Abstract
Long non-coding RNAs (lncRNAs) are transcripts without protein-coding potential but having a pivotal role in numerous biological functions. Long non-coding RNAs act as regulators at different levels of gene expression including chromatin organization, transcriptional regulation, and post-transcriptional control. Misregulation of lncRNAs expression has been found to be associated to cancer and other human disorders. Here, we review the different types of lncRNAs, their mechanisms of action on genome formatting and expression and emphasized on the multifaceted action of the H19 lncRNA.
Keywords: lncRNAs, H19, chromatin organization, transcriptional regulation, post-transcriptional control
The advent of DNA tilling arrays and deep sequencing technologies has revealed that a much larger part of the genome is transcribed into RNAs than previously assumed. It is estimated that up to 70% of the genome is transcribed but only 2% of the human genome codes for proteins (Bertone et al., 2004; Birney et al., 2007; Kapranov et al., 2007; ENCODE Project Consortium, 2012) and RNAs without coding potential are collectively referred as non-coding RNAs (ncRNAs).
Non-coding RNAs include the well-known ribosomal (r) RNAs, ribozymes, transfer (t) RNAs, small nuclear (sn) RNAs, telomere-associated RNAs (TERRA, TERC), as well as a plethora of far less characterized RNAs. Based on their size, these ncRNAs are subdivided into two groups: small ncRNAs (<200 nt) and long ncRNAs [lncRNA (>200 nt)]. Small ncRNAs, such as microRNAs (miRs), small interfering RNAs (siRNAs), or PIWI-interacting RNAs (piRNAs) received much attention and were shown to mainly act as negative regulators of gene expression. In contrast, lncRNAs represent a more functionally diverse class of transcripts. LncRNAs are found in a large diversity of animals species (Guttman et al., 2009; Jia et al., 2010; Pauli et al., 2012), but also in plants (Swiezewski et al., 2009), yeast (Houseley et al., 2008), and even in prokaryotes (Bernstein et al., 1993) and viruses (Reeves et al., 2007). LncRNAs remains poorly conserved among species (Pang et al., 2006; Derrien et al., 2012). However, accumulating evidences indicate that this RNA class plays an important role in a variety of biological processes and may be involved in cancer and other human diseases (Wapinski and Chang, 2011; Tano and Akimitsu, 2012).
Majority of lncRNAs are 5′ capped, 3′ polyadenylated, multi-exonic and are subjected to transcriptional regulation as coding mRNAs (Carninci et al., 2005; Guttman et al., 2010; Cabili et al., 2011; Derrien et al., 2012). Some of the lncRNAs such as XIST, MALAT1, or NEAT1 are almost exclusively localized in the nucleus (Brown et al., 1992; Hutchinson et al., 2007), whereas others are mostly found in the cytoplasm (Coccia et al., 1992; Yoon et al., 2012). In term of genomic organization, lncRNAs can be classified according to their proximity to protein coding genes into five categories: sense, when overlapping one or more exons of another transcript; antisense, when overlapping one or more exons of another transcript on the opposite strand; bidirectional, when its expression and the expression of the neighboring coding transcript on the opposite strand are initiated in close proximity; intronic, when raising from an intron of another transcript; or intergenic, when produced from an independent transcription unit in the interval between two protein coding genes. This crude classification illustrates that lncRNA expression may be controlled by different molecular mechanisms, but it does account neither for their modes of action nor for their cellular functions.
While only a limited number of lncRNAs has been studied, numerous evidences indicate that lncRNAs interact with a plethora of proteins. Furthermore, homologous Watson–Crick base pairing provides an efficient way by which lncRNAs may selectively interact with other nucleic acid species. It is believed that lncRNAs are involved in a diversity of cellular functions through gene expression regulation at different levels including chromatin organization, transcriptional regulation, and post-transcriptional mRNA processing (Mercer et al., 2009; Wilusz et al., 2009).
To complicate matters further, Anderson et al. (2015) recently described that a conserved micropeptide is encoded by a skeletal muscle-specific RNA previously annotated as a putative long non-coding RNA. This finding leads to the proposal that several lncRNAs could also have a biological function through the production of micropeptides.
LncRNAs in the Control of mRNA Processing
The ability of lncRNAs to recognize complementary sequences allows the regulation of mRNA processing at various steps, including degradation, splicing, translation, or transport (Figure 1).
FIGURE 1.
Functional mechanisms of long non-coding (lncRNA) action at the post-transcriptional levels. (A) mRNA stabilization. Base pairing between specific regions of a long non-coding antisense RNA and its sense transcript induces stabilization of the target mRNA and increases protein abundance. (B) mRNA degradation. Staufen double-stranded RNA-binding protein 1 (STAU1)-mediated mRNA decay is induced when base pairing is formed between the mRNA and a lncRNA. (C) Ribosome targeting. Through homologous base pairing with mRNAs and interactions with ribosomal proteins lncRNAs target transcripts to ribosomes or prevent translation. (D) Regulation of splicing. Base pairing between mRNAs and lncRNAs may prevent splicing by masking the splicing sites. In addition, lncRNAs are also implicated in the formation and maintenance of nuclear structures involved in alternative splicing of nascent transcripts. (E) miR sponge. By sequestering miRs through base pairing formations, lncRNAs affect the expression of the miR target genes. (F) Precursor of miRs. LncRNAs can serve as a source of miRs after processing. LncRNAs are shown in red, whereas mRNAs are in blue. See text for examples.
Base pairing between defined regions of the human β-site APP-cleaving enzyme 1 (BACE1) transcript and its antisense lncRNA BACE1-AS induces the mRNA stabilization and consequently the increase in BACE1 protein abundance (Faghihi et al., 2008). Similarly, the lncRNA TINCR (terminal differentiation-induced ncRNA) interacts with a range of differentiation mRNAs including FLG, LOR, ALOXE3, ALOX12B, ABCA12, CASP14, or ELOVL3, to increase their stability (Kretz et al., 2013). In contrast, the recognition of mRNAs by other lncRNAs, such as half-STAU1-binding site RNAs (1/2sbsRNAs) decrease target mRNA stability by inducing STAU1 recruitment and the STAU1-mediated mRNA decay pathway (Gong and Maquat, 2011).
The translational process may also be modulated positively or negatively by lncRNA–mRNA pairing. For example, the antisense lncRNA ULCH-AS1 (ubiquitin carboxy-terminal hydrolase L1 antisense RNA 1) enhances ULCH mRNA translation (Carrieri et al., 2012), whereas lincRNA-p21 or pseudo-NOS suppress target mRNA translation (Korneev et al., 1999; Yoon et al., 2012).
The lncRNA MALAT1 (metastasis associated lung adenocarcinoma transcript 1) regulates pre-mRNA alternative splicing by modulating active serine/arginine splicing factors levels (Tripathi et al., 2010). In this case, the modulation of the mRNA processing is not achieved by a lncRNA–mRNA pairing mechanism but rather by the MALAT1-mediated modulation of the distribution of various splicing factors in nuclear speckle domains. However, antisense transcripts may also affect alternative splicing of their sense transcripts by virtue of masking splice sites by base complementarity (Krystal et al., 1990; Khochbin et al., 1992; Beltran et al., 2008). For example, a specific isoform of the lncRNA NPPA-AS is capable of down-regulating the intron-retained NPPA (atriuretic peptide precursor A) mRNA variant through RNA duplex formation between the sense and antisense transcripts (Annilo et al., 2009).
LncRNAs and the Connection with the MicroRNA World
Some lncRNAs act on post-transcriptional regulation through the modulation of the microRNA (miR) pathways. MiRs, a large class of small ncRNA, function by annealing to complementary sites in the coding sequences or 3′-untranslated regions (UTRs) of target mRNAs where they favor the recruitment of protein factors that impair translation and/or promote transcript degradation leading to a decrease in protein abundance (Baek et al., 2008; Bartel, 2009). Specifically, one mechanism by which the BACE1-AS lncRNA enhances BACE1 sense mRNA stability could be by masking the binding site for miR-485-5p (Faghihi et al., 2010). Rather than competing for miR-binding sites, a number of lncRNAs contain miR-binding sites in their sequence and therefore act as “sponges” to sequester miRs away from their mRNA targets. The pseudogene PTENP1 previously considered as biologically inactive was found to sequester miRs, consequently affecting their action on target gene regulation (Poliseno et al., 2010). In particular, the 3′-UTR of the PTENP1 lncRNA binds the same set of miRs targeting the tumor suppressor gene PTEN, then reducing the downregulation of this transcript and thus enhancing PTEN protein abundance. A number of other lncRNAs, including KRASP1, linc-MD1, HULC, or linc-ROR were shown to control mRNA activity through a miR sponge mechanism (Poliseno et al., 2010; Wang et al., 2010, 2013; Cesana et al., 2011). These examples illustrate that lncRNAs could counteract miR actions, but lncRNAs can themselves give rise to miRs and thus favor post-translational control by miR pathways as it is the case for the mouse Dlk1–Dio3 cluster or the BIC lncRNA (Eis et al., 2005; Hagan et al., 2009). Within the Dlk1–Dio3 cluster, Meg3/Gtl2 contains in its last intron the evolutionarily conserved microRNA miR-770 whereas Meg8 transcripts have the intron-encoded miR-341, miR-1188, and miR-370. Similarly, miR-155 is processed from sequences present in BIC lncRNA that accumulates in lymphoma cells.
LncRNAs in the Transcriptional Control
A number of evidences indicate that lncRNAs can act at the level of transcription either negatively or positively through a variety of molecular mechanisms (Figure 2). The dihydrofolate reductase (DHFR) gene contains a major and a minor promoter. The minor promoter gives rise to a lncRNA that forms a stable triplex lncRNA-DNA association at the major DHFR promoter and interacts with the general transcription factor II B (TFIIB) leading to the dissociation of the transcriptional preinitiation complex at this major promoter and then reducing DHFR expression (Martianov et al., 2007).
FIGURE 2.
Functional mechanism of action at the levels of transcriptional regulation. (A) LncRNA may regulate transcription by virtue of RNA–DNA triplex formation preventing the formation of the transcription initiation complex at promoters. (B) LncRNAs can act as decoys by titrating transcription factors away from their cognate promoters. (C) LncRNAs can regulate transcription through the targeting of transcription factors to promoters or acting as co-factors involved in transcription factor activity. (D) LncRNA can also control transcription factor trafficking. LncRNAs are shown in red.
Other lncRNAs act as decoys to negatively control transcription by titrating transcription factors away from their cognate promoters. The lncRNA PANDAR (promoter of CDKN1A antisense DNA damage activated RNA) is induced in a TP53-dependent manner and inhibits apoptotic gene expression to favor cell-cycle arrest through direct interaction with, and sequestration of NFYA, a transcription factor controlling the apoptotic program upon DNA damage (Hung et al., 2011). Similarly, the lncRNA GAS5 (growth arrest-specific 5) contains an RNA motif derived from a stem-loop structure mimicking a DNA motif corresponding to the glucocorticoid response element. GAS5 binds to the DNA-binding domain of the glucocorticoid receptor, acts as a decoy glucocorticoid response element and is thus competing with DNA sites for binding to the glucocorticoid receptor (Kino et al., 2010).
Rather than acting as molecular decoys, lncRNA could modulate transcription by recruiting factors at target gene promoters or acting as transcription factor co-activators. For example, a lncRNA produced at the 5′ regulatory region of the cyclin D1 (CCND1) gene in response to genotoxic stress tethers and modulates the activity of the RNA-binding protein TLS (translocated in liposarcoma) which in turn inhibits the activity of the histone acetyltransferases CBP (CREB binding protein) and EP300, leading to CCND1 transcriptional repression (Wang et al., 2008). The lncRNA Evf-2 (DLX6-AS1) forms a stable complex with the homeodomain-containing protein DLX2 to induce expression of the adjacent genes at the DLX5/6 locus (Feng et al., 2006). In this later case, the Evf-2 lncRNA functions as a co-factor regulating transcription factor activity.
Other lncRNAs regulate transcription by controlling transcription factor trafficking. As such, the lncRNA NRON (non-protein coding RNA, repressor of NFAT) interacts with importin-beta family members to inhibit nuclear translocation of the inactive dephosphorylated nuclear factor of activated T cells (NFAT) trans-activator (Willingham et al., 2005).
LncRNAs and Epigenetics
LncRNAs have been implicated in the control of gene expression through the recruitment of epigenetic modifiers at specific genomic loci. In eukaryotic chromatin, epigenetic regulation is conveyed by covalent modifications of DNA (methylation, hydroxymethylation), modifications of histone tails (acetylation, methylation, phosphorylation, ubiquitinylation), and the incorporation of various histone variants. These modifications locally change chromatin organization and regulate gene expression without changes in the DNA sequence. A number of evidences indicate that lncRNAs, acting as guides targeting enzymes involved in chromatin modifications, are part of this picture (Figure 3).
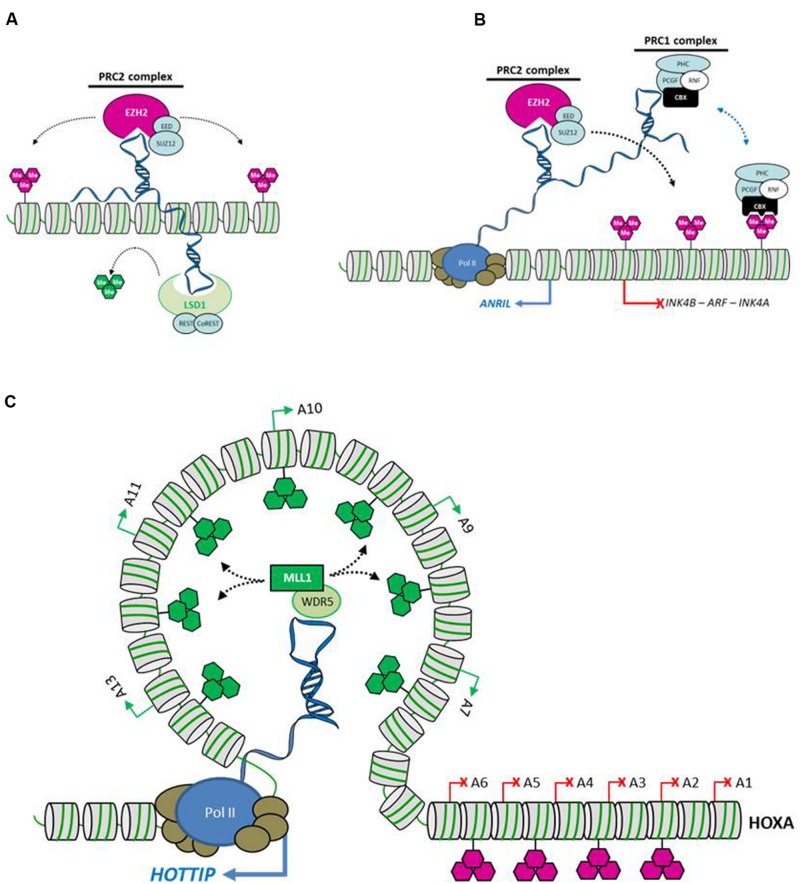
FIGURE 3.
Examples of lncRNAs controlling chromatin organization. (A) HOTAIR (HOX transcript antisense RNA) represses transcription in trans by recruiting two different chromatin modifying activities. The Polycomb Repressive Complex 2 (PRC2) produces the repressive H3K27me3 marks, whereas the LSD1-CoREST complex is responsible for the removal of the active H3K4me2/3 marks. (B) The ANRIL lncRNA represses transcription in cis at the INK4B/ARF/INK4A locus by recruiting the Polycomb repressive complexes PRC1 and PRC2. (C) The HOTTIP (HOXA transcript at the distal tip) lncRNA activates genes by recruiting the histone modifier complex WDR5-MLL which is responsible for H3K4me3 methylation, and by mediating long-range chromatin looping at one extremity of the HOXA locus. Purple hexagons represent H3K27me3 repressive marks, whereas green hexagons illustrate H3K4me3 activating marks.
The lncRNA HOTAIR (HOX transcript antisense RNA) is transcribed from the HOXC locus and targets Polycomb Repressive Complex 2 (PRC2) to silence distantly located genes, including genes at the HOXD locus and 100s of other genes on various chromosomes (Rinn et al., 2007; Zhang et al., 2015). Components of PRC2 trimethylate lysine 27 of histone H3 (H3K27me3) establishing the silent chromatin state (Völkel and Angrand, 2007; Völkel et al., 2015). Interestingly, HOTAIR also binds the LSD1–CoREST complex which possesses a lysine 4 of histone H3 demethylase activity, thus removing an active H3K4me2 chromatin mark (Tsai et al., 2010). Furthermore, deletion analysis of HOTAIR revealed that distinct parts of the lncRNA interact with PRC2 and LSD1 indicating that HOTAIR is able to bridge two independent chromatin modifying activities at a target locus. Indeed, the knockdown of HOTAIR is responsible for the concomitant loss of occupancy of PRC2 and LSD1, and concurrent loss of H3K27me3 and gain of H3K4me2 at target loci. Then, HOTAIR acts as an RNA scaffold targeting two different histone modification activities involved in heterochromatin formation.
The interplay between one lncRNA and different chromatin modifying complexes is also found at the INK4A tumor-suppressor locus. The antisense lncRNA ANRIL (antisense non-coding RNA in the INK4 locus, CDKN2B-AS) which is produced by the INK4B/ARF/INK4A locus binds specifically two Polycomb proteins, CBX7 (PRC1) and SUZ12 (PRC2). Disruption of interaction with both PRC1 and PRC2 proteins impacts the transcriptional repression at the INK4B locus in cis (Yap et al., 2010; Kotake et al., 2011). As another example, the lncRNA KCNQ1OT1 (KCNQ1 opposite strand/antisense transcript 1) mediates bidirectional silencing by interacting with chromatin and recruiting the PRC2 complex, as well as the histone methyltransferase G9a (EHMT2), resulting in an increase in the repressive histone modifications H3K27me3 and H3K9me3 at the KCNQ1 domain (Pandey et al., 2008). Thus, similar to HOTAIR and ANRIL, KCNQ1OT1 represents a prototype of a scaffold RNA recruiting multiple sets of chromatin modifying activities involved in target gene silencing. Approximately 20% of lncRNAs, including HOTAIR, ANRIL, KCNQ1OT1, but also XIST, RepA, HEIH, PCAT-1, H19, or linc-UBC1 (Zhao et al., 2008; Maenner et al., 2010; Prensner et al., 2011; Yang et al., 2011; Luo et al., 2013; He et al., 2013), are believed to guide PRC2 activity to target genes, indicating that lncRNA-mediated targeting of PRC2 at chromatin is a widely used strategy to repress gene expression through a chromatin reorganization mechanism (Khalil et al., 2009).
In contrast, the lncRNA HOTTIP (HOXA transcript at the distal tip) mediates transcriptional activation by controlling chromatin modification and organization (Wang et al., 2011). HOTTIP is produced from the 5′-end of the HOXA locus, downstream of HOXA13. The knockdown of HOTTIP decreases expression of HOXA genes in cis, with an efficacy that correlates with the proximity of the HOXA genes relative to the HOTTIP transcriptional unit. At the target genes, knockdown of HOTTIP results in the loss of activating H3K4me3 and H3K4me2 epigenetic marks, together with the decreases in occupancy of the MLL1 protein complex responsible for the establishment of these histone modifications. Furthermore, chromosome conformation capture carbon copy (C5) assays revealed abundant long-range looping interactions, bridging the transcribed target HOXA genes into proximity of the HOTTIP transcriptional unit. Thus, the mechanism by which the lncRNA HOTTIP controls HOXA expression relies on its potential to guide the histone methyltransferase MLL1 at target HOXA gene promoters, and on the formation of chromatin loops that connect distantly expressed HOXA genes to HOTTIP transcripts.
A role of lncRNAs in chromatin loop formation has also been described for the lncRNA CCAT1-L (Xiang et al., 2014). Indeed, CCAT1-L, is transcribed from a locus upstream of MYC and plays a role in MYC transcriptional regulation by promoting long-range chromatin looping.
Thus, lncRNAs, through the recruitment of chromatin modifiers and/or the induction of chromatin loops will modulate the chromatin conformation and will format the genome in a particular configuration. This lncRNA-mediated genome formatting emerges as a crucial and fundamental mechanism by which lncRNA may act on gene expression programs.
H19, a Prototype of a Multitask lncRNA
As discussed above, lncRNAs can regulate genome expression through different molecular mechanisms. However, several lncRNAs use multiple strategies that, in combination, may be required for their biological function. The action of the lncRNA H19 on gene expression illustrates the complexity of the combinatorial mechanisms of regulation achieved by a single lncRNA. H19 was the first lncRNA discovered (Brannan et al., 1990). Furthermore, H19 and its neighboring IGF2 gene located at position 11p15.5 are subjected to genomic imprinting and the study of the gene regulation at this locus serves as a model for understanding the molecular mechanisms involved in this genomic regulation. In addition, alterations of gene expression at the H19/IGF2 locus are associated to malignancies and developmental disorders. Loss of heterozygosity including loss of imprinting could be responsible for a loss of expression or a biallelic expression of these genes. Patients suffering from Beckwith–Wiedemann syndrome (BWS, OMIM 130650; Choufani et al., 2010) exhibit a loss of H19 expression and a biallelic expression of IGF2. BWS is associated with fetal and postnatal overgrowth and increased risk of embryonic or childhood cancers such as Wilm’s tumors. Loss of IGF2 expression with a biallelic H19 expression is responsible for 20 to 60% of cases of Silver–Russel syndrome (SRS, OMIM 180860; Penaherrera et al., 2010). SRS is an intrauterine growth delay associated to an altered postnatal growth with facial dysmorphia and corporal asymmetry. Numerous studies including ours indicate that H19 may play a key role in tumorigenesis and could contribute to tumor progression and aggressiveness. H19 overexpression has also been reported in various cancer tissues including breast (Adriaenssens et al., 1998; Lottin et al., 2002), bladder (Cooper et al., 1996), lung (Kondo et al., 1995), and esophageal cancers (Hibi et al., 1996). Several lines of evidence indicate that H19 could play a role in tumor invasion and angiogenesis. In breast cancer, the oncogenic role of H19 has been well established (Berteaux et al., 2005), even if the precise molecular mechanisms involved in tumorigenesis are not yet fully understood.
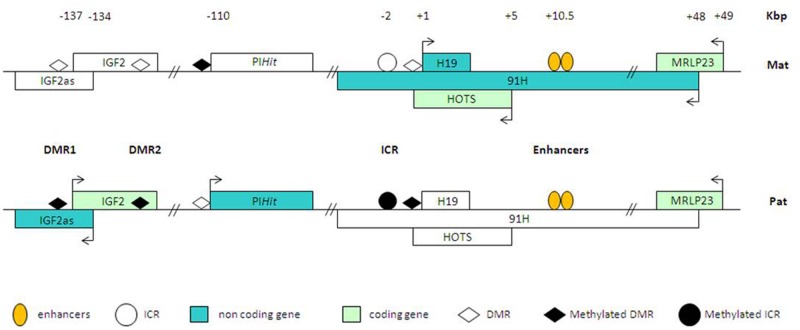
At the H19/IGF2 locus, both genes share a common set of enhancers located downstream of the H19 gene (Figure 4). The ICR (imprinting control region), located 2 kbp upstream of the H19 promoter, controls the monoallelic expression of H19 and IGF2 by insulating communication between the 3′ enhancers and the IGF2 promoter. The chromatin insulator property of the H19/IGF2 ICR is regulated by the insulator CTCF (CCTC-binding factor), which binds specifically to the unmethylated maternal allele. On the paternal allele, the ICR methylation does not allow CTCF binding and leads to IGF2 expression (reviewed in Lewis and Murrell, 2004). The H19/IGF2 locus contains other differentially methylated regions (DMRs), with DMR1 being a methylation-sensitive silencer and DMR2 being a methylation-sensitive activator (Constancia et al., 2000; Murrell et al., 2004). CTCF binding to the maternal ICR regulates its interaction with matrix attachment region 3 (MAR3) and DMR1 at IGF2, thus forming a tight loop around the maternal IGF2 locus which may contribute to its silencing. These interactions restrict the physical access of distal enhancers to the IGF2 promoter (Weber et al., 2003; Murrell et al., 2004; Kurukuti et al., 2006). Furthermore, several lncRNAs are produced at the H19/IGF2 locus adding further complexity to the locus regulation. The first antisense transcript at the H19/IGF2 locus is the lncRNA IGF2-AS (3–4 kb) discovered in 1991 in chicken (Rivkin et al., 1993; Moore et al., 1997). IGF2-AS and IGF2 are coregulated at the transcriptional levels but the function of this IGF2-AS lncRNA remains unclear. The lncRNA 91H (about 120 kb) is transcribed from the maternal allele (Berteaux et al., 2008). Recently, at the same position, a new protein coding gene HOTS (6 kbp) has been described (Onyango and Feinberg, 2011) but the relationship between the HOTS and 91H is still not clear. However, these two transcripts are transcribed in an antisense orientation compared to H19. An additional lncRNA produced by the H19/IGF2 locus has been identified (Court et al., 2011). This PIHit (paternally expressed IGF2/H19 intergenic transcript) lncRNA is a 5–6 kb transcript expressed from the paternal allele after birth. Thus, the genomic organization of coding and non-coding transcripts illustrates the complexity of the interleaved networks of lncRNAs expressed from the H19/IGF2 locus.
FIGURE 4.
Schematic representation of the transcriptional complexity at the H19/IGF2 locus. Non-coding transcripts at the H19/IGF2 are shown as blue squares when they are expressed. Coding genes are in green, when expressed. The differences in gene expression between the paternal and maternal alleles are shown. The DNA methylation status of the regulatory elements ICR (imprinting control region) and DMRs (differentially methylated regions) is indicated for the paternal and maternal alleles.
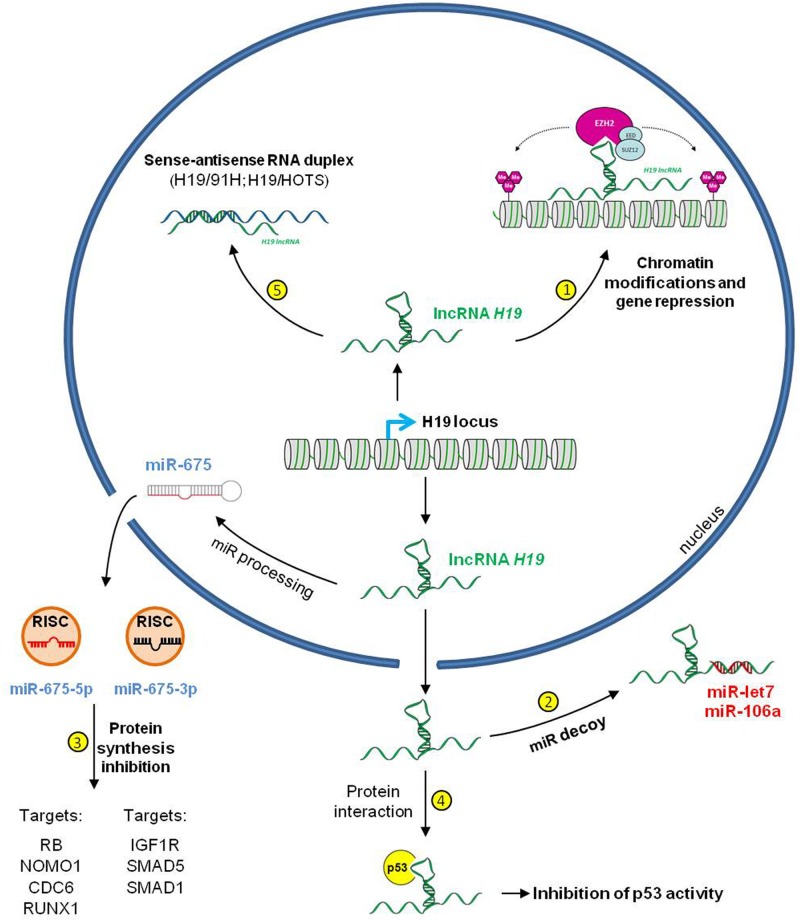
To complicate matters further, H19 lncRNA mechanisms of action appear to be extremely diverse, acting at various levels (Figure 5). H19 has been shown to guide chromatin modifying enzymes to specific loci. In particular, Luo et al. (2013) have shown that H19 binds to and recruit the histone methyltransferase EZH2 at the E-cadherin promoter, leading to an increase in H3K27me3 repressive marks and to the silencing of the E-cadherin gene in bladder cancer. PRC2 protein members are not the only chromatin modifying factors interacting with H19 since it has been shown that this lncRNA physically binds to the methyl-CpG-binding domain protein 1 (MBD1). The H19-MBD1 complex is then recruited at several imprinted genes including IGF2, SLC38A4, and PEG1 (Monnier et al., 2013). This recruitment also induces methylation at lysine 9 of histone H3 (H3K9me3), probably via the additional interaction with an H3K9 histone methylransferase.
FIGURE 5.
The multifaceted action of the lncRNA H19. The lncRNA H19 controls genome expression at multiple levels. H19 acts on chromatin organization through the recruitment of chromatin modifying complex PRC2 (1) and on post-transcriptional control as a miR decoys sequestering miR-106a and miR-let7 (2) or as a precursor for miR-675-5p and miR-675-3p (3) H19 also interact with p53 (TP53) and inactivate the tumor suppressor protein action (4) Furthermore, possible base pairing between H19 and the antisense transcripts 91H and HOTS may have biological outcomes (5).
The multifaceted action of H19 is also illustrated by its dual interaction with miR pathways. On one hand, the lncRNA H19 acts as miR sponge to sequester miR-106a as well as the mir-let7 family members (Kallen et al., 2013; Imig et al., 2015). On the other end, H19 serves as a precursor of miR-675 that will in turn, post-translationally regulate a number of targets involved in cell tumorigenicity, including RB, IGFR1, SMAD1, SMAD5, CDC6, NOMO1, or RUNX1 (Cai and Cullen, 2007; Tsang et al., 2010; Gao et al., 2012; Keniry et al., 2012; Dey et al., 2014; Zhuang et al., 2014). The role of H19 in tumor progression could also be mediated through its interaction with the tumor-suppressor TP53 protein. This association results in partial TP53 inactivation (Yang et al., 2012).
Several evidences also indicate that the H19 lncRNA controls IGF2 expression at the translational and/or post-translational levels (Li et al., 1998), suggesting that other mechanisms by which H19 exerts its action remain to be deciphered. Similarly, the possible role of RNA duplex formation between H19 and the antisense transcripts 91H and HOTS requires investigations.
LncRNAs in Human Diseases
Given the wide range of molecular actions achieved by the lncRNAs and their roles in various physiological processes, it is not surprising that they have been shown to be involved in many human diseases. A number of data indicate that alterations of lncRNA expression lead to tumorigenesis through changes at the chromatin, transcriptional or post-transcriptional levels that impact target genes expression (Table 1). Since lncRNAs are regulating a different cellular pathways, growing evidences suggest that they could play a role in a large number of other human disorders including metabolic diseases, neurodegenerative and psychiatric disorders, cardiovascular and immune dysfunctions (Taft et al., 2010; Esteller, 2011; Harries, 2012; Shi et al., 2013; Clark and Blackshaw, 2014).
Table 1.
Examples of long non-coding RNAs (lncRNAs) associated with human disorders.
| lncRNA | Cancer/disease | Mechanisms of action | Reference |
|---|---|---|---|
| ANRIL | Neurofibromatosis type 1, prostate cancer, melanoma, acute lymphoblastic leukemia | Chromatin modification via the recruitment of the Polycomb Repressive Complex 2 (PRC2) at the INKB/ARF/INK4A tumor suppressor locus | Pasmant et al. (2007), Pasmant et al. (2011), Yap et al. (2010), Iacobucci et al. (2011) |
| HOTAIR | Hepatocellular carcinoma, colorectal cancer, breast cancer, glioblastomas | Chromatin modification via the recruitment of PRC2 and LSD1 in trans. | Gupta et al. (2010), Kogo et al. (2011), Yang et al. (2011), Zhang et al. (2015) |
| H19 | Colorectal, gastric, breast, lung, esophageal, bladder, pancreas, ovary cancers | Chromatin modification via the recruitment of PRC2; Decoy for miR-Let-7; source of miR-675; TP53 inactivation | Kondo et al. (1995), Cooper et al. (1996), Hibi et al. (1996), Lottin et al. (2002), Berteaux et al. (2005), Tsang et al. (2010), Yang et al. (2012), Luo et al. (2013), Ma et al. (2014), Zhuang et al. (2014) |
| HEIH | Hepatocellular carcinoma | Chromatin modification via the recruitment of PRC2 | Yang et al. (2011) |
| PCAT-1 | Prostate cancer | Chromatin modification via the recruitment of PRC2 | Prensner et al. (2011) |
| linc-UBC1 | Bladder cancer | Chromatin modification via the recruitment of PRC2 | He et al. (2013) |
| BACE1-AS | Alzheimer’s disease | Increase in mRNA stability | Faghihi et al. (2008) |
| GAS5 | Breast, bladder cancers | Decoy for the glucocorticoid receptor; regulation of CDK6 expression | Mourtada-Maarabouni et al. (2009), Kino et al. (2010), Liu et al. (2013) |
| PTENP1 | Prostate cancer | miR decoy | Poliseno et al. (2010) |
| KCNA2-AS | Neuropathic pain | Decrease of KCNA2 expression | Zhao et al. (2013) |
| MIAT | Schizophrenia | Component of the nuclear matrix involved in mRNA splicing | Barry et al. (2014), Ishizuka et al. (2014) |
| MALAT1 | Lung cancer | Alternative splicing regulation | Schmidt et al. (2011) |
Perspectives and Concluding Remarks
LncRNAs represent a large part of the transcriptome and a very heterogeneous class of transcripts in terms of genomic organization and modes of action. Many of them are considered as key regulators of gene expression and thus, lncRNAs constitute an additional layer controlling the cellular programs. LncRNAs regulate diverse expression steps at the levels of chromatin rearrangement, transcriptional control, and/or post-transcriptional processing. By these actions, lncRNAs are involved in numerous physiological functions and in many cases lncRNA alterations are associated with human disorders.
The fact that lncRNAs can be deregulated in tumors and other human pathologies, make them attractive candidates as biomarkers and as targets for therapy. LncRNAs may be down-regulated at the RNA levels by targeting their sequence. As so, short interfering RNAs (siRNAs) designed to perfectly match exact stretches of nucleotides, guarantee a high degree of specificity leading to lncRNA degradation. The power of the siRNA approach is illustrated by the success of a number of preclinical studies where siRNAs targeted mRNAs (Kaur et al., 2014). Similar approaches can thus be envisioned to target non-coding RNAs. Indeed, siRNAs have also been used to target miRs, leading to heart regeneration in an in vivo mouse model (Aguirre et al., 2014) and the use of siRNAs has been proposed in a therapeutic strategy targeting the lncRNA HOTAIR in endometrial carcinoma (Huang et al., 2014). Similarly, antisense oligonucleotides, single-strand DNA, or RNA molecules of 8 to 50 nucleotides can be used to target lncRNA. Specifically, in vivo and in vitro experiments revealed that antisense oligonucleotides directed against the lncRNA MALAT1 inhibit its expression and drastically reduce lung cancer metastasis (Gutschner et al., 2013; Tripathi et al., 2013).
In this context, further exploration in the complexity of the lncRNA world promises the emergence of novel therapeutic opportunities.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work is supported by Inserm, the University of Lille, and grants from INCa (PLBio 2010), le Comité du Nord de la Ligue Contre le Cancer and l’ITMO Biologie Cellulaire, Développement et Evolution (BCDE).
References
- Adriaenssens E., Dumont L., Lottin S., Bolle D., Leprêtre A., Delobelle A., et al. (1998). H19 overexpression in breast adenocarcinoma stromal cells is associated with tumor values and steroid receptor status but independent of p53 and Ki-67 expression. Am. J. Pathol. 153 1597–1607 10.1016/S0002-9440(10)65748-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A., Montserrat N., Zacchigna S., Nivet E., Hishida T., Krause M. N., et al. (2014). In vivo activation of a conserved microRNA program induces mammalian heart regeneration. Cell Stem Cell 15 589–604 10.1016/j.stem.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. M., Anderson K. M., Chang C. L., Makarewich C. A., Nelson B. R., McAnally J. R., et al. (2015). A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 160 595–606 10.1016/j.cell.2015.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annilo T., Kepp K., Laan M. (2009). Natural antisense transcript of natriuretic peptide precursor A (NPPA): structural organization and modulation of NPPA expression. BMC Mol. Biol. 10:81 10.1186/1471-2199-10-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek D., Villén J., Shin C., Camargo F. D., Gygi S. P., Bartel D. P. (2008). The impact of microRNAs on protein output. Nature 455 64–71 10.1038/nature07242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G., Briggs J. A., Vanichkina D. P., Poth E. M., Beveridge N. J., Ratnu V. S., et al. (2014). The long non-coding RNA Gomafu is acutely regulated in response to neuronal activation and involved in schizophrenia-associated alternative splicing. Mol. Psychiatry 19 486–494 10.1038/mp.2013.45 [DOI] [PubMed] [Google Scholar]
- Bartel D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell 136 215–233 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran M., Puig I., Peña C., García J. M., Alvarez A. B., Peña R., et al. (2008). A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev. 22 756–769 10.1101/gad.455708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H. D., Zopf D., Freymann D. M., Walter P. (1993). Functional substitution of the signal recognition particle 54-kDa subunit by its Escherichia coli homolog. Proc. Natl. Acad. Sci. U.S.A. 90 5229–5233 10.1073/pnas.90.11.5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berteaux N., Aptel N., Cathala G., Genton C., Coll J., Daccache A., et al. (2008). A novel H19 antisense RNA overexpressed in breast cancer contributes to paternal IGF2 expression. Mol. Cell. Biol. 28 6731–6745 10.1128/MCB.02103-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berteaux N., Lottin S., Monté D., Pinte S., Quatannens B., Coll J., et al. (2005). H19 mRNA-like noncoding RNA promotes breast cancer cell proliferation through positive control by E2F1. J. Biol. Chem. 280 29625–22936 10.1074/jbc.M504033200 [DOI] [PubMed] [Google Scholar]
- Bertone P., Stolc V., Royce T. E., Rozowsky J. S., Urban A. E., Zhu X., et al. (2004). Global identification of human transcribed sequences with genome tiling arrays. Science 306 2242–2246 10.1126/science.1103388 [DOI] [PubMed] [Google Scholar]
- Birney E., Stamatoyannopoulos J. A., Dutta A., Guigó R., Gingeras T. R., Margulies E. H., et al. (2007). Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447 799–816 10.1038/nature05874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan C. I., Dees E. C., Ingram R. S., Tilghman S. M. (1990). The product of the H19 gene may function as an RNA. Mol. Cell. Biol. 10 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. J., Hendrich B. D., Rupert J. L., Lafrenière R. G., Xing Y., Lawrence J., et al. (1992). The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 71 527–542 10.1016/0092-8674(92)90520-M [DOI] [PubMed] [Google Scholar]
- Cabili M. N., Trapnell C., Goff L., Koziol M., Tazon-Vega B., Regev A, et al. (2011). Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 25 1915–1927 10.1101/gad.17446611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Cullen B. R. (2007). The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA 13 313–316 10.1261/rna.351707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carninci P., Kasukawa T., Katayama S., Gough J., Frith M. C., Maeda N., et al. (2005). FANTOM consortium; RIKEN genome exploration research group and genome science group (genome network project core group). The transcriptional landscape of the mammalian genome. Science 309 1559–1563. [DOI] [PubMed] [Google Scholar]
- Carrieri C., Cimatti L., Biagioli M., Beugnet A., Zucchelli S., Fedele S., et al. (2012). Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature 491 454–457 10.1038/nature11508 [DOI] [PubMed] [Google Scholar]
- Cesana M., Cacchiarelli D., Legnini I., Santini T., Sthandier O., Chinappi M., et al. (2011). A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147 358–369 10.1016/j.cell.2011.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choufani S., Shuman C., Weksberg R. (2010). Beckwith-Wiedemann syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 154C 343–354 10.1002/ajmg.c.30267 [DOI] [PubMed] [Google Scholar]
- Clark B. S., Blackshaw S. (2014). Long non-coding RNA-dependent transcriptional regulation in neuronal development and disease. Front. Genet. 5:164 10.3389/fgene.2014.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia E. M., Cicala C., Charlesworth A., Ciccarelli C., Rossi G. B., Philipson L., et al. (1992). Regulation and expression of a growth arrest-specific gene (gas5) during growth, differentiation, and development. Mol. Cell. Biol. 12 3514–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constancia M., Dean W., Lopes S., Moore T., Kelsey G., Reik W. (2000). Deletion of a silencer element in Igf2 results in loss of imprinting independent of H19. Nat. Genet. 26 203–206 10.1038/79930 [DOI] [PubMed] [Google Scholar]
- Cooper M. J., Fischer M., Komitowski D., Shevelev A., Schulze E., Ariel I., et al. (1996). Developmentally imprinted genes as markers for bladder tumor progression. J. Urol. 155 2120–2127 10.1016/S0022-5347(01)66120-2 [DOI] [PubMed] [Google Scholar]
- Court F., Baniol M., Hagege H., Petit J. S., Lelay-Taha M. N., Carbonell F., et al. (2011). Long-range chromatin interactions at the mouse Igf2/H19 locus reveal a novel paternally expressed long non-coding RNA. Nucleic Acids Res. 39 5893–5906 10.1093/nar/gkr209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien T., Johnson R., Bussotti G., Tanzer A., Djebali S., Tilgner H., et al. (2012). The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 22 1775–1189 10.1101/gr.132159.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey B. K., Pfeifer K., Dutta A. (2014). The H19 long noncoding RNA gives rise to microRNAs miR-675-3p and miR-675-5p to promote skeletal muscle differentiation and regeneration. Genes Dev. 28 491–501 10.1101/gad.234419.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eis P. S., Tam W., Sun L., Chadburn A., Li Z., Gomez M. F., et al. (2005). Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc. Natl. Acad. Sci. U.S.A. 102 3627–3632 10.1073/pnas.0500613102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium. (2012). An integrated encyclopedia of DNA elements in the human genome. Nature 489 57–74 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. (2011). Non-coding RNAs in human disease. Nat. Rev. Genet. 12 861–874 10.1038/nrg3074 [DOI] [PubMed] [Google Scholar]
- Faghihi M. A., Modarresi F., Khalil A. M., Wood D. E., Sahagan B. G., Morgan T. E., et al. (2008). Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feedforward regulation of β-secretase. Nat. Med. 14 723–730 10.1038/nm1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghihi M. A., Zhang M., Huang J., Modarresi F., Van der Brug M. P., Nalls M. A., et al. (2010). Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biol. 11 R56. 10.1186/gb-2010-11-5-r56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Bi C., Clark B. S., Mady R., Shah P., Kohtz J. D. (2006). The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 20 1470–1484 10.1101/gad.1416106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W. L., Liu M., Yang Y., Yang H., Liao Q., Bai Y., et al. (2012). The imprinted H19 gene regulates human placental trophoblast cell proliferation via encoding miR-675 that targets Nodal Modulator 1 (NOMO1). RNA Biol. 9 1002–1010 10.4161/rna.20807 [DOI] [PubMed] [Google Scholar]
- Gong C., Maquat L. E. (2011). lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3’ UTRs via Alu elements. Nature 470 284–288 10.1038/nature09701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. A., Shah N., Wang K. C., Kim J., Horlings H. M., Wong D. J., et al. (2010). Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464 1071–1076 10.1038/nature0897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschner T., Hämmerle M., Eissmann M., Hsu J., Kim Y., Hung G., et al. (2013). The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 73 1180–1189 10.1158/0008-5472.CAN-12-2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M., Amit I., Garber M., French C., Lin M. F., Feldser D., et al. (2009). Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458 223–227 10.1038/nature07672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M., Garber M., Levin J. Z., Donaghey J., Robinson J., Adiconis X., et al. (2010). Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat. Biotechnol. 28 503–510 10.1038/nbt.1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan J. P., O’Neill B. L., Stewart C. L., Kozlov S. V., Croce C. M. (2009). At least ten genes define the imprinted Dlk1-Dio3 cluster on mouse chromosome 12qF1. PLoS ONE 4:e4352 10.1371/journal.pone.0004352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries L. W. (2012). Long non-coding RNAs and human disease. Biochem. Soc. Trans. 40 902–906 10.1042/BST20120020 [DOI] [PubMed] [Google Scholar]
- He W., Cai Q., Sun F., Zhong G., Wang P., Liu H., et al. (2013). linc-UBC1 physically associates with polycomb repressive complex 2 (PRC2) and acts as a negative prognostic factor for lymph node metastasis and survival in bladder cancer. Biochim. Biophys. Acta 1832 1528–1537 10.1016/j.bbadis.2013.05.010 [DOI] [PubMed] [Google Scholar]
- Hibi K., Nakamura H., Hirai A., Fujikake Y., Kasai Y., Akiyama S., et al. (1996). Loss of H19 imprinting in esophageal cancer. Cancer Res. 56 480–482. [PubMed] [Google Scholar]
- Houseley J., Rubbi L., Grunstein M., Tollervey D., Vogelauer M. (2008). A ncRNA modulates histone modification and mRNA induction in the yeast GAL gene cluster. Mol. Cell 32 685–695 10.1016/j.molcel.2008.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Ke P., Guo L., Wang W., Tan H., Liang Y., et al. (2014). Lentivirus-mediated RNA interference targeting the long noncoding RNA HOTAIR inhibits proliferation and invasion of endometrial carcinoma cells in vitro and in vivo. Int. J. Gynecol. Cancer 24 635–642 10.1097/IGC.0000000000000121 [DOI] [PubMed] [Google Scholar]
- Hung T., Wang Y., Lin M. F., Koegel A. K., Kotake Y., Grant G. D., et al. (2011). Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat. Genet. 43 621–629 10.1038/ng.848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson J. N., Ensminger A. W., Clemson C. M., Lynch C. R., Lawrence J. B., Chess A. (2007). A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics 8:39 10.1186/1471-2164-8-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobucci I., Sazzini M., Garagnani P., Ferrari A., Boattini A., Lonetti A., et al. (2011). A polymorphism in the chromosome 9p21 ANRIL locus is associated to Philadelphia positive acute lymphoblastic leukemia. Leuk. Res. 35 1052–1059 10.1016/j.leukres.2011.02.020 [DOI] [PubMed] [Google Scholar]
- Imig J., Brunschweiger A., Brümmer A., Guennewig B., Mittal N., Kishore S., et al. (2015). miR-CLIP capture of a miRNA targetome uncovers a lincRNA H19-miR-106a interaction. Nat. Chem. Biol. 11 107–114 10.1038/nchembio.1713 [DOI] [PubMed] [Google Scholar]
- Ishizuka A., Hasegawa Y., Ishida K., Yanaka K., Nakagawa S. (2014). Formation of nuclear bodies by the lncRNA Gomafu-associating proteins Celf3 and SF1. Genes Cells 19 704–721 10.1111/gtc.12169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H., Osak M., Bogu G. K., Stanton L. W., Johnson R., Lipovich L. (2010). Genome-wide computational identification and manual annotation of human long noncoding RNA genes. RNA 16 1478–1487 10.1261/rna.1951310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen A. N., Zhou X. B., Xu J., Qiao C., Ma J., Yan L., et al. (2013). The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell 52 101–112 10.1016/j.molcel.2013.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapranov P., Willingham A. T., Gingeras T. R. (2007). Genome-wide transcription and the implications for genomic organization. Nat. Rev. Genet. 8 413–423 10.1038/nrg2083 [DOI] [PubMed] [Google Scholar]
- Kaur I. P., Chopra K., Rishi P., Puri S., Sharma G. (2014). Small RNAs: the qualified candidates for gene manipulation in diverse clinical pathologies. Crit. Rev. Ther. Drug. Carrier Syst. 31 305–329 10.1615/CritRevTherDrugCarrierSyst.2014007943 [DOI] [PubMed] [Google Scholar]
- Keniry A., Oxley D., Monnier P., Kyba M., Dandolo L., Smits G., et al. (2012). The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat. Cell Biol. 14 659–665 10.1038/ncb2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A. M., Guttman M., Huarte M., Garber M., Raj A., Rivea Morales D., et al. (2009). Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. U.S.A. 106 11667–11672 10.1073/pnas.0904715106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khochbin S., Brocard M. P., Grunwald D., Lawrence J. J. (1992). Antisense RNA and p53 regulation in induced murine cell differentiation. Ann. N. Y. Acad. Sci. 660 77–87 10.1111/j.1749-6632.1992.tb21060.x [DOI] [PubMed] [Google Scholar]
- Kino T., Hurt D. E., Ichijo T., Nader N., Chrousos G. P. (2010). Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 3:ra8 10.1126/scisignal.2000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogo R., Shimamura T., Mimori K., Kawahara K., Imoto S., Sudo T., et al. (2011). Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 71 6320–6326 10.1158/0008-5472.CAN-11-1021 [DOI] [PubMed] [Google Scholar]
- Kondo M., Suzuki H., Ueda R., Osada H., Takagi K., Takahashi T., et al. (1995). Frequent loss of imprinting of the H19 gene is often associated with its overexpression in human lung cancers. Oncogene 10 1193–1198 10.1378/chest.13-2736u7.13 [DOI] [PubMed] [Google Scholar]
- Korneev S. A., Park J. H., O’Shea M. (1999). Neuronal expression of neural nitric oxide synthase (nNOS) protein is suppressed by an antisense RNA transcribed from an NOS pseudogene. J. Neurosci. 19 7711–7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake Y., Nakagawa T., Kitagawa K., Suzuki S., Liu N., Kitagawa M., et al. (2011). Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene 30 1956–1962 10.1038/onc.2010.568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz M., Siprashvili Z., Chu C., Webster D. E., Zehnder A., Qu K., et al. (2013). Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature 493 231–235 10.1038/nature11661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal G. W., Armstrong B. C., Battey J. F. (1990). N-myc mRNA forms an RNA-RNA duplex with endogenous antisense transcripts. Mol. Cell. Biol. 10 4180–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurukuti S., Tiwari V. K., Tavoosidana G., Pugacheva E., Murrell A., Zhao Z., et al. (2006). CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc. Natl. Acad. Sci. U.S.A. 103 10684–10649 10.1073/pnas.0600326103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A., Murrell A. (2004). Genomic imprinting: CTCF protects the boundaries. Curr. Biol. 14 R284–R286 10.1016/j.cub.2004.03.026 [DOI] [PubMed] [Google Scholar]
- Li Y. M., Franklin G., Cui H. M., Svensson K., He X. B., Adam G., et al. (1998). The H19 transcript is associated with polysomes and may regulate IGF2 expression in trans. J. Biol. Chem. 273 28247–28252 10.1074/jbc.273.43.28247 [DOI] [PubMed] [Google Scholar]
- Liu Z., Wang W., Jiang J., Bao E., Xu D., Zeng Y., et al. (2013). Downregulation of GAS5 promotes bladder cancer cell proliferation, partly by regulating CDK6. PLoS ONE 8:e73991 10.1371/journal.pone.0073991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottin S., Adriaenssens E., Dupressoir T., Berteaux N., Montpellier C., Coll J., et al. (2002). Overexpression of an ectopic H19 gene enhances the tumorigenic properties of breast cancer cells. Carcinogenesis 23 1885–1895 10.1093/carcin/23.11.1885 [DOI] [PubMed] [Google Scholar]
- Luo M., Li Z., Wang W., Zeng Y., Liu Z., Qiu J. (2013). Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 333 213–221 10.1016/j.canlet.2013.01.033 [DOI] [PubMed] [Google Scholar]
- Ma C., Nong K., Zhu H., Wang W., Huang X., Yuan Z., et al. (2014). H19 promotes pancreatic cancer metastasis by derepressing let-7’s suppression on its target HMGA2-mediated EMT. Tumour Biol. 35 9163–9169 10.1007/s13277-014-2185-5 [DOI] [PubMed] [Google Scholar]
- Maenner S., Blaud M., Fouillen L., Savoye A., Marchand V., Dubois A., et al. (2010). 2-D structure of the A region of Xist RNA and its implication for PRC2 association. PLoS Biol. 8:e1000276 10.1371/journal.pbio.1000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martianov I., Ramadass A., Serra Barros A., Chow N., Akoulitchev A. (2007). Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature 445 666–670 10.1038/nature05519 [DOI] [PubMed] [Google Scholar]
- Mercer T. R., Dinger M. E., Mattick J. S. (2009). Long noncoding RNAs: insights into functions. Nat. Rev. Genet. 10 155–159 10.1038/nrg2521 [DOI] [PubMed] [Google Scholar]
- Monnier P., Martinet C., Pontis J., Stancheva I., Ait-Si-Ali S., Dandolo L. (2013). H19 lncRNA controls gene expression of the Imprinted Gene Network by recruiting MBD1. Proc. Natl. Acad. Sci. U.S.A. 110 20693–20698 10.1073/pnas.1310201110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T., Constancia M., Zubair M., Bailleul B., Feil R., Sasaki H., et al. (1997). Multiple imprinted sense and antisense transcripts, differential methylation and tandem repeats in a putative imprinting control region upstream of mouse Igf2. Proc. Natl. Acad. Sci. U.S.A. 94 12509–12514 10.1073/pnas.94.23.12509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourtada-Maarabouni M., Pickard M. R., Hedge V. L., Farzaneh F., Williams G. T. (2009). GAS5 a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene 28 195–208 10.1038/onc.2008.373 [DOI] [PubMed] [Google Scholar]
- Murrell A., Heeson S., Reik W. (2004). Interaction between differentially methylated regions partitions the imprinted genes Igf2 and H19 into parent-specific chromatin loops. Nat. Genet. 36 889–893 10.1038/ng1402 [DOI] [PubMed] [Google Scholar]
- Onyango P., Feinberg A. P. (2011). A nucleolar protein, H19 opposite tumor suppressor (HOTS), is a tumor growth inhibitor encoded by a human imprinted H19 antisense transcript. Proc. Natl. Acad. Sci. U.S.A. 108 16759–16764 10.1073/pnas.1110904108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R. R., Mondal T., Mohammad F., Enroth S., Redrup L., Komorowski J., et al. (2008). Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell 32 232–246 10.1016/j.molcel.2008.08.022 [DOI] [PubMed] [Google Scholar]
- Pang K. C., Frith M. C., Mattick J. S. (2006). Rapid evolution of noncoding RNAs: lack of conservation does not mean lack of function. Trends Genet. 22 1–5 10.1016/j.tig.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Pasmant E., Laurendeau I., Héron D., Vidaud M., Vidaud D., Bièche I. (2007). Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Res. 67 3963–3969 10.1158/0008-5472.CAN-06-2004 [DOI] [PubMed] [Google Scholar]
- Pasmant E., Sabbagh A., Masliah-Planchon J., Ortonne N., Laurendeau I., Melin L., et al. (2011). Role of noncoding RNA ANRIL in genesis of plexiform neurofibromas in neurofibromatosis type 1. J. Natl. Cancer Inst. 103 1713–1722 10.1093/jnci/djr416 [DOI] [PubMed] [Google Scholar]
- Pauli A., Valen E., Lin M. F., Garber M., Vastenhouw N. L., Levin J. Z., et al. (2012). Systematic identification of long noncoding RNAs expressed during zebrafish embryogenesis. Genome Res. 22 577–591 10.1101/gr.133009.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penaherrera M. S., Weindler S., Van Allen M. I., Yong S. L., Metzger D. L., McGillivray B., et al. (2010). Methylation profiling in individuals with Russell-Silver syndrome. Am. J. Med. Genet. 152A 347–355 10.1002/ajmg.a.33204 [DOI] [PubMed] [Google Scholar]
- Poliseno L., Salmena L., Zhang J., Carver B., Haveman W. J., Pandolfi P. P. (2010). A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465 1033–1038 10.1038/nature09144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prensner J. R., Iyer M. K., Balbin O. A., Dhanasekaran S. M., Cao Q., Brenner J. C., et al. (2011). Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1 an unannotated lincRNA implicated in disease progression. Nat. Biotechnol. 29 742–749 10.1038/nbt.1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M. B., Davies A. A., McSharry B. P., Wilkinson G. W., Sinclair J. H. (2007). Complex I binding by a virally encoded RNA regulates mitochondria-induced cell death. Science 316 1345–1348 10.1126/science.1142984 [DOI] [PubMed] [Google Scholar]
- Rinn J. L., Kertesz M., Wang J. K., Squazzo S. L., Xu X., Brugmann S. A., et al. (2007). Functional demarcation of active and silent chroatin domains in human HOX loci by noncoding RNAs. Cell 129 1311–1323 10.1016/j.cell.2007.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkin M., Rosen K. M., Villa-Komaroff L. (1993). Identification of an antisense transcript from the IGF-II locus in mouse. Mol. Reprod. Dev. 35 394–397 10.1002/mrd.1080350413 [DOI] [PubMed] [Google Scholar]
- Schmidt L. H., Spieker T., Koschmieder S., Schäffers S., Humberg J., Jungen D., et al. (2011). The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J. Thorac. Oncol. 6 1984–1992 10.1097/JTO.0b013e3182307eac [DOI] [PubMed] [Google Scholar]
- Shi X., Sun M., Liu H., Yao Y., Song Y. (2013). Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 339 159–166 10.1016/j.canlet.2013.06.013 [DOI] [PubMed] [Google Scholar]
- Swiezewski S., Liu F., Magusin A., Dean C. (2009). Coldinduced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature 462 799–802 10.1038/nature08618 [DOI] [PubMed] [Google Scholar]
- Taft R. J., Pang K. C., Mercer T. R., Dinger M., Mattick J. S. (2010). Non-coding RNAs: regulators of disease. J. Pathol. 220 126–139 10.1002/path.2638 [DOI] [PubMed] [Google Scholar]
- Tano K., Akimitsu N. (2012). Long non-coding RNAs in cancer progression. Front. Genet. 3:219 10.3389/fgene.2012.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi V., Ellis J. D., Shen Z., Song D. Y., Pan Q., Watt A. T., et al. (2010). The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell 39 925–938 10.1016/j.molcel.2010.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi V., Shen Z., Chakraborty A., Giri S., Freier S. M., Wu X., et al. (2013). Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLoS Genet. 9:e1003368 10.1371/journal.pgen.1003368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M. C., Manor O., Wan Y., Mosammaparast N., Wang J. K., Lan F., et al. (2010). Long noncoding RNA as modular scaffold of histone modification complexes. Science 329 689–693 10.1126/science.1192002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang W. P., Ng E. K., Ng S. S., Jin H., Yu J., Sung J. J., et al. (2010). Oncofetal H19-derived miR-675 regulates tumor suppressor RB in human colorectal cancer. Carcinogenesis 31 350–358 10.1093/carcin/bgp181 [DOI] [PubMed] [Google Scholar]
- Völkel P., Angrand P. O. (2007). The control of histone lysine methylation in epigenetic regulation. Biochimie 89 1–20 10.1016/j.biochi.2006.07.009 [DOI] [PubMed] [Google Scholar]
- Völkel P., Dupret B., Le Bourhis X., Angrand P. O. (2015). Diverse involvement of EZH2 in cancer epigenetics. Am. J. Transl. Res. 7 175–193. [PMC free article] [PubMed] [Google Scholar]
- Wang J., Liu X., Wu H., Ni P., Gu Z., Qiao Y., et al. (2010). CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 38 5366–5383 10.1093/nar/gkq285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. C., Yang Y. W., Liu B., Sanyal A., Corces-Zimmerman R., Chen Y., et al. (2011). A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472 120–124 10.1038/nature09819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Arai S., Song X., Reichart D., Du K., Pascual G., et al. (2008). Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature 454 126–130 10.1038/nature06992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Xu Z., Jiang J., Xu C., Kang J., Xiao L., et al. (2013). Endogenous miRNA sponge lincRNA-RoR regulates Oct4 Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev. Cell. 25 69–80 10.1016/j.devcel.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Wapinski O., Chang H. Y. (2011). Long noncoding RNAs and human disease. Trends Cell. Biol. 21 354–361 10.1016/j.tcb.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Weber M., Hagège H., Murrell A., Brunel C., Reik W., Cathala G., et al. (2003). Genomic imprinting controls matrix attachment regions in the Igf2 gene. Mol. Cell. Biol. 23 8953–8959 10.1128/MCB.23.24.8953-8959.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham A. T., Orth A. P., Batalov S., Peters E. C., Wen B. G., Aza-Blanc P., et al. (2005). A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science 309 1570–1573 10.1126/science.1115901 [DOI] [PubMed] [Google Scholar]
- Wilusz J. E., Sunwoo H., Spector D. L. (2009). Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 23 1494–1504 10.1101/gad.1800909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J. F., Yin Q. F., Chen T., Zhang Y., Zhang X. O., Wu Z., et al. (2014). Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res 24 513–531 10.1038/cr.2014.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Bi J., Xue X., Zheng L., Zhi K., Hua J., et al. (2012). Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells. FEBS J. 279 3159–3165 10.1111/j.1742-4658.2012.08694.x [DOI] [PubMed] [Google Scholar]
- Yang F., Zhang L., Huo X. S., Yuan J. H., Xu D., Yuan S. X., et al. (2011). Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology 54 1679–1689 10.1002/hep.24563 [DOI] [PubMed] [Google Scholar]
- Yap K. L., Li S., Muñoz-Cabello A. M., Raguz S., Zeng L., Mujtaba S., et al. (2010). Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol. Cell 38 662–674 10.1016/j.molcel.2010.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. H., Abdelmohsen K., Srikantan S., Yang X., Martindale J. L., De S., et al. (2012). LincRNA-p21 suppresses target mRNA translation. Mol. Cell 47 648–655 10.1016/j.molcel.2012.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Sun X., Zhou X., Han L., Chen L., Shi Z., et al. (2015). Long non-coding RNA HOTAIR promotes glioblastoma cell cycle progression in an EZH2 dependent manner. Oncotarget 6 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Sun B. K., Erwin J. A., Song J. J., Lee J. T. (2008). Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322 750–756 10.1126/science.1163045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Tang Z., Zhang H., Atianjoh F. E., Zhao J. Y., Liang L., et al. (2013). A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat. Neurosci. 16 1024–1031 10.1038/nn.3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang M., Gao W., Xu J., Wang P., Shu Y. (2014). The long non-coding RNA H19-derived miR-675 modulates human gastric cancer cell proliferation by targeting tumor suppressor RUNX1. Biochem. Biophys. Res. Commun. 448 315–322 10.1016/j.bbrc.2013.12.126 [DOI] [PubMed] [Google Scholar]