Abstract
Background
Medication-overuse headache (MOH) is common in the general population. We investigated effectiveness of brief intervention (BI) for achieving drug withdrawal in primary care patients with MOH.
Methods
The study was double-blind, pragmatic and cluster-randomised controlled. A total of 25 486 patients (age 18–50) from 50 general practitioners (GPs) were screened for MOH. GPs defined clusters and were randomised to receive BI training (23 GPs) or to continue business as usual (BAU; 27 GPs). The Severity of Dependence Scale was applied as a part of the BI. BI involved feedback about individual risk of MOH and how to reduce overuse. Primary outcome measures were reduction in medication and headache days/month 3 months after the intervention and were assessed by a blinded clinical investigator.
Results
42% responded to the postal screening questionnaire, and 2.4% screened positive for MOH. A random selection of up to three patients with MOH from each GP were invited (104 patients), 75 patients were randomised and 60 patients included into the study. BI was significantly better than BAU for the primary outcomes (p<0.001). Headache and medication days were reduced by 7.3 and 7.9 (95% CI 3.2 to 11.3 and 3.2 to 12.5) days/month in the BI compared with the BAU group. Chronic headache resolved in 50% of the BI and 6% of the BAU group.
Conclusions
The BI method provides GPs with a simple and effective instrument that reduces medication-overuse and headache frequency in patients with MOH.
Trial registration number
Keywords: HEADACHE, MIGRAINE, PAIN
Introduction
Headache is among the top 10 causes of morbidity, measured as years of life lost to disability.1 Chronic headache, that is, headache ≥15 days/month affects 2–5% of the general population.2–5 About half of those with chronic headache have medication-overuse headache (MOH).2 5–7 MOH is defined as the use of headache medication ≥10–15 days/month (depending on type of medication) for at least 3 months.8 9 It has a large impact on quality of life, and is probably the most costly headache disorder.10 MOH is regarded as a challenge to treat. Based on sound reasoning and expert opinion, current consensus suggests that withdrawal of the overused medication(s) lead to improvement of the headache, after initial worsening for 1–2 weeks.8 9
However, because most patients with MOH consult their general practitioner (GP) and observational data support that simple advice may be effective, the primary care setting should be tested as an appropriate setting.11–16
MOH can be identified through screening for headache frequency and dependency-like behaviour using the five simple questions of the Severity of Dependence Scale (SDS).17 Brief intervention (BI) involves a short screening instrument followed by individual feedback including information on why and how to reduce using of the substance in question. BI has successfully been applied to manage overuse of alcohol and different drugs in general practice.18–20 We have tailored a BI for management of MOH. The aim of this study was to test the effectiveness of the BI method versus business as usual (BAU) for achieving drug withdrawal and headache improvement in patients with MOH in general practice.
Methods
Design and study setting
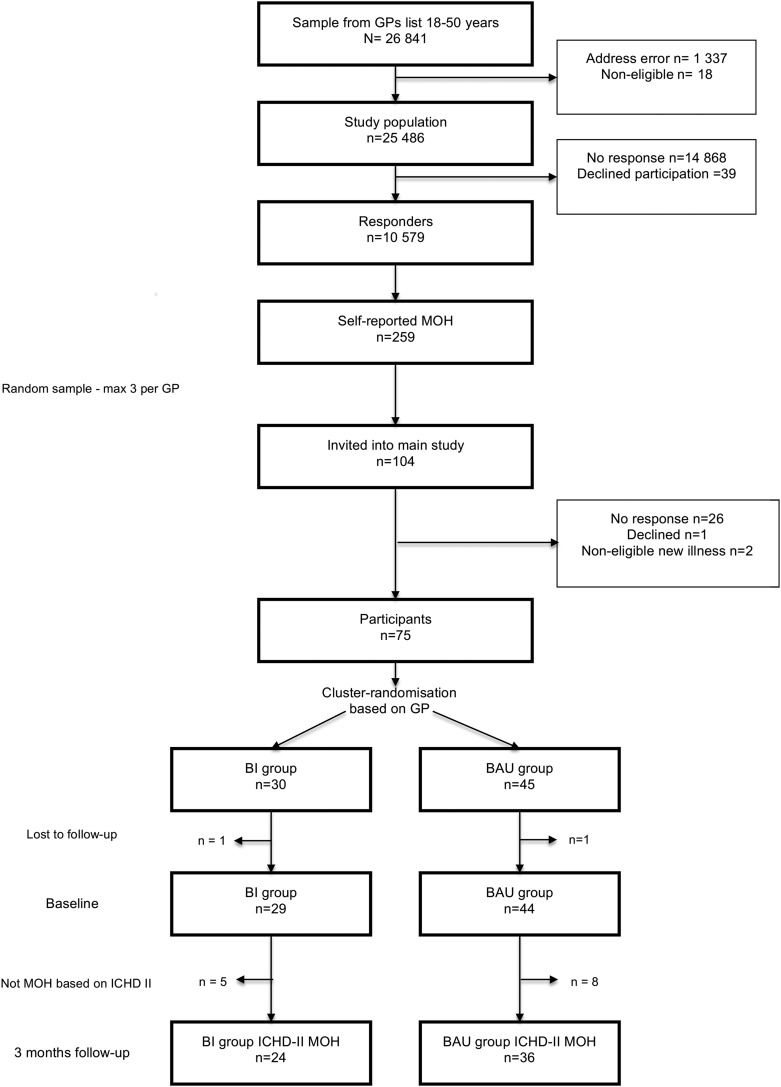
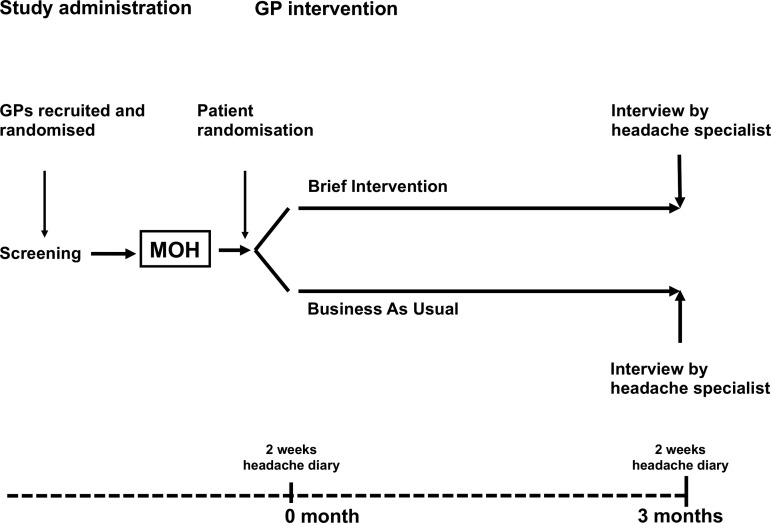
Double-blind pragmatic cluster randomised and parallel controlled study in primary care. The CONSORT-specified flow diagram and flow chart of the study design are shown in figures 1 and 2. A detailed study protocol has been published elsewhere.21 The study was undertaken in South-eastern Norway in 2011 and 2012.
Figure 1.
Flow diagram of the study. BI versus BAU. BAU, business as usual; BI, brief intervention; GP, general practitioner; ICHD-II, The International Classification of Headache Disorders, Second edition; MOH, medication-overuse headache.
Figure 2.
Flow chart of the study design. Figure illustrates main time line as well as timing of various moments for the patients, GPs and investigator group. Main outcome time point at 3 months. GP, general practitioner; MOH, medication-overuse headache.
Participants
General practitioners
In Norway, all GP specialists participate in mandatory peer continuous medical education (CME) groups. From a list of 35 such groups within 2 h driving distance from Oslo, Norway, we invited 18 groups to a clinical training course on the management of headache in primary care.
Patients
A short headache-screening questionnaire (frequency, intensity and medication use) was posted to all 18–50-year-old patients on the 50 participating GPs’ patient lists. Non-responders received two reminders.
Patients with self-reported chronic headache (≥15 days/month) and headache medication overuse ≥10 days/month, that is, self-reported MOH, were eligible for invitation to the study. Inclusion into the trial required that the diagnostic criteria of the International Classification of Headache Disorders (ICHD-II) for MOH were fulfilled after a clinical interview (box 1).22–25 The only exclusion criterion was insufficient Norwegian language skills.
Box 1. The International Classification of Headache Disorders, 2nd edition (ICHD—II) criteria for medication-overuse headache (MOH)22–25.
Medication-overuse headache (MOH)
Headache present on ≥15 days/month.
- Regular overuse for >3 months of one or more drugs that can be taken for acute and/or symptomatic treatment of headache.
- Simple analgesics on >15 days/month on a regular basis for >3 months.
- Ergotamine, triptans, opioids or combination analgesics on >10 days/month on a regular basis for >3 months.
- Any combination of ergotamine, triptans, analgesics and/or opioids >15 days/month on a regular basis for >3 months without overuse of any single class alone.
Headache has developed or markedly worsened during medication overuse.
Intervention
Brief intervention course
The GPs received a 1-day course on headache management in the CME groups by headache specialists (CL and ESK). The course consisted of small group teaching sessions and a 2 h presentation of BI exemplified by role-play. Half of the GPs received the BI course initially, the remainder received it after the 3 months follow-up.
Brief intervention
GPs allocated to the BI arm invited screening-positive patients with MOH to a BI consultation. SDS was scored individually during the consultation.
The five questions of the SDS adapted for headache were: (1) Do you think your use of headache medication was out of control? (never/almost never=0, sometimes=1, often=2, always/nearly always=3); (2) Did the prospect of missing a dose make you anxious or worried? (scoring as for question 1); (3) Did you worry about your use of your headache medication? (scoring as for question 1); (4) Did you wish you could stop? (scoring as for question 1); (5) How difficult would you find it to stop or go without your headache medication? (not difficult=0, quite difficult=1, very difficult=2, impossible=3).17 26
Cut-off values of ≥5 for women at risk for MOH and ≥4 for men were used.17 Patients were, using a short structured scheme based on a flip-over presentation, given information about MOH and the association between medication overuse and chronic headache. Further, based on the individual SDS result, patients received feedback on their SDS score and risk of medication-induced headache. With a consultation in an empathic and collaborative manner, the BI aimed towards achieving a decision by the patient that he/she would cut down the offending medication, an agreement about how the GP could support and a concrete plan. Explicit recommendations were reduction in headache medication towards ‘safe levels’, and information about possible difficulties and gains including that MOH usually ‘gets worse before it improves’ 1–2 weeks after withdrawal. The estimated time for the BI procedure was 9 min in one single ordinary consultation. GPs allocated to the BAU arm continued business as usual.
Baseline and follow-up assessment
Baseline
A validated diagnostic headache diary was used to prospectively record headache frequency and intensity (VAS—Visual Analogue Scale) and medication use (figure 2).27 Other baseline data were collected retrospectively at the blinded 3 months follow-up (figure 2).
Follow-up
The participants were interviewed and examined 3 months after inclusion by a headache expert (ESK, KGV or CL; figure 2). The ICHD-II criteria with revisions were applied (box 1).22–25 Patients unable to meet at the clinic were interviewed by telephone. Another 2-week headache diary was completed prior to this follow-up.
Randomisation
To avoid carry-over effects between GPs in the same CME group, the CME groups were the randomisation units, although each GP and his/her patients defined one cluster. An external statistician did the computer-generated randomisation.
Blinding
GPs were recruited, enrolled and the CME groups randomised before patients were screened and enrolled. Both GPs and patients only received information that the study aimed to evaluate headache care in general practice. There was no information about this being an intervention study in the invitation letters. All GPs and patients were blinded to study design, group participation and outcome evaluation. Investigator group (including interviewers) were blinded to patient group, intervention and treatment. The study administration collected consent forms, screening questionnaires and baseline headache diaries before any study-related contact between patient and their GP, and independently of the interviewers.
Outcomes
Outcomes were prespecified in the study protocol.21 Primary outcomes were numbers of headache and medication days/month comparing the two trial arms, as well as change compared with baseline. Secondary outcomes were numbers of patients at follow-up who no longer had chronic headache and medication overuse, numbers of patients with 25% and 50% reduction in headache days/month and difference in headache index (mean headache days/month×mean headache hours/day×mean pain intensity). In addition, data from headache diaries (change in headache days, medication days and VAS) were also secondary outcomes.
Sample size
Norwegian GPs have on average 1200 listed patients. It was estimated that each GP had approximately 30–40 chronic headache patients and at least 10 patients with MOH.
Using 80% power for the detection of a difference in medication days similar to a previous study,16 an intraclass correlation coefficient (ICC) of 0.5 and a 5% significance level, sufficient power would have been reached with 18 patients or 5 clusters (GPs) per arm. For analyses of proportion of patients with chronic headache, calculations suggested 30 patients or 8 GPs per arm. We thus originally assumed a sample size of at least 20 GPs (100 patients assuming 5 patients per GP) to be sufficient.21 Since the pilot study suggested that more than three patients per GP was not feasible,28 we increased the number of GPs to 50 to have sufficient power on the individual patient level. If one GP had more than three screening-positive patients, a random sample of three was drawn to avoid GPs declining to participate in the study due to workload.
Statistics
Clinical characteristics were presented as frequencies or means, and SDs or 95% CIs. Differences between BI and BAU groups were assessed by χ2 tests for categorical variables, and independent samples t tests for continuous variables. A hierarchical linear regression model (SAS MIXED procedure) with random effects for intercepts was fitted to the continuous outcome variables to take possible correlations between members of the same cluster (GP) into account. First, crude regression coefficients were calculated by bivariate analyses. The coefficients were then adjusted for age, gender and migraine status in a multivariate model. All statistical analyses were conducted using SAS V.9.2 and SPSS V.20.0. Significance level of p<0.025 was used for the two primary outcomes (Bonferroni corrected). For all other outcomes, the level of significance was set at 5%.
Registration of data from the interviews was made using Snap Survey (Snap Survey, London, UK).
Financial incentives
GPs earned CME credits for participating in the study. The participating patients received a free clinical examination by a headache specialist. The study covered the normal patient fee to the GP for the BI consultation.
Ethics and data security
Patients received written information and GPs received oral and written information before they consented. Data were anonymised and secured on a research server at Akershus University Hospital. The authors had full access to the study data.
Results
Sample characteristics
General practitioners
Out of 18 invited CME groups, 10 CME groups with 50 GPs were included. Reasons for groups not participating were: no response (3), time constraints (3), lack of interest (1) and no reason given (1). The included GPs were comparable with average national figures in terms of practice localisation (urban/suburban vs rural) and age distribution, while female GPs were over-represented (table 1).
Table 1.
GP characteristics
| Brief intervention | Business as usual | |
|---|---|---|
| Continuous medical education groups | 5 | 5 |
| General practitioners | 23 | 27 |
| Specialists in GP/family medicine | 20 | 25 |
| Mean age (95 % CI) | 46.3 (42.5 to 50.1) | 51.9 (49.0 to 54.8) |
| Gender (% (n)) | ||
| Women | 43 (10) | 59 (16) |
| Men | 57 (13) | 41 (11) |
| Mean number of 18–50 years old list patients/GP list (95% CI) | 561 (474 to 648) | 516 (473 to 559) |
| Total number of 18–50 years old list patients | 12 907 | 13 932 |
GP, general practitioner.
Patients
The CONSORT-specified flow diagram summarises the study (figure 1). The responder rate of the screening questionnaire was 42% (10 579/25 486), with a preponderance of older patients and women.
Two hundred and fifty-nine (2.4%) of the responders screened positive for MOH. A random selection of up to three self-reported patients with MOH from each GP were invited (104 patients). Reasons for non-participation are given in figure 1.
Each group lost one patient to follow-up, both were excluded from the analyses due to lack of any data. The randomised sample thus included 73 patients. The analysed sample was further reduced to 60 patients, since 13 patients did not meet the inclusion criteria for a clinically defined diagnosis of MOH (box 1). All included patients completed the 3 months follow-up. The mean age of the 60 patients was 42.1 (95% CI 40.2 to 43.9) years, 87% (76% to 93%) were women and 70% (58% to 80%) had co-occurrence of migraine. The mean duration of chronic headache was 16.6 (13.9 to 19.3) years while duration of medication overuse was 8.7 (7.3 to 10.2) years.
The majority of the participants had a face-to-face interview, 10% were interviewed by telephone. The headache diagnoses at baseline were independent of randomisation, type of interview or interviewer.
The BI and BAU groups had similar sociodemographics (marital status, education and income: data not shown), headache and medication characteristics at baseline (tables 2 and 3). The mean SDS score was 5 in the BI group.
Table 2.
Baseline characteristics of the brief intervention and business as usual groups
| Brief intervention (N=24) | Business as usual (N=36) | |
|---|---|---|
| Age, mean (95% CI) | 43.0 (40.6 to 45.5) | 41.4 (38.8 to 44.0) |
| Gender | ||
| Women (% (n)) | 92 (22) | 83 (30) |
| Men (% (n)) | 8 (2) | 17 (6) |
| Co-occurrence of migraine, % (n) | 67 (16) | 72 (26) |
| Mean years of chronic headache (95% CI) | 16.4 (12.3 to 20.6) | 16.8 (13.1 to 20.4) |
| Mean years of medication overuse (95% CI) | 8.8 (6.9 to 10.8) | 8.6 (6.5 to 10.8) |
Table 3.
Three months follow-up unadjusted data of the brief intervention and business as usual groups
| Baseline | Three months follow-up | ||||
|---|---|---|---|---|---|
| Brief intervention (N=24) | Business as usual (N=36) | Brief intervention (N=24) | Business as usual (N=36) | p Value (between arms at 3 months) | |
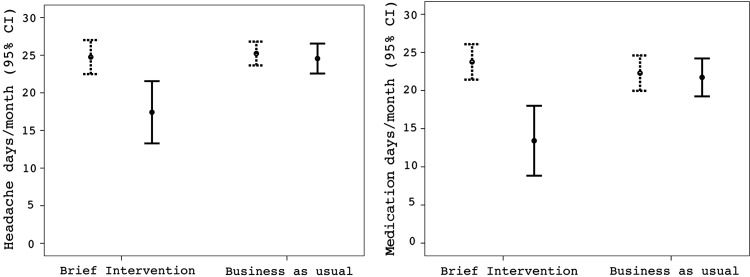
| Headache days/month, mean (95% CI) | 24.8 (22.5 to 27.0) | 25.2 (23.6 to 26.8) | 17.4 (13.2 to 21.5) | 24.6 (22.6 to 26.6) | 0.001 |
| Medication days/month, mean (95% CI) | 23.8 (21.4 to 26.1) | 22.3 (20.0 to 24.6) | 13.4 (8.8 to 18.0) | 21.7 (19.2 to 24.2) | 0.001 |
| Headache index, mean (95% CI) | NA | NA | 1691 (1112 to 2269) | 2233 (1926 to 2586) | 0.07 |
| Prophylactic headache medication, % (n) | 13 (3) | 19 (7) | 17 (4) | 22 (8) | 0.60 |
| Main headache diagnoses, % (n) | |||||
| Medication-overuse headache | 100 (24) | 100 (36) | 33 (8) | 94 (34) | <0.001 |
| Chronic tension-type headache | 0 (0) | 0 (0) | 17 (4) | 0 (0) | 0.02 |
| Chronic migraine | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Episodic tension-type headache and/or migraine | 0 (0) | 0 (0) | 50 (12) | 6 (2) | <0.001 |
| Main type overused medication, % (n) | |||||
| Simple analgesics | 63 (15) | 56 (20) | 17 (4) | 56 (20) | 0.03 |
| Triptans | 13 (3) | 22 (8) | 0 (0) | 22 (8) | 0.017 |
| Combination analgesics | 21 (5) | 11 (4) | 13 (3) | 11 (4) | 1.00 |
| Combination of acute analgesics | 0 (0) | 6 (2) | 0 (0) | 3 (1) | 1.00 |
| Opioids | 4 (1) | 6 (2) | 4 (1) | 6 (2) | 1.00 |
| Number of patients (%) | |||||
| Without medication overuse | 0 (0) | 0 (0) | 16 (67) | 1 (3) | <0.001 |
| Without chronic headache | 0 (0) | 0 (0) | 12 (50) | 2 (6) | <0.001 |
| With ≥25% reduction in headache days/month relative to baseline | 0 (0) | 0 (0) | 14 (58) | 2 (6) | <0.001 |
| With ≥50% reduction in headache days/month relative to baseline | 0 (0) | 0 (0) | 8 (33) | 2 (6) | 0.004 |
NA, not available.
Outcomes
Unadjusted outcome analyses
BI was significantly more effective than BAU in reducing headache days/month and medication days/month (table 3). Figure 3 shows the crude headache days/month and medication days/month at baseline and follow-up.
Figure 3.
Crude headache and medication data at baseline (dotted) and at 3 months follow-up (solid) in the brief intervention and business as usual.
The mean reduction in medication days in the BI group was 11 days (range 0–30) for simple analgesics, 8 days (range 0–26) for triptans and 14 days (range 4–22) for combination analgesics.
No patient had headache deterioration, except from temporary withdrawal headache after detoxification. Otherwise no adverse events or side effects were reported.
About 16–20% used prophylactic medication at baseline and follow-up with no significant difference between the study arms (table 3).
Adjusted primary outcomes analyses
BI was significantly better than BAU for both primary outcomes (table 4). Adjusted effect sizes were a difference of −7.3 (95% CI −11.3 to −3.2) headache days and −7.9 (−12.5 to −3.2) medication days between the groups in favour of BI. Headache and medication days were also reduced significantly more from baseline to follow-up in the BI than BAU group (table 4). There were no significant differences in patient-related primary outcomes depending on the individual GP.
Table 4.
Primary outcomes analysed by linear regression
| Outcome | ICC (%) | Crude | Adjusted* | ||
|---|---|---|---|---|---|
| Coeff (95% CI) | p Value | Coeff (95% CI) | p Value | ||
| Headache days/month | 22.3 | −7.2 (−11.2 to −3.1) | <0.001 | −7.3 (−11.3 to −3.2) | <0.001 |
| Medication days/month | 10.6 | −8.4 (−13.4 to −3.4) | 0.002 | −7.9 (−12.5 to −3.2) | 0.001 |
| Change in headache days/month | 26.1 | −6.7 (−9.6 to −3.9) | <0.001 | −6.8 (−9.6 to −3.7) | <0.001 |
| Change in medication days/month | 10.4 | −9.8 (−13.1 to −6.6) | <0.001 | −9.5 (−12.8 to −6.2) | <0.001 |
Coeff, regression coefficient in linear mixed model, showing average difference (number of days) between business as usual (coded as 0) and brief intervention (coded as 1) groups; ICC, intraclass correlation coefficient.
*Regression coefficients adjusted for age, gender and co-occurrence of migraine.
Secondary outcomes analyses
At follow-up, 67% (16/24) in the BI group were without medication overuse compared with 3% (1/36) in the BAU group. Chronic headache resolved in 50% (12/24) of the BI group and 6% (2/36) in the BAU group (table 3). In addition, in the BI group, one-third had a reduction of headache days/month of more than 50% and nearly 60% improved by at least 25% (table 3). Data from the prospective headache diaries showed a significant reduction in number of medication days in favour of the BI group, but not in headache days (table 5). In addition, there was a significant change in headache index in the BI group.
Table 5.
Secondary outcomes, diary data analysed by linear regression
| Outcome | ICC (%) | Crude | Adjusted* | ||
|---|---|---|---|---|---|
| Coeff (95% CI) | p Value | Coeff (95% CI) | p Value | ||
| Change in headache days/4 weeks | 63.7 | −1.5 (−4.8 to 1.7) | 0.34 | −1.4 (−4.8 to 1.9) | 0.38 |
| Change in medication days/4 weeks | 24.2 | −9.8 (−13.1 to −6.6) | <0.001 | −9.5 (−12.8 to −6.2) | <0.001 |
| Change in Visual Analogue Scale | 35.4 | −8.4 (−20.7 to 4.0) | 0.16 | −8.8 (−21.6 to 4.0) | 0.17 |
| Change in headache index | 7.2 | −300 (−574 to −25) | 0.03 | −304 (−590 to −18) | 0.04 |
Coeff, regression coefficient in linear mixed model, showing average difference (number of days) between business as usual (coded as 0) and brief intervention (coded as 1) groups; ICC: intraclass correlation coefficient.
*Regression coefficients adjusted for age, gender and co-occurrence of migraine.
Excluded patients
The 13 patients who were excluded since they did not have MOH (mean headache days/month at baseline 16.2 (95% CI 10.6 to 21.8), mean medication days/month at baseline 3.4 (1.6 to 5.2)) did not change their number of headache or medication days.
Discussion
Principal findings
BI provided by GPs is an effective treatment for MOH. Clinically significant effects were observed in primary and most secondary outcomes in the BI group, while no effects were observed in the BAU group.
Strengths and weaknesses of the study
Strengths are the blinded controlled design, the high external and internal validity with inclusion of representative GPs and patients from a large population sample. Our randomised controlled trial (RCT) adheres to the CONSORT statement for cluster randomised, pragmatically designed and non-pharmacological intervention studies.29–32 Outcomes were predefined and followed guidelines from the International Headache Society.33–35 The feasibility and logistics of the intervention were tested in a pilot study.28 The methods have been thoroughly discussed elsewhere.21 28
All Norwegian citizens are listed with a GP, and the included 50 GPs were representative of Norwegian GPs. The patient population of almost 27 000 was assumed to be population based, although the 42% screening questionnaire response rate might skew the sample. However, our data correspond well with a previous Norwegian epidemiological survey regarding patients’ gender, headache diagnoses and medication use, emphasising its representativeness.2 5 Intervention studies require willingness to cooperate and may lead to selection bias of GPs and patients, but the prestudy invitation and information did not mention any intervention. In addition, participants in the two study arms were comparable. Thus, selection bias is probably of minor significance.
The sample of included patients may seem small, but met pre-required power calculations. Thus, our results are most likely representative and valid.
The 18–50 years age range of patients was chosen in order to target a high number of patients with chronic headache without comorbidity of other interfering non-headache medication and disorders.
We chose the BI scheme as a structured form of simple advice that is easy to implement in a busy general practice. The intervention was conducted during a single consultation and it took an average of 9 min to complete.28 The BI was based on the individual SDS score which is different from other simple advice strategies. The SDS score was used to distinguish between chronic headache with and without medication overuse.17 This personal feedback on risk has been suggested to be one important factor which makes the BI more effective than only general advice in other types of overuse.36
Collecting data from baseline, using retrospective information at the 3 months follow-up, might have led to recall bias. We attempted to counteract this by, in addition, having prospective headache diaries collected independently of the follow-up.
The gold standard for diagnosing headache is an interview and a clinical examination by a physician experienced in headache diagnostics. Similar proportions of the different headache diagnoses made by the three interviewers suggest that interobserver variation was small. The ICHD-II was recently revised to ICHD-III edition β, but this does not affect the MOH diagnosis.37
A limitation with the study was small clusters (few patients per GP), however, all results are adjusted for ICC. Furthermore, results on individual patient levels met power calculations.
Results discussion
The RCT literature on withdrawal strategies for MOH is scant. To the best of our knowledge, this is the first double-blind RCT of MOH treatment in primary care.
In the present study, BI was significantly better than BAU and only the BI group improved compared with baseline. We regard the size of the change as clinically significant, especially taken into consideration that these patients had received no other treatment than a single BI during an ordinary GP consultation.
Other studies have also indicated effects of simple advice for MOH. However, none of these studies were blinded or had a control group without active intervention. Two Italian observational studies from neurology departments reported 78–92% of patients with simple MOH to be without chronic headache and medication overuse after 2 months.11 12 Our figures are not quite as impressive, and this may reflect the unselected diverse patients with MOH included in our study. In addition, patients referred to a specialist centre may be more motivated and the authority of headache specialists and the setting may play a role. However, our results are in accordance with reports regarding patients with complicated MOH in neurology outpatient setting.13 In our previous study, simple information on medication use led to similar improvement, albeit over 1–2 years, and the mean duration of MOH suggests that this was not a spurious finding. However, that study had no control group.16 The present controlled RCT confirms these findings. It is worth noting that simple advice and education was enough to detoxify most of the patients in these studies.11–13 16
Prophylactic medication was used by 16–20% of participants and equally frequent in both groups, thus it cannot explain our results. Whether or not initially to detoxify patients with MOH and whether prophylactic headache medication should be initiated immediately at withdrawal or after completing withdrawal therapy is debated.8 9 Our results support an initial withdrawal attempt, since it in itself has a clear and clinically significant effect. We suggest that prophylactic headache medication should be restricted to patients who do not benefit sufficiently from withdrawal or have other complicating conditions.
Clinical implications
Focus on MOH in primary care is important for early diagnosis, treatment and prevention.
In most European countries, overuse of simple analgesics, triptans and codeine-containing combination medications dominates.6 7 14 15 Patients overusing more centrally acting drugs with more pronounced physical abstinence profiles may be different. Taking care of uncomplicated cases in primary care may free more resources for referrals to neurologists for complicated cases. In contrast to the simple and inexpensive BI, most other withdrawal strategies undertaken in headache centres are more complex interventions based on inpatient treatment including different rescue medication, prophylaxis and continued support.8 9
The gain from effective management of MOH in primary care thus benefits patients and society and may reduce economical costs.
The BI provides the GPs with a powerful, time efficient instrument for managing MOH. The treatment is behavioural, simple and inexpensive and has no side effects.
Acknowledgments
The authors want to express their sincere gratitude to all participating patients and GPs, without them the study would not have been possible. Thanks also for logistic help from the research administration at Akershus University Hospital.
Footnotes
Contributors: CL had the original idea for the study and together with JS, MBR and ESK planned the overall design. ESK and CL carried out all the brief intervention courses. ESK, CL and KGV conducted the 3 months follow-up interview. ESK prepared the initial draft, and was the main author of the present manuscript. JS and MBR supported in the design of the protocol and scientific input. JŠB planned the statistics methodology and was involved in the experimental design and all analyses. All authors have read, revised and approved the final manuscript.
Funding: This study is supported by grants from the University of Oslo, the Research Centre at Akershus University Hospital and the South-Eastern Norway Regional Health Authority.
Competing interests: None.
Ethics approval: The study was approved by the Regional Committee for Medical Research Ethics (08-41-07332b 1.2007.2691), the Norwegian Social Science Data Services (NSD) and the Norwegian Directorate for Health.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aaseth K, Grande RB, Kvaerner KJ, et al. Prevalence of secondary chronic headaches in a population-based sample of 30–44-year-old persons. The Akershus study of chronic headache. Cephalalgia 2008;28:705–13. [DOI] [PubMed] [Google Scholar]
- 3.Castillo J, Munoz P, Guitera V, et al. Kaplan Award 1998. Epidemiology of chronic daily headache in the general population. Headache 1998;39:190–6. [DOI] [PubMed] [Google Scholar]
- 4.Lantéri-Minet M, Auray JP, El Hasnaoui A, et al. Prevalence and description of chronic daily headache in the general population in France. Pain 2003;102:143–9. [DOI] [PubMed] [Google Scholar]
- 5.Grande RB, Aaseth K, Gulbrandsen P, et al. Prevalence of primary chronic headache in a population-based sample of 30- to 44-year-old persons. The Akershus study of chronic headache. Neuroepidemiology 2008;30:76–83. [DOI] [PubMed] [Google Scholar]
- 6.Colás R, Muñoz P, Temprano R, et al. Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology 2004;62:1338–42. [DOI] [PubMed] [Google Scholar]
- 7.Jonsson P, Hedenrud T, Linde M. Epidemiology of medication overuse headache in the general Swedish population. Cephalalgia 2011;31:1015–22. [DOI] [PubMed] [Google Scholar]
- 8.Evers S, Jensen R, EFNS. Treatment of medication overuse headache—guideline of the EFNS headache panel. Eur J Neurol 2011;18:1115–21. [DOI] [PubMed] [Google Scholar]
- 9.Evers S, Marziniak M. Clinical features, pathophysiology, and treatment of medication-overuse headache. Lancet Neurol 2010;9:391–401. [DOI] [PubMed] [Google Scholar]
- 10.Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol 2012;19:703–11. [DOI] [PubMed] [Google Scholar]
- 11.Rossi P, Di Lorenzo C, Faroni J, et al. Advice alone vs. structured detoxification programmes for medication overuse headache: a prospective, randomized, open-label trial in transformed migraine patients with low medical needs. Cephalalgia 2006;26:1097–105. [DOI] [PubMed] [Google Scholar]
- 12.Rossi P, Faroni JV, Nappi G. Short-term effectiveness of simple advice as a withdrawal strategy in simple and complicated medication overuse headache. Eur J Neurol 2011;18:396–401. [DOI] [PubMed] [Google Scholar]
- 13.Rossi P, Faroni JV, Tassorelli C, et al. Advice alone versus structured detoxification programmes for complicated medication overuse headache (MOH): a prospective, randomized, open-label trial. J Headache Pain 2013;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristoffersen ES, Grande RB, Aaseth K, et al. Management of primary chronic headache in the general population: the Akershus study of chronic headache. J Headache Pain 2012;13:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonsson P, Linde M, Hensing G, et al. Sociodemographic differences in medication use, health-care contacts and sickness absence among individuals with medication-overuse headache. J Headache Pain 2012;13:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grande RB, Aaseth K, Benth JS, et al. Reduction in medication-overuse headache after short information. The Akershus study of chronic headache. Eur J Neurol 2011;18:129–37. [DOI] [PubMed] [Google Scholar]
- 17.Grande RB, Aaseth K, Benth JS, et al. The Severity of Dependence Scale detects people with medication overuse: the Akershus study of chronic headache. J Neurol Neurosurg Psychiatry 2009;80:784–9. [DOI] [PubMed] [Google Scholar]
- 18.Babor TF, Higgins-Biddle JC. Brief intervention for hazardous and harmful drinking—a manual for use in primary care. Geneva: World Health Organization, 2001. [Google Scholar]
- 19.Kaner EF, Beyer F, Dickinson HO, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev 2007;(2): CD004148. [DOI] [PubMed] [Google Scholar]
- 20.Humeniuk R, Ali R, Babor T, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction 2012;107:957–66. [DOI] [PubMed] [Google Scholar]
- 21.Kristoffersen ES, Straand J, Benth JS, et al. Study protocol: brief intervention for medication overuse headache—a double-blinded cluster randomised parallel controlled trial in primary care. BMC Neurol 2012;12:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24(Suppl 1):9–160. [DOI] [PubMed] [Google Scholar]
- 23.Olesen J, Bousser MG, Diener HC, et al. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia 2006;26:742–6. [DOI] [PubMed] [Google Scholar]
- 24.Silberstein SD, Olesen J, Bousser MG, et al. The International Classification of Headache Disorders, 2nd Edition (ICHD-II)—revision of criteria for 8.2 medication-overuse headache. Cephalalgia 2005;25:460–65. [DOI] [PubMed] [Google Scholar]
- 25.Erratum Cephalalgia 2006;26:360. [Google Scholar]
- 26.Gossop M, Darke S, Griffiths P, et al. The Severity of Dependence Scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction 1995;90:607–14. [DOI] [PubMed] [Google Scholar]
- 27.Russell MB, Rasmussen BK, Brennum J, et al. Presentation of a new instrument: the diagnostic headache diary. Cephalalgia 1992;12:369–74. [DOI] [PubMed] [Google Scholar]
- 28.Kristoffersen ES, Straand J, Russell MB, et al. Feasibility of a brief intervention for medication-overuse headache in primary care—a pilot study. BMC Res Notes 2014;7:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell MK, Piaggio G, Elbourne DR, et al. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345:e5661. [DOI] [PubMed] [Google Scholar]
- 31.Zwarenstein M, Treweek S, Gagnier JJ, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337:a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boutron I, Moher D, Altman DG, et al. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295–310. [DOI] [PubMed] [Google Scholar]
- 33.Bendtsen L, Bigal ME, Cerbo R, et al. Guidelines for controlled trials of drugs in tension-type headache: second edition. Cephalalgia 2010;30:1–16. [DOI] [PubMed] [Google Scholar]
- 34.Silberstein S, Tfelt-Hansen P, Dodick DW, et al. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia 2008;28:484–95. [DOI] [PubMed] [Google Scholar]
- 35.Hagen K, Jensen R, Boe MG, et al. Medication overuse headache: a critical review of end points in recent follow-up studies. J Headache Pain 2010;11:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babor TF, McRee BG, Kassebaum PA, et al. Screening, Brief Intervention, and Referral to Treatment (SBIRT): toward a public health approach to the management of substance abuse. Subst Abus 2007;28:7–30. [DOI] [PubMed] [Google Scholar]
- 37.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. [DOI] [PubMed] [Google Scholar]