Abstract
Lung cancer represents an increasingly frequent cancer diagnosis worldwide. An increasing awareness on smoking cessation as an important mean to reduce lung cancer incidence and mortality, an increasing number of therapy options and a steady focus on early diagnosis and adequate staging have resulted in a modestly improved survival. For early diagnosis and precise staging, imaging, especially positron emission tomography combined with CT (PET/CT), plays an important role. Other functional imaging modalities such as dynamic contrast-enhanced CT (DCE-CT) and diffusion-weighted MR imaging (DW-MRI) have demonstrated promising results within this field. The purpose of this review is to provide the reader with a brief and balanced introduction to these three functional imaging modalities and their current or potential application in the care of patients with lung cancer.
Keywords: diagnosis; diffusion magnetic resonance imaging/methods; lung neoplasms/diagnosis; neoplasm staging; positron emission tomography and computed tomography/methods; tomography, x-ray computed/methods
Introduction
Lung cancer, of which non-small-cell lung cancer accounts for 80–90% (D'addario et al., 2010; Pastorino, 2010), represents the leading cause of cancer mortality worldwide, each year causing the death of approximately 1·2 million people. The incidence of lung cancer is closely related to smoking patterns, and thus, a dramatically increasing incidence and mortality have been observed in China (Yang et al., 2004), while the incidence in Western countries has been expected to decrease, but keeps slowly increasing – mainly due to the increased incidence among females and the elderly (Palshof & Jakobsen, 2010).
During the last three decades, the approach towards lung cancer has changed from defeatistic to slightly optimistic, with focus on early diagnosis and precise staging, as the fundament for a growing number of therapy options. These advances have, however, resulted in only slightly improved survival; for example, in Denmark, overall survival for patients with lung cancer has improved with a total of 6 months during the last 10 years (Palshof & Jakobsen, 2010; Coleman et al., 2011). This improvement is mainly attributed to the increased number of therapy option and improved surgical techniques, but also faster diagnosis and improved staging are thought to play a role (Palshof & Jakobsen, 2010).
The purpose of this report is to review and discuss current literature on functional imaging in lung cancer, by functional imaging meaning modalities that provide us with more information than simple anatomy. We will try to provide the reader with a balanced introduction to one well-known and two newer functional imaging modalities in lung cancer (Table1), namely integrated positron emission tomography and CT with 18F-FDG (FDG-PET/CT) assessing tissue metabolism, dynamic contrast-enhanced CT (DCE-CT) estimating tumour blood flow and blood volume and diffusion-weighted magnetic resonance imaging (DW-MRI) exploring cellular density (cellularity and the integrity of cell membranes.
Table 1.
Summary of technical details and comparison of PET/CT, DCE-CT and DW-MRI.
| Modality | FDG-PET/CT | DCE-CT | DW-MRI |
|---|---|---|---|
| Principle | Describing tumour metabolism using the radioactive tracer FDG | Visualizes and measures tumour blood flow and blood volume. | Shows diffusion of water molecules within the tissue conditioned by cellular density |
| Patient preparation | 4- to 6-h fast 1-h rest after injection |
30-min rest after injection | No preparation |
| Examination time | 20 min | 2 min | 15 min (per bed position) |
| Postprocessing | 10 min | 10-15 min | 10 min |
| Quantification | Possible (SUV), but not necessary | Possible, but unreliable | Possible (ADC), but not very useful |
| Radiation dose | 5–15 mSv, dependant on CT protocol and FDG dose | 10–20 mSv, dependant on protocol | No radiation |
| Strength | High throughput Well validated Very sensitive |
Functional modality High accuracy |
No radiation No preparation No contrast Sensitive |
| Limitations | FDG not specific for cancer Radiation dose |
Respiratory motion Reproducibility Radiation dose |
Low SNR Respiratory motion and cardiac pulsation cause artefacts |
DCE-CT, dynamic contrast-enhanced CT; DW-MRI, diffusion-weighted magnetic resonance imaging; FDG-PET/CT, integrated positron emission tomography and CT with 18F-FDG; ADC, apparent diffusion coefficient; SUV, standardized uptake value.
Techniques and limitations
Integrated positron emission tomography and CT with 18F-FDG
From the 1990s, PET has been extensively studied for use in the diagnosis and staging of cancer, especially lung cancer (Fischer et al., 2001). Initially as a single modality technique, it requires acquisition of both emission scan and transmission (for the purpose of attenuation correction), resulting in a PET scan from the skull base to upper thigh lasting approximately 45 min. In year 2000, the PET/CT scanner was introduced in the United States, and the first European scanners were installed in Copenhagen and Zürich in 2001. The introduction of the hybrid PET/CT scanner combining functional information from the PET scanner with anatomy and data for attenuation correction obtained by CT (Beyer et al., 2000) was a game changer for the PET technology. A whole-body PET/CT scan on modern scanners lasts approximately 20 min. Today, PET/CT is recommended in many oncological settings, especially in the diagnosis and staging of lung cancer. The PET technique is based on the tracer principle, and in this respect, [18F]-fluorodeoxyglucose (FDG) is by far the most commonly used PET tracer, exploiting the increased glucose uptake and metabolism in malignant cells (Pauwels et al., 2000). In the following text referring to PET and PET/CT, it is implicit that the tracer in question is FDG unless otherwise mentioned. The PET technology is inherently extremely sensitive, however, movement of the positron before annihilation and slight variations in the angle between the two photons limit the spatial resolution of current clinical systems to 4–5 mm (Sandler, 2003). This, combined with the unavoidable respiratory movements (PET acquisition takes approximately 3 min per bed position) have resulted in the rule of thumb that in tumours, smaller than 10 mm, the PET technique is considered to be less sensitive.
Using PET in the diagnosis of lung cancer, a false-positive rate as high as 20–25% has been reported (Stroobants et al., 2003). This is mainly due to increased uptake of FDG in inflammatory cells (Fischer & Mortensen, 2006; Baxter et al., 2011). Also pulmonary embolism or iatrogenic microembolism can cause FDG uptake mimicking malignancy (but without correlate on CT) (Schreiter et al., 2011). Iatrogenic procedures might also induce false-positive results: of relevance in lung cancer is placement of chest tubes, percutaneous needle biopsy, mediastinoscopy, talc pleurodesis (may persist long after the procedure (Kwek et al., 2004)), radiation pneumonitis and esophagitis (Truong et al., 2005). Detailed knowledge of patient history and the use of integrated PET/CT (with side-by-side reading by nuclear medicine physician and radiologist) can help to distinguish malignant FDG uptake from uptake due to benign causes and improve specificity. False-negative results (Fig.1) are less common and mainly due to small size or well-differentiated malignancies, such as bronchiolo-alveolar adenocarcinomas and carcinoids (Marom et al., 2002; Higashi et al., 2003).
Figure 1.
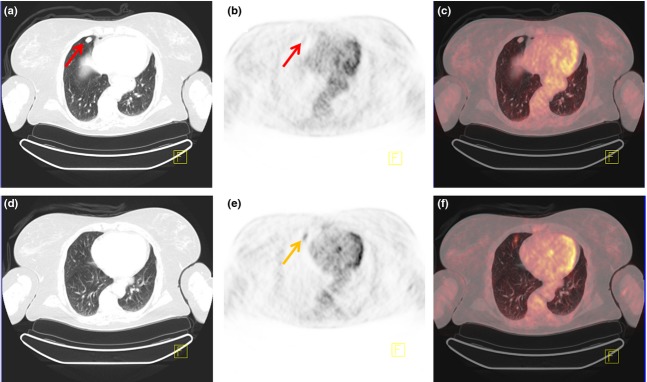
PET/CT scan of a 60-year-old female with a tumour anterior in the right lung, easily visible on CT (a). However, on PET/CT, FDG uptake was hardly visible in the area of the tumour (b,c). Taking a closer look at the slices above the tumour (d–f), moderately focal increased FDG uptake was visible here (SUVmax = 4). This uptake corresponds to the tumour, but as a result of respiratory movements during PET acquisition, there is a misalignment between CT and FDG-PET. The patient was diagnosed with a carcinoid tumour, which explains the relatively low FDG uptake.
PET can be evaluated visually and/or semi-quantitatively by means of the standardized uptake value (SUV). SUV is the activity concentration in the lesion normalized for the injected dose and the weight or body surface area of the patient (Thie, 2004). SUV is highly dependent on a number of factors related to the patient (e.g. length of fast, period between injection of FDG and scan time), the type of scanner and reconstruction algorithm, making it unsuitable for uncritical comparison between different scanners, centres and time periods. A common SUV threshold suggesting malignancy cannot be recommended (Nguyen et al., 2011), and a PET report should always rely mainly on the visual analysis and only include information on the SUV to support the conclusion and if considered clinically relevant (Boellaard et al., 2010).
The CT part of the PET/CT scan is often performed as a whole-body low-dose scan without intravenous contrast (Pfannenberg et al., 2007a,2007b). By doing this, it is possible to keep radiation dose from the CT scan at a minimum and avoid the use of IV contrast. It is, however, our experience and have also been demonstrated by recent studies that the diagnostic accuracy and clinical value of the PET/CT are markedly improved by applying a standard dose contrast-enhanced CT, also in the staging of patients with lung cancer, especially with regard to assessment of lymph nodes and for obtaining an accurate description of tumour extent (Pfannenberg et al., 2007b; Cronin et al., 2010).
Dynamic contrast-enhanced CT
Dynamic contrast-enhanced CT is a functional imaging modality, which, in theory, can quantify the perfusion of tissues by calculating the delivery of contrast agent and therefore blood to these tissues (Miles et al., 1991, 1993, 1997). This is expected to be clinically useful, and accordingly, studies investigating the use of DCE-CT in oncology are increasingly reported in literature (Miles, 1999; Miles et al., 2000; Yi et al., 2004).
The fundamental principle of DCE-CT is based on the temporal changes in tissue density following intravenous administration of iodinated contrast media. By obtaining in quick succession a series of images of a particular tissue of interest, it is possible to record the temporal changes in the tissue attenuation occurring after intravenous injection of contrast. The quantification of perfusion recorded by CT is carried out using mathematical modelling techniques.
Dynamic contrast-enhanced CT has four fundamental requirements: (i) Intravenous administration of a contrast agent with a high flow rate, (ii) Repeated CT scans of the same volume of tissue. These scans must be obtained before, during and immediately after the intravenous administration of the contrast agent, to study the variation in tissue attenuation, (iii) Input of an arterial region of interest (ROI) to construct an arterial time–attenuation curve and (iv) Input of a tissue ROI to construct a tissue time–attenuation curve. The two curves are compared to obtain perfusion parameter measurements for the tissue interstitium of interest (Petralia et al., 2010a).
Postprocessing of DCE-CT generates colour maps for both quantitative parameters, that is tumour blood flow and tumour blood volume, and semi-quantitative parameters, that is tumour peak enhancement intensity. In quantitative analysis, the operator places a ROI in the tumour, and dedicated perfusion software is then used to calculate numeric perfusion values for the ROI. This numeric value represents the mean of the numeric perfusion values for each voxel within the ROI, and as such, it provides an estimate of the total perfusion of the selected tumour volume (Fig.2). In qualitative analysis, the colour maps yield a visual impression of the blood flow and blood volume within the tissue being studied, allowing for quick identification of the areas with the highest or lowest blood flow and blood volume (Coche, 2012) (Fig.3).
Figure 2.
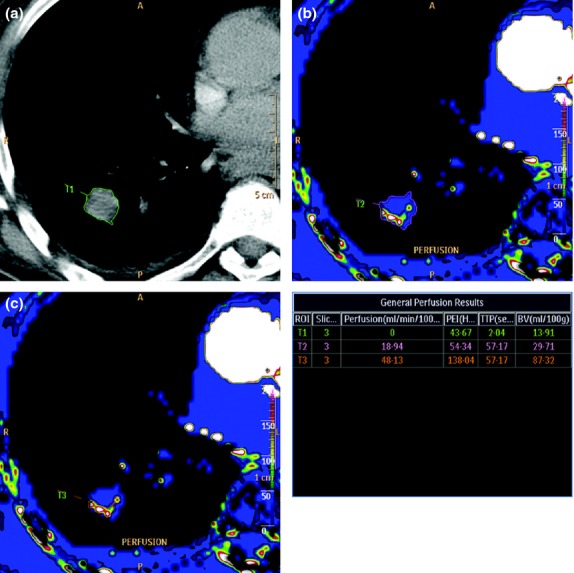
Examples of three different DCE-CT tumour ROIs using the quantitative method in a 69-year-old male with an adenocarcinoma in the right lung: (a) T1, a large ROI comprising the entire tumour. The ROI is drawn on a morphological image; (b) T2, a large ROI comprising the same entire tumour. This time, the ROI is drawn on a perfusion map; and (c) T3, a small ROI comprising only the maximally perfused parts of the tumour. The ROI is drawn on a perfusion map. These examples illustrate the significance of choosing the right ROI method and the importance of stating the chosen method in the report.
Figure 3.
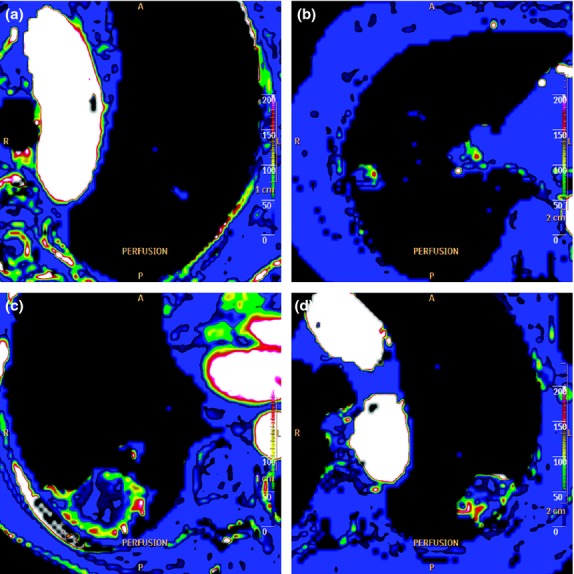
Four examples of enhancement patterns using a qualitative method: (a) No perfusion. The patient was a 79-year-old male with a squamous cell carcinoma. (b) Partial ring perfusion. The patient was a 66-year-old female with an adenocarcinoma. (c) Complete ring perfusion. The patient was a 67-year-old female with an adenocarcinoma. (d) Heterogeneous perfusion. The patient was a 78-year-old male with a squamous cell carcinoma.
The most important technical limitation of DCE-CT is respiratory motion, which can lead to image misregistration and errors in calculation of both quantitative and semi-quantitative parameters. Respiratory motion is a challenge for the actual parameters as well as for the reproducibility. This was evaluated in a study with 11 patients with lung tumour by Ng et al. (2011) using 16-detector row CT. The authors found that the quantitative parameters were significantly influenced by respiratory motion and the duration of data acquisition. Currently, the preferred method to minimize respiratory motion is to instruct patients to hold their breath or to use shallow breathing. However, motion correcting software is beginning to emerge both in descriptive as well as in comparative studies (Sauter et al., 2012a,b, 2013). In the future, the use of modern respiratory-gated, 256- or 320-detector row CT may improve misregistration through more extensive coverage, while reducing respiratory artefacts.
Another important limitation in DCE-CT concerns the radiation dose delivered to the patient and the use of potentially toxic intravenous iodinated contrast material. Fraioli et al. (2011a) measured the radiation dose to 21·7 ± 1·6 mSv using 64-detector row dual-source CT with tube voltage at 100 kV and tube current at 120 mAs. This is a substantial dose, similar to that of combined PET/CT.
Diffusion-weighted magnetic resonance imaging
Recently, diffusion-weighted magnetic resonance imaging (DW-MRI) has become a widely used imaging modality, applied to evaluate tissue characterization in terms of its cellular density (tissue cellularity and the integrity of cellular membranes). DW-MRI is based on diffusion of water molecules in tissues. Water movement would be completely random in an unrestricted environment, a phenomenon known as Brownian motion (Koh & Padhani, 2006; Kwee et al., 2008). Within biological tissues, water molecules are distributed among intravascular, intracellular and extracellular spaces, and their motion is impeded by interaction with tissue compartment, cell membranes and intracellular organelles. In short, the more the viable cells, the higher the restriction of water diffusion.
The most common approach to render magnetic resonance imaging (MRI) sensitive to diffusion is by applying two strong symmetrical diffusion gradients on either side of the 180° refocusing pulse in the spin-echo sequence (Nasu et al., 2004). All water molecules of the imaged tissues will be affected by the first diffusion gradient, altering the phase shift of the protons in the water molecules; the second gradient reverses this phase shift, but only for those protons that have no spatial displacement during the acquisition time. Thus, if movement of the water molecule between application of the first and the second gradient pulses occurs, complete rephasing cannot happen leading to a loss of signal from this spatial location (Charles-Edwards & deSouza, 2006). On the other hand, in tissues with limited or almost no water diffusion, that is due to high cellularity as in malignant tumours, the MR signal will be retained. The sensitivity of the DWI sequence to water motion can be varied by changing the gradient amplitude, the duration of the applied gradient and the time interval between the diffusion gradients. The parameter proportional to these three factors is known as the b-value (Kwee et al., 2008; Qayyum, 2009). Diffusion-weighted imaging is performed with at least two b-values. By applying different b-values, quantitative analysis – known as the apparent diffusion coefficient (ADC) – is possible. Application of greater number of b-values improves the accuracy of ADC but increases the scanning time. As in all other existing modalities, false-positive and false-negative results do occur on DWI; with the most commonly occurring pitfalls being ‘T2 shine-through’ effect (delusions from slow flowing blood). Whereas DW-MRI measures cell density, FDG-PET/CT measures cell metabolism – thus, both modalities are prone to some of the same pitfalls hampering specificity, that is inflammation. DW-MRI has not yet been widely applied to diagnose, stage and therapy evaluation of patients with lung cancer due to artefacts from respiratory and cardiac motion. Low spatial resolution of DW-MRI images makes it difficult to evaluate a primary lesion and its relation to adjacent structures. However, use of standard MRI could help to delineate eventual spread into mediastinum, pleura and bone structures (Hochhegger et al., 2011; Biederer et al., 2012a,b). Scanning time for DWI is relatively short (the total imaging time could be around 228–265 s) (Hasegawa et al., 2008; Nomori et al., 2008; Nakayama et al., 2010) Concerning the procedure of DW imaging, using breath-hold technique can improve sensitivity (Biederer et al., 2012a,b), small nodules could, theoretically, be better visualized, and quantitative assessment of diffusion could be more accurately measured, but is still very dependent on the respiratory and heart rates, and data are currently scarce.
Clinical results
Lesion characterization
A solitary pulmonary nodule (SPN) is often the first depictable sign of lung cancer. However, an SPN is by no means equal to an early lung cancer. SPN is defined as a lesion smaller than 3 cm in diameter (larger than 3 cm is a mass) and completely surrounded by lung tissue (Wahidi et al., 2007). The probability of malignancy in an SPN varies significantly dependent on patient history (an incidental finding or a patient presenting with symptoms?), smoking history, age, radiological findings, etc. (Gould et al., 2007b). Assessing the pretest probability of malignancy in a given SPN facilitates clinical decision-making when selecting and interpreting the results of diagnostic imaging (e.g. PET) and invasive tests (Gould et al., 2007a).
Integrated positron emission tomography and CT with 18F-FDG
Early systematic reviews comparing the diagnostic value of PET compared to CT in discriminating malignant from benign pulmonary nodules or masses found PET to be highly sensitive (approximately 95%) but less specific (75–80%) (Fischer et al., 2001; Gould et al., 2001) and with positive and negative predictive values around 90%. The latter depends on the prevalence or pretest probability of cancer. By applying the likelihood ratio, it can be seen that a negative PET scan (LR = 0·05) results in a large gain of knowledge from pre- to post-test probability of cancer as compared to a negative CT (LR = 0·2) (Fischer et al., 2001). In short, in the setting of pulmonary nodules, PET is better to exclude than confirm malignancy. More recent studies comparing CT and PET/CT are scarce but can reproduce a significant difference between CT and PET/CT, in favour of the latter (Yi et al., 2006; Kim et al., 2007; Jeong et al., 2008; Kagna et al., 2009). This difference is seen both with regard to sensitivity and specificity, but especially the difference in specificity decreases (Harders et al., 2012), when comparing PET/CT with standard contrast-enhanced CT. These studies are, as well as the earlier studies on the diagnosis of lung nodules, hampered by a relatively high prevalence of cancer (40–70%) (Fischer & Mortensen, 2012). Thus, the high diagnostic accuracy of PET/CT for diagnosing SPN found in these studies may not be valid in the present population where an increasing number of patients are referred with an incidental finding of a solitary pulmonary nodule, for exmaple, after performing a cardiac CT due to angina or chest X-ray during a health examination or as a result of participation in a lung cancer screening trial. The ability of PET to rule out a malignant diagnosis can potentially reduce the number of invasive procedures resulting from, for example, cardiac CT and screening trials (Pastorino et al., 2003). However, in a screening population, an increased frequency of small and relatively low metabolic tumours can be expected, hampering the sensitivity and specificity of the PET technique (Lindell et al., 2005; Bar-Shalom et al., 2008). This problem can be addressed by combining the information of tumour growth rate (e.g. after 3 months) with FDG uptake (Bastarrika et al., 2005; Ashraf et al., 2011), making PET/CT a valuable second-step test. Large and well-designed trials addressing this issue are still scarce.
Dynamic contrast-enhanced CT
Early reports (Zhang & Kono, 1997; Yi et al., 2004) described how blood flow and peak enhancement intensity were higher for malignant and inflammatory tumours than for benign ones. A subsequent meta-analysis found the diagnostic accuracy of DCE-CT, DCE-MRI, FDG-PET and 99mTc depreotide single-photon emission CT (SPECT) for the evaluation of solitary pulmonary nodules to be comparable, with only negligible differences in performance between the tests (Cronin et al., 2008).
Since these reports, DCE-CT methods have been sophisticated, and quantitative and semi-quantitative methods of analysis have been further developed to better characterize the nature of lung nodules and tumours (Chae et al., 2008, 2010; Li et al., 2010; Ohno et al., 2011a,2011b). Thus, in a recent report, Sitartchouk et al. (2008) demonstrated higher blood flow, blood volume and extraction fraction values in malignant lung nodules compared with benign ones, and in another report, Ohno et al. (2011a,2011b) used 256- and 320-detector row CT to acquire dynamic data within a 16-cm area every 2 s without helical scanning. In 50 patients with 76 lung nodules, the authors showed that first-pass area-detector DCE-CT had the potential to be more specific and accurate than FDG-PET/CT for differentiating between malignant and benign lung nodules.
Diffusion-weighted magnetic resonance imaging
Only few studies have assessed the value of DW-MRI for solitary pulmonary nodules: Ohba et al. (2009) found that DWI clearly identified the malignant nodules in a fashion similar to PET imaging. Similarly, Mori et al. (2008) compared the performance of PET and DW-MRI using cut-off values for respectively SUV and ADC. They found the two modalities to be equally sensitive (0·72 and 0·70, respectively), whereas DW-MRI was significantly more specific (0·97 and 0·79, respectively). At the moment, there is no standard ADC threshold for differentiation benign from malignant lesion. On high b-values (1000 s mm−2), tissues with restricted diffusion appear bright on DW images and dark on the ADC map; if several high b-values are applied, malignant lesions will show increased signal intensity (SI) with increasing b-values (Fig.4). By some authors this correlation between increasing b-values and SI is considered to be a useful marker of malignancy, that is, when differentiating metastases from haemangioma in the liver (Parikh et al., 2008; Inan et al., 2010). In a recent study, SI was significantly different between malignant and benign lung lesions, whereas there was no significant difference in ADC values between malignant and benign lesions (Gumustas et al., 2012).
Figure 4.
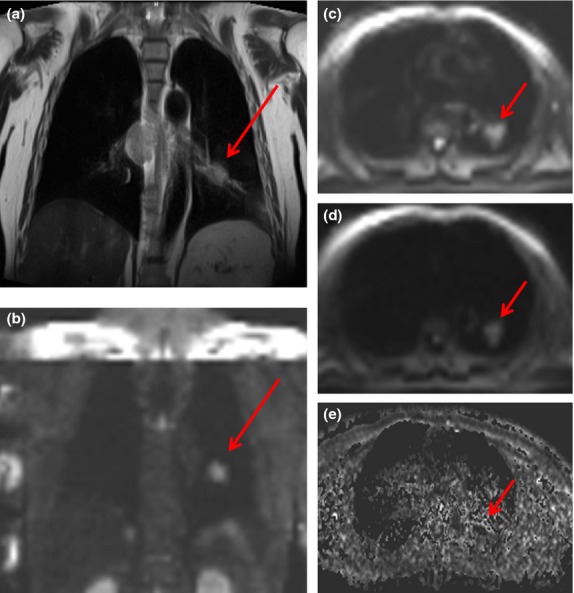
MR scans of a 54-year-old patient with adenocarcinoma in the left lung. During initial examination on coronal T2-weighted images, (a) primary tumour (red arrow) is seen as being isointense to soft tissue. Correspondent coronal DW image displays tumour as a hyperintense zone (b). On the transaxial images, the SI (signal intensity) of the primary tumour (on b-value of 50 and 1000 s mm−2) is seen to stay relatively high and only decrease slightly together with lesion diameter if b-value is elevated (c and d). On ADC (e) map, the primary lesion shows low SI, corresponding to the malignant nature of the tumour.
Recommendations
In accordance with recent recommendations, PET/CT should be used for evaluation of patients with pulmonary nodules with low to moderate risk of malignancy (solid nodules 8–30 mm in size and indeterminate on CT) (Gould et al., 2013). Recent data indicate that DCE-CT could be a possible alternative to PET/CT for this indication, but more studies are needed to confirm this. Data on DW-MRI are promising but scarce, and the field lacks standardization.
Lung cancer staging
Treatment options for patients with lung cancer are highly dependent on the stage of the disease, making accurate and fast staging pivotal. Non-small-cell lung cancer is staged according to the TNM system as initially suggested by Mountain (Mountain, 1986, 1997) and recently revised by the International Association of Lung Cancer (Detterbeck et al., 2009). Staging is used to predict survival and to guide the patient towards the most appropriate treatment regimen or clinical trial. The most significant division is between those patients who are candidates for surgery and those who may benefit from chemotherapy, radiation therapy or both. Only patients with localized disease (TNM stage I-IIb evt. IIIA) will be candidates for primary curative surgery. For most patients with advanced disease (stage IV), palliative treatment with chemotherapy will be the only option. Thus, to allocate the patient to the correct treatment, accurate description of (i) distant metastases and (ii) mediastinal spread (N) is mandatory, whereas the T-stage will substantially influence the treatment choice only in the case of tumour invasion making resection impossible.
Integrated positron emission tomography and CT with 18F-FDG
Single modality PET is insufficient for an accurate description of T-stage, whereas combined PET/CT is significantly more accurate than both PET (Lardinois et al., 2003; Cerfolio et al., 2004; Halpern et al., 2005) and standard CT (diagnostic quality with intravenous contrast) for T-staging (Antoch et al., 2003; de Wever et al., 2007). Mediastinal staging is, in patients without distant metastases, the most significant factor for treatment planning as mediastinal spread (N2–N3 disease) excludes the patient from primary surgery. Initial reports on PET reported very high accuracy with regard to N-staging and significantly higher than the accuracy of CT (Fischer et al., 2001; Reed et al., 2003). In more recent studies, this difference seems to narrow down, but still a staging strategy including PET/CT appears more sensitive with regard to mediastinal disease (de Wever et al., 2007; Fischer et al., 2011). It has been suggested that mediastinoscopy or other invasive staging can be omitted in cases where mediastinum is PET negative (Detterbeck et al., 2007; de Leyn et al., 2007). But by doing this, 16% of the patients have occult N2 disease (Al-Sarraf et al., 2008). To avoid this, patients with central tumours, enlarged lymph nodes on CT and/or N1 disease on PET/CT should perform a confirmatory invasive examination (Fischer et al., 2011). Distant metastases (M1 disease) in otherwise operable patients are reported in approximately 5–15% of patients performing PET or PET/CT (Fig.5) (van Tinteren et al., 2002; Lardinois et al., 2003; Reed et al., 2003; Fischer et al., 2009). Assessing the overall diagnostic accuracy of PET/CT, PET and CT with regard to M-stage is hampered by limited relevant literature as well as protocol variations. A high diagnostic value of PET/CT for diagnosing bone metastases is well documented, and PET/CT is found to be more sensitive than bone scan and CT (Fischer et al., 2007; Song et al., 2009). Similarly PET, and recently PET/CT, is effective in discriminating between malignant and benign adrenal masses (Metser et al., 2006; Ozcan Kara et al., 2011). Isolated PET-positive lesions should however be confirmed to avoid deeming a patient inoperable on a false-positive basis. Due to the high background signal caused by physiological cerebral FDG uptake, PET performs poorly in the detection of brain metastases, especially compared to cerebral MRI. Kruger et al. (2011) found a sensitivity for brain metastases of 27% in 104 lung cancer patients with neurological symptoms. PET/CT can detect brain metastases, but a negative PET/CT scan does not exclude brain metastases, especially not in case of neurological symptoms.
Figure 5.
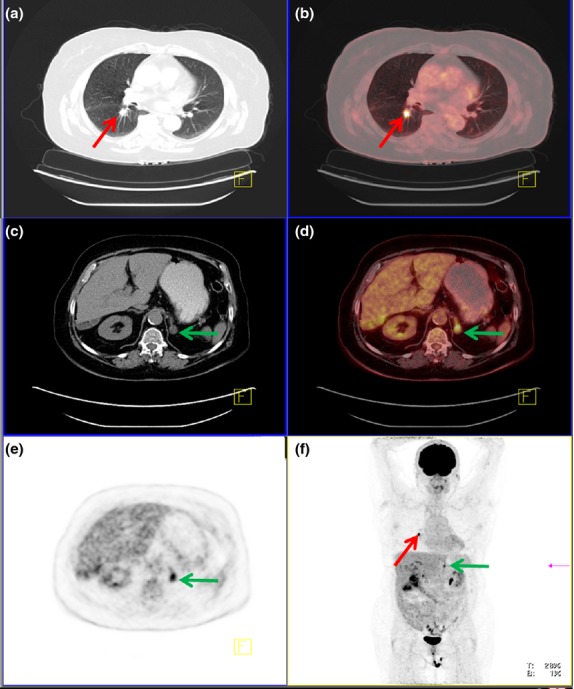
PET/CT scan of a 66-year-old female with a central tumour in the right lung (red arrow), easily visible on CT (a). Tumour was highly FDG-avid indicative of malignancy (SUVmax = 16) (b). An enlarged left adrenal gland was visible on CT (green arrow) (c) and similar to the primary tumour highly FDG-avid, corresponding to a metastasis (SUVmax = 9) (d,e). Both findings can be seen on the multi-intensity projection (MIP, f) and was confirmed to be adenocarcinoma.
The clinical value of PET/CT for pre-operative staging has been demonstrated by two randomized studies. Both studies found that pre-operative staging with PET/CT significantly reduces the frequency of futile thoracotomies without affecting overall survival (Fischer et al., 2009; Maziak et al., 2009). The question of whether or not the use of PET/CT improves survival, apart from improved stage-specific survival as a result of stage migration, has recently been discussed (Dinan et al., 2012; Gregory et al., 2012; Hofman et al., 2013). As mentioned above, one of the important effects of applying PET and PET/CT to lung cancer staging is the detection of unrecognized metastases and upstaging of patients. This results in more patients receiving palliative treatment instead of potentially curative therapy, that is surgery. If PET inappropriately upstages patients, overall survival should decrease. Thus, as stated by Hofman et al., finding that all-cause survival does not change significantly with the increasing use of PET supports a conclusion that PET may reduce morbidity associated with futile therapies without negatively affecting overall patient outcomes.
Dynamic contrast-enhanced CT
Standard contrast-enhanced CT is the most widely available and commonly used non-invasive modality for the evaluation of the mediastinum in lung cancer. Forty-three studies evaluating the accuracy of CT scanning for staging the mediastinum were analysed in the American College of Chest Physicians (ACCP) guidelines from 2013 (Silvestri et al., 2013). The pooled sensitivity and specificity of CT scanning for identifying mediastinal lymph node metastasis were 55% and 81%, respectively. Although the combined estimates should be interpreted with caution as the studies were statistically heterogeneous, these findings closely mirrored previous analyses addressing the accuracy of CT scanning for staging the mediastinum in NSCLC by Gould et al. (2003) and by Dwamena et al. (1999). While it remains the best overall anatomical study available for the thorax, CT is clearly an imperfect means of staging the mediastinum. Thus, CT both overstages as well as understages the mediastinal nodes. Nonetheless, CT continues to play an important and necessary role in the evaluation of these patients.
Dynamic contrast-enhanced CT has been studied as a means of identifying patients with lung cancer at risk of malignant nodal infiltration. However, conflicting results have been reported so far. In a retrospective study of 130 patients with NSCLC who underwent pre-operative DCE-CT followed by surgical resection, Tateishi et al. (2002) reported that tumour peak enhancement intensity was significantly higher in patients with lymph node involvement compared with patients without nodal involvement. Likewise, preliminary studies by Li et al. (2008a,2008b) involving patients with surgically resected lung cancer showed a trend towards higher tumour blood flow and peak enhancement intensity when nodal infiltration was present. Unfortunately, however, these results were not statistically significant when a larger cohort of patients were analysed (Li et al., 2008b). At present, DCE-CT has no role in the detection of extra thoracic metastases.
Diffusion-weighted magnetic resonance imaging
In recent years, DW-MRI has been brought forward as a potential tool in oncology to differentiate benign from malignant lymph nodes (Qayyum, 2009; Malayeri et al., 2011; Bonekamp et al., 2012). Several studies have demonstrated that this technique could be used to distinguish metastatic from non-metastatic lymph nodes in patients with NSCLC (Hasegawa et al., 2008; Nomori et al., 2008; Nakayama et al., 2010). On high b-values, malignant nodes could have slightly different SI (signal intensity) compared to the primary lesion. In general, under quantitative assessment of DW-MR images of lymph nodes, the ADC is found to be significantly lower for metastatic lymph nodes; however, no broadly accepted ADC threshold exists (Ohno et al., 2011a,b). In a recent meta-analysis comparing DW-MRI and FDG-PET/CT, the authors found equal sensitivity (PET/CT 0·75 and DW-MRI 0·72), but a higher specificity for DW-MRI compared to PET/CT (0·95 and 0·89, respectively) (Wu et al., 2012). Two recent clinical trials could not reproduce any difference in the diagnostic value of PET/CT and DW-MRI for staging of NSCLC (Pauls et al., 2012; Sommer et al., 2012), thus, for the moment, DW-MRI might be considered as a supplement or maybe even a substitution technique for FDG-PET/CT in staging of patients with lung cancer.
Recommendations
For staging of patients with lung cancer imaging, assessing potential locoregional as well as extrathoracal spread is needed. Staging by means of PET/CT (supplemented with invasive examination of eventual mediastinal spread) is currently the state of art (Silvestri et al., 2013). Whether invasive mediastinal staging can be omitted in patients with small tumours and negative mediastinum on PET and CT is likely but still controversial. Data on whole-body DW-MRI for lung cancer staging are emerging and promising but needs confirmation in larger studies. As for now, there is no role for DCE-CT for staging of lung cancer.
Treatment monitoring
The definition of tumour response using the WHO (Miller et al., 1981) and RECIST (Eisenhauer et al., 2009) criteria is based upon an experiment performed 30 years ago determining the accuracy with which sixteen experienced oncologists could measure tumour size by palpation (Weber, 2005). Structural imaging techniques such as chest radiographs and CT provide excellent anatomical details and are essential tools in the care of patients with lung cancer. However, whether structural imaging is the most valid measure of tumour response is uncertain (Vansteenkiste et al., 2004), and data suggest that measurement of lung tumour size on CT scans is often inconsistent (Erasmus et al., 2003). Furthermore, structural changes after surgery and/or radiotherapy can be difficult to discern from local tumour relapse and new generations of targeted drugs does not necessarily result in significant structural decreases, despite clinical effect.
Integrated positron emission tomography and CT with 18F-FDG
Data on several solid tumours, including non-small-cell lung cancer, indicate a possible advantage of response evaluation by FDG-PET during and after chemotherapy (Juweid & Cheson, 2006; Herrmann et al., 2011). As FDG preferentially accumulates in viable tumour cells and not in fibrotic or necrotic tissue (Higashi et al., 1993), a change in FDG uptake on PET could be a better way to monitor response and perhaps even to assess response before structural changes occur. Current evidence indicates that FDG-PET response has a variable correlation with CT response, but probably is more accurate than CT response (Maziak et al., 2009). The first clinical study on PET for therapy evaluation in patients with NSCLC was published in 2003. Weber et al. (2003) examined 57 patients before and after one cycle of chemotherapy, demonstrating that changes in tumour FDG retention was closely correlated to prognosis. The principle of using PET for (early) therapy evaluation has since been tested in numerous studies and settings, including conventional cytotoxic chemotherapy, radiotherapy and molecular-targeted therapy, that is EGFR tyrosine kinase inhibitors (Hoekstra et al., 2002; Mac Manus et al., 2005; van Baardwijk et al., 2007; Kong et al., 2007; Maziak et al., 2009; Benz et al., 2011). All studies conclude that PET is potentially useful for therapy planning and evaluation; however, larger controlled trials assessing the clinical effect is still lacking as is necessary standardization of SUV measurements (Weber & Figlin, 2007).
Dynamic contrast-enhanced CT
Several studies have suggested that DCE-CT may be potentially useful in the assessment of patients undergoing chemotherapy, radiation therapy and ablation therapy (Kiessling et al., 2004; Wang et al., 2009; Bellomi et al., 2010; Hegenscheid et al., 2010; Lind et al., 2010; Petralia et al., 2010a,b; Tacelli et al., 2010; Fraioli et al., 2011a,b). Thus, case studies have revealed changes in quantitative and semi-quantitative parameters in patients with NSCLC who were treated with ‘non-vascular targeting’ agents. In preliminary studies, Wang et al. (2009) found a significant decrease in tumour blood flow and blood volume in a patient following two cycles of chemo- and radiotherapy, while Kiessling et al. (2004) described a reduction in tumour blood flow in a patient after two cycles of chemotherapy. The effects of combined chemotherapy and anti-angiogenic agents have also been investigated (Lind et al., 2010; Fraioli et al., 2011a). Angiogenesis and epidermal growth factor receptor inhibitors were evaluated in a study including 23 patients who received dual-source CT at baseline and 3 and 6 weeks after treatment. In their report, Lind et al. described how mean tumour blood flow decreased significantly from 39·2 ml per 100 g min−1 at baseline to 15·1 ml per 100 g min−1 at week 3–9·4 ml per 100 g min−1 at week 6. Tumour blood flow was lower in RECIST responders versus non-responders at week 3 and 6, respectively (Lind et al., 2010). In another study, Fraioli et al. (2011a) assessed 45 patients with non-resectable NSCLC > 20 mm. Subjects underwent DCE-CT at baseline and 40 days after treatment with chemotherapy and anti-angiogenic agents. The authors showed how treatment-induced changes in perfusion could be identified using DCE-CT. They also found that tumour blood flow, blood volume and permeability values were lower in responders compared with non-responders. Of particular interest, they observed discrepancies between quantitative and semi-quantitative assessments, and RECIST criteria evaluations. The authors emphasized the fact that macroscopic changes in tumour size did not necessarily reflect the biological changes induced by therapy. It is thus possible that DCE-CT performed shortly after initiating therapy may be useful for therapy planning, as it may provide a better evaluation of physiological changes than the conventional size assessment obtained using RECIST criteria.
Diffusion-weighted magnetic resonance imaging
Apparent diffusion coefficient seems to be a promising tool for an early tumour response assessment. Derived from DW-MRI, ADC has been shown to be a useful biological marker for early detection and prediction of tumour response to chemotherapy and chemoradiotherapy in different malignant tumours, including head and neck, brain tumours, hepatic metastasis and gynaecological tumours (Harry et al., 2008; Kim et al., 2009). A study dedicated to early response detection to chemotherapy by DCE- and DW-MRI showed that an increase in ADC after one course of chemotherapy (increase by more than 25% from initial ADC value) correlated with longer progression-free survival (Biederer et al., 2012a,b). Results of this study suggest that early response to therapy could be predicted by means of ADC change, despite the absence of any obvious morphological changes (e.g. shrinkage of tumour size or anatomical changes in tumour structure); this could, probably, be explained by early alterations of tumour cellular density under anticancer drugs therapy, which increases volume of tumour extracellular spaces by means of cells necrosis and apoptosis (Koh & Padhani, 2006; Hochhegger et al., 2011). It has been hypothesized that tumours with low baseline pretreatment ADC values respond better to chemo- or radiotherapy than tumours with high ADC values. Tumours with high pretreatment ADC values are more likely to be necrotic, leading to decreased sensitivity to treatment. This is confirmed in a recent study by Ohno et al. (2012) including 64 patients with NSCLC, in which the ability of DW-MRI (ADC values) and FDG-PET/CT (SUVmax), respectively, to predict response to chemo radiotherapy was assessed. The authors found that both ADC and SUVmax could significantly differentiate between responders and non-responders. However, performing a ROC analysis, the area under the curve (AUC) was significantly larger when using ADC compared to SUV, suggesting that DW-MRI might have better potential for prediction of tumour response than FDG-PET/CT.
Recommendations
Data on functional imaging in therapy evaluation of patients with lung cancer are characterized by many smaller and promising studies, but randomized studies or studies assessing the potential impact on survival of functional imaging for this indication are lacking. Structural assessment by RECIST still is the gold standard, but with targeted therapy, targeted or tailored evaluation should follow, for example using DCE-CT for the evaluation of treatment with anti-angiogenic drugs, DW-MRI for drugs causing apoptosis and PET/CT for drugs targeting metabolism and proliferation. Common for all three modalities is an urgent need for standardization.
Discussion
In this paper, we have described three functional imaging modalities for use or potential use in the care of patients with lung cancer (Table1). The amount of evidence on the value of PET and PET/CT for diagnosing and staging lung cancer is huge, and PET now has its place in several recommendations and guidelines for this indication. In many of the studies cited in this paper, PET/CT is used as a reference standard for comparison with the newer modalities such as DCE-CT and DW-MRI. It is intriguing to compare these functional modalities, but it should be stressed that while all three modalities are indeed functional they measure different tissue characteristics, that is metabolism, perfusion and cellularity. By focusing only on their respective diagnostic value, we might miss important information from the combined assessment of different tissue characteristic (Kim et al., 2012).
Dynamic contrast-enhanced CT is a promising imaging modality in lung cancer. A number of smaller studies have successfully identified patients with lung cancer, patients with nodal involvement and patients who might benefit from specific treatment regimens. Only few reports have compared F-18-FDG-PET/CT with DCE-CT. A complex relationship between tumour glucose metabolism and tumour blood flow has been hypothesized (Miles & Williams, 2008). Thus, in a study of standardized uptake value (SUV) measured using F-18-FDG-PET and standardized perfusion value (SPV) measured using DCE-CT, Miles et al. reported a positive correlation between the ratio of SUV to SPV in lung cancer with higher values found in larger tumours. The authors also reported a significant correlation between SUV and SPV for tumours smaller than 4·5 cm2 (Miles et al., 2006).
Dynamic contrast-enhanced CT is challenged by technical difficulties, lack of reproducibility and a rather high radiation dose. Therefore, at present, the modality cannot be recommended for standard clinical use in suspected or known lung cancer. Future research and development should preferably include automatic segmentation of tumours with an acceptable reproducibility and tools to reduce motion artefacts. Until then, DCE-CT remains experimental and should only be used in research and in special situations.
Diffusion-weighted magnetic resonance imaging has already shown its usefulness in differentiation, response assessment and recurrence disease identification in primarily brain, head and neck, abdominal and pelvic tumours. Diagnostic value of lung MR imaging is still interrogative, but lack of radiation and no need for contrast material to be used in DW-MRI makes this technique very attractive, especially in cases where repetitive examinations should be performed. DWI could also be applied for patients with renal dysfunction. Early changes of the ADC values before evident morphological tumour tissue alterations could help to conduct chemo- and chemoradiotherapy in a more efficient way. Low spatial resolution is still considered to be a problem and probably could be overcome with the help of fusion T2 and DW images; in these terms, new techniques to subdue challenging MR respiratory and cardiac motion artefacts should be found.
More and more studies comparing DW-MRI and FDG-PET/CT are emerging. In a recent study comparing the performance of DW-MRI and FDG-PET/CT in mediastinal staging, the specificity of both DW-MRI and FDG-PET was hampered by the occurrence of inflammatory lymph nodes. However, only FDG-PET was false positive in the presence of anthracosilicosis, whereas DW-MRI was false positive in the presence of lymphatic oedemas and coagulation necrosis (Ohno et al., 2011a,b). Exciting data derived from a rat glioma model examining the treatment-associated inflammatory response by DW-MRI and FDG-PET suggest less profound effect of chemotherapy-associated immunological response on tumour diffusion compared to tumour FDG uptake after therapy (Galban et al., 2010). PET/MR would be the method of choice for a more thorough assessment of the differences and correlation between FDG-PET and DW-MRI, and implications for diagnosis and response evaluation.
Conclusion and perspectives
PET/CT still is a rational first choice for diagnosing and staging patients with lung cancer, however, data are emerging that DCE-CT could be a reasonable alternative or supplement for assessment of pulmonary nodules and perhaps mediastinal staging. Data are emerging that staging by DW-MRI could be a valuable alternative to PET/CT. For therapy evaluation, data are less mature for all three modalities, but for future clinical trials in this field, we should try to consider the three modalities not as competitors, but as complements evaluating different hallmarks of cancer biology. Especially within the field of therapy prediction and evaluation, an intelligent combination of different functional imaging modalities could prove very valuable (Reed et al., 2003), but also the addition of an extra functional parameter could increase diagnostic accuracy in many settings (Kim et al., 2012). With a more sophisticated use of combined PET/CT scanners and the emergence of PET/MR, this is not a pipe dream but can be done now.
Conflict of interests
The authors have no conflict of interest.
References
- Al-Sarraf N, Aziz R, Gately K, Lucey J, Wilson L, McGovern E, Young V. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg. 2008;33:104–109. doi: 10.1016/j.ejcts.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Antoch G, Stattaus J, Nemat AT, Marnitz S, Beyer T, Kuehl H, Bockisch A, Debatin JF, Freudenberg LS. Non-small cell lung cancer: dual-modality PET/CT in preoperative staging. Radiology. 2003;229:526–533. doi: 10.1148/radiol.2292021598. [DOI] [PubMed] [Google Scholar]
- Ashraf H, Dirksen A, Loft A, Bertelsen AK, Bach KS, Hansen H, Pedersen JH, Mortensen J. Combined use of positron emission tomography and volume doubling time in lung cancer screening with low-dose CT scanning. Thorax. 2011;66:315–319. doi: 10.1136/thx.2010.136747. [DOI] [PubMed] [Google Scholar]
- van Baardwijk A, Bosmans G, Dekker A, van Kroonenburgh M, Boersma L, Wanders S, Ollers M, Houben R, Minken A, Lambin P, de Ruysscher D. Time trends in the maximal uptake of FDG on PET scan during thoracic radiotherapy. A prospective study in locally advanced non-small cell lung cancer (NSCLC) patients. Radiother Oncol. 2007;82:145–152. doi: 10.1016/j.radonc.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Bar-Shalom R, Kagna O, Israel O, Guralnik L. Noninvasive diagnosis of solitary pulmonary lesions in cancer patients based on 2-fluoro-2-deoxy-D-glucose avidity on positron emission tomography/computed tomography. Cancer. 2008;113:3213–3221. doi: 10.1002/cncr.23928. [DOI] [PubMed] [Google Scholar]
- Bastarrika G, Garcia-Velloso MJ, Lozano MD, Montes U, Torre W, Spiteri N, Campo A, Seijo L, Alcaide AB, Pueyo J, Cano D, Vivas I, Cosin O, Dominguez P, Serra P, Richter JA, Montuenga L, Zulueta JJ. Early lung cancer detection using spiral computed tomography and positron emission tomography. Am J Respir Crit Care Med. 2005;171:1378–1383. doi: 10.1164/rccm.200411-1479OC. [DOI] [PubMed] [Google Scholar]
- Baxter CG, Bishop P, Low SE, Baiden-Amissah K, Denning DW. Pulmonary aspergillosis: an alternative diagnosis to lung cancer after positive [18F]FDG positron emission tomography. Thorax. 2011;66:638–640. doi: 10.1136/thx.2010.155515. [DOI] [PubMed] [Google Scholar]
- Bellomi M, Viotti S, Preda L, D'andrea G, Bonello L, Petralia G. Perfusion CT in solid body-tumours. Part II: Clinical applications and future development. Radiol Med. 2010;115:858–874. doi: 10.1007/s11547-010-0545-9. [DOI] [PubMed] [Google Scholar]
- Benz MR, Herrmann K, Walter F, Garon EB, Reckamp KL, Figlin R, Phelps ME, Weber WA, Czernin J, Allen-Auerbach MS. (18)F-FDG PET/CT for monitoring treatment responses to the epidermal growth factor receptor inhibitor erlotinib. J Nucl Med. 2011;52:1684–1689. doi: 10.2967/jnumed.111.095257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, Jerin J, Young J, Byars L, Nutt R. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–1379. [PubMed] [Google Scholar]
- Biederer J, Beer M, Hirsch W, Wild J, Fabel M, Puderbach M, van Beek EJ. MRI of the lung (2/3). Why.. when.. how? Insights Imaging. 2012a;3:355–371. doi: 10.1007/s13244-011-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer J, Mirsadraee S, Beer M, Molinari F, Hintze C, Bauman G, Both M, van Beek EJ, Wild J, Puderbach M. MRI of the lung (3/3)-current applications and future perspectives. Insights Imaging. 2012b;3:373–386. doi: 10.1007/s13244-011-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boellaard R, O'doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, Oyen WJ, Kotzerke J, Hoekstra OS, Pruim J, Marsden PK, Tatsch K, Hoekstra CJ, Visser EP, Arends B, Verzijlbergen FJ, Zijlstra JM, Comans EF, Lammertsma AA, Paans AM, Willemsen AT, Beyer T, Bockisch A, Schaefer-Prokop C, Delbeke D, Baum RP, Chiti A, Krause BJ. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:181–200. doi: 10.1007/s00259-009-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonekamp S, Corona-Villalobos CP, Kamel IR. Oncologic applications of diffusion-weighted MRI in the body. J Magn Reson Imaging. 2012;35:257–279. doi: 10.1002/jmri.22786. [DOI] [PubMed] [Google Scholar]
- Cerfolio RJ, Ojha B, Bryant AS, Raghuveer V, Mountz JM, Bartolucci AA. The accuracy of integrated PET-CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancer. Ann Thorac Surg. 2004;78:1017–1023. doi: 10.1016/j.athoracsur.2004.02.067. ; discussion 1017-23. [DOI] [PubMed] [Google Scholar]
- Chae EJ, Song JW, Seo JB, Krauss B, Jang YM, Song KS. Clinical utility of dual-energy CT in the evaluation of solitary pulmonary nodules: initial experience. Radiology. 2008;249:671–681. doi: 10.1148/radiol.2492071956. [DOI] [PubMed] [Google Scholar]
- Chae EJ, Song JW, Krauss B, Song KS, Lee CW, Lee HJ, Seo JB. Dual-energy computed tomography characterization of solitary pulmonary nodules. J Thorac Imaging. 2010;25:301–310. doi: 10.1097/RTI.0b013e3181e16232. [DOI] [PubMed] [Google Scholar]
- Charles-Edwards EM, deSouza NM. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging. 2006;6:135–143. doi: 10.1102/1470-7330.2006.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coche E. Advances and perspectives in lung cancer imaging using multidetector row computed tomography. Expert Rev Anticancer Ther. 2012;12:1313–1326. doi: 10.1586/era.12.112. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, Nur U, Tracey E, Coory M, Hatcher J, McGahan CE, Turner D, Marrett L, Gjerstorff ML, Johannesen TB, Adolfsson J, Lambe M, Lawrence G, Meechan D, Morris EJ, Middleton R, Steward J, Richards MA ICBP Module 1 Working Group. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–138. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin P, Dwamena BA, Kelly AM, Carlos RC. Solitary pulmonary nodules: meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy. Radiology. 2008;246:772–782. doi: 10.1148/radiol.2463062148. [DOI] [PubMed] [Google Scholar]
- Cronin CG, Prakash P, Blake MA. Oral and IV contrast agents for the CT portion of PET/CT. AJR Am J Roentgenol. 2010;195:W5–W13. doi: 10.2214/AJR.09.3844. [DOI] [PubMed] [Google Scholar]
- D'addario G, Fruh M, Reck M, Baumann P, Klepetko W, Felip E ESMO Guidelines Working Group. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl. 5):v116–v119. doi: 10.1093/annonc/mdq189. [DOI] [PubMed] [Google Scholar]
- Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA American College of Chest Physicians. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(Suppl. 3):202S–220S. doi: 10.1378/chest.07-1362. [DOI] [PubMed] [Google Scholar]
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- Dinan MA, Curtis LH, Carpenter WR, Biddle AK, Abernethy AP, Patz EF, Jr, Schulman KA, Weinberger M. Stage migration, selection bias, and survival associated with the adoption of positron emission tomography among medicare beneficiaries with non-small-cell lung cancer, 1998-2003. J Clin Oncol. 2012;30:2725–2730. doi: 10.1200/JCO.2011.40.4392. [DOI] [PubMed] [Google Scholar]
- Dwamena BA, Sonnad SS, Angobaldo JO, Wahl RL. Metastases from non-small cell lung cancer: mediastinal staging in the 1990s–meta-analytic comparison of PET and CT. Radiology. 1999;213:530–536. doi: 10.1148/radiology.213.2.r99nv46530. [DOI] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Erasmus JJ, Gladish GW, Broemeling L, Sabloff BS, Truong MT, Herbst RS, Munden RF. Interobserver and intraobserver variability in measurement of non-small-cell carcinoma lung lesions: implications for assessment of tumor response. J Clin Oncol. 2003;21:2574–2582. doi: 10.1200/JCO.2003.01.144. [DOI] [PubMed] [Google Scholar]
- Fischer BM, Mortensen J. The future in diagnosis and staging of lung cancer: positron emission tomography. Respiration. 2006;73:267–276. doi: 10.1159/000092080. [DOI] [PubMed] [Google Scholar]
- Fischer BM, Mortensen J. The role of positron emission tomography for evaluation of lung nodules and staging lung cancer. Curr Respir Care Rep. 2012;1:30. [Google Scholar]
- Fischer BM, Mortensen J, Hojgaard L. Positron emission tomography in the diagnosis and staging of lung cancer: a systematic, quantitative review. Lancet Oncol. 2001;2:659–666. doi: 10.1016/S1470-2045(01)00555-1. [DOI] [PubMed] [Google Scholar]
- Fischer BM, Mortensen J, Langer SW, Loft A, Berthelsen AK, Petersen BI, Daugaard G, Lassen U, Hansen HH. A prospective study of PET/CT in initial staging of small-cell lung cancer: comparison with CT, bone scintigraphy and bone marrow analysis. Ann Oncol. 2007;18:338–345. doi: 10.1093/annonc/mdl374. [DOI] [PubMed] [Google Scholar]
- Fischer B, Lassen U, Mortensen J, Larsen S, Loft A, Bertelsen A, Ravn J, Clementsen P, Hogholm A, Larsen K, Rasmussen T, Keiding S, Dirksen A, Gerke O, Skov B, Steffensen I, Hansen H, Vilmann P, Jacobsen G, Backer V, Maltbaek N, Pedersen J, Madsen H, Nielsen H, Hojgaard L. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med. 2009;361:32–39. doi: 10.1056/NEJMoa0900043. [DOI] [PubMed] [Google Scholar]
- Fischer BM, Mortensen J, Hansen H, Vilmann P, Larsen SS, Loft A, Bertelsen AK, Ravn J, Clementsen P, Hoegholm A, Larsen KR, Dirksen A, Skov BG, Krasnik M, Hojgaard L, Lassen U. Multimodality approach to mediastinal staging in non-small cell lung cancer. Faults and benefits of PET-CT: a randomised trial. Thorax. 2011;66:294–300. doi: 10.1136/thx.2010.154476. [DOI] [PubMed] [Google Scholar]
- Fraioli F, Anzidei M, Zaccagna F, Mennini ML, Serra G, Gori B, Longo F, Catalano C, Passariello R. Whole-tumor perfusion CT in patients with advanced lung adenocarcinoma treated with conventional and antiangiogenetic chemotherapy: initial experience. Radiology. 2011a;259:574–582. doi: 10.1148/radiol.11100600. [DOI] [PubMed] [Google Scholar]
- Fraioli F, Vetere S, Anile M, Venuta F. Computed tomography perfusion: a new method to evaluate response to therapy in lung cancer. J Thorac Oncol. 2011b;6:1599–1600. doi: 10.1097/JTO.0b013e3182259207. [DOI] [PubMed] [Google Scholar]
- Galban CJ, Bhojani MS, Lee KC, Meyer CR, van Dort ME, Kuszpit KK, Koeppe RA, Ranga R, Moffat BA, Johnson TD, Chenevert TL, Rehemtulla A, Ross BD. Evaluation of treatment-associated inflammatory response on diffusion-weighted magnetic resonance imaging and 2-[18F]-fluoro-2-deoxy-D-glucose-positron emission tomography imaging biomarkers. Clin Cancer Res. 2010;16:1542–1552. doi: 10.1158/1078-0432.CCR-08-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001;285:914–924. doi: 10.1001/jama.285.7.914. [DOI] [PubMed] [Google Scholar]
- Gould MK, Kuschner WG, Rydzak CE, Maclean CC, Demas AN, Shigemitsu H, Chan JK, Owens DK. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med. 2003;139:879–892. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- Gould MK, Ananth L, Barnett PG Veterans Affairs Snap Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007a;131:383–388. doi: 10.1378/chest.06-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould MK, Fletcher J, Iannettoni MD, Lynch WR, Midthun DE, Naidich DP, Ost DE American College of Chest Physicians. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007b;132(Suppl. 3):108S–130S. doi: 10.1378/chest.07-1353. [DOI] [PubMed] [Google Scholar]
- Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, Wiener RS. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(Suppl. 5):e93S–e120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory DL, Hicks RJ, Hogg A, Binns DS, Shum PL, Milner A, Link E, Ball DL, Mac Manus MP. Effect of PET/CT on management of patients with non-small cell lung cancer: results of a prospective study with 5-year survival data. J Nucl Med. 2012;53:1007–1015. doi: 10.2967/jnumed.111.099713. [DOI] [PubMed] [Google Scholar]
- Gumustas S, Inan N, Akansel G, Ciftci E, Demirci A, Ozkara SK. Differentiation of malignant and benign lung lesions with diffusion-weighted MR imaging. Radiol Oncol. 2012;46:106–113. doi: 10.2478/v10019-012-0021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern BS, Schiepers C, Weber WA, Crawford TL, Fueger BJ, Phelps ME, Czernin J. Presurgical staging of non-small cell lung cancer: positron emission tomography, integrated positron emission tomography/CT, and software image fusion. Chest. 2005;128:2289–2297. doi: 10.1378/chest.128.4.2289. [DOI] [PubMed] [Google Scholar]
- Harders SW, Madsen HH, Hjorthaug K, Arveschoug AK, Rasmussen TR, Meldgaard P, Andersen JB, Pilegaard HK, Hager H, Rehling M, Rasmussen F. Characterization of pulmonary lesions in patients with suspected lung cancer: computed tomography versus [(1)(8)F] fluorodeoxyglucose-positron emission tomography/computed tomography. Cancer Imaging. 2012;12:437–446. doi: 10.1102/1470-7330.2012.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry VN, Semple SI, Gilbert FJ, Parkin DE. Diffusion-weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol Oncol. 2008;111:213–220. doi: 10.1016/j.ygyno.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Hasegawa I, Boiselle PM, Kuwabara K, Sawafuji M, Sugiura H. Mediastinal lymph nodes in patients with non-small cell lung cancer: preliminary experience with diffusion-weighted MR imaging. J Thorac Imaging. 2008;23:157–161. doi: 10.1097/RTI.0b013e318166d2f5. [DOI] [PubMed] [Google Scholar]
- Hegenscheid K, Behrendt N, Rosenberg C, Kuehn JP, Ewert R, Hosten N, Puls R. Assessing early vascular changes and treatment response after laser-induced thermotherapy of pulmonary metastases with perfusion CT: initial experience. AJR Am J Roentgenol. 2010;194:1116–1123. doi: 10.2214/AJR.09.2810. [DOI] [PubMed] [Google Scholar]
- Herrmann K, Benz MR, Krause BJ, Pomykala KL, Buck AK, Czernin J. (18)F-FDG-PET/CT in evaluating response to therapy in solid tumors: where we are and where we can go. Q J Nucl Med Mol Imaging. 2011;55:620–632. [PubMed] [Google Scholar]
- Higashi K, Clavo AC, Wahl RL. Does FDG uptake measure proliferative activity of human cancer cells? In vitro comparison with DNA flow cytometry and tritiated thymidine uptake. J Nucl Med. 1993;34:414–419. [PubMed] [Google Scholar]
- Higashi K, Matsunari I, Ueda Y, Ikeda R, Guo J, Oguchi M, Tonami H, Yamamoto I. Value of hole-body FDG PET in management of lung cancer. Ann Nucl Med. 2003;17:1–14. doi: 10.1007/BF02988253. [DOI] [PubMed] [Google Scholar]
- Hochhegger B, Marchiori E, Sedlaczek O, Irion K, Heussel CP, Ley S, Ley-Zaporozhan J, Soares Souza A, Jr, Kauczor HU. MRI in lung cancer: a pictorial essay. Br J Radiol. 2011;84:661–668. doi: 10.1259/bjr/24661484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra CJ, Hoekstra OS, Stroobants SG, Vansteenkiste J, Nuyts J, Smit EF, Boers M, Twisk JW, Lammertsma AA. Methods to monitor response to chemotherapy in non-small cell lung cancer with 18F-FDG PET. J Nucl Med. 2002;43:1304–1309. [PubMed] [Google Scholar]
- Hofman MS, Ball DL, Mac Manus MP, Ware R, Hicks RJ. Let's get SEERious: more accurate staging with consequent high management impact is not just stage migration. J Clin Oncol. 2013;31:819. doi: 10.1200/JCO.2012.46.5872. [DOI] [PubMed] [Google Scholar]
- Inan N, Kilinc F, Sarisoy T, Gumustas S, Akansel G, Demirci A. Diffusion weighted MR imaging in the differential diagnosis of haemangiomas and metastases of the liver. Radiol Oncol. 2010;44:24–29. doi: 10.2478/v10019-010-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SY, Lee KS, Shin KM, Bae YA, Kim BT, Choe BK, Kim TS, Chung MJ. Efficacy of PET/CT in the characterization of solid or partly solid solitary pulmonary nodules. Lung Cancer. 2008;61:186–194. doi: 10.1016/j.lungcan.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 2006;354:496–507. doi: 10.1056/NEJMra050276. [DOI] [PubMed] [Google Scholar]
- Kagna O, Solomonov A, Keidar Z, Bar-Shalom R, Fruchter O, Yigla M, Israel O, Guralnik L. The value of FDG-PET/CT in assessing single pulmonary nodules in patients at high risk of lung cancer. Eur J Nucl Med Mol Imaging. 2009;36:997–1004. doi: 10.1007/s00259-009-1061-9. [DOI] [PubMed] [Google Scholar]
- Kiessling F, Boese J, Corvinus C, Ederle JR, Zuna I, Schoenberg SO, Brix G, Schmahl A, Tuengerthal S, Herth F, Kauczor HU, Essig M. Perfusion CT in patients with advanced bronchial carcinomas: a novel chance for characterization and treatment monitoring? Eur Radiol. 2004;14:1226–1233. doi: 10.1007/s00330-004-2288-2. [DOI] [PubMed] [Google Scholar]
- Kim SK, Allen-Auerbach M, Goldin J, Fueger BJ, Dahlbom M, Brown M, Czernin J, Schiepers C. Accuracy of PET/CT in characterization of solitary pulmonary lesions. J Nucl Med. 2007;48:214–220. [PubMed] [Google Scholar]
- Kim S, Loevner L, Quon H, Sherman E, Weinstein G, Kilger A, Poptani H. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res. 2009;15:986–994. doi: 10.1158/1078-0432.CCR-08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YN, Yi CA, Lee KS, Kwon OJ, Lee HY, Kim BT, Choi JY, Kim SW, Chung MP, Han J, Kim TS, Chung MJ, Shim YM. A proposal for combined MRI and PET/CT interpretation criteria for preoperative nodal staging in non-small-cell lung cancer. Eur Radiol. 2012;22:1537–1546. doi: 10.1007/s00330-012-2388-3. [DOI] [PubMed] [Google Scholar]
- Koh DM, Padhani AR. Diffusion-weighted MRI: a new functional clinical technique for tumour imaging. Br J Radiol. 2006;79:633–635. doi: 10.1259/bjr/29739265. [DOI] [PubMed] [Google Scholar]
- Kong FM, Frey KA, Quint LE, ten Haken RK, Hayman JA, Kessler M, Chetty IJ, Normolle D, Eisbruch A, Lawrence TS. A pilot study of [18F]fluorodeoxyglucose positron emission tomography scans during and after radiation-based therapy in patients with non small-cell lung cancer. J Clin Oncol. 2007;25:3116–3123. doi: 10.1200/JCO.2006.10.3747. [DOI] [PubMed] [Google Scholar]
- Kruger S, Mottaghy FM, Buck AK, Maschke S, Kley H, Frechen D, Wibmer T, Reske SN, Pauls S. Brain metastasis in lung cancer. Comparison of cerebral MRI and 18F-FDG-PET/CT for diagnosis in the initial staging. Nuklearmedizin. 2011;50:101–106. doi: 10.3413/Nukmed-0338-10-07. [DOI] [PubMed] [Google Scholar]
- Kwee TC, Takahara T, Ochiai R, Nievelstein RA, Luijten PR. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol. 2008;18:1937–1952. doi: 10.1007/s00330-008-0968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwek BH, Aquino SL, Fischman AJ. Fluorodeoxyglucose positron emission tomography and CT after talc pleurodesis. Chest. 2004;125:2356–2360. doi: 10.1378/chest.125.6.2356. [DOI] [PubMed] [Google Scholar]
- Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, von Schulthess GK, Steinert HC. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–2507. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- de Leyn P, Lardinois D, van Schil PE, Rami-Porta R, Passlick B, Zielinski M, Waller DA, Lerut T, Weder W. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg. 2007;32:1–8. doi: 10.1016/j.ejcts.2007.01.075. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang ZG, Chen TW, Chen HJ, Sun JY, Lu YR. Peripheral lung carcinoma: correlation of angiogenesis and first-pass perfusion parameters of 64-detector row CT. Lung Cancer. 2008a;61:44–53. doi: 10.1016/j.lungcan.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang ZG, Chen TW, Deng YP, Yu JQ, Li ZL. Whole tumour perfusion of peripheral lung carcinoma: evaluation with first-pass CT perfusion imaging at 64-detector row CT. Clin Radiol. 2008b;63:629–635. doi: 10.1016/j.crad.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang ZG, Chen TW, Yu JQ, Sun JY, Chen HJ. First-pass perfusion imaging of solitary pulmonary nodules with 64-detector row CT: comparison of perfusion parameters of malignant and benign lesions. Br J Radiol. 2010;83:785–790. doi: 10.1259/bjr/58020866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind JS, Meijerink MR, Dingemans AM, van Kuijk C, Ollers MC, de Ruysscher D, Postmus PE, Smit EF. Dynamic contrast-enhanced CT in patients treated with sorafenib and erlotinib for non-small cell lung cancer: a new method of monitoring treatment? Eur Radiol. 2010;20:2890–2898. doi: 10.1007/s00330-010-1869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell RM, Hartman TE, Swensen SJ, Jett JR, Midthun DE, Nathan MA, Lowe VJ. Lung cancer screening experience: a retrospective review of PET in 22 non-small cell lung carcinomas detected on screening chest CT in a high-risk population. AJR Am J Roentgenol. 2005;185:126–131. doi: 10.2214/ajr.185.1.01850126. [DOI] [PubMed] [Google Scholar]
- Mac Manus MP, Hicks RJ, Matthews JP, Wirth A, Rischin D, Ball DL. Metabolic (FDG-PET) response after radical radiotherapy/chemoradiotherapy for non-small cell lung cancer correlates with patterns of failure. Lung Cancer. 2005;49:95–108. doi: 10.1016/j.lungcan.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Malayeri AA, el Khouli RH, Zaheer A, Jacobs MA, Corona-Villalobos CP, Kamel IR, Macura KJ. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics. 2011;31:1773–1791. doi: 10.1148/rg.316115515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marom EM, Sarvis S, Herndon JE, II, Patz EF., Jr T1 lung cancers: sensitivity of diagnosis with fluorodeoxyglucose PET. Radiology. 2002;223:453–459. doi: 10.1148/radiol.2232011131. [DOI] [PubMed] [Google Scholar]
- Maziak DE, Darling GE, Inculet RI, Gulenchyn KY, Driedger AA, Ung YC, Miller JD, Gu CS, Cline KJ, Evans WK, Levine MN. Positron emission tomography in staging early lung cancer: a randomized trial. Ann Intern Med. 2009;151:221–228. doi: 10.7326/0003-4819-151-4-200908180-00132. , W-48. [DOI] [PubMed] [Google Scholar]
- Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E. 18F-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med. 2006;47:32–37. [PubMed] [Google Scholar]
- Miles KA. Tumour angiogenesis and its relation to contrast enhancement on computed tomography: a review. Eur J Radiol. 1999;30:198–205. doi: 10.1016/s0720-048x(99)00012-1. [DOI] [PubMed] [Google Scholar]
- Miles KA, Williams RE. Warburg revisited: imaging tumour blood flow and metabolism. Cancer Imaging. 2008;8:81–86. doi: 10.1102/1470-7330.2008.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles KA, Hayball M, Dixon AK. Colour perfusion imaging: a new application of computed tomography. Lancet. 1991;337:643–645. doi: 10.1016/0140-6736(91)92455-b. [DOI] [PubMed] [Google Scholar]
- Miles KA, Hayball MP, Dixon AK. Functional images of hepatic perfusion obtained with dynamic CT. Radiology. 1993;188:405–411. doi: 10.1148/radiology.188.2.8327686. [DOI] [PubMed] [Google Scholar]
- Miles K, Dawson PH, Blomley M. Functional Computed Tomography. Oxford: Isis Medical Media; 1997. [Google Scholar]
- Miles KA, Charnsangavej C, Lee FT, Fishman EK, Horton K, Lee TY. Application of CT in the investigation of angiogenesis in oncology. Acad Radiol. 2000;7:840–850. doi: 10.1016/s1076-6332(00)80632-7. [DOI] [PubMed] [Google Scholar]
- Miles KA, Griffiths MR, Keith CJ. Blood flow-metabolic relationships are dependent on tumour size in non-small cell lung cancer: a study using quantitative contrast-enhanced computer tomography and positron emission tomography. Eur J Nucl Med Mol Imaging. 2006;33:22–28. doi: 10.1007/s00259-005-1932-7. [DOI] [PubMed] [Google Scholar]
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Mori T, Nomori H, Ikeda K, Kawanaka K, Shiraishi S, Katahira K, Yamashita Y. Diffusion-weighted magnetic resonance imaging for diagnosing malignant pulmonary nodules/masses: comparison with positron emission tomography. J Thorac Oncol. 2008;3:358–364. doi: 10.1097/JTO.0b013e318168d9ed. [DOI] [PubMed] [Google Scholar]
- Mountain CF. A new international staging system for lung cancer. Chest. 1986;89(Suppl. 4):225S–233S. doi: 10.1378/chest.89.4_supplement.225s. [DOI] [PubMed] [Google Scholar]
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- Nakayama J, Miyasaka K, Omatsu T, Onodera Y, Terae S, Matsuno Y, Cho Y, Hida Y, Kaga K, Shirato H. Metastases in mediastinal and hilar lymph nodes in patients with non-small cell lung cancer: quantitative assessment with diffusion-weighted magnetic resonance imaging and apparent diffusion coefficient. J Comput Assist Tomogr. 2010;34:1–8. doi: 10.1097/RCT.0b013e3181a9cc07. [DOI] [PubMed] [Google Scholar]
- Nasu K, Kuroki Y, Kuroki S, Murakami K, Nawano S, Moriyama N. Diffusion-weighted single shot echo planar imaging of colorectal cancer using a sensitivity-encoding technique. Jpn J Clin Oncol. 2004;34:620–626. doi: 10.1093/jjco/hyh108. [DOI] [PubMed] [Google Scholar]
- Ng CS, Chandler AG, Wei W, Anderson EF, Herron DH, Charnsangavej C, Kurzrock R. Reproducibility of perfusion parameters obtained from perfusion CT in lung tumors. AJR Am J Roentgenol. 2011;197:113–121. doi: 10.2214/AJR.10.5404. [DOI] [PubMed] [Google Scholar]
- Nguyen NC, Kaushik A, Wolverson MK, Osman MM. Is there a common SUV threshold in oncological FDG PET/CT, at least for some common indications? A retrospective study. Acta Oncol. 2011;50:670–677. doi: 10.3109/0284186X.2010.550933. [DOI] [PubMed] [Google Scholar]
- Nomori H, Mori T, Ikeda K, Kawanaka K, Shiraishi S, Katahira K, Yamashita Y. Diffusion-weighted magnetic resonance imaging can be used in place of positron emission tomography for N staging of non-small cell lung cancer with fewer false-positive results. J Thorac Cardiovasc Surg. 2008;135:816–822. doi: 10.1016/j.jtcvs.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Ohba Y, Nomori H, Mori T, Ikeda K, Shibata H, Kobayashi H, Shiraishi S, Katahira K. Is diffusion-weighted magnetic resonance imaging superior to positron emission tomography with fludeoxyglucose F 18 in imaging non-small cell lung cancer? J Thorac Cardiovasc Surg. 2009;138:439–445. doi: 10.1016/j.jtcvs.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Koyama H, Matsumoto K, Onishi Y, Takenaka D, Fujisawa Y, Yoshikawa T, Konishi M, Maniwa Y, Nishimura Y, Ito T, Sugimura K. Differentiation of malignant and benign pulmonary nodules with quantitative first-pass 320-detector row perfusion CT versus FDG PET/CT. Radiology. 2011a;258:599–609. doi: 10.1148/radiol.10100245. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Koyama H, Yoshikawa T, Nishio M, Aoyama N, Onishi Y, Takenaka D, Matsumoto S, Maniwa Y, Nishio W, Nishimura Y, Itoh T, Sugimura K. N stage disease in patients with non-small cell lung cancer: efficacy of quantitative and qualitative assessment with STIR turbo spin-echo imaging, diffusion-weighted MR imaging, and fluorodeoxyglucose PET/CT. Radiology. 2011b;261:605–615. doi: 10.1148/radiol.11110281. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Koyama H, Yoshikawa T, Matsumoto K, Aoyama N, Onishi Y, Sugimura K. Diffusion-weighted MRI versus 18F-FDG PET/CT: Performance as predictors of tumor treatment response and patient survival in patients with non-small cell lung cancer receiving chemoradiotherapy. AJR Am J Roentgenol. 2012;198:75–82. doi: 10.2214/AJR.11.6525. [DOI] [PubMed] [Google Scholar]
- Ozcan Kara P, Kara T, Kara Gedik G, Kara F, Sahin O, Ceylan Gunay E, Sari O. The role of fluorodeoxyglucose-positron emission tomography/computed tomography in differentiating between benign and malignant adrenal lesions. Nucl Med Commun. 2011;32:106–112. doi: 10.1097/MNM.0b013e32834199e7. [DOI] [PubMed] [Google Scholar]
- Palshof T, Jakobsen E. Lungecancer årsrapport 2011. Dansk Lunge Cancer Gruppe; 2010. http://www.lungecancer.dk. [Google Scholar]
- Parikh T, Drew SJ, Lee VS, Wong S, Hecht EM, Babb JS, Taouli B. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008;246:812–822. doi: 10.1148/radiol.2463070432. [DOI] [PubMed] [Google Scholar]
- Pastorino U. Lung cancer screening. Br J Cancer. 2010;102:1681–1686. doi: 10.1038/sj.bjc.6605660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino U, Bellomi M, Landoni C, de Fiori E, Arnaldi P, Picchio M, Pelosi G, Boyle P, Fazio F. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet. 2003;362:593–597. doi: 10.1016/S0140-6736(03)14188-8. [DOI] [PubMed] [Google Scholar]
- Pauls S, Schmidt SA, Juchems MS, Klass O, Luster M, Reske SN, Brambs HJ, Feuerlein S. Diffusion-weighted MR imaging in comparison to integrated [(1)(8)F]-FDG PET/CT for N-staging in patients with lung cancer. Eur J Radiol. 2012;81:178–182. doi: 10.1016/j.ejrad.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Pauwels EK, Sturm EJ, Bombardieri E, Cleton FJ, Stokkel MP. Positron-emission tomography with [18F]fluorodeoxyglucose. Part I. Biochemical uptake mechanism and its implication for clinical studies. J Cancer Res Clin Oncol. 2000;126:549–559. doi: 10.1007/PL00008465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia G, Bonello L, Viotti S, Preda L, D'andrea G, Bellomi M. CT perfusion in oncology: how to do it. Cancer Imaging. 2010a;10:8–19. doi: 10.1102/1470-7330.2010.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia G, Preda L, D'andrea G, Viotti S, Bonello L, de Filippi R, Bellomi M. CT perfusion in solid-body tumours. Part I: technical issues. Radiol Med. 2010b;115:843–857. doi: 10.1007/s11547-010-0519-y. [DOI] [PubMed] [Google Scholar]
- Pfannenberg AC, Aschoff P, Brechtel K, Muller M, Bares R, Paulsen F, Scheiderbauer J, Friedel G, Claussen CD, Eschmann SM. Low dose non-enhanced CT versus standard dose contrast-enhanced CT in combined PET/CT protocols for staging and therapy planning in non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2007a;34:36–44. doi: 10.1007/s00259-006-0186-3. [DOI] [PubMed] [Google Scholar]
- Pfannenberg AC, Aschoff P, Brechtel K, Muller M, Klein M, Bares R, Claussen CD, Eschmann SM. Value of contrast-enhanced multiphase CT in combined PET/CT protocols for oncological imaging. Br J Radiol. 2007b;80:437–445. doi: 10.1259/bjr/34082277. [DOI] [PubMed] [Google Scholar]
- Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics. 2009;29:1797–1810. doi: 10.1148/rg.296095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CE, Harpole DH, Posther KE, Woolson SL, Downey RJ, Meyers BF, Heelan RT, Macapinlac HA, Jung SH, Silvestri GA, Siegel BA, Rusch VW American College Of Surgeons Oncology Group Z0050 Trial. Results of the American College of Surgeons Oncology Group Z0050 trial: the utility of positron emission tomography in staging potentially operable non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;126:1943–1951. doi: 10.1016/j.jtcvs.2003.07.030. [DOI] [PubMed] [Google Scholar]
- Sandler MP. Diagnostic Nuclear Medicine. 4th edn. Philadelphia: Lippincott/Williams & Wilkins; 2003. [Google Scholar]
- Sauter AW, Merkle A, Schulze M, Spira D, Hetzel J, Claussen CD, Horger MS. Intraobserver and interobserver agreement of volume perfusion CT (VPCT) measurements in patients with lung lesions. Eur J Radiol. 2012a;81:2853–2859. doi: 10.1016/j.ejrad.2011.06.047. [DOI] [PubMed] [Google Scholar]
- Sauter AW, Winterstein S, Spira D, Hetzel J, Schulze M, Mueller M, Pfannenberg C, Claussen CD, Klotz E, Hann von Weyhern C, Horger MS. Multifunctional profiling of non-small cell lung cancer using 18F-FDG PET/CT and volume perfusion CT. J Nucl Med. 2012b;53:521–529. doi: 10.2967/jnumed.111.097865. [DOI] [PubMed] [Google Scholar]
- Sauter AW, Spira D, Schulze M, Pfannenberg C, Hetzel J, Reimold M, Klotz E, Claussen CD, Horger MS. Correlation between [(1)(8)F]FDG PET/CT and volume perfusion CT in primary tumours and mediastinal lymph nodes of non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2013;40:677–684. doi: 10.1007/s00259-012-2318-2. [DOI] [PubMed] [Google Scholar]
- Schreiter N, Nogami M, Buchert R, Froeling V, Brenner W, Diekmann F. Pulmonary FDG uptake without a CT counterpart - a pitfall in interpreting PET/CT images. Acta Radiol. 2011;52:513–515. doi: 10.1258/ar.2011.100448. [DOI] [PubMed] [Google Scholar]
- Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, Harris LJ, Detterbeck FC. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(Suppl. 5):e211S–e250S. doi: 10.1378/chest.12-2355. [DOI] [PubMed] [Google Scholar]
- Sitartchouk I, Roberts HC, Pereira AM, Bayanati H, Waddell T, Roberts TP. Computed tomography perfusion using first pass methods for lung nodule characterization. Invest Radiol. 2008;43:349–358. doi: 10.1097/RLI.0b013e3181690148. [DOI] [PubMed] [Google Scholar]
- Sommer G, Wiese M, Winter L, Lenz C, Klarhofer M, Forrer F, Lardinois D, Bremerich J. Preoperative staging of non-small-cell lung cancer: comparison of whole-body diffusion-weighted magnetic resonance imaging and 18F-fluorodeoxyglucose-positron emission tomography/computed tomography. Eur Radiol. 2012;22:2859–2867. doi: 10.1007/s00330-012-2542-y. [DOI] [PubMed] [Google Scholar]
- Song JW, Oh YM, Shim TS, Kim WS, Ryu JS, Choi CM. Efficacy comparison between (18)F-FDG PET/CT and bone scintigraphy in detecting bony metastases of non-small-cell lung cancer. Lung Cancer. 2009;65:333–338. doi: 10.1016/j.lungcan.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Stroobants S, Verschakelen J, Vansteenkiste J. Value of FDG-PET in the management of non-small cell lung cancer. Eur J Radiol. 2003;45:49–59. doi: 10.1016/s0720-048x(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Tacelli N, Remy-Jardin M, Copin MC, Scherpereel A, Mensier E, Jaillard S, Lafitte JJ, Klotz E, Duhamel A, Remy J. Assessment of non-small cell lung cancer perfusion: pathologic-CT correlation in 15 patients. Radiology. 2010;257:863–871. doi: 10.1148/radiol.10100181. [DOI] [PubMed] [Google Scholar]
- Tateishi U, Kusumoto M, Nishihara H, Nagashima K, Morikawa T, Moriyama N. Contrast-enhanced dynamic computed tomography for the evaluation of tumor angiogenesis in patients with lung carcinoma. Cancer. 2002;95:835–842. doi: 10.1002/cncr.10730. [DOI] [PubMed] [Google Scholar]
- Thie JA. Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med. 2004;45:1431–1434. [PubMed] [Google Scholar]
- van Tinteren H, Hoekstra OS, Smit EF, van den Bergh JH, Schreurs AJ, Stallaert RA, van Velthoven PC, Comans EF, Diepenhorst FW, Verboom P, van Mourik JC, Postmus PE, Boers M, Teule GJ. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002;359:1388–1393. doi: 10.1016/s0140-6736(02)08352-6. [DOI] [PubMed] [Google Scholar]
- Truong MT, Erasmus JJ, Macapinlac HA, Marom EM, Mawlawi O, Gladish GW, Sabloff BS, Bruzzi JF, Munden RF. Integrated positron emission tomography/computed tomography in patients with non-small cell lung cancer: normal variants and pitfalls. J Comput Assist Tomogr. 2005;29:205–209. doi: 10.1097/01.rct.0000159510.13694.8c. [DOI] [PubMed] [Google Scholar]
- Vansteenkiste J, Fischer BM, Dooms C, Mortensen J. Positron-emission tomography in prognostic and therapeutic assessment of lung cancer: systematic review. Lancet Oncol. 2004;5:531–540. doi: 10.1016/S1470-2045(04)01564-5. [DOI] [PubMed] [Google Scholar]
- Wahidi MM, Govert JA, Goudar RK, Gould MK, McCrory DC American College of Chest Physicians. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(Suppl. 3):94S–107S. doi: 10.1378/chest.07-1352. [DOI] [PubMed] [Google Scholar]
- Wang J, Wu N, Cham MD, Song Y. Tumor response in patients with advanced non-small cell lung cancer: perfusion CT evaluation of chemotherapy and radiation therapy. AJR Am J Roentgenol. 2009;193:1090–1096. doi: 10.2214/AJR.08.1367. [DOI] [PubMed] [Google Scholar]
- Weber WA. Use of PET for monitoring cancer therapy and for predicting outcome. J Nucl Med. 2005;46:983–995. [PubMed] [Google Scholar]
- Weber WA, Figlin R. Monitoring cancer treatment with PET/CT: does it make a difference? J Nucl Med. 2007;48(Suppl. 1):36S–44S. [PubMed] [Google Scholar]
- Weber WA, Petersen V, Schmidt B, Tyndale-Hines L, Link T, Peschel C, Schwaiger M. Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21:2651–2657. doi: 10.1200/JCO.2003.12.004. [DOI] [PubMed] [Google Scholar]
- de Wever W, Ceyssens S, Mortelmans L, Stroobants S, Marchal G, Bogaert J, Verschakelen JA. Additional value of PET-CT in the staging of lung cancer: comparison with CT alone, PET alone and visual correlation of PET and CT. Eur Radiol. 2007;17:23–32. doi: 10.1007/s00330-006-0284-4. [DOI] [PubMed] [Google Scholar]
- Wu LM, Xu JR, Gu HY, Hua J, Chen J, Zhang W, Haacke EM, Hu J. Preoperative mediastinal and hilar nodal staging with diffusion-weighted magnetic resonance imaging and fluorodeoxyglucose positron emission tomography/computed tomography in patients with non-small-cell lung cancer: which is better? J Surg Res. 2012;178:304–314. doi: 10.1016/j.jss.2012.03.074. [DOI] [PubMed] [Google Scholar]
- Yang L, Parkin DM, Li LD, Chen YD, Bray F. Estimation and projection of the national profile of cancer mortality in China: 1991-2005. Br J Cancer. 2004;90:2157–2166. doi: 10.1038/sj.bjc.6601813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CA, Lee KS, Kim EA, Han J, Kim H, Kwon OJ, Jeong YJ, Kim S. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology. 2004;233:191–199. doi: 10.1148/radiol.2331031535. [DOI] [PubMed] [Google Scholar]
- Yi CA, Lee KS, Kim BT, Choi JY, Kwon OJ, Kim H, Shim YM, Chung MJ. Tissue characterization of solitary pulmonary nodule: comparative study between helical dynamic CT and integrated PET/CT. J Nucl Med. 2006;47:443–450. [PubMed] [Google Scholar]
- Zhang M, Kono M. Solitary pulmonary nodules: evaluation of blood flow patterns with dynamic CT. Radiology. 1997;205:471–478. doi: 10.1148/radiology.205.2.9356631. [DOI] [PubMed] [Google Scholar]


