Abstract
Intracellular ATP is the most vital source of cellular energy for biologic systems, whereas extracellular ATP is a multifaceted mediator of several cell functions via its interaction, in an autocrine or paracrine manner, with P2 purinergic receptors expressed on the cell surface. These ionotropic and metabotropic P2 purinergic receptors modulate a variety of physiologic events upon the maintenance of a highly sensitive “set point,” the derangement of which may lead to the development of key pathogenic mechanisms during acute and chronic diseases. Growing evidence suggests that extracellular ATP signaling via P2 purinergic receptors may be involved in different renal pathologic conditions. For these reasons, investigators and pharmaceutical companies are actively exploring novel strategies to antagonize or block these receptors with the goal of reducing extracellular ATP production or accelerating extracellular ATP clearance. Targeting extracellular ATP signaling, particularly through the P2X7 receptor, has considerable translational potential, given that novel P2X7-receptor inhibitors are already available for clinical use (e.g., CE224,535, AZD9056, and GSK1482160). This review summarizes the current evidence regarding the involvement of extracellular ATP and its P2 purinergic receptor–mediated signaling in physiologic and pathologic processes in the kidney; potential therapeutic options targeting extracellular ATP purinergic receptors are analyzed as well.
Keywords: extracellular ATP, purinergic receptors, diabetic nephropathy, type 1 diabetes, type 2 diabetes, kidney transplantation
ATP is the most important source of energy for intracellular reactions,1 including synthesis and degradation of biologic molecules, muscle contraction, and membrane transport.2 Intracellular ATP (iATP) levels, which are regulated by mitochondrial oxidative phosphorylation,3 reflect cell activity and viability, and a decline in iATP levels is associated with cell death.4 ATP is then exported from the mitochondrial matrix to the cytoplasm, across the inner mitochondrial membrane by the ADP/ATP carrier protein.3 The release of cytoplasmic ATP occurs as a physiologically regulated mechanism; indeed, ATP, given that it is a highly charged molecule, does not cross the plasma membrane.5,6 Only a small fraction of ATP is released from the cells into the extracellular space through a series of finely tuned processes. For instance, in nonexcitable cells, ATP release occurs through exocytosis, ion channels, gap junction hemichannels, nucleotide transporters, and the cystic fibrosis transmembrane conductance regulator.7 The extracellular release of ATP can be triggered by a wide range of stimuli such as mechanical stress, cell membrane damage, inflammation, hypoxia, and excitation of neural tissue, and by cell growth and death.7 Although the concentration of ATP extracellularly is 1000-fold lower compared with the intracellular space, it can still exert precise, albeit partially unknown, signaling function on the cell membrane, by binding and activating membrane-anchored ionotropic P2X (P2XRs) and metabotropic P2Y (P2YRs), purinergic receptors that are likely ubiquitous. The eight G protein–coupled P2YRs (P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11–P2Y14) are activated by a range of native agonists (e.g., ATP, ADP, UTP, and UDP). Aside from the crucial role exerted by P2Y12 in platelet aggregation, the targeting of which has yielded successful therapeutic strategies,8 additional functions of P2Y12 (e.g., influencing microglial motility and stimulating ciliary movement and secretion of epithelial cells) have been discovered.9 In addition to the activation of purinergic signaling, the effect of extracellular ATP (eATP) relies upon the activity of ectonucleotidases (e.g., CD39 and CD73), which degrade eATP to ADP, AMP, and adenosine, all of which are capable themselves of exerting P1R- and P2R-mediated functions.10 The P2XRs (ligand-gated ion channels), of which seven have been identified thus far (P2X1–P2X7), bind ATP as their principal ligand and are involved in a variety of biologic responses, mainly related to inflammation, tissue damage and cell proliferation, and the graft-versus-host response.10 Among these P2XRs, P2X7R appears particularly intriguing due to its emerging role in NLRP3/ASC/caspase1 inflammasome activation11,12 as well as its involvement in acute allograft rejection.13 eATP-P2Rs signaling modulates several aspects of normal kidney function, but it is also implicated in the development of renal damage during chronic diseases such as diabetes and hypertension, as well as in inherited conditions including polycystic kidney disease (PKD), although the precise mechanisms underlying the involvement of these receptors is not fully understood.14 The present review addresses the pathophysiologic roles exerted in the kidney by eATP and by P2 purinergic receptors.
The eATP/P2Rs Axis in the Kidney
eATP Production in the Kidney
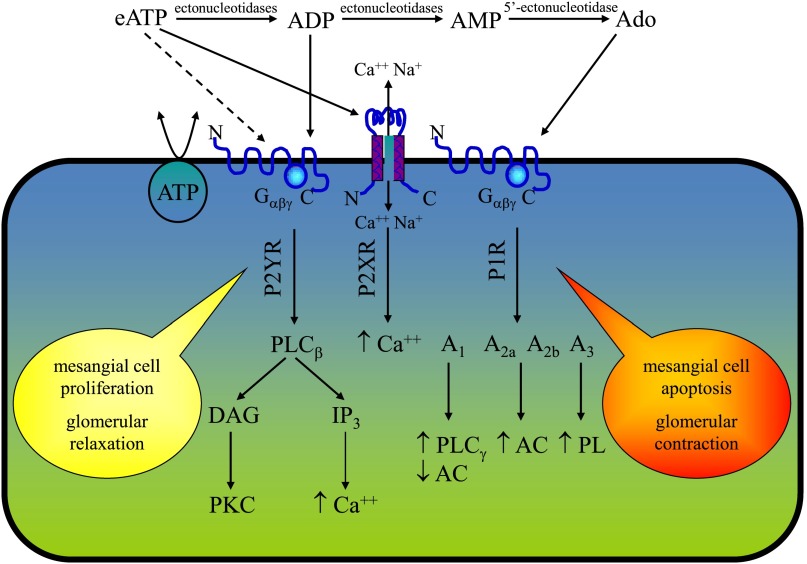
Sources of eATP in the kidney include perivascular and peritubular nerve terminals, aggregating platelets, circulating erythrocytes, and resident endothelial and epithelial cells.15 In renal epithelial cells, derived from human nephron segments, ATP release occurs in both the apical and basolateral membrane, with apical release predominating.16 eATP has been also found in the interstitial fluid of canine renal cortex, possibly released by macula densa cells in response to BP-induced changes in renal vascular resistance.17 The effective amount of eATP found in the extracellular space could be influenced by several factors, including the activity of the hydrolyzing enzymes ectoapyrases18 (Figure 1).
Figure 1.
The purinergic system in the glomerular cells. eATP has a short life depending on the activity of ectonucleotidases that degrade eATP to generate ADP, AMP, and adenosine. The purinergic receptors P2YR, P2XR, and P1R bind extracellular nucleotides and nucleosides, and their activation causes a cascade of signaling. Such an effect may promote cellular proliferation or apoptosis of mesangial cells. Ado, adenosine; PLC, phospholipase C; DAG, diacylglycerol; PKC, protein kinase C; IP3, inositol 1,4,5-triphosphate; AC, adenylate cyclase; PL, phospholipase.
Expression of Purinergic Receptors in the Kidney
eATP exerts effects on the kidney in both a paracrine and autocrine manner, via the activation of all P2Xs and some P2Ys receptors.15 These receptors are expressed in the cortical and in the medullary renal compartments14,15; however, upon stringent analysis, some of these results appear controversial and mainly based on mRNA expression, which does not necessarily imply an increased functional activity of these receptors. The lack of availability of adequate antibodies as well as the complexity of the P2R structure may have caused uncertainty in their immunohistochemical evaluation. It can be also hypothesized that some of these receptors, particularly P2X7R, are scarcely expressed in physiologic conditions but can be upregulated during inflammation. The localization of ionotropic and metabotropic receptors in the kidney is reported in Table 1.
Table 1.
P2XR and P2YR localization in the kidney
| Receptor | Species | RNA/Protein | Renal Cell | Reference |
|---|---|---|---|---|
| P2X1 | Mouse | Yes/no | Medullary thick ascending limb, inner strip of outer medulla | 72 |
| Rat | No/yes | Vascular networks | 73 | |
| Pig | Yes/no | Epithelial cell line (LLC-PK1) | 74 | |
| Human | Yes/no | Mesangial cell culture | 36 | |
| P2X2 | Mouse | Yes/no | Inner strip of outer medulla | 72 |
| Human | Yes/no | Mesangial cell culture | 36 | |
| P2X3 | Mouse | Yes/no | Inner strip of outer medulla, inner medullary collecting duct cell line | 72,75 |
| P2X4 | Mouse | Yes/no | Inner medullary collecting duct cell line, distal convoluted tubule immortalized cells, inner stripe of the outer medulla, isolated tubules in medullary thick ascending limb | 72,75,76 |
| Human | Yes/no | Mesangial cell culture | 36 | |
| P2X5 | Mouse | Yes/no | Distal convoluted tubule immortalized cells, inner stripe of the outer medulla, isolated tubules in medullary thick ascending limb | 72,76 |
| Human | Yes/no | Mesangial cell culture | 36 | |
| P2X6 | Human | Yes/no | Mesangial cell culture | 36 |
| P2X7 | Mouse | Yes/no | Inner stripe of the outer medulla | 72 |
| Rat | Yes/yes | Mesangial cells and glomeruli | 77 | |
| Madin-Darby canine | Yes/yes | Epithelial cells | 78 | |
| Human | Yes/no | Mesangial cell culture | 36 | |
| P2Y1 | Mouse | Yes/no | Inner medullary collecting duct cells | 75 |
| Rat | Yes/yes | Podocytes, proximal convoluted tubule, descending and thin ascending limb of Henle’s loop, outer medullary collecting duct | 31,79 | |
| Madin- | Yes/no | Epithelial cells | 80 | |
| Darby canine | ||||
| Human | Yes/no | Mesangial cell culture, epithelial cell line (A498) | 36,81 | |
| P2Y2 | Mouse | Yes/no | Medullary thick ascending limb, inner stripe of the outer medulla, distal convoluted tubule cells, cortical collecting duct cells | 72,76,82 |
| Rat | Yes/yes | Proximal convoluted tubule, descending and thin ascending limb of Henle’s loop, outer medullary collecting duct, mesangial cells and glomeruli, inner medulla collecting duct | 68,77,79,83 | |
| Human | Yes/no | Mesangial cell culture, epithelial cell line (A498) | 36,81 | |
| P2Y4 | Rat | Yes/no | Mesangial cells and glomeruli, proximal convoluted tubule, thin ascending limb of Henle’s loop, outer medullary collecting duct | 77,79 |
| Human | Yes/no | Mesangial cell culture | 36 | |
| P2Y6 | Mouse | Yes/no | Medullary thick ascending limb, inner strip of the outer medulla | 72 |
| Rat | Yes/no | Mesangial cells and glomeruli, proximal convoluted and straight tubule, thin descending limb of Henle’s loop, medullary and cortical thick ascending limb, outer medullary and cortical collecting duct | 68,84 | |
| Human | Yes/no | Mesangial cell culture | 36 | |
| P2Y11 | Madin- | Yes/no | Epithelial cells | 80 |
| Darby canine | ||||
| Human | Yes/no | Mesangial cell culture, epithelial cell line (A498) | 36,81 | |
| P2Y12 | Mouse | Yes/no | Inner strip of the outer medulla | 72 |
| Human | Yes/no | Mesangial cell culture | 36 | |
| P2Y13, P2Y14 | Mouse | Yes/no | Inner strip of the outer medulla | 72 |
Effects of eATP Signaling via Purinergic Receptors
Effects on Renal Hemodynamics
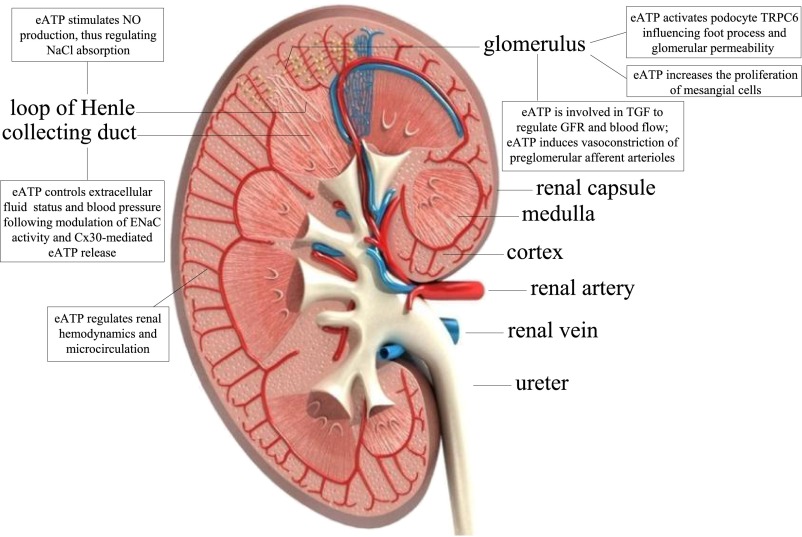
eATP exerts a fundamental role in the regulation of renal hemodynamics and microcirculation19 (Figure 2). For instance, the intrarenal infusion of ATP in canine kidneys in vivo produces vasodilation through P2XR and P2YR activation and stimulation of endothelial release of nitric oxide.20 However, some studies suggest that eATP-mediated vasoconstriction may occur in juxtamedullary nephron preparations in vitro and that eATP may induce preglomerular vasoconstriction of afferent arterioles.21 Another eATP-mediated effect on renal hemodynamics may stem from tubuloglomerular feedback, which is operated by the macula densa. Indeed, macula densa releases eATP in response to increased luminal NaCl concentration, thus regulating the GFR and renal blood flow22 (Figure 2).
Figure 2.
eATP signaling effects along the nephron. NO, nitric oxide; ENaC, epithelial sodium channel; TGF, tubuloglomerular feedback; TRPC6, transient receptor potential cation channel, subfamily C, member 6.
Effects on Tubular Transport Function
Mechanical stimulation of renal tubules, either by cell swelling or by an increase in tubular flow, promotes ATP release by renal epithelial cells, which in turn activates P2 receptors in the apical and basolateral membrane, thus modulating renal tubular transport.23,24 In the absence of fluid flow, primary cilium, expressed by renal epithelial cells, protrudes perpendicularly into the tubular lumen, while fluid flow triggers cilium bending that elicits tubular release of eATP and inhibits the renal tubular transport of solute and water.24 In the thick ascending limb, ATP release may activate basolateral (via P2XRs) and luminal (via P2YRs) membrane and stimulate nitric oxide production, thus regulating NaCl absorption25 and potentially modulating the activity of the epithelial sodium channel in the collecting duct26 (Figure 2). eATP may therefore control extracellular fluid volume status and BP,26,27 and maintenance of these regulatory functions appears to be performed by the connexin 30 hemichannel (Cx30) in the collecting duct.28 Cx30 knockout (KO) mice, with a salt retention phenotype in response to acute elevations in BP, have a reduced ability to excrete urinary salt and water.28 Interestingly, Cx30 KO mice showed reduced luminal ATP release in vitro28 and in vivo, suggesting that Cx30-dependent purinergic intracellular calcium signaling may control the regulation of salt and water reabsorption.29 Consistent with the aforementioned observations, upon injection of ATP agonists (α,β-methylene ATP and β,γ-methylene ATP) into the femoral vein of rat, there is an increase in diuresis and sodium excretion,30 while pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), a nonselective P2 antagonist,30 abolished this effect. In conclusion, taken together, these findings confirm that eATP has an important role in the regulation of renal hemodynamics, salt, and water reabsorption, thus contributing to the maintenance of normal BP, body fluid, and electrolyte balance (Figure 2).
Effects on Permeation
Recent relevant contributions have identified and characterized P2YRs in podocytes.31,32 eATP activates podocyte TRPC6 Ca2+-permeable channels, thus influencing foot process effacement and subsequent changing in glomerular basement membrane permeability.33 Mechanical-induced podocyte injuries appear to be mediated, at least in part, by P2Rs, including P2Y2.32
Effects on Cell Proliferation and Extracellular Matrix Deposition
Extracellular nucleotides may regulate renal fibroblast proliferation and activity, thus influencing fibroblast-to-myofibroblast transformation, confirming the existence of an interesting P2R-mediated cross-talk between epithelial cells and fibroblasts.34,35 eATP also increases the proliferation of human mesangial cells, via P2YR-mediated activation of the Ras-Raf-MAPK42/44 signal transduction pathway.36
Pathologic Role of eATP in Kidney Diseases
Diabetic Nephropathy
Preclinical studies on the role of eATP signaling in diabetic nephropathy are limited by the lack of suitable murine models of the disease.37,38 In the CD39 (the main vascular ectonucleotidase)–null diabetic mouse model, glomerulosclerosis is more severe than in age-matched diabetic wild-type animals, thus suggesting a protective role against inflammation of this eATP-hydrolyzing enzyme.39 More recently, the genetic deletions of the adenosine A2B receptor and of CD73 (a key enzyme that produces extracellular adenosine) in mice have been found to be associated with more severe diabetic nephropathy.40 Increased eATP signaling via P2X4R due to hyperglycemia induces activation of the NLRP3 inflammasome, thus stimulating IL-1β and IL-18 release, with development of tubulointerstitial inflammation41,42 (Table 2). Two studies revealed that in individuals with type 2 diabetes and diabetic nephropathy, P2X4R, P2X7R, and NLPR3 are upregulated compared with controls, and that tubular P2X4R expression colocalized in confocal analysis with NLRP3, IL-1β, and IL-18 expression.12,41 This is in agreement with the results from in vitro studies in HK-2 cells, in which high-glucose challenge increased protein expression of NLRP3 and augmented the release of IL-1β and IL-1841 (Table 2). Thus, the activation of eATP signaling may play a crucial role in the development of diabetic nephropathy by enhancing inflammation.
Table 2.
Kidney diseases in which a putative role for eATP signaling is claimed
| Disease | Model | eATP Signaling | Reference |
|---|---|---|---|
| Diabetic nephropathy | In vitro | Hyperglycemia induces an increase in eATP signaling via P2X4R with NLRP3 activation that stimulates IL-1β and IL-18 release and development of tubulointerstitial inflammation | 41,42 |
| In vitro | P2X4R, P2X7R, and NLRP3 are upregulated in individuals with T2D and diabetic nephropathy, tubular P2X4R expression colocalizes with NLRP3, IL-1β, and IL-18 | 12,41 | |
| In vitro | In HK-2 cells, high-glucose challenge increases expression of NLRP3, cleaves caspase-1, and IL-1β and the release of IL-1β and IL-18 | 41 | |
| Hypertension | In vivo | In a murine model of hypertension, P2X7R KO mice show reduced BP, renal injury, urinary albumin excretion, and renal interstitial fibrosis | 43 |
| In vivo | There is an association between BP and the rs591874 polymorphism of the P2X7R gene | 46 | |
| GN | In vivo | In rat and murine models of GN and in renal biopsies of individuals with lupus nephritis, an increase in glomerular P2X7R expression is observed | 48 |
| In vivo | In a model of GN using P2X7R KO mice, P2X7R deficiency is renoprotective | 49 | |
| PKDs | In vivo | In Han:SPRD rats, renal P2Y2R, P2Y6R, and P2X7R expression is increased | 53 |
| In vitro | Epithelial cells generated from individuals with PKD release a higher amount of eATP compared with healthy controls as well as in a murine model of PKD | 16,54 | |
| Kidney allograft rejection | In vivo | No direct evidence of eATP signaling in kidney transplantation | |
| In murine models of heart, islet, and lung transplantation P2X1R expression increases in syngenic and allogeneic graft, P2X7R upregulation is during allograft rejection | 56 | ||
| Other kidney diseases | In vitro | P2X7R is implicated in interstitial inflammation and fibrosis, tubular atrophy, and renal cell apoptosis | 59 |
T2D, type 2 diabetes; NLRP3, NOD-like receptor-family protein 3.
Hypertension
Some studies have suggested that P2X7Rs may be relevant in the onset of hypertension by inducing a proinflammatory setting, in addition to influencing NaCl and water reabsorption.18,43,44 P2X7R KO mice were used to investigate the role of P2X7R in a deoxycorticosterone acetate high-salt diet–induced hypertension murine model.43 Although P2X7R mRNA and protein expression increased after treatment in the kidney of wild-type mice, P2X7R KO mice showed reduced BP, renal injury, urinary albumin excretion, and renal interstitial fibrosis compared with controls43 (Table 2). Furthermore, a recent article suggested that CD73 and adenosine A2B receptor mRNA/protein and mRNA expression, respectively, were augmented in kidneys of angiotensin II–infused mice.45 Despite these interesting observations, very few human studies, mostly genetic, have thus far explored the link between purinergic signaling and BP control. An association between BP and the single nucleotide polymorphism rs591874 in the first intron of the P2X7R gene was found46 (Table 2). However, another study found no relationship between two single nucleotide polymorphisms of the P2X7R gene, Glu496Ala and His155Tyr, and endothelial function or arterial stiffness in essential hypertensive patients.47 Taken together, these studies suggest that altered eATP signaling may contribute to hypertension by controlling vascular tone and by favoring Na+ retention (Table 2).
GN
An increase in glomerular P2X7R mRNA and protein expression was observed in rat and murine models of GN compared with controls, as well as in renal biopsies obtained from individuals with lupus nephritis.48 P2X7R deficiency was shown to be renoprotective in an experimental model of antibody-mediated GN, with P2X7R KO mice displaying a reduction in glomerular thrombosis by 60%, in proteinuria by 52% and in serum creatinine by 38% compared with wild-type49 (Table 2). Renal expression of the P2X7/NLRP3 inflammasome pathway and IL-1β are significantly increased in a mouse model of lupus nephritis, and P2X7 inhibition reduces immune complex deposition in the kidneys as well as the levels of circulating anti–double-stranded DNA antibodies.50 In a rat model of GN, the activation of adenosine A2A receptors prevents renal infiltration of macrophages and arrests the progression of kidney disease, by inhibiting incoming fibrosis.51 Therefore, eATP signaling is likely involved in the pathogenesis of GN; however, the underlying mechanisms and its functional importance need to be further elucidated.
PKD
Accumulating evidence suggests that eATP signaling via P2XRs and P2YRs could be detrimental with regard to progression of PKD.52 In Han:SPRD rats which developed polycystic kidneys, increased P2Y2R, P2Y6R, and P2X7R renal mRNA expression was evident compared with control animals,53 suggesting a possible role of these receptors in the disease (Table 2). Renal epithelial cells from individuals with PKD release higher amounts of ATP (from 0.5 μM to 2 μM) compared with those obtained from healthy controls, which then may be trapped in the lumen of the cyst.16 Interestingly, cyst fluid from PKD kidneys contained large amounts of ATP (up to 10 μM),16 and eATP may augment PKD cyst widening through stimulation of salt and water secretion across PKD cystic epithelia.16 It is possible that a defect in the degradation of eATP in epithelial cells, due to lack and/or mislocalization of ecto-ATPases, ectoapyrases, and ectonucleotidases, occurs in individuals with PKD; the eATP signal may therefore last longer in the PKD microenvironment.54 Indeed, although normal renal epithelial cells degraded 50% of the total ATP in the medium in 20 minutes, 50% of the total ATP was degraded by 3 hours in PKD epithelial cells.54 As far as the involvement of the adenosine receptor in polycystic kidney disease, PKD1-mutated cystic cells express increased levels of adenosine A3A receptors.55 Thus, cyst growth and expansion in polycystic kidney disease may be worsened by eATP and adenosine-mediated signaling, but it remains unclear whether this may lead to a clinically relevant therapeutic strategy.
Kidney Allograft Rejection
Although the literature is lacking in direct studies on the role of eATP in kidney transplantation, preclinical and clinical studies are available for other models of allotransplantation.10 An increased release of ATP is likely to occur in allograft transplantation, possibly due to ischemia/reperfusion or upon the activation of immune cells, thus acting as a danger signal that may modulate the alloimmune response.10 In models of murine heart, islet, and lung transplantation, P2X1R expression was increased in both syngeneic and allogeneic grafts, whereas intragraft P2X7R upregulation was observed only during allograft rejection56 (Table 2). This may suggest that P2X7R upregulation is specifically associated with the alloimmune response, whereas P2X1R expression is more related to a peritransplant inflammatory response and possibly ischemia/reperfusion events.13,57 Interestingly, adenosine A2A receptor signaling may attenuate ischemia-reperfusion injury and allogeneic T-cell recognition, thus delaying allograft rejection.58 The aforementioned data suggested that eATP is involved in the regulation of cellular and immunologic process that occurs during allograft organ rejection.
Other Kidney Diseases
P2X7R is implicated in the onset of interstitial inflammation, tubular fibrosis, and atrophy that rapidly occur after unilateral ureteral obstruction,59 whereas P2X4R has recently unveiled protective properties in the above-described setting.60 Extracellular adenosine has recently been regarded as an intriguing novel modulator of AKI through its renal signaling. The A1A, A2A, A3A adenosine receptors have different beneficial effects against ischemia-reperfusion injury and AKI, by modulating metabolic demand and leukocyte-mediated renal inflammation, as well as by decreasing necrosis, apoptosis, and tissue damage.61 The activation of adenosine receptors is needed for efficient kidney protection, and the clinical availability of adenosine may suggest a fast-track for this compound to be translated into the clinical setting when supplementation is needed.
Targeting eATP and Its Receptors in the Kidney
Diabetic Nephropathy
The enzyme apyrase, the P2 receptor antagonist suramin, the P2X4R selective antagonist 5-BDBD, and silencing of the P2X4R gene are all able to nearly normalize NLRP3 expression and reduce the release of IL-1β and IL-18, which is increased by high glucose in HK-2 cells in vitro41 (Table 3). In another study, the increased expression of NLRP3 and IL-18 release were attenuated by P2X7R silencing in murine podocytes.12 Furthermore, the P2X7R inhibitor periodate-oxidized ATP reduced TGF-β and mesangial extracellular matrix production by rat mesangial cell culture under high-glucose conditions42 (Table 3). Targeting of eATP signaling may reduce the inflammatory component of diabetic nephropathy and thus may be considered a future novel therapeutic tool for diabetic nephropathy.41
Table 3.
Preclinical targeting of eATP signaling in different kidney diseases
| Target | Antagonist | Preclinical Effect | Reference |
|---|---|---|---|
| P2Xs/P2Ys | PPADS/Suramin | Reduces cyst size in Madin-Darby canine kidney-derived cysts | 70 |
| P2Xs/P2Ys | PPADS | Reduces glomerular mesangial cell proliferation in the anti-Thy1 rat model of GN | 68 |
| P2Xs/P2Ys | PPADS | Prevents afferent arteriolar thickening development in angiotensin II infusion in rat | 62 |
| Reduces necrosis-associated inflammation | 35 | ||
| P2Xs/P2Ys | Suramin | Normalizes NLRP3 expression, reducing the release of IL-1β and IL-18 increased by high glucose in HK-2 cells | 41 |
| Promotes renal repair after injury when administered with adult renal progenitor cells | 71 | ||
| P2Xs | NF279 | Prevents vasoconstriction of afferent arteriolar diameter, driving an increase in renal perfusion pressure in rodents | 63 |
| P2Xs | TNP-ATP | Normalizes NLRP3 expression, reducing the release of IL-1β and IL-18 increased by high glucose in HK-2 cells | 41 |
| P2X1 | PPADS/IP5I | Inhibits autoregulatory control of whole-kidney blood flow | 64 |
| P2X4 | 5-BDBD gene silencing | Normalizes NLRP3 expression, reducing the release of IL-1β and IL-18 increased by high glucose in HK-2 cells | 41 |
| P2X7 | A-438079 | Decreases glomerular thrombosis, glomerular macrophage infiltration and proteinuria in a rat model of GN | 49 |
| P2X7 | Brilliant Blue G | Causes a reduction in arterial BP and a decrease in renal vascular resistance in the Fischer rat | 65,66 |
| P2X7 | A-438079 | Diminishes renal fibroblast death | 35 |
| P2X7 | A-438079/oATP | Decreases the frequency of cystic phenotype | 69 |
| P2X7 | oATP | Reduces the mesangial extracellular matrix and TGF-β upregulation in rat mesangial cell culture under high-glucose conditions | 42 |
| P2X7 | oATP | Prolongs heart and islet graft survival in mouse | 13,57 |
| P2X7 | P2X7R silencing | In murine podocytes, attenuates upregulated expression of NLRP3, pro-caspase 1, and release of IL-18 | 12 |
| Diminishes renal fibroblast death | 35 | ||
| eATP | apyrase | Normalizes NLRP3 expression, reducing the release of IL-1β and IL-18 increased by high glucose in HK-2 cells | 41 |
| Reduces necrosis-associated inflammation | 35 | ||
| eATP | CD39-CD73 genetic deletion | Enhances the hypoxic injury in kidney transplantation murine models and reduces cardiac allograft survival | 10 |
NF279, 8,8′-(carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino))bis(1,3,5-naphthalenetrisulfonic acid); TNP-ATP, 2′,3′-O-(2,4,6-trinitrophenyl)adenosine 5′-triphosphate; IP5I, P1,P5-di(-inosine-5-pentaphosphate) pentasodium salt; 5-BDBD, 5-(3-bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one); A-438079, 3-[[5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine; oATP, periodate-oxidized ATP; NLRP3, NOD-like receptor-family protein 3.
Hypertension
The involvement of the P2 receptorial system in the modulation of vascular tone and arterial pressure is complex and still under investigation. Administration of PPADS, a nonselective P2 antagonist, significantly prevented the development of afferent arteriolar thickening during angiotensin II infusion in rats, as well as reduced the increase in α-actin expression in mesangial cells62 (Table 3). In vitro exposure to a P2XR antagonist (NF279) prevented vasoconstriction of afferent arteriolar diameter, increasing renal perfusion pressure in rodents.63 In vivo inhibition of P2X1R with PPADS or IP5I inhibits autoregulatory control of whole-kidney blood flow,64 whereas the infusion of Brilliant Blue G, a P2X7R antagonist, to Fischer rats and Dahl salt-sensitive rats reduced arterial BP and decreased renal vascular resistance.65,66 On the other hand, P2Y2R modulates, via paracrine signaling, renal sodium excretion in the distal nephron in mice, thus playing a pivotal role in maintaining arterial BP within a normal range.67 In conclusion, although it is difficult to anticipate the net effect of modulating eATP signaling on arterial BP, a reduction in vasoconstriction tone and in renal salt and water homeostasis suggests a potential benefit in hypertension.
GN
PPADS, a nonselective P2 antagonist, injected intravenously and intraperitoneally in vivo in the anti-Thy1 model of rat mesangial proliferative GN reduced glomerular mesangial cell proliferation, without affecting the proliferative activity of non-mesangial cells (i.e., mainly endothelial cells and monocytes/macrophages).68 In a rat model of antibody-mediated GN, administration of A-438079 decreased glomerular thrombosis and reduced glomerular macrophage infiltration and proteinuria compared with the vehicle-treated group49 (Table 3). The robust proinflammatory role of eATP signaling suggests a potential effective therapeutic option for its targeting in GN.
PKD
In a zebrafish model of PKD, oxidized ATP and A-438079, P2X7R antagonists, decreased the frequency of the cystic phenotype.69 In the growth of Madin-Darby canine kidney-derived cysts, the use of P2XR/P2YR inhibitors (suramin and PPADS) significantly reduced cyst size.70 When ATP was removed from the culture medium using higher concentrations of apyrase, cyst growth was also reduced significantly70 (Table 3). eATP signaling may thus promote cyst growth and its targeting may slow the progression of the disease.
Kidney Allograft Rejection
In heart, islet, and lung transplantation models, targeting of eATP/P2X7R signaling has been associated with long-term graft function, thus potentially representing an intriguing therapeutic target in kidney transplantation.10,56 Heart grafts of periodate-oxidized ATP–treated mice showed mild graft lymphocyte infiltration and reduced coronaropathy compared with untreated animals.13 Analogous results were obtained in a murine model of islet transplantation, wherein treatment with eATP targeting showed a significant synergism with rapamycin in reducing the alloimmune response57 (Table 3). CD39 and CD73 ectoenzymes may represent future therapeutic targets as well.10 Genetic ablation of CD39 and CD73 enhanced hypoxic injury in kidney and liver transplantation murine models and reduced cardiac allograft survival10 (Table 3). Clinical studies in kidney transplant recipients are underway and will clarify whether eATP can serve as a therapeutic target.
Other Kidney Diseases
Depletion of eATP with apyrase or inhibition of the P2XR with PPADS has been shown to reduce the extent of necrosis-associated inflammation.35 Furthermore, treatment with A438079, a highly selective P2X7R inhibitor, or knockdown of the P2X7R with small interference RNA diminished renal fibroblast death induced by supernatant collected from necrotic cells.35 Mice treated with suramin and murine adult renal progenital cells underwent extensive renal repair after injury.71 Both studies suggested that blocking eATP may be a promising therapeutic treatment for AKI.
In conclusion, knowledge and understanding of eATP function in the renal system has grown in recent years. eATP signaling may modulate renal hemodynamics, microcirculation, BP, and tubular transport of solute and fluid. Furthermore, eATP signaling may play an important role in increasing kidney damage and inflammation in diabetic nephropathy, as well as in other systemic diseases characterized by a relevant degree of renal inflammatory response. It is also involved in the pathogenesis of hypertension, GN, and PKD, as well as in the regulation of cellular and immunologic processes that occur during allograft organ rejection.10,52 P2XR inhibitors (e.g., CE224,535, AZD9056, and GSK1482160) are available for clinical use and are under evaluation as immunomodulatory agents.10 Thus, eATP signaling may be targeted in a wide range of pathologic renal conditions and may lead to the discovery of clinically relevant therapeutic strategies.
Disclosures
None.
Acknowledgments
P.F. was the recipient of a Juvenile Diabetes Research Foundation Career Development Award, an American Society of Nephrology Career Development Award, and an American Diabetes Association Mentor-Based Fellowship grant. P.F. was also supported by a Translational Research Program grant from Boston Children's Hospital, a Harvard Stem Cell Institute grant (“Diabetes Program” DP-0123-12-00), and Italian Ministry of Health grants (RF-2010-2303119, RF-2010-2314794, and “Staminali” RF-FSR-2008-1213704).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Burnstock G: Purinergic signalling: Its unpopular beginning, its acceptance and its exciting future. BioEssays 34: 218–225, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Imamura H, Nhat KP, Togawa H, Saito K, Iino R, Kato-Yamada Y, Nagai T, Noji H: Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci U S A 106: 15651–15656, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clémençon B, Babot M, Trézéguet V: The mitochondrial ADP/ATP carrier (SLC25 family): Pathological implications of its dysfunction. Mol Aspects Med 34: 485–493, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Feldenberg LR, Thevananther S, del Rio M, de Leon M, Devarajan P: Partial ATP depletion induces Fas- and caspase-mediated apoptosis in MDCK cells. Am J Physiol 276: F837–F846, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Gordon JL: Extracellular ATP: Effects, sources and fate. Biochem J 233: 309–319, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falzoni S, Donvito G, Di Virgilio F: Detecting adenosine triphosphate in the pericellular space. Interface Focus 3: 20120101, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fields RD: Nonsynaptic and nonvesicular ATP release from neurons and relevance to neuron-glia signaling. Semin Cell Dev Biol 22: 214–219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang XF, Fan JY, Meng J, Jin C, Yuan JQ, Yang YJ: Impact of new oral or intravenous P2Y12 inhibitors and clopidogrel on major ischemic and bleeding events in patients with coronary artery disease: A meta-analysis of randomized trials. Atherosclerosis 233: 568–578, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Erlinge D: P2Y receptors in health and disease. Adv Pharmacol 61: 417–439, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Vergani A, Tezza S, Fotino C, Visner G, Pileggi A, Chandraker A, Fiorina P: The purinergic system in allotransplantation. Am J Transplant 14: 507–514, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Baldini C, Rossi C, Ferro F, Santini E, Seccia V, Donati V, Solini A: The P2X7 receptor-inflammasome complex has a role in modulating the inflammatory response in primary Sjögren’s syndrome. J Intern Med 274: 480–489, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Solini A, Menini S, Rossi C, Ricci C, Santini E, Blasetti Fantauzzi C, Iacobini C, Pugliese G: The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: Possible role of NLRP3 inflammasome activation. J Pathol 231: 342–353, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Vergani A, Tezza S, D’Addio F, Fotino C, Liu K, Niewczas M, Bassi R, Molano RD, Kleffel S, Petrelli A, Soleti A, Ammirati E, Frigerio M, Visner G, Grassi F, Ferrero ME, Corradi D, Abdi R, Ricordi C, Sayegh MH, Pileggi A, Fiorina P: Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7. Circulation 127: 463–475, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birch RE, Schwiebert EM, Peppiatt-Wildman CM, Wildman SS: Emerging key roles for P2X receptors in the kidney. Front Physiol 4: 262, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallon V: P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol 294: F10–F27, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Wilson PD, Hovater JS, Casey CC, Fortenberry JA, Schwiebert EM: ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys. J Am Soc Nephrol 10: 218–229, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Nishiyama A, Majid DS, Taher KA, Miyatake A, Navar LG: Relation between renal interstitial ATP concentrations and autoregulation-mediated changes in renal vascular resistance. Circ Res 86: 656–662, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Schwiebert EM, Kishore BK: Extracellular nucleotide signaling along the renal epithelium. Am J Physiol Renal Physiol 280: F945–F963, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Inscho EW: ATP, P2 receptors and the renal microcirculation. Purinergic Signal 5: 447–460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majid DS, Inscho EW, Navar LG: P2 purinoceptor saturation by adenosine triphosphate impairs renal autoregulation in dogs. J Am Soc Nephrol 10: 492–498, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Inscho EW, Mitchell KD, Navar LG: Extracellular ATP in the regulation of renal microvascular function. FASEB J 8: 319–328, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Komlosi P, Peti-Peterdi J, Fuson AL, Fintha A, Rosivall L, Bell PD: Macula densa basolateral ATP release is regulated by luminal [NaCl] and dietary salt intake. Am J Physiol Renal Physiol 286: F1054–F1058, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J: Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18: 2062–2070, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Praetorius HA, Leipziger J: Intrarenal purinergic signaling in the control of renal tubular transport. Annu Rev Physiol 72: 377–393, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Silva G, Beierwaltes WH, Garvin JL: Extracellular ATP stimulates NO production in rat thick ascending limb. Hypertension 47: 563–567, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD: Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem 283: 36599–36607, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajagopal M, Kathpalia PP, Widdicombe JH, Pao AC: Differential effects of extracellular ATP on chloride transport in cortical collecting duct cells. Am J Physiol Renal Physiol 303: F483–F491, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J: Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol 20: 1724–1732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svenningsen P, Burford JL, Peti-Peterdi J: ATP releasing connexin 30 hemichannels mediate flow-induced calcium signaling in the collecting duct. Front Physiol 4: 292, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jankowski M, Szamocka E, Kowalski R, Angielski S, Szczepańska-Konkel M: The effects of P2X receptor agonists on renal sodium and water excretion in anaesthetized rats. Acta Physiol (Oxf) 202: 193–201, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Ilatovskaya DV, Palygin O, Levchenko V, Staruschenko A: Pharmacological characterization of the P2 receptors profile in the podocytes of the freshly isolated rat glomeruli. Am J Physiol Cell Physiol 305: C1050–C1059, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burford JL, Villanueva K, Lam L, Riquier-Brison A, Hackl MJ, Pippin J, Shankland SJ, Peti-Peterdi J: Intravital imaging of podocyte calcium in glomerular injury and disease. J Clin Invest 124: 2050–2058, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roshanravan H, Dryer SE: ATP acting through P2Y receptors causes activation of podocyte TRPC6 channels: Role of podocin and reactive oxygen species. Am J Physiol Renal Physiol 306: F1088–F1097, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Ponnusamy M, Liu N, Gong R, Yan H, Zhuang S: ERK pathway mediates P2X7 expression and cell death in renal interstitial fibroblasts exposed to necrotic renal epithelial cells. Am J Physiol Renal Physiol 301: F650–F659, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponnusamy M, Ma L, Gong R, Pang M, Chin YE, Zhuang S: P2X7 receptors mediate deleterious renal epithelial-fibroblast cross talk. Am J Physiol Renal Physiol 300: F62–F70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vonend O, Grote T, Oberhauser V, Von Kügelgen I, Rump LC: P2Y-receptors stimulating the proliferation of human mesangial cells through the MAPK42/44 pathway. Br J Pharmacol 139: 1119–1126, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doria A, Niewczas MA, Fiorina P: Can existing drugs approved for other indications retard renal function decline in patients with type 1 diabetes and nephropathy? Semin Nephrol 32: 437–444, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiorina P, Vergani A, Bassi R, Niewczas MA, Altintas MM, Pezzolesi MG, D’Addio F, Chin M, Tezza S, Ben Nasr M, Mattinzoli D, Ikehata M, Corradi D, Schumacher V, Buvall L, Yu CC, Chang JM, La Rosa S, Finzi G, Solini A, Vincenti F, Rastaldi MP, Reiser J, Krolewski AS, Mundel PH, Sayegh MH: Role of podocyte B7-1 in diabetic nephropathy. J Am Soc Nephrol 25: 1415–1429, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman DJ, Rennke HG, Csizmadia E, Enjyoji K, Robson SC: The vascular ectonucleotidase ENTPD1 is a novel renoprotective factor in diabetic nephropathy. Diabetes 56: 2371–2379, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Tak E, Ridyard D, Kim JH, Zimmerman M, Werner T, Wang XX, Shabeka U, Seo SW, Christians U, Klawitter J, Moldovan R, Garcia G, Levi M, Haase V, Ravid K, Eltzschig HK, Grenz A: CD73-dependent generation of adenosine and endothelial Adora2b signaling attenuate diabetic nephropathy. J Am Soc Nephrol 25: 547–563, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen K, Zhang J, Zhang W, Zhang J, Yang J, Li K, He Y: ATP-P2X4 signaling mediates NLRP3 inflammasome activation: A novel pathway of diabetic nephropathy. Int J Biochem Cell Biol 45: 932–943, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Solini A, Iacobini C, Ricci C, Chiozzi P, Amadio L, Pricci F, Di Mario U, Di Virgilio F, Pugliese G: Purinergic modulation of mesangial extracellular matrix production: Role in diabetic and other glomerular diseases. Kidney Int 67: 875–885, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Ji X, Naito Y, Weng H, Endo K, Ma X, Iwai N: P2X7 deficiency attenuates hypertension and renal injury in deoxycorticosterone acetate-salt hypertension. Am J Physiol Renal Physiol 303: F1207–F1215, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Vonend O, Turner CM, Chan CM, Loesch A, Dell’Anna GC, Srai KS, Burnstock G, Unwin RJ: Glomerular expression of the ATP-sensitive P2X receptor in diabetic and hypertensive rat models. Kidney Int 66: 157–166, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Zhang Y, Wang W, Dai Y, Ning C, Luo R, Sun K, Glover L, Grenz A, Sun H, Tao L, Zhang W, Colgan SP, Blackburn MR, Eltzschig HK, Kellems RE, Xia Y: Elevated ecto-5′-nucleotidase-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension. Circ Res 112: 1466–1478, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palomino-Doza J, Rahman TJ, Avery PJ, Mayosi BM, Farrall M, Watkins H, Edwards CR, Keavney B: Ambulatory blood pressure is associated with polymorphic variation in P2X receptor genes. Hypertension 52: 980–985, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Ghiadoni L, Rossi C, Duranti E, Santini E, Bruno RM, Salvati A, Taddei S, Solini A: P2X7 receptor polymorphisms do not influence endothelial function and vascular tone in neo-diagnosed, treatment-naive essential hypertensive patients. J Hypertens 31: 2362–2369, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Turner CM, Tam FW, Lai PC, Tarzi RM, Burnstock G, Pusey CD, Cook HT, Unwin RJ: Increased expression of the pro-apoptotic ATP-sensitive P2X7 receptor in experimental and human glomerulonephritis. Nephrol Dial Transplant 22: 386–395, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Taylor SR, Turner CM, Elliott JI, McDaid J, Hewitt R, Smith J, Pickering MC, Whitehouse DL, Cook HT, Burnstock G, Pusey CD, Unwin RJ, Tam FW: P2X7 deficiency attenuates renal injury in experimental glomerulonephritis. J Am Soc Nephrol 20: 1275–1281, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao J, Wang H, Dai C, Wang H, Zhang H, Huang Y, Wang S, Gaskin F, Yang N, Fu SM: P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum 65: 3176–3185, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia GE, Truong LD, Chen JF, Johnson RJ, Feng L: Adenosine A(2A) receptor activation prevents progressive kidney fibrosis in a model of immune-associated chronic inflammation. Kidney Int 80: 378–388, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Rangan G: Role of extracellular ATP and P2 receptor signaling in regulating renal cyst growth and interstitial inflammation in polycystic kidney disease. Front Physiol 4: 218, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner CM, Ramesh B, Srai SK, Burnstock G, Unwin RJ: Altered ATP-sensitive P2 receptor subtype expression in the Han:SPRD cy/+ rat, a model of autosomal dominant polycystic kidney disease. Cells Tissues Organs 178: 168–179, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Schwiebert EM, Wallace DP, Braunstein GM, King SR, Peti-Peterdi J, Hanaoka K, Guggino WB, Guay-Woodford LM, Bell PD, Sullivan LP, Grantham JJ, Taylor AL: Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am J Physiol Renal Physiol 282: F763–F775, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Aguiari G, Varani K, Bogo M, Mangolini A, Vincenzi F, Durante C, Gessi S, Sacchetto V, Catizone L, Harris P, Rizzuto R, Borea PA, Del Senno L: Deficiency of polycystic kidney disease-1 gene (PKD1) expression increases A(3) adenosine receptors in human renal cells: Implications for cAMP-dependent signalling and proliferation of PKD1-mutated cystic cells. Biochim Biophys Acta 1792: 531–540, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Liu K, Vergani A, Zhao P, Ben Nasr M, Wu X, Iken K, Jiang D, Su X, Fotino C, Fiorina P, Visner GA: Inhibition of the purinergic pathway prolongs mouse lung allograft survival. Am J Respir Cell Mol Biol 51: 300–310, 2014 [DOI] [PubMed] [Google Scholar]
- 57.Vergani A, Fotino C, D’Addio F, Tezza S, Podetta M, Gatti F, Chin M, Bassi R, Molano RD, Corradi D, Gatti R, Ferrero ME, Secchi A, Grassi F, Ricordi C, Sayegh MH, Maffi P, Pileggi A, Fiorina P: Effect of the purinergic inhibitor oxidized ATP in a model of islet allograft rejection. Diabetes 62: 1665–1675, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sevigny CP, Li L, Awad AS, Huang L, McDuffie M, Linden J, Lobo PI, Okusa MD: Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. J Immunol 178: 4240–4249, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Gonçalves RG, Gabrich L, Rosário A, Jr, Takiya CM, Ferreira ML, Chiarini LB, Persechini PM, Coutinho-Silva R, Leite M, Jr: The role of purinergic P2X7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int 70: 1599–1606, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Kim MJ, Turner CM, Hewitt R, Smith J, Bhangal G, Pusey CD, Unwin RJ, Tam FW: Exaggerated renal fibrosis in P2X4 receptor-deficient mice following unilateral ureteric obstruction. Nephrol Dial Transplant 29: 1350–1361, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yap SC, Lee HT: Adenosine and protection from acute kidney injury. Curr Opin Nephrol Hypertens 21: 24–32, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Graciano ML, Nishiyama A, Jackson K, Seth DM, Ortiz RM, Prieto-Carrasquero MC, Kobori H, Navar LG: Purinergic receptors contribute to early mesangial cell transformation and renal vessel hypertrophy during angiotensin II-induced hypertension. Am J Physiol Renal Physiol 294: F161–F169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ: Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest 112: 1895–1905, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Osmond DA, Inscho EW: P2X(1) receptor blockade inhibits whole kidney autoregulation of renal blood flow in vivo. Am J Physiol Renal Physiol 298: F1360–F1368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menzies RI, Unwin RJ, Dash RK, Beard DA, Cowley AW, Jr, Carlson BE, Mullins JJ, Bailey MA: Effect of P2X4 and P2X7 receptor antagonism on the pressure diuresis relationship in rats. Front Physiol 4: 305, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ji X, Naito Y, Hirokawa G, Weng H, Hiura Y, Takahashi R, Iwai N: P2X(7) receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertens Res 35: 173–179, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Vallon V, Rieg T: Regulation of renal NaCl and water transport by the ATP/UTP/P2Y2 receptor system. Am J Physiol Renal Physiol 301: F463–F475, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rost S, Daniel C, Schulze-Lohoff E, Bäumert HG, Lambrecht G, Hugo C: P2 receptor antagonist PPADS inhibits mesangial cell proliferation in experimental mesangial proliferative glomerulonephritis. Kidney Int 62: 1659–1671, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Chang MY, Lu JK, Tian YC, Chen YC, Hung CC, Huang YH, Chen YH, Wu MS, Yang CW, Cheng YC: Inhibition of the P2X7 receptor reduces cystogenesis in PKD. J Am Soc Nephrol 22: 1696–1706, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turner CM, King BF, Srai KS, Unwin RJ: Antagonism of endogenous putative P2Y receptors reduces the growth of MDCK-derived cysts cultured in vitro. Am J Physiol Renal Physiol 292: F15–F25, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Han X, Zhao L, Lu G, Ge J, Zhao Y, Zu S, Yuan M, Liu Y, Kong F, Xiao Z, Zhao S: Improving outcomes of acute kidney injury using mouse renal progenitor cells alone or in combination with erythropoietin or suramin. Stem Cell Res Ther 4: 74, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marques RD, de Bruijn PI, Sorensen MV, Bleich M, Praetorius HA, Leipziger J: Basolateral P2X receptors mediate inhibition of NaCl transport in mouse medullary thick ascending limb (mTAL). Am J Physiol Renal Physiol 302: F487–F494, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Chan CM, Unwin RJ, Bardini M, Oglesby IB, Ford AP, Townsend-Nicholson A, Burnstock G: Localization of P2X1 purinoceptors by autoradiography and immunohistochemistry in rat kidneys. Am J Physiol 274: F799–F804, 1998 [DOI] [PubMed] [Google Scholar]
- 74.Filipovic DM, Adebanjo OA, Zaidi M, Reeves WB: Functional and molecular evidence for P2X receptors in LLC-PK1 cells. Am J Physiol 274: F1070–F1077, 1998 [DOI] [PubMed] [Google Scholar]
- 75.McCoy DE, Taylor AL, Kudlow BA, Karlson K, Slattery MJ, Schwiebert LM, Schwiebert EM, Stanton BA: Nucleotides regulate NaCl transport in mIMCD-K2 cells via P2X and P2Y purinergic receptors. Am J Physiol 277: F552–F559, 1999 [DOI] [PubMed] [Google Scholar]
- 76.Dai LJ, Kang HS, Kerstan D, Ritchie G, Quamme GA: ATP inhibits Mg(2+) uptake in MDCT cells via P2X purinoceptors. Am J Physiol Renal Physiol 281: F833–F840, 2001 [DOI] [PubMed] [Google Scholar]
- 77.Harada H, Chan CM, Loesch A, Unwin R, Burnstock G: Induction of proliferation and apoptotic cell death via P2Y and P2X receptors, respectively, in rat glomerular mesangial cells. Kidney Int 57: 949–958, 2000 [DOI] [PubMed] [Google Scholar]
- 78.Jalilian I, Spildrejorde M, Seavers A, Curtis BL, McArthur JD, Sluyter R: Functional expression of the damage-associated molecular pattern receptor P2X7 on canine kidney epithelial cells. Vet Immunol Immunopathol 150: 228–233, 2012 [DOI] [PubMed] [Google Scholar]
- 79.Bailey MA, Imbert-Teboul M, Turner C, Marsy S, Srai K, Burnstock G, Unwin RJ: Axial distribution and characterization of basolateral P2Y receptors along the rat renal tubule. Kidney Int 58: 1893–1901, 2000 [DOI] [PubMed] [Google Scholar]
- 80.Post SR, Rump LC, Zambon A, Hughes RJ, Buda MD, Jacobson JP, Kao CC, Insel PA: ATP activates cAMP production via multiple purinergic receptors in MDCK-D1 epithelial cells. Blockade of an autocrine/paracrine pathway to define receptor preference of an agonist. J Biol Chem 273: 23093–23097, 1998 [DOI] [PubMed] [Google Scholar]
- 81.Säve S, Persson K: Extracellular ATP and P2Y receptor activation induce a proinflammatory host response in the human urinary tract. Infect Immun 78: 3609–3615, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cuffe JE, Bielfeld-Ackermann A, Thomas J, Leipziger J, Korbmacher C: ATP stimulates Cl- secretion and reduces amiloride-sensitive Na+ absorption in M-1 mouse cortical collecting duct cells. J Physiol 524: 77–90, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kishore BK, Ginns SM, Krane CM, Nielsen S, Knepper MA: Cellular localization of P2Y(2) purinoceptor in rat renal inner medulla and lung. Am J Physiol Renal Physiol 278: F43–F51, 2000 [DOI] [PubMed] [Google Scholar]
- 84.Bailey MA, Imbert-Teboul M, Turner C, Srai SK, Burnstock G, Unwin RJ: Evidence for basolateral P2Y(6) receptors along the rat proximal tubule: Functional and molecular characterization. J Am Soc Nephrol 12: 1640–1647, 2001 [DOI] [PubMed] [Google Scholar]