Abstract
Percutaneous transluminal renal angioplasty/stenting (PTRAS) is frequently used to treat renal artery stenosis and renovascular disease (RVD); however, renal function is restored in less than one half of the cases. This study was designed to test a novel intervention that could refine PTRAS and enhance renal recovery in RVD. Renal function was quantified in pigs after 6 weeks of chronic RVD (induced by unilateral renal artery stenosis), established renal damage, and hypertension. Pigs with RVD then underwent PTRAS and were randomized into three groups: placebo (RVD+PTRAS), chronic endothelin-A receptor (ET-A) blockade (RVD+PTRAS+ET-A), and chronic dual ET-A/B blockade (RVD+PTRAS+ET-A/B) for 4 weeks. Renal function was again evaluated after treatments, and then, ex vivo studies were performed on the stented kidney. PTRAS resolved renal stenosis, attenuated hypertension, and improved renal function but did not resolve renal microvascular rarefaction, remodeling, or renal fibrosis. ET-A blocker therapy after PTRAS significantly improved hypertension, microvascular rarefaction, and renal injury and led to greater recovery of renal function. Conversely, combined ET-A/B blockade therapy blunted the therapeutic effects of PTRAS alone or PTRAS followed by ET-A blockade. These data suggest that ET-A receptor blockade therapy could serve as a coadjuvant intervention to enhance the outcomes of PTRAS in RVD. These results also suggest that ET-B receptors are important for renal function in RVD and may contribute to recovery after PTRAS. Using clinically available compounds and techniques, our results could contribute to both refinement and design of new therapeutic strategies in chronic RVD.
Keywords: renal artery stenosis, endothelin-1, angioplasty, microcirculation, imaging
Chronic renovascular disease (RVD) increases the risk of cardiovascular morbidity and mortality and may progressively induce renal injury, leading to ESRD.1 Percutaneous transluminal renal angioplasty/stenting (PTRAS) is a frequently used therapeutic strategy to treat patients with chronic RVD. Targeting the renal stenosis is a logical choice for treating RVD, because the resolution of the vascular obstruction followed by restoration of blood flow to the site of injured tissues should play an important role to initiate successful repairing responses. The use of PTRAS in RVD grew significantly during the past 20 years,2 with tremendous progress in successfully resolving renal stenosis and restoring blood flow (>95% of the cases).3 However, despite the high technical success of PTRAS, improvement in renal function is still observed in a relatively small portion of the cases.4 The reasons for the persistent poor outcomes after PTRAS in RVD are still unclear. Furthermore, the dissociation between the technical success of PTRAS and renal outcomes underscores a pressing need to identify more effective therapeutic strategies in RVD.
Endothelin-1 (ET-1) is a powerful renal vasoconstrictor and mitogenic peptide that plays important roles in controlling BP and renal function. The hemodynamic effects of ET-1 occur through activation of its specific receptors: ET-A mediates vasoconstriction and proliferation of smooth muscle cells, and ET-B mediates clearance of ET-1 and vasodilation. However, overactivation of the renal ET system may contribute to the initiation and progression of CKD in diabetes, hypertension, and GN. In addition to its hemodynamic actions, we and others have shown that ET-1 (mainly through ET-A) can contribute to the development and progression of renal inflammation, fibrosis, and vascular remodeling.5–7 Furthermore, circulating and renal ET-1 increases in patients with renal dysfunction and renal ischemia, and activation of ET-A receptors promotes short- and long-term ischemic renal injury.8,9
Using a well established swine model of chronic RVD, we recently showed that the ET-1/ET-A pathway is upregulated in RVD both systemically and in the stenotic kidney and that chronic ET-A blockade in experimental RVD improves renal function, decreases renal damage, and protects the renal microvasculature of the stenotic kidney.10,11 These studies showed the role of the ET-1/ET-A pathways in the development and the progression of renal injury in RVD. We also showed that PTRAS in this model of RVD is feasible and effective in resolving the stenosis while mimicking the inadequate responses observed in human RVD.12 This model of RVD, therefore, provides an opportunity to not only test interventions that could refine PTRAS but also, increase the understanding of underlying mechanisms. This study was designed to test the hypothesis that chronic specific blockade of the ET-A receptors in RVD after successful PTRAS will improve the function and long-term outcome of the stenotic kidney beyond what might occur with PTRAS alone. Furthermore, although ET-A and ET-B receptors have specific and likely opposing roles, in some clinical situations, they may lead to similar pathologic effects,13 and unforeseen synergistic effect may occur by silencing both receptors.14 Thus, our study will also determine the effects of combined ET-A and ET-B receptor antagonism on the recovery of renal function after PTRAS.
Results
At 6 Weeks: Pre-PTRAS
Pigs after 6 weeks of untreated RVD showed a similar and significant degree of renal artery stenosis and increase in BP compared with normal controls (Figures 1 and 2, Table 1). Body weights were similar among the groups, whereas circulating ET-1 levels were similarly elevated in all pigs after 6 weeks of RVD (Table 1). Serum creatinine was increased as renal blood flow (RBF) and GFR substantially decreased (between 30% and 50%) compared with normal controls, which we have previously observed10–12 (Figure 3, Table 1). The deterioration in renal hemodynamics and function was evident at baseline and also after endothelium-dependent challenge by acetylcholine (data not shown), suggesting a significant renal microvascular (MV) endothelial dysfunction.
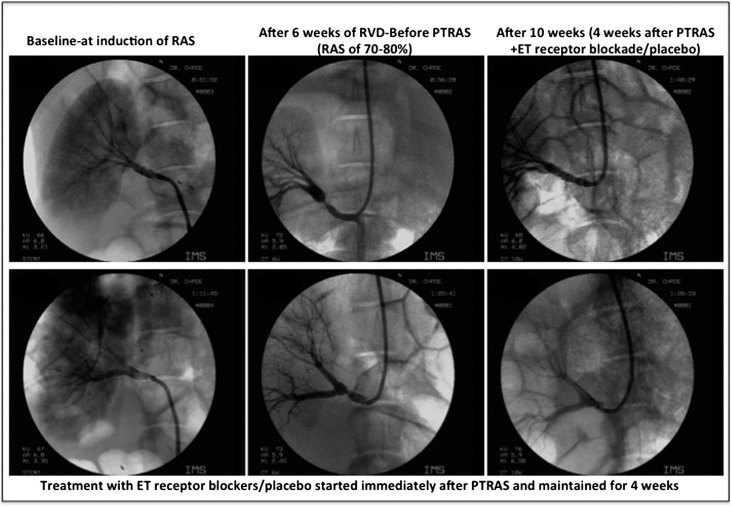
Figure 1.
Renal angiography before and after PTRAS. Representative renal angiography showing the main renal artery (left panels) at baseline, (center panels) 6 weeks after induction of renal artery stenosis (RAS), and (right panels) 4 weeks after PTRAS. PTRAS was similarly effective in resolving RAS in all pigs, with a similar vascular patency 4 weeks after angioplasty. Residual vascular stenosis, when present, was between 6% and 11% at 10 weeks (not significant). Treatment with ET receptor blockers after PTRAS did not modify the success of the intervention.
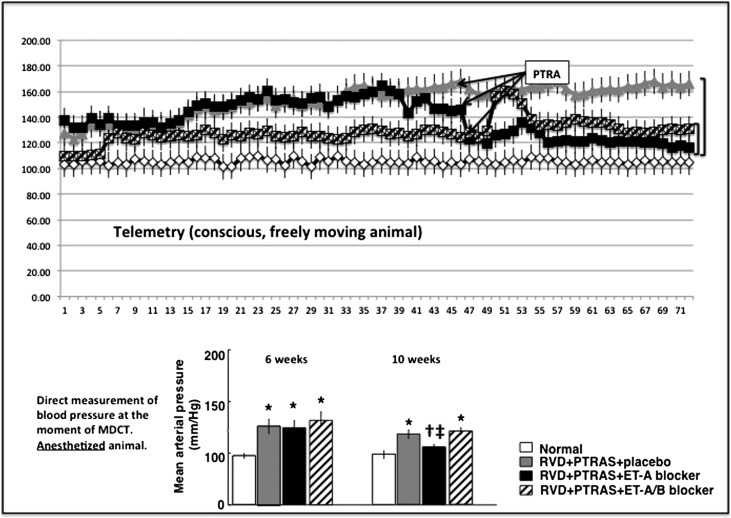
Figure 2.
Blood pressure before and after treatments. (Upper panel) Graph showing the development of hypertension and changes (telemetry) after PTRAS followed by ET receptor blocker therapy. (Lower panel) Bar graph showing measurements of BP in anesthetized animals (direct intra-arterial measurement) during in vivo CT studies at 6 weeks (before PTRAS) and at 10 weeks (4 weeks after PTRAS and treatment with ET blockers or placebo) in normal (n=7), RVD (RVD+PTRAS; n=7), and RVD+PTRAS treated with ET-A (n=7) or ET-A/B (n=5) blockers for 4 weeks. Reduction of BP was significant in pigs treated with ET-A blockers after PTRAS. Pigs treated with PTRAS alone or combined PTRAS+ET-A/B blockers did not show significant reductions in BP compared with pre-PTRAS values. *P<0.05 versus normal. †P<0.05 versus RVD+PTRAS. ‡P<0.05 versus 6 weeks.
Table 1.
Body weight, degree of stenosis, PRA, renal vascular resistance, ET-1 levels (renal vein blood stenotic kidney), and renal cortical and medullary volumes (means±SEMs) in normal pigs (n=7), RVD pigs (n=7), and RVD pigs before PTRAS and treatment with ET-A (n=7) and ET-A/B (n=5) receptor blockers
| Parameter | Normal | RVD before PTRAS | RVD before PTRAS+ET-A | RVD before PTRAS+ET-A/B |
|---|---|---|---|---|
| Body weight (kg) | 49.4±1.8 | 48.6±2.8 | 44.4±4.6 | 50.7±3.4 |
| Degree of stenosis (%) | 0.0±0.0 | 76.3±5.9a | 73.8±8.9a | 73.8±10.2a |
| PRA (ng/ml per hour) | 0.26±0.02 | 0.31±0.07 | 0.28±0.04 | 0.29±0.07 |
| RVR (mmHg/ml per minute) | 0.19±0.05 | 0.40±0.14a | 0.52±0.15a | 0.43±0.18a |
| ET-1 (pg/ml) | 0.87±0.06 | 2.3±0.3a | 2.0±0.2a | 1.9±0.3a |
| Cortical volume (cc) | 127.1±7.9 | 67.8±6.4a | 64.2±2.3a | 80.3±10.5a |
| Medullary volume (cc) | 36.8±2.3 | 26.3±1.7a | 19.2±5.7a | 21.1±1.3a |
Parameters were obtained after 6 weeks of observation and no treatments. RVR, renal vascular resistance.
P<0.05 versus normal.
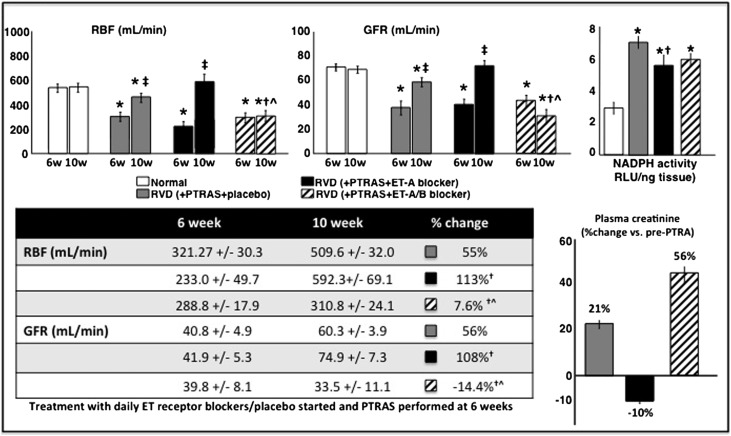
Figure 3.
Renal hemodynamics and function before and after treatments. (Upper left and upper center panels) Average quantification (in bar graphs; mean±SEM) of RBF and GFR after 6 and 10 weeks of observation (post-PTRAS and ET blockers/placebo). (Upper right panel) Activity of NADP(H) oxidase in the stenotic kidney measured by lucigenin luminescence and expressed in relative light units (RLUs) per nanogram of tissue. (Lower left panel) Table showing absolute values of RBF and GFR at 6 and 10 weeks and the changes (expressed in percentage) after PTRAS followed by 4 weeks of ET receptor blockers treatment or placebo. (Lower right panel) Average quantification (in bar graphs; mean±SEM) showing the changes in plasma creatinine after PTRAS followed by ET receptor blockers or placebo in normal (n=7), RVD (RVD+PTRAS; n=7), and RVD+PTRAS treated with ET-A (n=7) or ET-A/B (n=5) blockers for 4 weeks. Administration of ET-A receptor blockers for 4 weeks resulted in a greater recovery of renal function, decreased oxidative stress, and reduction in plasma creatinine compared with PTRAS alone. However, combined blockade of the ET-A and ET-B receptors significantly blunted the beneficial effects of PTRAS and/or ET-A blockade. *P<0.05 versus normal. †P<0.05 versus RVD+PTRAS. ‡P<0.05 versus 6 weeks. ^P<0.05 versus RVD+PTRAS+ET-A.
At 10 Weeks: Post-PTRAS and PTRAS Followed by ET Blocker Therapy
PTRAS was effective in resolving the stenosis in all animals with RVD. Residual stenosis was between 6% and 11% at 10 weeks and similar in all PTRAS-treated animals, regardless of the ET blocker therapy that they received (Figure 1, Table 2). PTRAS resulted in a reduction of BP, but it was distinctly greater in those animals in which PTRAS was followed by 4 weeks of ET-A blockade compared with those that received placebo or ET-A/B therapy (Figure 2). Dual ET-A/B blockade further increased circulating ET-1 levels (Table 2), suggesting effective blockade of ET-B receptors and a consequent reduction in the renal clearance of ET-1. Furthermore, PTRAS alone improved basal RBF and GFR, but such improvements were much greater in PTRAS followed by ET-A blockade for 4 weeks, which resulted in a greater decrease in renal vascular resistance (Table 2) and almost a full recovery of basal renal hemodynamics and function (Figure 3). The difference in effect between PTRAS and PTRAS+ET-A blockade was also reflected by greater improvements in plasma creatinine (Figure 3), nephrinuria (Table 2), and renal expression of phosphorylated endothelial nitric oxide synthase (p-eNOS; RVD+PTRAS: 0.68±0.04; RVD+PTRAS+ET-A: 0.89±0.06; P<0.05 versus RVD+PTRAS, NS versus normal). However, combination of ET-A/B blocker therapy after PTRAS did not improve renal hemodynamics, function, plasma creatinine, nephrinuria, or p-eNOS (RVD+PTRAS+ET-A/B: 0.66±0.03; P<0.05 versus RVD+PTRAS+ET-A and normal, NS versus RVD+PTRAS). In fact, dual ET-A/B blockade diminished the benefits of both PTRAS and ET-A blockade therapy in RVD (Figure 3). Responses to acetylcholine were similarly blunted in all PTRAS groups (data not shown).
Table 2.
Body weight, degree of stenosis, PRA, renal vascular resistance, ET-1 levels (renal vein blood stenotic kidney), excretion of nephrin (urine), and renal cortical and medullary volumes (means±SEMs) in normal pigs (n=7), RVD pigs (n=7), and RVD pigs before PTRAS and treatment with ET-A (n=7) and ET-A/B (n=5) receptor blockers
| Parameter | Normal | RVD+PTRAS | RVD+PTRAS+ET-A | RVD+PTRAS+ET-A/B |
|---|---|---|---|---|
| Body weight (kg) | 55.6±1.8 | 59.2±2.3 | 48.4±7.5 | 59.5±2.7 |
| Degree of stenosis (%) | 0.0±0.0 | 6.7±3.7 | 9.6±5.2 | 10.6±5.8 |
| PRA (ng/ml per hour) | 0.23±0.05 | 0.28±0.04 | 0.29±0.02 | 0.26±0.09 |
| RVR (mmHg/ml per minute) | 0.19±0.06 | 0.25±0.04 | 0.17±0.08a | 0.39±0.07b,c,d |
| ET-1 (pg/ml) | 0.91±0.05 | 2.6±0.5b | 2.2±0.2b | 8.7±0.6b,c,d |
| Nephrin (μg/ml urine) | 0.4±0.2 | 3.2±0.6b | 1.1±0.4c | 2.7±0.6b,d |
| Cortical volume (cc) | 114.7±4.0 | 94.1±1.2b | 118.9±21.6c | 91.1±10.1a,b |
| Medullary volume (cc) | 32.9±1.3 | 29.4±2.7 | 35.7±7.9 | 23.8±3.7b,c,d |
Parameters were obtained 4 weeks after PTRAS and placebo/ET receptor blocker therapy at 10 weeks. RVR, renal vascular resistance.
P>0.05 but P<0.10 versus RVD+PTRAS or RVD+PTRAS+ET-A (trend).
P<0.05 versus normal.
P<0.05 versus RVD+PTRAS.
P<0.05 versus RVD+PTRAS+ET-A.
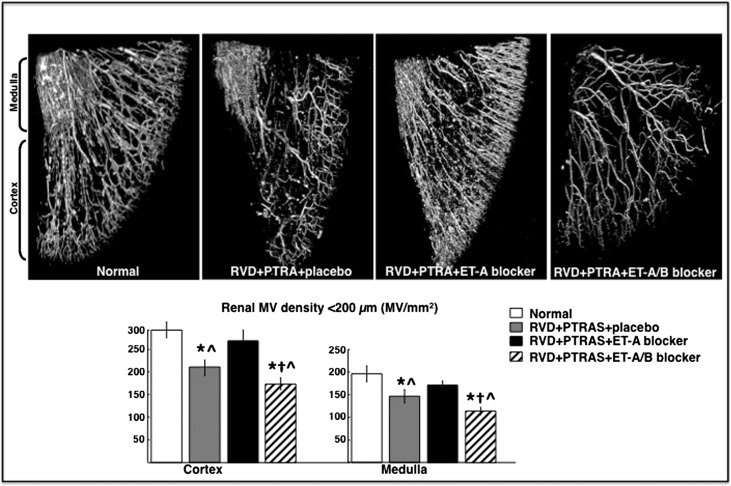
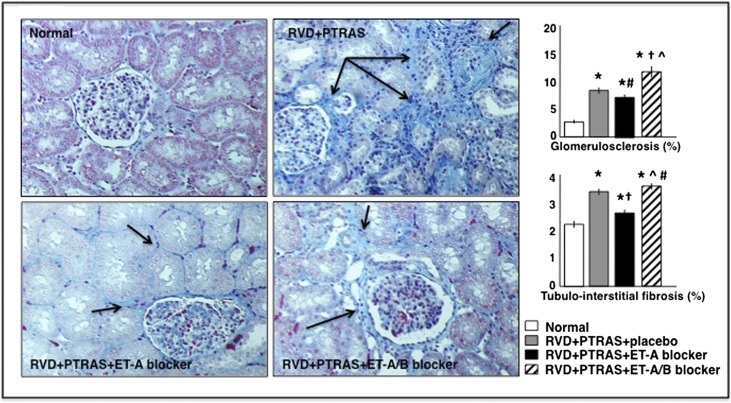
In addition, PTRAS alone or followed by ET-A/B blockade did not resolve renal MV rarefaction despite a full resolution of the stenosis, unlike ET-A blocker therapy, which led to an increase of the cortical and medullary MV densities (Figure 4), suggesting a distinct effect of ET-A blockade on renal microvessels. Finally, ET-A blocker therapy for 4 weeks also reduced renal fibrosis (mainly evident in the tubulointerstitium) compared with PTRAS alone- and PTRAS+ET-A/B–treated kidneys (Figure 5).
Figure 4.
Renal microvascular density after treatments. Representative three-dimensional micro-CT (upper panel) reconstruction and (lower panel) quantification of the cortical and medullary MV densities (diameters under 500 μM) of the stenotic kidney 4 weeks after PTRAS and placebo, ET-A, or ET-A/B blocker therapy. PTRAS alone did not resolve MV rarefaction in the previously stenotic kidney, unlike those kidneys that underwent PTRAS followed by ET-A blockers, which showed a significant increase in cortical and medullary MV densities. However, PTRAS followed by ET-A/B blockers showed an additional decrease in renal MV density compared with PTRAS alone. *P<0.05 versus normal. †P<0.05 versus RVD+PTRAS. ^P<0.05 versus RVD+PTRAS+ET-A.
Figure 5.
Renal morphology after treatments. Representative trichrome pictures (from stenotic kidneys) of the glomeruli, tubules, and tubulointerstitial regions (×20; shown as examples to illustrate renal damage) and quantification of (upper right panel) glomerulosclerosis and (lower right panel) tubulointerstitial fibrosis in normal, RVD (RVD+PTRAS), and RVD+PTRAS treated with ET-A or ET-A/B blockers for 4 weeks. Chronic ET-A blocker after PTRAS reduced renal fibrosis (although it was more evident in the tubulointerstitial space; arrows) to a greater extent compared with PTRAS+placebo or ET-A/B blockers. *P<0.05 versus normal. †P<0.05 versus RVD+PTRAS. ^P<0.05 versus RVD+PTRAS+ET-A. #P>0.05 but P<0.10 versus RVD+PTRAS.
Untreated Controls
An additional group of animals with RVD that did not undergo PTRAS or receive ET antagonists was observed in parallel for 10 weeks and served as untreated controls. We observed a significant and persistent reduction in RBF and GFR in the stenotic kidney accompanied by a marked cortical and medullary MV rarefaction and renal fibrosis (Table 3), which concurred with our previous reports in this model.10,11,15,16
Table 3.
Renal hemodynamics (stenotic kidney) at 6 and 10 weeks, MV density, and renal fibrosis (stenotic kidney) at 10 weeks in pigs with RVD (n=5) and no additional treatments
Discussion
Our study shows that chronic ET-A receptor blocker therapy after successful PTRAS in RVD improved the renal outcomes compared with PTRAS alone. Although PTRAS fully resolved the stenosis, improvements in renal function were smaller compared with in animals that received PTRAS followed by ET-A blockers, in which almost a full recovery of renal hemodynamics and function of the previously stenotic kidney was achieved. These observations suggest greater renoprotection with this combined approach. Furthermore, PTRAS alone did not resolve renal fibrosis or MV rarefaction like ET-A blockade did, implying a direct effect of ET-A blockade to attenuate renal tissue damage, enhance MV proliferation, and possibly, repair pre-existing vessels. These effects, in turn, support an important role for MV integrity in the progression of renal injury and implicate its importance for the response to treatments in RVD. This study also shows that a combined ET-A/B receptor antagonism markedly attenuated the beneficial effects of both PTRAS and single ET-A blockade, implying that a functional ET-1/ET-B pathway may play a role in the recovery of renal function after therapeutic interventions in RVD.
Recent evidence supports the notion that both medical- and catheter-based therapies may be similarly effective,17 which has decreased the use of PTRAS as the first choice to treat RVD.18,19 However, the majority of the data from clinical studies is still controversial regarding the advantages of one or the other treatment in RVD.20 More importantly, the reason for the limited success after PTRAS in one half of the patient population remains unclear.20,21 Factors such as heterogeneity of the sample population included in some of the studies (e.g., different degrees of renal damage) combined with the lack of accurate markers of renal injury or predictors of therapeutic success may play a role in the absence of supporting evidence for a definitive therapeutic strategy in this disease.
Because renal damage is progressive, its extent and severity in the poststenotic kidney may play a role in the outcomes of RVD.22,23 However, there is still a shortage of techniques that can accurately determine the severity of renal damage pre-PTRAS and therefore, define its role in the responses to therapy. It is possible that there is a tipping point at which irreversible renal damage may render any intervention futile. Furthermore, the initiating events leading to progressive damage of the poststenotic kidney are still not completely known. Therefore, enhancing the protection of the renal parenchyma might contribute to increasing the success of PTRAS in recovering renal function in RVD.
We have shown that the progressive renal functional and structural damage in the stenotic renal parenchyma is concomitant to progressive MV dysfunction, damage, and loss. Damage of the microvasculature initially affects the renal cortex but later, extends to the renal medulla,12,24,25 underscoring the progressive pattern of renal vascular injury in the stenotic kidney. Our studies also showed that therapeutic interventions that protect the renal microcirculation also improve renal function, emphasizing the importance of MV integrity in renal function and damage.12,24,25 We recently showed the important role that the ET pathway plays on the progressive injury of the stenotic kidney in RVD, which is mainly mediated by the ET-A receptor.10,11 Blockade of the ET-A receptor, indeed, significantly improved renal function, decreased MV and tissue damage, and improved MV proliferation and repair, even without resolving renal artery stenosis,10,11 suggesting that renal injury in the stenotic kidney is progressive but potentially reversible through targeted ET antagonism. Thus, this model offers a unique opportunity to determine whether therapeutic actions of ET-A receptors blockade could play a role in renal outcomes after PTRAS in RVD.
In this study, PTRAS was similarly effective in resolving the vascular obstruction in all animals with RVD, and specific ET-A or combined ET-A/B blockade did not modify vascular patency after stenting. However, only the combination PTRAS+ET-A blockade improved basal RBF and GFR, leading to an almost full recovery of renal function. These changes were accompanied by improvement in the renal redox status and augmented p-eNOS (not observed after PTRAS alone or PTRAS+ET-A/B), which might have resulted in improved renal bioavailability of nitric oxide after ET-A antagonism. However, the absence of a significant improvement in renal hemodynamics after infusion of acetylcholine may argue against a significant increase in nitric oxide and warrants additional studies. Beneficial effects on renal hemodynamics were also accompanied by a greater decrease in BP, reduced renal fibrosis, and improved MV density in both cortex and medulla only in PTRAS+ET-A–treated animals. The reduction in the urinary excretion of nephrin further supports the notion of distinct protective effects of ET-A blockade (not achieved by PTRAS alone or the combination PTRAS+ET-A/B) in renal podocytes that may have played a role in the greater recovery of renal function.26 In turn, the persistent increase in nephrin excretion after addition of ET-B blockade may also suggest a role of ET-B receptors in podocyte health and function in RVD.27 The greater improvements in renal function, MV, and parenchymal damage in the stented kidney after ET-A blockade that were not achieved by PTRAS alone suggest pre-existing (and possibly progressive) damage that cannot be resolved by the sole resolution of the vascular obstruction. These results extend our previous work12 and suggest that severity of the damage in the poststenotic kidney in human RVD may be partly responsible for the disparate recovery of renal function after PTRAS, suggesting that at a certain point, renal injury may become irreversible and not possible to retreat by revascularization alone.
A combined strategy that targets the renal parenchyma in addition to resolution of the stenosis offers a promising approach that may be of clinical interest. Our study unravels a positive interaction between PTRAS and ET-A blockade to further recover renal function and supports a novel use for an existing clinically available compound, which opens the possibility for a refinement of current PTRAS strategies for patients with RVD. Although we are aware that the effects of the treatments were evaluated at one time point and interventions were performed at a relatively early stage of RVD, these results offer promising data for additional development of this combined strategy for severe cases of renal dysfunction. The progressive nature of RVD supports the notion that the additional protection of ET-A blockade could enhance the efficacy of PTRAS, which could help the transition into clinical studies of this approach to recuperate the kidney.
This study extends our previous observations10,11 by highlighting the distinct renoprotective effects of chronic ET-A receptor antagonism in RVD and also supporting the notion that an intact ET-1/ET-B pathway may play an important role for renal recovery after PTRAS. We observed that, despite a successful resolution of the stenosis, a combined ET-A/B blockade strategy after PTRAS did not improve renal function, MV damage, or nephrinuria. On the contrary, our results suggest that ET-A/B blocker therapy significantly diminished the beneficial effects of PTRAS or ET-A receptor blockade on the stented kidney, implying that ET-B receptors are necessary to protect the kidney and may contribute to renal recovery after PTRAS. Furthermore, BP shows a transient increase during the first 10 days after PTRAS and initiation of ET-A/B blockers but overall, shows a similar pattern compared with PTRAS alone and no major improvements compared with pre-PTRAS values. The reasons for the transient increase in BP in PTRAS+ET-A/B–treated pigs are not clear. A reduction in BP after ET-A/B blockade has not always been observed.28 It is possible that addition of ET-B receptor antagonism after PTRAS might have triggered AKI29 or exacerbated eventual reperfusion injury30 that was minimized in this model by single ET-A blockade, suggesting a potential interaction between both receptors in controlling renal damage.9 Another possibility is that the addition of ET-B blockade exacerbated MV remodeling31 and thus, intrarenal MV resistance in the poststenotic kidney, which may have also played a role later in the minimal or even negative changes in RBF or GFR 4 weeks after PTRAS. A prolonged and possibly exacerbated vasoconstriction29 after ET-B antagonism may have also helped to aggravate MV rarefaction, which may have contributed to the persistence of renal fibrosis. Therefore, because ET-A blockade had a significant hemodynamic effect on the PTRAS-treated kidney that led to recover renal function, it is possible that the combination of vasoconstriction and enhanced progrowth activity after addition of ET-B blockade may have expanded renal damage,32,33 which may have, consequently, blunted the effect of PTRAS.
Major strengths of the swine model of RVD are that it shows several features usually observed in chronic human RVD, such as hypertension,2 progressive deterioration of renal function, and development of renal injury.34 Furthermore, our model of unilateral RVD shows deterioration of renal function in the stenotic kidney alone with relatively preserved function in the nonstenotic contralateral kidney,25 which is usually observed in humans. However, some limitations of this study should be recognized. Previous clinical studies reported normal plasma renin activity (PRA) levels in 25%–37% of patients with RVD35,36 (suggesting PRA as an unreliable index of RVD37), whereas the swine model show an early but transient increase that goes back to baseline levels after 4–6 weeks of RVD.10,38 Because of the growth pattern and size limitations, the animals used in the study are relatively young and consequently, lack some common comorbid diseases often observed associated with human CKD, such as obesity39,40 or diabetes,41 that may compromise renal outcomes. It should also be recognized that interventions were performed at a relatively early stage of the disease, and it will be necessary to evaluate these interventions for a longer period and also, at more advanced stages of RVD (e.g., greater renal fibrosis) before moving forward into the design of clinical studies.
The dose of atrasentan was based on our previous studies. However, we are aware that the dose selected was relatively higher than previously tested doses in patients.42 We did not observe any adverse effects (e.g., fluid retention assessed by differences in body weight between groups after treatments) frequently observed in patients, which may have reflected species differences. Because of the high dose selected, it is possible that some nonspecific ET-B blockade may have occurred, which may help to explain the incomplete resolution of renal injury after ET-A blockade as well as the exacerbated functional and structural renal damage after ET-A/B blockade. Future studies testing lower doses may help to address this issue.
In summary, this study shows that chronic ET-A receptor blockade therapy could serve as a coadjuvant intervention to enhance the outcomes of PTRAS in RVD. ET-A blocker therapy after PTRAS led to almost a full recovery of renal function of the previously stenotic kidney and greater reduction of BP and renal damage. Our data suggest distinct protective effects of ET-A blockade on the renal parenchyma that likely contributed to greater renal recovery compared with PTRAS alone in an additive or possibly synergistic fashion. These results also support the notion that ET-B receptors are important to renal function in RVD and may contribute to recovery after PTRAS, because a concomitant blockade of ET-A/B receptors blunted the therapeutic effects of our interventions. Our study provides supporting data that may help in designing new therapeutic approaches and might contribute to the refining of PTRAS and the development of a novel intervention that could improve long-term recovery in RVD.
Concise Methods
The Institutional Animal Care and Use Committee at the University of Mississippi Medical Center approved all of the procedures. Thirty-one prejuvenile domestic pigs (Sus scrofa domestica) were used for the study, which lasted a total of 10 weeks. In 24 pigs, unilateral renal artery stenosis was induced at baseline by placing a local irritant copper coil (on day 1 of the study) inside the main renal artery constituting a surrogate of RVD as previously shown.15,25 BP was continuously measured by telemetry (PhysioTel; Data Sciences International) and averaged for each 24-hour period as described.15,25,43 Additional animals were used as normal controls (normal; n=7).
Six weeks after induction of RVD, all pigs were anesthetized with intramuscular telazol (5 mg/kg) and xylazine (2 mg/kg), intubated, and mechanically ventilated on room air. Anesthesia was maintained with a mixture of ketamine (0.2 mg/kg per minute) and xylazine (0.03 mg/kg per minute) in normal saline and administered through an ear vein cannula (0.05 ml/kg per minute). Pigs then underwent renal angiography to quantify the degree of renal artery stenosis as described.10,11,44 After angiography, the catheter was positioned in the superior vena cava, and in vivo helical multidetector computer tomography (MDCT) flow studies were performed for quantification of single-kidney RBF, perfusion, and GFR at baseline and after intrarenal infusion of the endothelium-dependent vasodilator acetylcholine as previously described.10
Immediately after completion of the in vivo MDCT studies at 6 weeks and while still under anesthesia, 19 animals with RVD underwent PTRAS under fluoroscopic guidance using a balloon catheter and tantalum stent deployment to optimize vascular patency for revascularization (matched to the size of the renal artery and length of stenosis) as recently described.12 Immediately after PTRAS, the RVD pigs were then randomized (double blind and placebo-controlled) into three groups: placebo (RVD+PTRAS; n=7), those chronically treated with a specific ET-A receptor blocker (ABT 627; 0.75 mg/kg per day, oral; RVD+PTRAS+ET-A; n=7), and those treated with specific ET-A and ET-B receptor blockers (A-192621; 1 mg/kg per day, oral; RVD+PTRAS+ET-A/B; n=5). The doses were selected on the basis of studies from our laboratory and other laboratories that showed them to be effective in vivo and in vitro.10,11,45,46 Treatment or placebo was maintained for 4 additional weeks, BP was continuously monitored by telemetry, and at 10 weeks, MDCT in vivo studies were repeated as done at 6 weeks. The remaining five animals with RVD did not receive any additional treatments and served as untreated controls.
Renal vascular resistance was calculated at 6 and 10 weeks as recently described.24 Blood from the inferior vena cava and renal veins (from the stenotic kidney) and urine were collected (at 6 and 10 weeks) to measure PRA, serum creatinine (ELISA; BioAssay Systems), circulating ET-1 (ELISA; R&D Systems) from stenotic kidney vein blood, and nephrin in urine (suggestive of podocyte damage; ELISA; Exocell, PA) following the vendors’ instructions.
On completion of all of the in vivo studies, the pigs were allowed 2 days to recover and then euthanized by an intravenous overdose of sodium pentobarbital (100 mg/kg). Kidneys were then removed and immersed in heparinized saline (10 units/ml) before preparation for ex vivo studies. A kidney lobe was used for microcomputer tomography (micro-CT) reconstruction. Another lobe was removed, snap-frozen in liquid nitrogen, and stored at −80°C, and another portion was preserved in 10% formalin and used to investigate renal morphology in midhilar renal cross-sections stained with trichrome and hematoxylin/eosin. Renal tissue was also used to quantify the superoxide production in the stenotic kidney by lucigenin luminescence as shown.10,47
MDCT Analyses
Manually traced regions of interest were selected in MDCT images in the aorta, renal cortex, medulla, and papilla; their densities were sampled, and time-density curves were generated. The area under each segment of the curve and its first moment were calculated using curve-fitting parameters and used to calculate single-kidney RBF (milliliters per minute), GFR (milliliters per minute), and renal perfusion (milliliters per minute per cubic centimeter of tissue) using previously validated methods.15,44,48
Micro-CT
The stenotic kidney was perfused with an intravascular contrast agent (Microfil MV122; Flow Tech, Inc., Carver, MA), and samples were scanned at 0.3° increments using a micro-CT scanner and reconstructed for subsequent analysis as described.25 The cortex and medulla were tomographically divided, and the spatial density, distribution of microvessels (diameters<500 μm), and images were then analyzed with Analyze (Biomedical Imaging Resource; Mayo Clinic, Rochester, MN) as described.25,49
Western Blotting
Standard blotting protocols in renal cortical tissue homogenates were followed as previously described10,16 using specific polyclonal antibodies against p-eNOS (Santa Cruz Biotechnology). β-Actin (1:500; Sigma-Aldrich, St. Louis, MO) was used as a loading control.
Histology
Midhilar 5-µm cross-sections of each kidney (one per animal) were examined. In each slide, trichrome staining was semiautomatically quantified in 15–20 fields using a computer-aided image analysis program (NIS Element 3.0; Nikon Instruments, Melville, NY) and expressed as percentage of staining of total surface area, and the results from all fields were averaged. Glomerular score was assessed by recording the number of sclerotic glomeruli of 100 counted glomeruli as described.10
Statistical Analyses
Results are expressed as means±SEMs. Comparisons were performed within groups using the paired t test and among groups using one-way ANOVA with Bonferroni correction for multiple comparisons. Statistical significance was accepted for P≤0.05.
Disclosures
None.
Acknowledgments
We thank Dr. Timothy C. McCowan, Chair of the Department of Radiology at University of Mississippi Medical Center, and his staff for their assistance on in vivo computer tomography studies.
This work was supported by National Institutes of Health Grants HL095638, HL51971, and GM104357, American Heart Association Grant 18490005, and an unrestricted grant from Abbott (AbbVie) Laboratories.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Safian RD, Madder RD: Refining the approach to renal artery revascularization. JACC Cardiovasc Interv 2: 161–174, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Garovic VD, Textor SC: Renovascular hypertension and ischemic nephropathy. Circulation 112: 1362–1374, 2005 [DOI] [PubMed] [Google Scholar]
- 3.White CJ, Olin JW: Diagnosis and management of atherosclerotic renal artery stenosis: Improving patient selection and outcomes. Nat Clin Pract Cardiovasc Med 6: 176–190, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Textor SC: Ischemic nephropathy: Where are we now? J Am Soc Nephrol 15: 1974–1982, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Chade AR, Best PJ, Rodriguez-Porcel M, Herrmann J, Zhu X, Sawamura T, Napoli C, Lerman A, Lerman LO: Endothelin-1 receptor blockade prevents renal injury in experimental hypercholesterolemia. Kidney Int 64: 962–969, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Chade AR, Krier JD, Textor SC, Lerman A, Lerman LO: Endothelin-a receptor blockade improves renal microvascular architecture and function in experimental hypercholesterolemia. J Am Soc Nephrol 17: 3394–3403, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Schneider MP, Boesen EI, Pollock DM: Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annu Rev Pharmacol Toxicol 47: 731–759, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhaun N, Goddard J, Kohan DE, Pollock DM, Schiffrin EL, Webb DJ: Role of endothelin-1 in clinical hypertension: 20 years on. Hypertension 52: 452–459, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Zager RA, Johnson AC, Andress D, Becker K: Progressive endothelin-1 gene activation initiates chronic/end-stage renal disease following experimental ischemic/reperfusion injury. Kidney Int 84: 703–712, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chade AR, Stewart NJ, Peavy PR: Disparate effects of single endothelin-A and -B receptor blocker therapy on the progression of renal injury in advanced renovascular disease. Kidney Int 85: 833–844, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelsen S, Hall JE, Chade AR: Endothelin-A receptor blockade slows the progression of renal injury in experimental renovascular disease. Am J Physiol Renal Physiol 301: F218–F225, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chade AR, Kelsen S: Renal microvascular disease determines the responses to revascularization in experimental renovascular disease. Circ Cardiovasc Interv 3: 376–383, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haynes WG, Strachan FE, Webb DJ: Endothelin ETA and ETB receptors cause vasoconstriction of human resistance and capacitance vessels in vivo. Circulation 92: 357–363, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Ozaki S, Ohwaki K, Ihara M, Ishikawa K, Yano M: Coexpression studies with endothelin receptor subtypes indicate the existence of intracellular cross-talk between ET(A) and ET(B) receptors. J Biochem 121: 440–447, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO: Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 106: 1165–1171, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO: Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol 23: 1295–1301, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB, Sr., Dworkin LD, CORAL Investigators : Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370: 13–22, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrmann SM, Saad A, Textor SC: Management of atherosclerotic renovascular disease after Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) [published online ahead of print April 9, 2014]. Nephrol Dial Transplant [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritchie J, Green D, Chrysochou C, Chalmers N, Foley RN, Kalra PA: High-risk clinical presentations in atherosclerotic renovascular disease: Prognosis and response to renal artery revascularization. Am J Kidney Dis 63: 186–197, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Textor SC, Misra S, Oderich GS: Percutaneous revascularization for ischemic nephropathy: The past, present, and future. Kidney Int 83: 28–40, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J, ASTRAL Investigators : Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 361: 1953–1962, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Wright JR, Duggal A, Thomas R, Reeve R, Roberts IS, Kalra PA: Clinicopathological correlation in biopsy-proven atherosclerotic nephropathy: Implications for renal functional outcome in atherosclerotic renovascular disease. Nephrol Dial Transplant 16: 765–770, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Wright JR, Shurrab AE, Cheung C, Waldek S, O’Donoghue DJ, Foley RN, Mamtora H, Kalra PA: A prospective study of the determinants of renal functional outcome and mortality in atherosclerotic renovascular disease. Am J Kidney Dis 39: 1153–1161, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Chade AR, Kelsen S: Reversal of renal dysfunction by targeted administration of VEGF into the stenotic kidney: A novel potential therapeutic approach. Am J Physiol Renal Physiol 302: F1342–F1350, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iliescu R, Fernandez SR, Kelsen S, Maric C, Chade AR: Role of renal microcirculation in experimental renovascular disease. Nephrol Dial Transplant 25: 1079–1087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM: Endothelin receptor A-specific stimulation of glomerular inflammation and injury in a streptozotocin-induced rat model of diabetes. Diabetologia 54: 979–988, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto T, Hirohama T, Uemura H: Endothelin B receptor-like immunoreactivity in podocytes of the rat kidney. Arch Histol Cytol 65: 245–250, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Boffa JJ, Tharaux PL, Dussaule JC, Chatziantoniou C: Regression of renal vascular fibrosis by endothelin receptor antagonism. Hypertension 37: 490–496, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Goddard J, Johnston NR, Hand MF, Cumming AD, Rabelink TJ, Rankin AJ, Webb DJ: Endothelin-A receptor antagonism reduces blood pressure and increases renal blood flow in hypertensive patients with chronic renal failure: A comparison of selective and combined endothelin receptor blockade. Circulation 109: 1186–1193, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Arfian N, Emoto N, Vignon-Zellweger N, Nakayama K, Yagi K, Hirata K: ET-1 deletion from endothelial cells protects the kidney during the extension phase of ischemia/reperfusion injury. Biochem Biophys Res Commun 425: 443–449, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Kitada K, Yui N, Matsumoto C, Mori T, Ohkita M, Matsumura Y: Inhibition of endothelin ETB receptor system aggravates neointimal hyperplasia after balloon injury of rat carotid artery. J Pharmacol Exp Ther 331: 998–1004, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Forbes JM, Hewitson TD, Becker GJ, Jones CL: Simultaneous blockade of endothelin A and B receptors in ischemic acute renal failure is detrimental to long-term kidney function. Kidney Int 59: 1333–1341, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Saleh MA, Pollock JS, Pollock DM: Distinct actions of endothelin A-selective versus combined endothelin A/B receptor antagonists in early diabetic kidney disease. J Pharmacol Exp Ther 338: 263–270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung CM, Wright JR, Shurrab AE, Mamtora H, Foley RN, O’Donoghue DJ, Waldek S, Kalra PA: Epidemiology of renal dysfunction and patient outcome in atherosclerotic renal artery occlusion. J Am Soc Nephrol 13: 149–157, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Rossi GP, Pavan E, Chiesura-Corona M, Bader M, Paganini G, Cesari M, De Toni R, Feltrin GP, Ganten D, Pessina AC: Renovascular hypertension with low-to-normal plasma renin: Clinical and angiographic features. Clin Sci (Lond) 93: 435–443, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Vasilev T, Kiprov D, Puchlev A, Todorova L: Plasma renin activity in patients with renovascular hypertension. Cor Vasa 20: 35–43, 1978 [PubMed] [Google Scholar]
- 37.Herrmann SM, Textor SC: Diagnostic criteria for renovascular disease: Where are we now? Nephrol Dial Transplant 27: 2657–2663, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu XY, Urbieta Caceres VH, Favreau FD, Krier JD, Lerman A, Lerman LO: Enhanced endothelial progenitor cell angiogenic potency, present in early experimental renovascular hypertension, deteriorates with disease duration. J Hypertens 29: 1972–1979, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foster MC, Hwang SJ, Larson MG, Lichtman JH, Parikh NI, Vasan RS, Levy D, Fox CS: Overweight, obesity, and the development of stage 3 CKD: The Framingham Heart Study. Am J Kidney Dis 52: 39–48, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stenvinkel P, Zoccali C, Ikizler TA: Obesity in CKD—what should nephrologists know? J Am Soc Nephrol 24: 1727–1736, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGovern AP, Rusholme B, Jones S, van Vlymen JN, Liyanage H, Gallagher H, Tomson CR, Khunti K, Harris K, de Lusignan S: Association of chronic kidney disease (CKD) and failure to monitor renal function with adverse outcomes in people with diabetes: A primary care cohort study. BMC Nephrol 14: 198, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhaun N, Melville V, Blackwell S, Talwar DK, Johnston NR, Goddard J, Webb DJ: Endothelin-A receptor antagonism modifies cardiovascular risk factors in CKD. J Am Soc Nephrol 24: 31–36, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC: Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol 10: 1455–1465, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO: Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol 281: F630–F638, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Matsumura Y, Taira S, Kitano R, Hashimoto N, Kuro T: Selective antagonism of endothelin ET(A) or ET(B) receptor in renal hemodynamics and function of deoxycorticosterone acetate-salt-induced hypertensive rats. Biol Pharm Bull 22: 858–862, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Wettstein R, Mörsdorf P, Bächle A, Amon M, Pittet B, Menger MD, Harder Y: Selective blockade of endothelin-B receptor improves survival of critically perfused musculocutaneous flaps. Langenbecks Arch Surg 392: 331–338, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Kelsen S, He X, Chade AR: Early superoxide scavenging accelerates renal microvascular rarefaction and damage in the stenotic kidney. Am J Physiol Renal Physiol 303: F576–F583, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO: Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: Comparison with electron-beam CT. Radiology 243: 405–412, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO: Cortical microvascular remodeling in the stenotic kidney: Role of increased oxidative stress. Arterioscler Thromb Vasc Biol 24: 1854–1859, 2004 [DOI] [PubMed] [Google Scholar]