Abstract
Background
The aim of this study was to investigate the protective effects of L-glutamine (GLN) against liver and kidney injury caused by acute toxicity of deltamethrin (DLM).
Material/Methods
Thirty-two rats were indiscriminately separated into 4 groups with 8 rats each: control group (distilled water; 10 ml/kg, perorally [p.o.]), DLM group (35 mg/kg p.o. one dose.), GLN group (1.5 gr/kg, p.o. single dose.) and DLM (35 mg/kg p.o. one dose.) + GLN group (1.5 gr/kg, p.o. one dose after 4 hours.). Testing for total antioxidant status (TAS), total oxidant status (TOS), interleukin-1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) analyses were performed on tissue samples, and alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), urea, and creatinine were analyzed on serum samples. Liver and kidney samples were histopathologically analyzed.
Results
The TOS level in liver was significantly higher in the DLM group than in the control group, and the level in DLM+GLN group was considerably lower than in the DLM group. The TAS level in the DLM+GLN group was considerably higher than in the control and DLM groups. The TAS level in kidney tissues was considerably lower in the DLM group than in controls, but was similar to other groups. Histopathological analyses of liver tissues established a significant difference between DLM and DLM+GLN groups in terms of grade 2 hepatic injury. However, no significant difference was found between DLM and DLM+GLN groups in terms of kidney injury.
Conclusions
Glutamine leads to significant improvement in deltamethrin-induced acute hepatotoxicity in terms of histopathologic results, tissue oxidative stress parameters, and serum liver function marker enzymes.
Keywords: Acute Kidney Injury, Drug-Induced Liver Injury, Glutamic Acid, Oxidative Stress
Background
Deltamethrin (DLM) is a broad-spectrum synthetic pyrethroid insecticide mainly used for protecting agricultural crops, fruits, and vegetables against weevils, mites, beetles, and ants [1]. DLM is the most commonly used insecticide in most countries due to its fast metabolism and low toxicity to humans and other non-target animals, as well as its strong effect on many pests [2]. However, several side effects of DLM have been reported, including neurotoxicity, immunosuppression, allergy, hypertension, decreased testosterone levels, hepatotoxicity, and nephrotoxicity [3].
The acaricidal and insecticidal effects of DLM are mainly considered to arise from its binding to a distinct receptor site on voltage-gated sodium channels and extending the open state by constraining the inactivation and deactivation of channels. Nevertheless, the other effects of DLM on biological membranes are exerted at sites other than the voltage-dependent sodium channel because of the high hydrophobic profile of DLM [4,5]. In several reports, the liver was shown to collect a larger concentration of metabolites because it is the main site of DLM metabolism, and the kidney is reported as the main organ of excretion [6,7].
L-glutamine (GLN) is an essential amino acid which is vital for life activities and accounts for ~60% of free amino acids in the body. Recent studies have reported that GLN, as an effective immune nutrient, provides improvement in hepatic ischemia-reperfusion, reduces the release of inflammatory cytokines, regulates immunoreaction, and provides protection against oxidative stress [8–11]. In addition, GLN plays a vital role in the glutathione peroxidase (GSH) metabolism. Welbourne et al. reported that GLN functioned as a rate-limiter for the GSH synthesis under oxidative stress [12]. GSH is a potent ubiquitous antioxidant which functions as an important factor for many drugs and endogenous substance metabolisms [13,14].
The purpose of this study was to determine the hepato-renoprotective and antioxidant effects of GLN against DLM-induced acute oxidative stress and hepato-nephrotoxicity in rats.
Material and Methods
Chemicals
Deltamethrin (Butox® 50 mg/ml) was obtained as a trading product in clinical formulation from Intervet Co. (France), and glutamine was bought from Sigma Chemical Co. (St Louis, Missouri, USA).
Animals and drug treatment
Thirty-two male Wistar Albino rats weighing 250–300 g were randomly chosen among the rats treated and controlled at Dicle University Medical Faculty Health Sciences Research and Application Center (Diyarbakir, Turkey). The rats were kept in 8×9×14 cm wooden cages. Prior to the experiment, all rats were fed standard rat chow and water ad libitum and were housed in an air-conditioned room at 21°C with 12-h light/dark cycle and were treated humanely. Animals were fasted before the experiment. The study was approved by the local Animal Ethical Committee of Dicle University (2014/30), Diyarbakir, Turkey.
The rats were randomly divided into 4 groups with 8 rats each:
Control group: (distilled water; 10 ml/kg, perorally [p.o.]);
DLM group: a single dose of DLM 35 mg/kg body weight was diluted in distilled water and administered by a gastric tube;
GLN group: a single dose of GLN 1.5 g/kg was diluted in distilled water and administered perorally by a gastric tube;
DLM+GLN group: DLM was administered 35 mg/kg perorally single dose and GLN was administered 1.5 g/kg single dose after 4 hours [15,16].
The rats were anesthetized 24 h after drug administration, using ketamine hydrochloride (50 mg/mL, 10 mg/kg, Ketalar; Bayer, Leverkusen, Germany) and xylazine (2%, 0.1 mL/kg, Rompun; Bayer) in solution (2 mL/kg) 50 mg/kg, intramuscularly). The surgical procedure was performed in supine position, and laparotomy was applied through medial incision. Following the surgery, the rats were sacrificed by exsanguination. Tissue samples were obtained from liver and kidney, and blood samples were taken. Liver and kidney tissue samples were homogenized, and the centrifugation of homogenates was achieved at 3000 rpm for 10 min at 4°C, and the supernatants were removed and stored at −80°C until the parameter analyses of total antioxidant capacity (TAS), total oxidant capacity (TOS), interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) were performed. Serum samples were used to analyze alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), urea, and creatinine.
Biochemical analysis
Cytokines, TAS, TOS assays
IL-1β, IL-6, and TNF-α eBioscience (Austria), and TAS, TOS were purchased from Rel Assay Diagnostics (Gaziantep, Turkey). The analyses were performed using the guidelines of the manufacturer.
Biochemical parameters
The intracardiac route was used to draw the maximum volume of blood. This volume was then centrifuged and the sera were stored at −70°C until the biochemical analysis were performed. An Abbott Architect c16000 Autoanalyzer was used to assess serum AST and ALT, urea, creatinine, and LDH values.
Measurement of IL-1β, IL-6, and TNF-α
Commercially available rat enzyme-linked immunosorbent assay (ELISA) kits were used to assess the levels of IL-1β, IL-6 and TNF-α. The assessments were performed using the guidelines of the manufacturer.
Measurement of total oxidant status (TOS)
In the supernatant fragments, the TOS levels were measured through the new automated measurement method improved by Erel [17]. In this method, the oxidants in the specimen oxidize the ferrous ion-o-dianisidine complex to ferric ion. The glycerol molecules present in high amounts in the reaction medium contribute to the oxidation reaction. In an acidic medium, the ferric ion leads to the formation of a coloured complex with xylenol orange. The color intensity measured with the help of spectrophotometry depends on the total quantity of oxidant molecules in the specimen. Hydrogen peroxide is used for the calibration of the essay and the results are expressed in mmol Trolox equivalent/L.
Measurement of total antioxidant status (TAS)
The TAS of the supernatant phase was analyzed using the new automated measurement method improved by Erel [18]. Based on this method, the most potent biological radical – the hydroxyl radical – is produced. In the assay, the ferrous ion solution in Reagent 1 is mixed with the hydrogen peroxide in Reagent 2. The sequentially produced radicals, including the brown-coloured dianisidinyl radical cation produced by the hydroxyl radical, are also potent radicals. Present method helps to measure the antioxidative effect of the sample against the potent free radical reactions initiated by the produced hydroxyl radical. The precision of the analysis is below 3%. The obtained results are expressed as μmol H2O2 equivalent/L.
Histopathological analysis
Liver and kidney sections were obtained for histopathological analysis, and the sections were fixed in 10% buffered formalin, dehydrated in ethanol (50–100%), purified in xylene, and buried in paraffin. Sections (4–5 mm thick) were arranged and then painted with hematoxylin and eosin (H–E). The sections were studied to assess the histopathological changes in the liver and kidney.
The severity of hepatic damage was assessed using an ordinal grading system as follows:
Grade 0: minimal or no sign of damage,
Grade 1: mild damage accompanied by cytoplasmic vacuolation and focal nuclear pyknosis,
Grade 2: moderate damage with cytoplasmic vacuolization, confluent areas of hepatocyte ballooning with no obvious necrosis, sinusoidal dilatation and congestion, and loss of intercellular borders,
Grade 3: moderate to severe damage with areas of coagulative necrosis, cytoplasmic hypereosinophilia, widespread sinusoidal dilatation and congestion,
Grade 4: severe damage with severe confluent coagulative necrosis, and disintegration of and hemorrhage into hepatic chords, leading to the loss of tissue architecture [19].
Renal injury was graded as follows:
Grade 0: no diagnostic change;
Grade 1: tubular cell swelling, loss of brush borders, nuclear reduction with up to 1/3 of the tubular profile with nuclear loss;
Grade 2: in addition to Grade 1, greater nuclear reduction with up to 2/3 of the tubular profile with nuclear loss;
Grade 3: greater nuclear reduction with more than 2/3 of the tubular profile with nuclear loss [20].
Statistical analysis
Statistical analysis was performed using SPSS for Windows 18.0 (SPSS Inc., Chicago, IL, USA). In univariate analyses, categorical variables were evaluated by the chi-square test and numerical variables by the Student t test. The groups were compared using one-way ANOVA, and post-hoc analysis was made with Tukey’s test. The results were expressed as Mean ±SD. A p value of <0.05 was considered significant.
Results
Biochemical analysis
The results concerning the biochemical parameters for liver and kidney tissues are as follows:
The TAS level in liver tissues was significantly lower in DLM group compared to control group and DLM+GLN group, and the level in DLM+GLN group was significantly higher than in control group and DLM group. The TOS level was significantly higher in DLM group than in other groups, and the level in DLM+GLN group was significantly lower than in DLM group. The TNF-α level in DLM+GLN group was significantly lower than in DLM group. In terms of IL-1β level, no significant difference was found between DLM+GLN group and DLM group. The IL-6 level in DLM group was significantly higher than in other groups, and the levels in GLN group and DLM+GLN group were similar to each other.
The TAS level in kidney tissues was significantly lower in the DLM group than the control group, but similar to the levels in other groups. The TOS level in the DLM group was significantly higher than in other groups, and the level in the DLM+GLN group was significantly higher than in the GLN group. In terms of TNF-α level, the DLM group was significantly higher than other groups, and the other groups were all similar to each other. In terms of IL-1β level, the DLM group was significantly higher than the control group and GLN group, but similar to the DLM+GLN group. The IL-6 level in the DLM group was significantly higher than in other groups. Table 1 presents biochemical parameters for liver and kidney tissues in control and treated groups.
Table 1.
Biochemical parameters for liver and kidney tissues in control and treated groups.
| Tissue | Group | TAS | TOS | TNF-α | IL-1β | IL-6 |
|---|---|---|---|---|---|---|
| Liver | Control | 1.13±0.10 | 185.28±52.32 | 488.10±141.37 | 759.92±113.43 | 713.74±52.90a |
| DLM | 0.39±0.18a | 344.46±24.82a | 2554.38±251.19a | 1067.53±454.50b | 1527.46±650.45b | |
| GLN | 1.01±0.20b | 197.95±37.22b | 743.79±292.06a,b | 611.28±46.53c | 708.99±54.09b | |
| DLM+GLN | 0.86±0.12a | 225.67±19.53 | 1398.17±249.63a,b,c | 958.11±74.88 | 1043.56±118.33 | |
| Kidney | Control | 2.01±0.23 | 23.97±3.43 | 489.74±374.62 | 759.68±87.53a | 672.01±121.34 |
| DLM | 1.48±0.12a | 68.13±6.50a | 1223.51±248.06a | 1067.53±454.50 | 1280.30±103.1a | |
| GLN | 1.74±0.59 | 16.84±4.03a,b | 439.73±285.41b | 611.28±46.53b | 753.92±85.79b | |
| DLM+GLN | 1.51±0.38 | 29.64±11.71b,c | 770.40±133.50b | 958.11±74.88ac | 694.37±48.72b |
TAS – total antioxidant capacity (μmol H2O2 equivalent/L); TOS – total oxidant capacity (mmol Trolox equivalent/L); TNF-α – tumor necrosis factor-alpha (pg/mL); IL-1β – interleukin-1 beta (pg/mL); IL-6 – interleukin-6 (pg/mL); results are expressed as mean ±SD.
The result is significant when compared to Control group;
the result is significant when compared to DLM group;
the result is significant when compared to GLN group.
In serum analyses, the ALT level in the DLM group was significantly higher than in other groups. The AST level in the DLM group was higher than in other groups, and the level in the DLM+GLN group was significantly higher than in the GLN group. The LDH level in the DLM group was significantly higher than in other groups. In terms of urea level, the DLM group was significantly higher than the control group and the level in the DLM+GLN group was similar to the one in the control group. The creatinine level in the DLM group was significantly higher than in other groups. Table 2 presents the serum biochemical parameters in the control and treated groups.
Table 2.
Serum biochemical parameters in control and treated groups.
| Groups | AST (u/l) | ALT (u/l) | LDH (u/l) | Urea (mg/dl) | Cr (mg/dl) |
|---|---|---|---|---|---|
| Control | 77.6±23.1a | 34.4±4.2 | 92.1±17.6 | 57.5±5.3 | 1.05±0.2 |
| DLM | 470.7±248.9b | 343.8±159.1b | 1455.5±362.1a | 159.3±19.3a | 3.9±2.2a |
| GLN | 57.9±8.2b | 59.6±9.1b | 283.7±477.7b | 62.0±6.3b | 0.7±0.1b |
| DLM+GLN | 113.5±12.1 | 226.4±38.8a,b,c | 213.6±66.5b | 115.7±87.6 | 3.61±2.0a,c |
AST – aspartate aminotransferase; ALT – alanine aminotransferase; LDH – lactate dehydrogenase; Cr – creatinine, results are expressed as mean ±SD.
The result is significant when compared to Control group;
the result is significant when compared to DLM group;
the result is significant when compared to GLN group.
Histopathological analysis
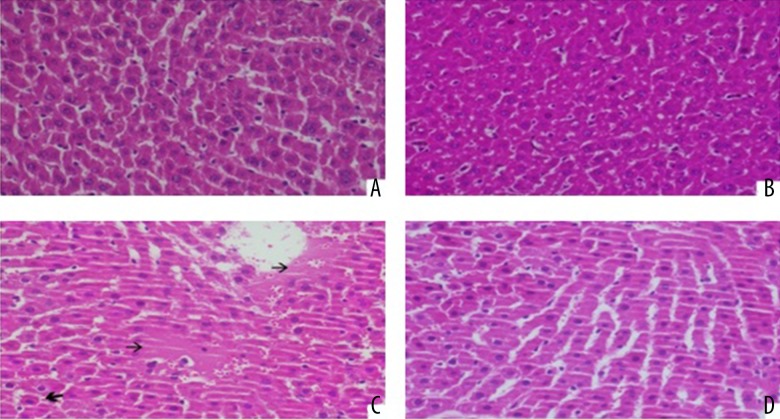
The results concerning the histopathological staging of liver tissues are as follows (Figure 1):
Figure 1.
(A) Hepatic section of control group (H&E ×400). (B, C) Deltametrin-intoxicated grade 1 hepatocyte cytoplasm vacuolization and focal nuclear pyknosis) and grade 3 (cytoplsamic vacuolization of hepatocytes as well as sinusoidal congestion and dilatation) hepatic injury (H&E ×400). (D) Deltametrin + L-glutamine treated protected hepatic lobule rats (H&E ×400).
In terms of Grade 0 and 2, a significant difference was found between the DLM group and the control group, and also between the DLM group and the GLN group. Another significant difference was established between the DLM group and the DLM+GLN group in terms of Grade 2. Also, the DLM+GLN group presented a significant difference with the control group and GLN group in terms of Grade 0 and Grade 1, but had no significant difference from the DLM group in terms of Grade 2.
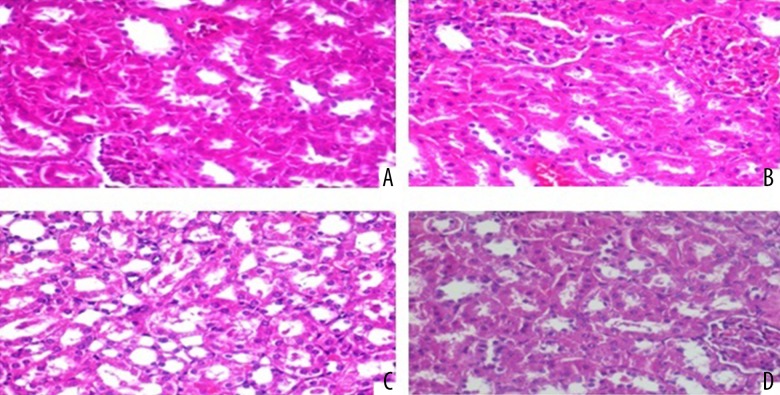
The results concerning the histopathological staging of kidney tissues are as follows (Figure 2):
Figure 2.
(A) Normal histological characteristic of the conrol group in the renal tubule (H&E ×200). (B, C) Deltametrin-intoxicated grade 1 (swelling of renal tubules, and mild loss in the nucleus) injury and grade 2 (swelling of renal tubules, and moderate loss in the nucleus) injury (H&E ×200). (D) Deltametrin + L-glutamine treated rats (H&E ×200).
No significant difference was established between the DLM group and DLM+GLN group in terms of Grade 2, and a significant difference was found between the DLM group and the control group in terms of Grade 0, 1, and 2. The GLN group also presented a significant difference with the DLM group in terms of Grade 0, 1, and 2. In addition, the DLM+GLN group established a significant difference with the control group and GLN group in terms of Grade 0 and 1. Table 3 presents the histopathologic grading of hepatic and renal injury in the control and treated groups.
Table 3.
Histopathologic grading of hepatic and renal injury in control and treated groups.
| Tissue | Pathologic grade | Control group | DLM group | GLN group | DLM+GLN group |
|---|---|---|---|---|---|
| Liver | (0) | 7 | 0a | 6b | 2a,c |
| (1) | 1 | 3 | 2 | 6a,c | |
| (2) | 0 | 4a | 0b | 0b | |
| (3) | 0 | 1 | 0 | 0 | |
| (4) | 0 | 0 | 0 | 0 | |
| Kidney | (0) | 8 | 0a | 8b | 0a,c |
| (1) | 0 | 4a | 0b | 6a,c | |
| (2) | 0 | 4a | 0b | 2 | |
| (3) | 0 | 0 | 0 | 0 |
The result is significant when compared to Control group;
the result is significant when compared to DLM group;
the result is significant when compared to GLN group. The numbers in parentheses indicate grade of liver and kidney, other figures indicate the number of rats in the table.
Discussion
The results revealed that GLN is an effective agent offering protection against DLM-induced liver injury. This effect was confirmed by the levels of tissue oxidative stress markers (TAS, TOS), serum biomarkers of hepatotoxicity (ALT, AST, LDH), and the grades of cellular injuries. Nevertheless, this effect was not verified for renal injury.
Hepatotoxicity may occur due to various infections and drugs [21]. Oxidative stress is one of the major mechanisms of pesticide-induced toxicities [22]. In some studies on pyrethroid insecticides, free radicals and oxidative stress-mediated injury resulting from lipid peroxidation have been reported [23,24]. Abdel-Daim et al. found that the administration of DLM increased lipid peroxidation through elevated hepatic and renal malondialdehyde (MDA) level, and decreased the hepatic and renal antioxidants(superoxide dismutase, catalase, GSH) [3]. As consistent with the literature, the DLM group in our study presented lower TAS levels and higher TOS levels compared to other groups.
Glutamine, although accepted as a non-essential amino acid, is called “conditionally essential” due to its important cellular function in critically ill patients [25]. Liver is the primary organ of GLN consumption. Since GLN is a precursor of GSH, its supplementation in the clinical diet may play a contributory role in maintaining high levels of GSH and in avoiding oxidative stress-induced injury [26]. Moreover, the hepatoprotective effect of GLN and its critical role in liver functions are reported [27]. Studies have found that GLN is a potent agent on the reduction of liver injury resulting from various toxic chemicals and also that GLN exerts these effects through its antioxidant effects [28,29]. In our study, we also found that the histopathological liver injury was significantly reduced in the DLM+GLN group. In addition to this improvement, in the same group, we found an increase in TAS and a significant decrease in TOS levels as well as a significant improvement in the serum markers of liver injury (AST, ALT, and LDH). These findings indicate both the critical role of oxidative stress in DLM-induced toxicity and also the hepatoprotective effect of glutamine, which is mediated by its antioxidant effect. The glutamine group in our study presented significant improvements in terms of TAS and TOS levels compared to the control group. This may be an indication that glutamine is likely to offer protective effects in healthy individuals as well.
Renal tubular cells and glomeruli are critical target organs for the synthetic pyrethroid pesticide compounds which produce various renal toxic effects [30]. El-Gerbed et al. histopathologically found glomerular congestion, tubular degeneration, necrosis, and swelling of tubules, and vacuolization at various sites along the kidney cortex in the rats administered with DLM [31]. Several studies also reported an increase in lipid peroxidation, oxidative stress, and proinflammatory cytokines (IL-1β, IL-6, TNF-α) in the kidney tissues of the rats with DLM-induced toxicity [3,31]. In an in vitro study, glutamine was found to suppress the TNF-α and IL-6 levels in human monocytes in a dose-dependent manner [32]. From these findings, it can be inferred that glutamine is theoretically able to provide renoprotective effects through its antioxidant and anti-inflammatory properties. On the contrary, Abraham et al. reported that glutamine provided no protection against cyclophosphamide nephrotoxicity although it led to improvement in oxidative stress parameters [33]. Similarly, Moraa et al. showed that glutamine had no protective effect against cisplatin-induced nephrotoxicity [34]. In our study, glutamine offered no significant renal improvement in terms of cytopathology, whereas it led to significant improvement in the TOS levels. Nevertheless, it had no contributory effect on the TAS levels and the renal functions such as urea and creatinine. Although these findings confirm the role of inflammation and oxidative stress in DLM-induced nephrotoxicity, they suggest that the protective role of glutamine remains very limited. From this situation it can be understood that the pathogenesis of DLM-induced nephrotoxicity and the nephroprotective effect mechanism of glutamine have yet to be investigated.
Interleukin-6, IL-1β, and TNF-α are major proinflammatory cytokines induced by oxidative stress and inflammation [35]. Some previous studies reported that the immunomodulating effects of GLN and alanine were dependent on the suppression of TNF-α, IL-6 and IL-1β in human peripheral blood mononuclear cells (PBMCs) [36,37]. Other studies revealed that glutamine provided a potent immunomodulatory effect by decreasing the expression of proinflammatory cytokines (TNF-α, IL-6, IL-1β) [32,38,39]. In our study, an increase was observed in the inflammatory cytokines in the rats administered with DLM. Moreover, in the DLM+GLN group, a significant decrease was detected in TNF-α and IL-6 levels, whereas no contributory effect was found in IL-1β levels. This result was the same both in liver and kidney tissues. These findings seem to be in line with the literature.
Conclusions
We conclude that glutamine leads to significant improvement in DLM-induced acute hepatotoxicity in terms of histopathological results, tissue oxidative stress parameters, and serum liver function marker enzymes, but it does not offer the same protective effect against kidney injury. More studies should be conducted to clarify and to determine the mechanism of damage of kidney by deltamethrin and the nephroprotective effects of glutamine.
Footnotes
Source of support: Departmental sources
References
- 1.Mehlhorn H, Schumacher B, Jatzlau A, et al. Efficacy of deltamethrin against nymphs and adults of ticks (Ixodes ricinus, Rhipicephalus sanguineus) in treated hair of cattle and sheep. Parasitol Res. 2011;108:963–71. doi: 10.1007/s00436-010-2141-2. [DOI] [PubMed] [Google Scholar]
- 2.Chargui I, Grissa I, Bensassi F, et al. Oxidative stress, biochemical and histopathological alterations in the liver and kidney of female rats exposed to low doses of deltamethrin (DM): a molecular assessment. Biomed Environ Sci. 2012;25:672–83. doi: 10.3967/0895-3988.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Daim MM, Abuzead SM, Halawa SM. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One. 2013;8(9):e72991. doi: 10.1371/journal.pone.0072991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chinn K, Narahashi T. Stabilization of sodium channel states by deltamethrin in mouse neuroblastoma cells. J Physiol. 1986;380:191–207. doi: 10.1113/jphysiol.1986.sp016280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michelangeli F, Robson MJ, East JM, Lee AG. The conformation of pyrethroids bound to lipid bilayers. Biochim Biophys Acta. 1990;1028:49–57. doi: 10.1016/0005-2736(90)90264-o. [DOI] [PubMed] [Google Scholar]
- 6.Anand SS, Bruckner JV, Haines WT, et al. Characterization of deltamethrin metabolism by rat plasma and liver microsomes. Toxicol Appl Pharmacol. 2006;212:156–66. doi: 10.1016/j.taap.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 7.El-Maghraby S. Metabolism of deltamethrin in rats. Biomed Environ Sci. 2007;20:212–16. [PubMed] [Google Scholar]
- 8.Stangl R, Szijártó A, Ónody P, et al. Reduction of liver ischemia reperfusion injury via glutamine pretreatment. J Surg Res. 2011;166:95–103. doi: 10.1016/j.jss.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 9.Zhang WX, Zhou LF, Zhang L, et al. Protective effects of glutamine preconditioning on ischemia reperfusion injury in rats. Hepatobiliary Pancreat Dis Int. 2011;10:78–82. doi: 10.1016/s1499-3872(11)60011-8. [DOI] [PubMed] [Google Scholar]
- 10.Peng HC, Chen YL, Chen JR, et al. Effects of glutamine administration on inflammatory responses in chronic ethanol fed rats. J Nutr Biochem. 2011;22:282–88. doi: 10.1016/j.jnutbio.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Kessel A, Toubi E, Pavlotzky E, et al. Treatment with glutamine is associated with down regulation of Toll like receptor 4 and myeloid differentiation factor 88 expression and decrease in intestinal mucosal injury caused by lipopolysaccharide endotoxaemia in a rat. Clin Exp Immunol. 2008;151:341–47. doi: 10.1111/j.1365-2249.2007.03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welbourne TC. Ammonia production and glutamine incorporation into glutathione in the functioning rat kidney. Can J Biochem. 1979;57:233–37. doi: 10.1139/o79-029. [DOI] [PubMed] [Google Scholar]
- 13.Anders MW. Glutathione-dependent bioactivation of xenobiotics: implications for mutagenicity and carcinogenicity. Princess Takamatsu Symp. 1990;21:89–99. [PubMed] [Google Scholar]
- 14.Dekant W, Vamvakas S. Glutathione-dependent bioactivation of xenobiotics. Xenobiotica. 1993;23:873–87. doi: 10.3109/00498259309059415. [DOI] [PubMed] [Google Scholar]
- 15.Weiner ML, Nemec M, Sheets L, et al. Comparative functional observational battery study of twelve commercial pyrethroid insecticides in male rats following acute oral exposure. Neurotoxicology. 2009;30(Suppl 1):S1–16. doi: 10.1016/j.neuro.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Yan H, Lv SG, et al. Effects of glycyl-glutamine dipeptide supplementation on myocardial damage and cardiac function in rats after severe burn injury. Int J Clin Exp Pathol. 2013;6(5):821–30. [PMC free article] [PubMed] [Google Scholar]
- 17.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–11. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–19. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Camargo CA, Jr, Madden JF, Gao W, et al. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997;26(6):1513–20. doi: 10.1002/hep.510260619. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee PK, Cuzzocrea S, Brown PA, et al. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000;58(2):658–73. doi: 10.1046/j.1523-1755.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 21.Salwa H, Abdallah H, Robert EB, et al. Bicalutamide-induced hepatotoxicity: A rare adverse effect. Am J Case Rep. 2014;15:266–70. doi: 10.12659/AJCR.890679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee BD, Seth V, Ahmed RS. Pesticide-induced oxidative stress: perspectives and trends. Rev Environ Health. 2001;16:1–40. doi: 10.1515/reveh.2001.16.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Sayeed I, Parvez S, Pandey S, et al. Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotox Environ Saf. 2003;56:295–301. doi: 10.1016/s0147-6513(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 24.Kale M, Rathore N, John S, Bhatnagar D. Lipid peroxidative damage on pyrethroid exposure and alterations in antioxidant status in rat erythrocytes: a possible involvement of reactive oxygen species. Toxicol Lett. 1999;105:197–205. doi: 10.1016/s0378-4274(98)00399-3. [DOI] [PubMed] [Google Scholar]
- 25.Lacey JM, Wilmore DW. Is glutamine a conditionally essential amino acid? Nut Rev. 1990;48:297–309. doi: 10.1111/j.1753-4887.1990.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 26.Amores-Sánchez MI1, Medina MA. Glutamine, as a precursor of glutathione, and oxidative stress. Mol Genet Metab. 1999;67:100–5. doi: 10.1006/mgme.1999.2857. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Ierapetritou MG, Roth CM. Effects of amino acid transport limitations on cultured hepatocytes. Biophys Chem. 2010;152:89–98. doi: 10.1016/j.bpc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Turkez H, Geyikoglu F, Yousef MI, et al. Ameliorative effect of supplementation with L-glutamine on oxidative stress, DNA damage, cell viability and hepatotoxicity induced by 2,3,7,8-tetrachlorodibenzop-dioxin in rat hepatocyte cultures. Cytotechnology. 2012;64:687–99. doi: 10.1007/s10616-012-9449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Z, Cai F, Lin N, et al. Effects of glutamine on oxidative stress and nuclear factor κB expression in the livers of rats with nonalcoholic fatty liver disease. Experımental and Therapeutıc Medicine. 2014;7:365–70. doi: 10.3892/etm.2013.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohamed M, Abdellatif MD, Sabar A, Elglammal MD. Sodium fluoride ion and renal function after prolonged sevoflurane or isoflurane anaesthesia. Br J Anaesth. 2003;19:79–83. [Google Scholar]
- 31.El-Gerbed MSA. Protective effect of lycopene on deltamethrin-induced histological and ultrastructural changes in kidney tissue of rats. Toxicol Ind Health. 2014;30(2):160–73. doi: 10.1177/0748233712448115. [DOI] [PubMed] [Google Scholar]
- 32.Raspé C, Czeslick E, Weimann A, et al. Glutamine and alanine-induced differential expression of intracellular IL-6, IL-8, and TNF-α in LPS-stimulated monocytes in human whole-blood. Cytokine. 2013;62(1):52–57. doi: 10.1016/j.cyto.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Abraham P, Isaac B. The effects of oral glutamine on cyclophosphamide-induced nephrotoxicity in rats. Hum Exp Toxicol. 2011;30:616–23. doi: 10.1177/0960327110376552. [DOI] [PubMed] [Google Scholar]
- 34.Moraa LO, Antunes LMG, Francescato HDC, Bianchi MLP. The effects of oral glutamine on cisplatin-induced nephrotoxicity in rats. Pharmacological Research. 2003;47:517–22. doi: 10.1016/s1043-6618(03)00040-9. [DOI] [PubMed] [Google Scholar]
- 35.Nijveldt RJ, Teerlink T, Van Der Hoven B, et al. Asymmetrical dimethylarginine(ADMA) in critically ill patients: high plasma ADMA concentration is an independent risk factor of ICU mortality. Clin Nutr. 2003;22:23–30. doi: 10.1054/clnu.2002.0613. [DOI] [PubMed] [Google Scholar]
- 36.Wischmeyer PE, Riehm J, Singleton KD, et al. Glutamine attenuates tumor necrosis factor-alpha release and enhances heat shock protein 72 in human peripheral blood mononuclear cells. Nutrition. 2003;19(1):1–6. doi: 10.1016/s0899-9007(02)00839-0. [DOI] [PubMed] [Google Scholar]
- 37.Wang LM, Pan LY, Zhang F, et al. Mechanism of glutamine downregulates the cytokine expression in lipopolysaccharide-stimulated human peripheral blood mononuclear cells. View Record in Scopus. 2009;47(20):1578–80. [PubMed] [Google Scholar]
- 38.Coeffier M, Miralles-Barrachina O, Le Pessot F, et al. Influence of glutamine on cytokine production by human gut in vitro. Cytokine. 2001;13:148–54. doi: 10.1006/cyto.2000.0813. [DOI] [PubMed] [Google Scholar]
- 39.Schuster H, Blanc MC, Neveux N, et al. Protective effects of regulatory amino acids on ischemia-reperfusion injury in the isolated perfused rat liver. Scand J Gastroenterol. 2006;41:1342–49. doi: 10.1080/00365520600682039. [DOI] [PubMed] [Google Scholar]