially enrolled in the vehicle group were split into serum clinical chemistry and systolic BP/diastolic BP matched groups (n=12), one of which began treatment with 3.0 mg/kg per day tenapanor (interventional arm). The remaining group remained on vehicle for the remainder of the study. Weekly 24-hour urine samples were analyzed for P and Na content by ion chromatography as described above. Serum and urine creatinine were measured by standard clinical chemistry analysis using an Olympus au400e analyzer (Beckman Coulter, Brea, CA) to calculate the fractional excretion of P.
Fecal Electrolytes in Rats
To examine the subchronic effect of an NHE3 inhibitor on fecal excretion of Na and P, NTX3572 was dosed to rats (n=9) through food admix for 4 days. Male Sprague–Dawley rats (8 weeks old) were fed 0.6% inorganic P diet (TD.84122; Harlan-Teklad) that also contains 0.6% Na for 1 week before study. Rats have previously been determined to eat, on average, 18 g/d of this diet ad libitum. Powdered NTX3572 was mixed in an electric mixer with powdered 0.6% P diet step-wise into larger volumes for 10 minutes at a time until NTX3572 content was 0.13 mg/g chow for the 10-mg/kg per day dose and 0.39 mg/g chow for the 30-mg/kg per day dose. Diet mixture was visually similar to parent diet (vehicle), and the homogeneity and drug content of the chow were confirmed by extraction analysis. On day 0, each rat was placed in metabolic caging and given 18 g/d vehicle chow or chow containing NTX3572 at the indicated doses and free access to water. Daily (24-hour) food and water consumption was measured as well as urine and feces collection over the same time period for 4 days. Using day 1 for acclimation to metabolic cages and limited food access, Na and P uptake and excretion were averaged for each 24-hour period from day 2 to 4. Urine samples were treated and analyzed by ion chromatography as described above. Dried fecal pellets or a representative sample from dried homogenized feces were digested with repeated additions of concentrated nitric acid and hydrogen peroxide over 2–3 hours at 65°C–95°C. The sample solutions were diluted with 1% nitric acid before analysis with an atomic emission spectrometer (Agilent 4100 MP-AES) at the following element emission wavelengths: Na (588.995 nm) and P (214.915 or 213.618 nm). A cesium solution was used as an ionization buffer and internal standard. Data analysis was performed using Agilent MP Expert software.
Studies in Rats with Induced Vascular Calcification
The therapeutic effects of tenapanor were investigated in surgically induced uremic rats designed to model CKD adapted from a procedure previously described by Lopez et al.24,29 Male Sprague–Dawley rats were purchased from Charles River Laboratories with 5/6th NPX surgical procedures performed by the vendor. The procedure consists of two surgeries: subtotal nephrectomy of the left kidney followed by a 1-week recovery before uninephrectomy of the right kidney. After a 3-day recovery period from the second surgery, the rats were transported to the Ardelyx, Inc. animal facility at 9 weeks of age. On arrival, the rats were fed a basal-purified diet consisting of 0.9% inorganic P and 0.6% Ca (TD.10809; Harlan-Teklad). On study initiation, a regimen of calcitriol administration 3 times per week was initiated. Calcitriol or 1,25-dihydroxyvitamin D3 (product BML-DM200; ENZO Life Sciences, Farmingdale, NY) was prepared weekly from aliquots of stock solution consisting of 50 µg/ml in ethanol stored at −80°C in opaque glass vessels. On each day of dosing, the stock solution was diluted 1000-fold with propylene glycol to 50 ng/ml for a target dose of 80 ng/kg. This solution was administered by intraperitoneal injection between 3 and 4 p.m. three times a week for the course of the study. The morning after the first calcitriol dosing, serum was obtained by retro-orbital bleeding and analyzed for serum creatinine by standard clinical chemistry analysis using the ACE Alera Clinical Chemistry System (Alfa Wassermann Diagnostic Technologies). Animals with serum creatinine levels of 0.8–1.5 mg/dl were enrolled to the study in two experimental groups (n=12) stratified based on serum creatinine and body weight. Enrolled rats were given the same diet or this basal diet supplemented with 0.065 mg tenapanor per 1 g chow, which gave a daily dosage of approximately 5 mg/kg per day calculated from a rat with the average body weight of 235 g eating 18 g chow per day. To minimize the effect of any initial difference in food uptake, food ramping occurred during the first 6 days of the study in metabolic cages, and afterward, rats were allowed to continue with the same assigned diets ad libitum for the rest of study (Supplemental Figure 1A shows the growth curve). The following measures of animal health were recorded: daily food and water consumption (when housed in metabolic cages), mortality, morbidity, and weekly body weights (Supplemental Figure 1B shows the study inclusion plot). Retro-orbital bleeds were conducted the morning after a calcitriol administration at weeks 0, 1, 2, and 3 for serum clinical chemistry analysis. On day 27, tissues were collected along with trunk plasma after decapitation. Serum samples were analyzed by standard clinical chemistry analysis as well as C-terminal FGF-23 (full-length) concentration determination by ELISA (Immutopics International, San Clemente, CA). Hearts, aortic arches, kidney remnants, and cleaned stomachs were all weighed after collection. Proximal jejunal sections were isolated (30-cm section beginning 10 cm distal to the pylorus) and gently flushed, and mucosal scrapings were collected.
To analyze the Ca and P content of the stomach and aortic arch, samples were prepared by acid extraction. Each tissue was placed in a tube with 2 ml 0.6 N HCl and rotated for 48 hours. After homogenization, the top liquid was removed for analysis by ion chromatography. For Ca analysis, sampling was performed with an AS autosampler (Dionex; Thermo Fisher Scientific) linked to a Dionex ICS-3000 ion chromatography system using an IonPac CS12A cation-exchange analytical column (2×250 mm, 8-µm particle size; Dionex; Thermo Fisher Scientific). Chromatography was performed using an isocratic method (25 mM methanesulfonic acid, 0.35 ml/min). For P analysis, sampling was performed with an AS autosampler (Dionex; Thermo Fisher Scientific) linked to a Dionex ICS-3000 ion chromatography system using an IonPac AS18 anion-exchange analytical column (2×250 mm, 7.5-µm particle size; Dionex; Thermo Fisher Scientific). Chromatography was performed using an isocratic method (35 mM KOH, 0.35 ml/min). Total masses of Ca and P were calculated per 1 g wet tissue.
NaPi2b, NHE3, and β-actin protein expressions were assessed in the brush border membrane of the proximal jejenum of vehicle- and tenapanor-treated animals. Brush border membrane vesicles were prepared from jejnunal mucosal scrapings using a magnesium differential centrifugation method that has been previously described.37 Twenty micrograms brush border membrane proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with antibodies against NaPi2b (generated for Ardelyx, Inc.; raised in rabbits against rat NaPi2b; amino acids 9–26, NAHPNPNKFIEGASGPQSC), NHE3 (AB3085; EMD Millipore, Billerica, MA), and β-actin (A4700; Sigma-Aldrich, St. Louis, MO). Imaging and densitometric analysis of the immunoblots were performed using Quantity One software (Bio-Rad, Hercules, CA).
Statistical Analyses
Data are presented as means±SEMs and were analyzed with GraphPad Prism version 6.03 for Windows (GraphPad Software, La Jolla, CA). Studies involving compound treatment over time were analyzed by two-way ANOVA followed by Dunnett multiple comparisons post hoc test. All other data were analyzed by one-way ANOVA followed by Dunnett multiple comparisons post hoc test or t test. P≤0.05 was considered statistically significant for all studies.
Disclosures
All authors are employees of and have a financial interest in Ardelyx, Inc.
Supplementary Material
Acknowledgments
The authors acknowledge Peter Greasley (Astra-Zeneca) for his review of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014030317/-/DCSupplemental.
References
- 1.Prié D, Ureña Torres P, Friedlander G: Latest findings in phosphate homeostasis. Kidney Int 75: 882–889, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Gilbertson DT, Chen SC: The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol 4[Suppl 1]: S5–S11, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens LA, Djurdjev O, Cardew S, Cameron EC, Levin A: Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: Evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol 15: 770–779, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Young EW, Akiba T, Albert JM, McCarthy JT, Kerr PG, Mendelssohn DC, Jadoul M: Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 44[Suppl 2]: 34–38, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Lynch KE, Lynch R, Curhan GC, Brunelli SM: Prescribed dietary phosphate restriction and survival among hemodialysis patients. Clin J Am Soc Nephrol 6: 620–629, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kestenbaum B: Phosphate metabolism in the setting of chronic kidney disease: Significance and recommendations for treatment. Semin Dial 20: 286–294, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Cozzolino M, Mazzaferro S, Brandenburg V: The treatment of hyperphosphataemia in CKD: Calcium-based or calcium-free phosphate binders? Nephrol Dial Transplant 26: 402–407, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Cozzolino M, Staniforth ME, Liapis H, Finch J, Burke SK, Dusso AS, Slatopolsky E: Sevelamer hydrochloride attenuates kidney and cardiovascular calcifications in long-term experimental uremia. Kidney Int 64: 1653–1661, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow GM: Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23: 1407–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chue CD, Townend JN, Moody WE, Zehnder D, Wall NA, Harper L, Edwards NC, Steeds RP, Ferro CJ: Cardiovascular effects of sevelamer in stage 3 CKD. J Am Soc Nephrol 24: 842–852, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marks J, Debnam ES, Unwin RJ: The role of the gastrointestinal tract in phosphate homeostasis in health and chronic kidney disease. Curr Opin Nephrol Hypertens 22: 481–487, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hattenhauer O, Traebert M, Murer H, Biber J: Regulation of small intestinal Na-P(i) type IIb cotransporter by dietary phosphate intake. Am J Physiol 277: G756–G762, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Katai K, Miyamoto K, Kishida S, Segawa H, Nii T, Tanaka H, Tani Y, Arai H, Tatsumi S, Morita K, Taketani Y, Takeda E: Regulation of intestinal Na+-dependent phosphate co-transporters by a low-phosphate diet and 1,25-dihydroxyvitamin D3. Biochem J 343: 705–712, 1999 [PMC free article] [PubMed] [Google Scholar]
- 16.Marks J, Srai SK, Biber J, Murer H, Unwin RJ, Debnam ES: Intestinal phosphate absorption and the effect of vitamin D: A comparison of rats with mice. Exp Physiol 91: 531–537, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Spencer AG, Labonte ED, Rosenbaum DP, Plato CF, Carreras CW, Leadbetter MR, Kozuka K, Kohler J, Koo-McCoy S, He L, Bell N, Tabora J, Joly KM, Navre M, Jacobs JW, Charmot D: Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med 6: 227ra36, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Charmot D Jacobs J Spencer A Raab M Rosenbaum D: RDX5791, a non-systemic NHE3 inhibitor for the treatment of fluid and sodium overload, shifts sodium excretion from urine to feces in healthy subjects. Presented at the ERA-EDTA Conference, Paris, April 26–29 2012 [Google Scholar]
- 19.Charmot D, Jacobs J, Leadbetter M, Navre M, Carreras C, Bell N: Compounds and methods for inhibiting NHE- mediated antiport in the treatment of disorders associated with fluid retention or salt overload and gastrointestinal tract disorders. World Intellectual Property Organization WO2010078449A2: (examples 202 and 203 for tenapanor and NTX792, resp.) 2010 [Google Scholar]
- 20.Bell N, Carreras C, Charmot D, Chen T, Leadbetter M, Jacobs J, Lewis J: Compounds and Methods for Inhibiting NHE-Mediated Antiport in the treatment of Disorders Associated with Fluid Retention or Salt Overload and Gastrointestinal Tract Disorders. World Intellectual Property Organization WO2014029984: 2014 [Google Scholar]
- 21.Tonelli M, Pannu N, Manns B: Oral phosphate binders in patients with kidney failure. N Engl J Med 362: 1312–1324, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Burke SK, Slatopolsky EA, Goldberg DI: RenaGel, a novel calcium- and aluminium-free phosphate binder, inhibits phosphate absorption in normal volunteers. Nephrol Dial Transplant 12: 1640–1644, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Pennick M, Poole L, Dennis K, Smyth M: Lanthanum carbonate reduces urine phosphorus excretion: Evidence of high-capacity phosphate binding. Ren Fail 34: 263–270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez I, Aguilera-Tejero E, Mendoza FJ, Almaden Y, Perez J, Martin D, Rodriguez M: Calcimimetic R-568 decreases extraosseous calcifications in uremic rats treated with calcitriol. J Am Soc Nephrol 17: 795–804, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Andrukhova O, Zeitz U, Goetz R, Mohammadi M, Lanske B, Erben RG: FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone 51: 621–628, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira RB, Cancela AL, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, Carvalho AB, Jorgetti V, Canziani ME, Moysés RM: Early control of PTH and FGF23 in normophosphatemic CKD patients: A new target in CKD-MBD therapy? Clin J Am Soc Nephrol 5: 286–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Mallamaci F, Zoccali C: Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: A randomized clinical trial. Am J Kidney Dis 59: 177–185, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Ohnishi M, Nakatani T, Lanske B, Razzaque MS: In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-dihydroxyvitamin d levels. Circ Cardiovasc Genet 2: 583–590, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez I, Mendoza FJ, Aguilera-Tejero E, Perez J, Guerrero F, Martin D, Rodriguez M: The effect of calcitriol, paricalcitol, and a calcimimetic on extraosseous calcifications in uremic rats. Kidney Int 73: 300–307, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Toussaint ND, Pedagogos E, Tan SJ, Badve SV, Hawley CM, Perkovic V, Elder GJ: Phosphate in early chronic kidney disease: Associations with clinical outcomes and a target to reduce cardiovascular risk. Nephrology (Carlton) 17: 433–444, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Parra E, Tuñón J, Egido J, Ortiz A: Phosphate: A stealthier killer than previously thought? Cardiovasc Pathol 21: 372–381, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Jimbo R, Kawakami-Mori F, Mu S, Hirohama D, Majtan B, Shimizu Y, Yatomi Y, Fukumoto S, Fujita T, Shimosawa T: Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of Klotho deficiency. Kidney Int 85: 1103–1111, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St. John Sutton M, Ojo A, Gadegbeku CDi Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paradiso AM, Tsien RY, Machen TE: Na+-H+ exchange in gastric glands as measured with a cytoplasmic-trapped, fluorescent pH indicator. Proc Natl Acad Sci U S A 81: 7436–7440, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandes I, Béliveau R, Friedlander G, Silve C: NaPO(4) cotransport type III (PiT1) expression in human embryonic kidney cells and regulation by PTH. Am J Physiol 277: F543–F551, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Bijsterbosch MK, Duursma AM, Bouma JM, Gruber M: The plasma volume of the Wistar rat in relation to the body weight. Experientia 37: 381–382, 1981 [DOI] [PubMed] [Google Scholar]
- 37.Labonté ED, Howles PN, Granholm NA, Rojas JC, Davies JP, Ioannou YA, Hui DY: Class B type I scavenger receptor is responsible for the high affinity cholesterol binding activity of intestinal brush border membrane vesicles. Biochim Biophys Acta 1771: 1132–1139, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.