Abstract
Aortic stiffening, assessed by carotid-femoral pulse wave velocity, is associated with CKD. Transmission of excessive flow pulsatility into the low-impedance renal microvasculature may mediate this association. However, direct analyses of macrovascular–microvascular relations in the kidney are limited. Using arterial tonometry, iohexol clearance, and magnetic resonance imaging, we related arterial stiffness, GFR, urinary albumin excretion, and potential mediators, including renal artery pulsatility index, renal vascular resistance, and arterial volume in the cortex, in 367 older adults (ages 72–92 years) participating in the Age, Gene/Environment Susceptibility-Reykjavik Study. In a model adjusted for age, sex, heart rate, and body size, aortic stiffness was related to GFR (Slope of regression B=−2.28±0.85 ml/min per SD, P=0.008) but not urine albumin (P=0.09). After accounting for pulsatility index, the relation between aortic stiffness and GFR was no longer significant (P=0.10). Mediation analysis showed that 34% of the relation between aortic stiffness and GFR was mediated by pulsatility index (95% confidence interval of indirect effect, −1.35 to −0.29). An additional 20% or 36% of the relation was mediated by lower arterial volume in the cortex or higher renal vascular resistance, respectively, when offered as mediators downstream from higher pulsatility index (95% confidence interval of indirect effect including arterial volume in the cortex, −2.22 to −0.40; 95% confidence interval of indirect effect including renal vascular resistance, −2.51 to −0.76). These analyses provide the first evidence that aortic stiffness may contribute to lower GFR by transferring excessive flow pulsatility into the susceptible renal microvasculature, leading to dynamic constriction or vessel loss.
Keywords: arteries, arteriosclerosis, clinical epidemiology, GFR, hemodynamics and vascular regulation
Carotid-femoral pulse wave velocity (CFPWV) is a well established marker of aortic stiffness and has been studied extensively as a risk factor for cardiovascular disease and mortality in CKD and related conditions.1–5 Increased aortic stiffness increases pressure, flow pulsatility, and transmission of excessive pulsatility into the peripheral vasculature.6 The kidneys are especially vulnerable to pressure and flow pulsatility because of the low impedance associated with obligate high renal blood flow.6,7
There are at least two potential mechanisms that could mediate a harmful effect of higher CFPWV on kidney function. First, increased pulsatility may cause constriction of small resistance vessels in the kidney.8 Second, increased pulsatility may cause structural damage and loss of small vessels, particularly in the cortex, where pressure and flow pulsatility are high. Both mechanisms would be expected to reduce the arterial volume in the cortex (AVC) and increase renal vascular resistance. Through a combination of applanation tonometry, magnetic resonance (MR) imaging, and kidney measures, we ascertained CFPWV, renal vascular resistance, renal artery pulsatility index (PI), AVC, GFR (by iohexol clearance), and urine albumin-to-creatinine ratio (ACR) in addition to demographic and cardiovascular risk factors in 367 older adults as part of the Age, Gene/Environment Susceptibility-Reykjavik (AGES) Study. We hypothesized that (1) higher CFPWV is associated with lower GFR and higher urine ACR and (2) lower GFR and higher urine ACR associated with greater CFPWV are mediated, in part, by higher PI and microvascular alterations. Although the cross-sectional nature of our study precludes direct proof of the causal mechanisms that we propose, statistical mediation provides a framework in which we may formally test the observed data for evidence of such mechanisms. Mediation can show whether some or all of the significance of the association between an exposure and an outcome statistically is explained by the influence of the exposure on the potential mediator. The analysis also gives an estimate of the amount of the observed effect of the exposure on the outcome that can be explained by the action of the exposure through the mediator if the hypothesized mechanism exists. Although this evidence is necessarily circumstantial, it can still provide important support and motivation for more definitive investigations.
Results
Characteristics of the Sample
Characteristics of the sample are presented in Table 1 and compared with the full AGES-II cohort in Supplemental Table 1. Compared with the full AGES-II cohort, participants included in the study were younger with higher hematocrit, higher GFR, and lower urine ACR, and they were less likely to be diabetic. They also had higher total cholesterol and were more likely to be treated for hypertension. Study participants were 72–92 years old (59% women), and 40% of participants had CKD on the basis of reduced GFR (<60 ml/min per 1.73 m2) or increased urine ACR (>30 mg/g creatinine). The mean systolic BP was in the hypertensive range, and three fourths of the participants were treated for hypertension (Table 1). Total renal blood flow was approximately 17% of cardiac output (Table 1).
Table 1.
Characteristics of the sample
| Variable | Study Sample (n=367) |
|---|---|
| Men, n (%) | 149 (41%) |
| Age, yr | 79±3 |
| Height, cm | 168±9 |
| Weight, kg | 77±14 |
| Heart rate, min−1 | 62±9 |
| BP, mmHg | |
| Systolic | 142±18 |
| Diastolic | 63±9 |
| MAP | 93±11 |
| CPP | 80±21 |
| Cardiac output, ml/s | 65±15 |
| CFPWV, m/s | 12.0 (10.4, 14.8) |
| Total kidney volume, ml | 331±77 |
| Cortex volume, ml | 199±51 |
| Renal blood flow, ml/s | 10.9 (8.7, 13.2) |
| Renal vascular resistance, dyne·s/cm5 | 11,506 (9253, 14,316) |
| Renal artery PI | 1.85 (1.62, 2.20) |
| AVC, ml | 48±19 |
| Body mass index, kg/m2 | 27±4 |
| Obese, n (%) | 82 (22%) |
| GFR, ml/min per 1.73 m2 | 65±15 |
| GFR<60 ml/min per 1.73 m2 | 133 (36%) |
| Urine ACR, mg/g | 7.14 (4.76, 13.00) |
| Urine ACR>30 mg/g | 31 (8%) |
| CKD | 148 (40%) |
Association of Systemic Hemodynamic Measures with Kidney Measures
Minimally adjusted associations of CFPWV, mean arterial pressure (MAP), and central pulse pressure (CPP), each examined individually, with separate renal artery flow variables are presented in Table 2. Higher CFPWV was associated with greater PI and renal vascular resistance and smaller AVC. Similar to CFPWV, CPP was associated positively with PI and renal vascular resistance and negatively with AVC. MAP was not associated with PI or AVC. PI and renal vascular resistance were positively related, and both of these quantities were negatively related to AVC. All associations were confirmed linear by univariate ANOVA for polynomial contrasts with no evidence of any threshold effects.
Table 2.
Individual associations between systemic and local renal hemodynamics
| Independent Variable | Renal artery PI, unitless | AVC, ml | RVR, dyne·s/cm5 | |||
|---|---|---|---|---|---|---|
| B±SEM | P Values | B±SEM | P Values | B±SEM | P Values | |
| CFPWV, m/s | 0.16±0.05 | 0.003 | −0.10±0.05 | 0.05 | 0.18±0.05 | <0.001 |
| MAP, mmHg | — | NS | — | NS | — | NAa |
| CPP, mmHg | 0.26±0.05 | <0.001 | −0.13±0.05 | 0.004 | 0.15±0.05 | <0.01 |
| Renal artery PI, unitless | — | NA | −0.28±0.05 | <0.001 | 0.37±0.05 | <0.001 |
| AVC, ml | −0.30±0.05 | <0.001 | — | NA | −0.47±0.05 | <0.001 |
| RVR, dyne·s/cm5 | 0.34±0.05 | <0.001 | −0.40±0.04 | <0.001 | — | NA |
Each entry represents a separate multivariable model adjusted for age, sex, body surface area, and heart rate. Effect sizes and SEMs are expressed in SDs of the dependent variable (columns) per SD of the independent variable (rows). All dependent variables have been log-transformed, and therefore, coefficients may be interpreted as a percent change per 1-SD increase in the independent variable. NS (P>0.05) associations are indicated. RVR, renal vascular resistance; NA, not applicable.
RVR and MAP cannot be compared because of mathematical dependencies.
Association of Systemic and Renal Hemodynamic Measures with GFR and Urine ACR
Table 3 shows minimally adjusted relations of individual systemic and renal hemodynamic measures with GFR and urine ACR. CFPWV was the only systemic hemodynamic measure related to GFR (P<0.01), whereas MAP was the only systemic hemodynamic measure related to ACR (P=0.03). Each of the renal hemodynamic variables was strongly correlated with GFR (P<0.001); individuals with greater flow pulsatility or renal vascular resistance and those with lower AVC had lower GFR. AVC had the strongest relation with GFR. None of the renal hemodynamic measures were related to ACR (P>0.30 for all three measures). The associations with GFR were confirmed linear by univariate ANOVA for polynomial contrasts with no evidence of threshold effects. The association of MAP with urine ACR had a significant quadratic component (P=0.03) because of a slight drop off in the third tertile, but no significant threshold effect was observed.
Table 3.
Individual associations of arterial stiffness and local renal hemodynamics with GFR and urine ACR
| Independent Variable | GFR, ml/min | Urine ACR, mg/g | ||
|---|---|---|---|---|
| B±SEM | P Values | B±SEM | P Values | |
| CFPWV, m/s | −2.3±0.8 | <0.01 | — | NS |
| MAP, mmHg | — | NS | 0.04±0.02 | 0.03 |
| CPP, mmHg | — | NS | — | NS |
| Renal artery PI, unitless | −5.0±0.8 | <0.001 | — | NS |
| AVC, ml | 8.2±0.7 | <0.001 | — | NS |
| Renal vascular resistance, dyne·s/cm5 | −7.1±0.7 | <0.001 | — | NS |
Each entry represents a separate multivariable model adjusted for age, sex, body surface area, and heart rate. Effect sizes and SEMs are expressed in units of the dependent variable (columns) per SD of the independent variable (rows). Urine ACR has been log-transformed; as a result, coefficients may be interpreted as a percent change per 1-SD increase in the independent variable. NS (P>0.05) associations are indicated.
The relation between CFPWV and GFR was attenuated and no longer significant after additionally adjusting the multivariable model for PI, renal vascular resistance, or AVC. On the basis of these observations, we performed mediation analysis to examine the extent of mediation of the relation between CFPWV and GFR by PI, renal vascular resistance, and AVC.
Mediation Analyses
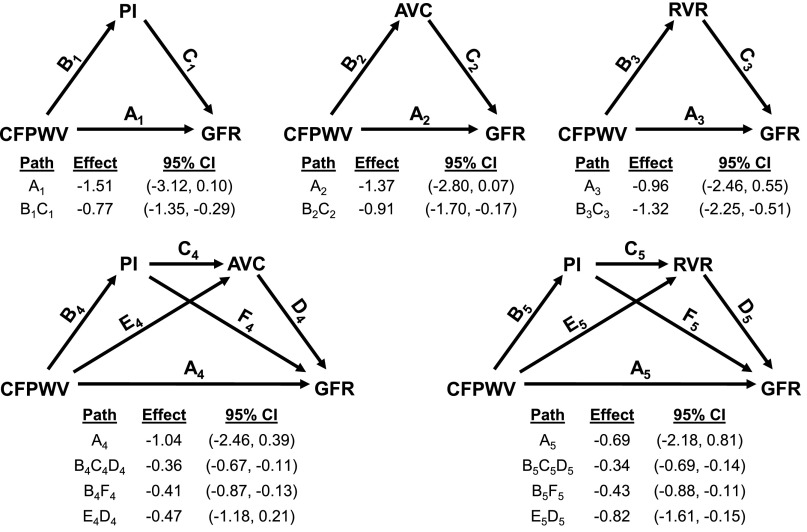
Results of mediation analyses are summarized in Figure 1. In separate analyses, a component of the relation between CFPWV and GFR was fully mediated by PI, AVC, and renal vascular resistance (models 1–3, respectively). In other words, the individual indirect effects of PI (model 1), AVC (model 2), and renal vascular resistance (model 3) were each significant and substantial enough to eliminate the significance of the residual direct effect of CFPWV on GFR (P>0.10 for direct effect for all models), explaining 34%, 40%, and 58% of the total observed effect, respectively. We also tested whether any of the indirect effect of CFPWV on GFR through PI was subsequently mediated by either lower AVC or higher renal vascular resistance (models 4 and 5, respectively). Considering the possible indirect effect of PI on GFR through reduced AVC explained an additional 20% of the total effect of CFPWV on GFR (95% confidence interval of indirect effect: −2.22, −0.40 ml/min per SD), whereas the indirect effect of PI on GFR through higher renal vascular resistance explained an additional 36% of the total effect (95% confidence interval of indirect effect: −2.51, −0.76 ml/min per SD). The component of the negative relation between CFPWV and GFR that was potentially mediated through lower AVC alone (model 4; path E4D4) was no longer significant after accounting for the intermediate higher PI.
Figure 1.
Mediation analyses of the association between CFPWV and GFR. Path models and mediation analyses describing mediation of the relation between CFPWV and GFR by renal artery PI, AVC, and renal vascular resistance (RVR). The total effect for the relation between CFPWV and GFR was −2.28 ml/min per SD (95% confidence limit, −3.95 to −0.61). Path effects are reported as the difference in GFR (milliliters per minute) observed per 1-SD difference in CFPWV along with 95% confidence intervals (95% CIs). Models are adjusted for age, sex, body surface area, and heart rate. Model numbers are denoted by path subscripts. Residual direct effects are labeled as path A in each model, and indirect effects are labeled with relevant groupings of letters B–F as needed.
Discussion
Increased aortic stiffness as assessed by CFPWV is frequently seen in individuals with CKD, and it is an established risk factor for cardiovascular complications and mortality in patients with CKD.1–5 Excessive aortic stiffness increases transmission of pulsatile power into the microcirculation, potentially resulting in damage or remodeling in small vessels. Although the associated risk of microvascular damage is systemic in nature, the necessarily low vascular impedance of obligate high-flow organs, such as the brain and kidneys, may uniquely predispose them to pulsatile damage.6,9 However, only limited research has examined the specific negative consequences of increased transmission of pulsatile energy into the renal vasculature in the presence of increased arterial stiffness. The AGES Study represents an unprecedented MR-enabled collection of hemodynamic, functional, and structural data on a large, well characterized, and relatively healthy older community-based cohort. Our study was intended to use the unique opportunity presented by the comprehensive AGES Study MR and tonometry data to test the hypothesis that excess pulsatility transmitted into the kidneys by a stiffened aorta contributes to microvascular damage and loss of function. To our knowledge, our study is the first to examine this hypothesis explicitly using a comprehensive assessment of local and systemic hemodynamics, sophisticated kidney imaging, measured GFR, urine ACR, and formal mediation analysis. We found a moderate relation between CFPWV and GFR in our older sample, despite highly prevalent elevation of CFPWV and a narrow age range. CFPWV was not related to urine ACR. The association between CFPWV and GFR was mediated, in part, by higher PI, lower AVC, and higher renal vascular resistance. Our findings provide evidence in support of the hypothesis that a stiffer aorta leads to increased delivery of pulsatile energy to the kidneys, resulting in microvascular rarefaction, increased renal vascular resistance, and reduction in GFR.
Numerous studies have shown that increased arterial stiffness may be associated with reduced GFR and increased albuminuria.10–14 Although CFPWV was not related to urine ACR in our study participants, greater CFPWV was associated with a lower GFR. Mediation analysis provided useful insights into potential specific mechanisms by which aortic stiffness may have affected the kidney microvasculature and reduced function in our sample. For example, a component of the relation between CFPWV and GFR that involves lower AVC and higher renal vascular resistance is partially attributable to an association between CFPWV and PI. A plausible explanation for these associations is that excessive flow pulsatility may reduce AVC, increase renal vascular resistance, and reduce function by either altering tone or remodeling, which may be reversible, or damaging the microcirculation of the kidney, leading to loss of vessels and glomeruli. It is also important to note that renal vascular resistance still had a substantial independent relation with GFR after adjusting for a potential upstream effect of PI, indicating that factors other than excessive flow pulsatility may contribute to increased renal vascular resistance and reduced kidney function in individuals with higher CFPWV.
Although CFPWV is the most common and widely accepted measure of aortic stiffness,15 several of the other variables that we analyzed merit some discussion. First, our models adjusted for heart rate and body surface area in addition to age and sex. Adjustment for body size was necessary, because GFR, AVC, and renal vascular resistance depend on kidney size and therefore, body size. We adjusted for heart rate, because it is a known confounder of CFPWV.16 We evaluated PI rather than resistive index,17 because previous analyses in the AGES cohort found robust relations between target organ damage in the brain and PI as measured in the carotid artery.9 In addition, although the resistive index has been used as a measure of flow pulsatility, it is primarily intended as a surrogate for renal vascular resistance, which we have measured directly. We measured GFR using an exogenous marker instead of estimating it using an endogenous marker, such as serum creatinine or cystatin C. This approach avoids the potential confounding effects of age-, sex-, and body size-dependent estimating equations and circumvents potential relations between vascular risk factors and non-GFR determinants of serum levels of endogenous markers used for GFR estimation, which may confound the relation between eGFR and CFPWV.18 Also, raw GFR rather than GFR indexed to body surface area was used in our analyses to adjust only one time for body size. Finally, this study is the first that we are aware of that evaluated AVC as a measure of renal vascularity. Although the estimate is somewhat crude, it is notable in that such a global in vivo measurement is impossible without imaging and arguably provides the exact information desired for our analysis.
It is important to note the respective roles played by MAP and CPP in these analyses. MAP is an established correlate of CFPWV and as a result, a potential confounder of the models presented.19 However, MAP was not associated with GFR in our sample and hence, not a potential confounder or mediator of the relation between CFPWV and GFR. However, the role of CPP is less certain. Although not correlated with GFR or urine ACR in adjusted models, CPP was as strongly related to our mediator variables as CFPWV. Statistically, this observation may indicate that CPP has a negative effect on GFR through the mediators that is offset by some unknown positive direct effect. In addition, it is worth noting that greater pressure pulsatility alone in the absence of accompanying flow pulsatility may not increase transmission of pulsatile power into the vascular bed and hence, may not produce microvascular damage.
There are several limitations to our study. First, the most obvious limitation is that our study was cross-sectional and therefore, cannot show causality. Mediation analysis provides evidence supportive of potential causal pathways that must then be confirmed in appropriate interventional studies in animal models or clinical trials. Although we adjusted the models for several known potential confounders, it is possible that the effects described may be attributable to unknown variables. Second, because of the small size of the resistance vessels relative to the resolution of dynamic contrast-enhanced MR imaging and lack of a provocative or interventional maneuver, we are not able to distinguish between the effects of vessel loss and vessel constriction in our measurements of AVC. Thus, although our measurement of the AVC provides structural information, it cannot differentiate permanent loss compared with functional reductions in vascularity. However, a recent longitudinal study of living kidney donors and recipients suggests that the renal modifications associated with increased arterial stiffness are persistent if not permanent.20 An acute intervention study may help to further refine this distinction. Third, the limited age range (72–92 years old) of the participants may limit the generalizability of the results. Although both arterial stiffness and reduced kidney function are very common in the elderly, there are a number of other age-related effects that could be altering the relations involved, including reduced cardiac output and reduced filtration demand. Survivor and selection bias may have played a role in attenuating the relations between aortic stiffness and urine ACR. In addition to the known excess risk of cardiovascular mortality associated with even modest elevations in urine ACR,21 it is possible that the eGFR-based exclusion criterion (eGFR<30 ml/min per 1.73 m2) for kidney imaging reduced both the prevalence and severity of albuminuria observed in the study sample (Supplemental Table 1).
The existence of a link between arterial stiffness and negative cardiovascular and kidney outcomes has been known for decades. However, modern hemodynamic and imaging techniques and large-scale studies using these techniques are only now allowing us to understand the potential causal mechanisms involved. This study provides evidence of one such previously hypothesized mechanism: that increased arterial stiffness may contribute to reduced kidney function through increased delivery of flow pulsatility to this low-impedance organ. We have shown that higher CFPWV, the standard measure of aortic stiffness, is negatively related to measured GFR and that this relation is substantially mediated by higher flow pulsatility through microvascular constriction or rarefaction and higher vascular resistance in functional kidney tissues. Although the effect size was modest, each 1 SD higher CFPWV was associated with lower GFR roughly equivalent to 2 years of aging.22 The persistence of an association between GFR and CFPWV in the absence of comparable relations between GFR and MAP or CPP suggests that aortic stiffness (as assessed by CFPWV) may be an important therapeutic target for the maintenance of adequate kidney function even beyond the potential beneficial effects of BP control.
Concise Methods
Participants
The rationale and design of the AGES Study have been presented in detail.23 The AGES Study originates from the Reykjavik Study, a community-based cohort established in 1967 to prospectively study cardiovascular disease in Iceland. Between 2008 and 2011, we conducted a second AGES examination (AGES-II) in which a total of 3411 men and women participated in detailed evaluations of cardiovascular, neurocognitive, musculoskeletal, and metabolic phenotypes; 3317 participants attended the tonometry visit when recruitment occurred for this study, and they comprise the base sample for this study. AGES-II was approved by the National Bioethics Committee in Iceland, which acts as the institutional review board for the Icelandic Heart Association, and the National Institute on Aging Intramural Institutional Review Board. All participants gave written informed consent.
A random subset of the AGES-II cohort was recruited to participate in a comprehensive MR imaging evaluation of cardiac, aortic, and kidney structure and function. A separate but overlapping subset was recruited to participate in the AGES-Kidney Study, wherein GFR was measured using plasma clearance of iohexol. Aortic and bilateral renal artery phase contrast images were attempted in 479 of 633 participants initially recruited into the MR study. As outlined in Supplemental Figure 1, 399 participants in this base sample had complete usable data. Of 399 complete studies, 367 participants belonged to the overlapping subsample on which GFR was also measured. The final sample (n=367) consisted of 218 women and 149 men with an age range of 72–92 years.
Laboratory Variables
Tonometry data were acquired with participants supine after 10 minutes of rest. Auscultatory BP was obtained by using a semiautomated computer-controlled device. Arterial tonometry and simultaneous electrocardiography were obtained from the brachial, radial, femoral, and carotid arteries with a custom transducer. Urine ACR was evaluated using an early morning spot urine sample. Urine albumin was measured using a nephelometric analyzer (ProSpec; Dade Behring GMBH, Marburg, Germany; coefficient of variation=3.2%). Urine creatinine was measured on a multiple analyzer (Modular P Chemistry Analyzer; Roche Diagnostics, Indianapolis, IN; coefficient of variation=4.3% at a concentration of 18 mg/dl and 1.5% at a concentration of 97 mg/dl). GFR was measured as previously described by plasma iohexol clearance using the Bröchner-Mortensen method and equation24,25 and reported without adjustment for body surface area to allow for uniform adjustment of hypothesized associations for body size. Iohexol assays were performed at the University of Minnesota.
Image Acquisition and Analysis
MR images were acquired using a 1.5T scanner (Signa Excite; GE Medical Systems, Waukesha, WI). An eight-channel phased array torso coil was placed over the participant lying in the supine position. Three-plane fast imaging using steady-state acquisition localizers was acquired to identify the root of the aorta and the renal arteries. Phase contrast MR images were acquired during free breathing in the proximal aorta, 1 cm distal to the sinotubular junction, and both renal arteries. The aortic imaging used velocity encoding at 150 cm/s with a minimum of 100 phases per cardiac cycle, and the renal artery imaging used velocity encoding at 100 cm/s with 30 phases per cardiac cycle. Both imaging sequences used a 256×256 acquisition matrix with 6-mm slices, the aortic images had a resolution of 1.32 mm/pixel, and the renal artery images had a resolution of 0.85 mm/pixel.
Cardiac output and renal blood flows were computed from phase contrast MR images. Arterial regions of interest (ROIs) in the proximal aorta and renal artery were semiautomatically defined by thresholding on the magnitude images, with trained analysts editing the ROIs as necessary. The ROIs were then superimposed onto the phase contrast images. Average instantaneous volume flow rate was computed by integrating pixel intensity in the ROI for each phase. Single-kidney vascular resistance was computed as MAP divided by mean renal blood flow in each renal artery, and total renal vascular resistance was computed as the reciprocal sum of two single-kidney values. The PI was computed as the amplitude of the flow waveform (maximum minus minimum) divided by the mean flow in each renal artery and reported as the average of the two single-kidney values.
The dynamic contrast-enhanced kidney imaging sequence was on the basis of that of Lee et al.26 and has been previously described in detail.27 Kidney ROIs were determined by thresholding on precontrast images, with trained analysts editing the ROIs as necessary. After manual exclusion of major cysts (>1 cm in diameter) from the kidney ROIs, total bilateral kidney volume was determined by voxel counting. The renal cortex was segmented from the kidney ROI using custom automated software on the basis of template matching of the signal intensity–time curves for each voxel in the kidney ROI.27 After segmentation, the volume of the cortex was computed using voxel counting. The mean vascular fraction (averaged over the entire cortex) was estimated by comparing the peak gadolinium contrast concentration immediately after contrast injection in the abdominal aorta with the peak concentration in the cortex during the early vascular phase. Gadolinium concentration was estimated using the ratio of the magnetization equations for the pre- and postcontrast images.28 The mean vascular fraction was then multiplied by the total cortical volume to yield an estimate of AVC. AVC was examined as both a hemodynamic variable and a structure variable, because it could be a marker of either vascular tone or microvascular rarefaction, and distinguishing between the two was not possible from the imaging data.
Statistical Analyses
Sample characteristics and hemodynamic and imaging measures were tabulated. Normality of data was assessed by quantile–quantile plots. Non-normal distributions were appropriately transformed as necessary by standard methods. Relations between systemic hemodynamic variables and kidney variables were assessed using linear regression analysis. Models were adjusted for age, sex, body surface area, and heart rate. To test for nonlinear trends in the data, we performed univariate ANOVA (also adjusted for age, sex, heart rate, and body surface area) testing for linear and quadratic contrasts with groupings defined by tertiles of CFPWV. We also performed Bonferroni-corrected post hoc comparisons to test for threshold effects. Exposures were entered as sex-specific residuals in all regression and mediation models. Effect sizes in mediation analyses are reported as difference in the dependent variable per SD difference in the independent variable with 95% confidence intervals. A P<0.05 was considered significant for linear regression, and α=0.05 was used for all mediation analyses.
Individual mediation analyses were performed when appropriate on the basis of the results of linear regression to examine hypothesized relations of systemic and local hemodynamic variables with kidney structure and function. Specifically, mediation analysis was performed when (1) the exposure was significantly correlated to the mediator and outcome (after adjustment for confounders) and (2) the mediator was significantly correlated to the outcome. Indirect effects and confidence intervals were estimated by bootstrapping with 10,000 resamples using the PROCESS Statistical Package for SPSS (SPSS, version 15.0, 2006; SPSS Inc., Chicago, IL).29 Statistically significant mediation is established when the indirect effect is significantly different from zero, with full mediation defined by the additional attenuation of the association between independent and dependent variables into nonsignificance after inclusion of the mediator variable or variables.
Disclosures
T.W., J.D.G., and A.A.T. are employees of Cardiovascular Engineering, Inc. G.F.M. is owner of Cardiovascular Engineering, Inc., a company that develops and manufactures devices to measure vascular stiffness, serves as a consultant to and receives honoraria from Novartis and Merck, and is funded by National Institutes of Health Research Grants HL094898, DK082447, HL107385, and HL104184.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant N01-AG-12100, the National Institute on Aging Intramural Research Program, Hjartavernd (the Icelandic Heart Association), the Althingi (the Icelandic Parliament), National Institutes of Health National Heart, Lung and Blood Institute Grant HL094898, and National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grant DK082447.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014050450/-/DCSupplemental.
References
- 1.Baumann M, Wassertheurer S, Suttmann Y, Burkhardt K, Heemann U: Aortic pulse wave velocity predicts mortality in chronic kidney disease stages 2-4. J Hypertens 32: 899–903, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness on survival in end-stage renal disease. Circulation 99: 2434–2439, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM: Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int 63: 1852–1860, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 103: 987–992, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Shoji T, Emoto M, Shinohara K, Kakiya R, Tsujimoto Y, Kishimoto H, Ishimura E, Tabata T, Nishizawa Y: Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol 12: 2117–2124, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Mitchell GF: Effects of central arterial aging on the structure and function of the peripheral vasculature: Implications for end-organ damage. J Appl Physiol (1985) 105: 1652–1660, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Rourke MF, Safar ME: Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 46: 200–204, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Loutzenhiser R, Bidani A, Chilton L: Renal myogenic response: Kinetic attributes and physiological role. Circ Res 90: 1316–1324, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ: Arterial stiffness, pressure and flow pulsatility and brain structure and function: The Age, Gene/Environment Susceptibility—Reykjavik study. Brain 134: 3398–3407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, Froissart M, Houillier P, Boutouyrie P: Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int 69: 350–357, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Guérin AP, Pannier B, Métivier F, Marchais SJ, London GM: Assessment and significance of arterial stiffness in patients with chronic kidney disease. Curr Opin Nephrol Hypertens 17: 635–641, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Kawamoto R, Kohara K, Tabara Y, Miki T, Ohtsuka N, Kusunoki T, Yorimitsu N: An association between decreased estimated glomerular filtration rate and arterial stiffness. Intern Med 47: 593–598, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Tomiyama H, Tanaka H, Hashimoto H, Matsumoto C, Odaira M, Yamada J, Yoshida M, Shiina K, Nagata M, Yamashina A: Arterial stiffness and declines in individuals with normal renal function/early chronic kidney disease. Atherosclerosis 212: 345–350, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Wang MC, Tsai WC, Chen JY, Huang JJ: Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis 45: 494–501, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T, Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries : Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 30: 445–448, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Cecelja M, Chowienczyk P: Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: A systematic review. Hypertension 54: 1328–1336, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Petersen LJ, Petersen JR, Talleruphuus U, Ladefoged SD, Mehlsen J, Jensen HA: The pulsatility index and the resistive index in renal arteries. Associations with long-term progression in chronic renal failure. Nephrol Dial Transplant 12: 1376–1380, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Mathisen UD, Melsom T, Ingebretsen OC, Jenssen T, Njølstad I, Solbu MD, Toft I, Eriksen BO: Estimated GFR associates with cardiovascular risk factors independently of measured GFR. J Am Soc Nephrol 22: 927–937, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim EJ, Park CG, Park JS, Suh SY, Choi CU, Kim JW, Kim SH, Lim HE, Rha SW, Seo HS, Oh DJ: Relationship between blood pressure parameters and pulse wave velocity in normotensive and hypertensive subjects: Invasive study. J Hum Hypertens 21: 141–148, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Bahous SA, Stephan A, Blacher J, Safar M: Cardiovascular and renal outcome in recipients of kidney grafts from living donors: Role of aortic stiffness. Nephrol Dial Transplant 27: 2095–2100, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE, Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group : Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106: 1777–1782, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Grewal GS, Blake GM: Reference data for 51Cr-EDTA measurements of the glomerular filtration rate derived from live kidney donors. Nucl Med Commun 26: 61–65, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V: Age, Gene/Environment Susceptibility-Reykjavik Study: Multidisciplinary applied phenomics. Am J Epidemiol 165: 1076–1087, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bröchner-Mortensen J: A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest 30: 271–274, 1972 [DOI] [PubMed] [Google Scholar]
- 25.Inker LA, Fan L, Okparavero A, Gudnason V, Eiriksdottir G, Andresdottir M, Gudmundsdottir H, Indridason O, Palsson R, Launer L, Harris TB, Mitchell GF, Levey AS: Comparing cystatin c and creatinine for estimating measured GFR and CKD prevalence in a community-based sample of the elderly [Abstract]. J Am Soc Nephrol 24: 43A–44A, 2013. 23184055 [Google Scholar]
- 26.Lee VS, Rusinek H, Bokacheva L, Huang AJ, Oesingmann N, Chen Q, Kaur M, Prince K, Song T, Kramer EL, Leonard EF: Renal function measurements from MR renography and a simplified multicompartmental model. Am J Physiol Renal Physiol 292: F1548–F1559, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Woodard T, Sigurdsson S, Gotal JD, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Gudnason V, Harris TB, Launer LJ, Levey AS, Mitchell GF: Segmental kidney volumes measured by dynamic contrast-enhanced magnetic resonance imaging and their association with ckd in older people [published online ahead of print July 10, 2014]. Am J Kidney Dis doi:10.1053/j.ajkd.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang JL, Rusinek H, Bokacheva L, Chen Q, Storey P, Lee VS: Use of cardiac output to improve measurement of input function in quantitative dynamic contrast-enhanced MRI. J Magn Reson Imaging 30: 656–665, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes A: Introduction to Mediation, Moderation, and Conditional Process Analysis, New York, Guilford Press, 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.