Abstract
Background and Objectives
A sharply contoured cryolipolysis vacuum applicator was developed to improve fit and tissue draw in the abdomen and flanks to better accommodate a range of body types and a variety of treatment sites. This study was carried out to evaluate the safety and efficacy of the new applicator for treatment of flank fat (“love handles”).
Study Design/Materials and Methods
A cryolipolysis vacuum applicator with a sharply contoured cup and curved cooling plates was used to treat 20 flanks. Two treatment cycles were delivered sequentially to each flank (60-minute cycle at a Cooling Intensity Factor of 41.6). Efficacy was evaluated 12 weeks post-treatment by physicians performing blinded, independent review of clinical photographs. Safety was assessed by the treating physician monitoring subjects for side effects and adverse events.
Results
Four blinded, independent physician reviewers properly identified the pre- and post-treatment photographs 94.4% of the time. Improvement was scored from 0 (none) to 10 (complete) and showed an average 4.3 point (43%) improvement. Side-effects were limited to erythema, edema, bruising, and numbness or tingling at the treatment site, and resolved without treatment.
Conclusions
Multiple treatment cycles from a new improved-fit cryolipolysis applicator are safe and effective for reduction of flank fat bulges. A high degree of improvement was reported by blinded, physician evaluation of standardized photographs. Laser Surg. Med. 46:731–735, 2014. © 2014 The Authors. Lasers in Surgery and Medicine published by Wiley Periodicals, Inc.
Keywords: cryolipolysis, flanks, multiple cycles, non-invasive body contouring, non-surgical fat reduction
Introduction
Cold-induced panniculitis, with subsequent atrophy is a well-known phenomenon in both children and adults [1–3]. Based upon observations of fat susceptibility to cold injury and a case report of popsicle panniculitis [4], researchers studied and developed controlled cryolipolysis; the controlled application of cooling to non-invasively reduce subcutaneous fat. Cryolipolysis was first shown to effectively reduce fat in a porcine model [5]. That study demonstrated fat reduction via examination by ultrasound and histopathologic evaluation; safety data were collected in this animal model by demonstrating no skin injury and a lack of change in serum lipid levels following treatment [5]. Since then, there have been follow-up porcine [6] and human clinical studies showing safe and effective cryolipolysis in a number of treatment areas including the abdomen, flanks, and thighs [7–13]. The safety of cryolipolysis treatments has also been demonstrated in clinical studies of cryolipolysis where serum lipid levels and liver function tests were performed [14], as well as peripheral nerve studies [15], all demonstrating no abnormalities following treatment. Cryolipolysis received U.S. Food and Drug Administration (FDA) clearance for fat reduction of the flanks in 2010, for abdominal fat reduction in 2012, and for fat reduction of the thighs in 2014. Studies have also demonstrated safety and effectiveness for treatment of undesirable fat in the back, arms, and chest [16–19]. Long-term persistence of fat reduction has been demonstrated in subjects treated to a single flank, to control for fluctuations in weight, for up to five years following a single treatment session [20].
Since flanks are perhaps the most commonly treated of all cryolipolysis sites, this study was intended to investigate improvements to flank treatment efficacy using a new cryolipolysis handpiece. Previously, a moderately-contoured applicator (CoolCurve, eZ App 6.2, Zeltiq Aesthetics, Inc., Pleasanton, CA) was used for flank treatments. A new applicator with a sharply contoured vacuum cup and curved cooling panels (CoolCurve+, Zeltiq Aesthetics, Inc.) was used for the current study. Design changes were intended to provide improved patient fit and tissue draw for sharply contoured treatment sites such as flanks. This study investigates the safety and efficacy of two overlapping treatment cycles to the flanks using a sharply-contoured cryolipolysis vacuum applicator.
Materials and Methods
This institutional review board (IRB)-approved clinical study investigated the safety and efficacy of treatment with two cycles per flank, administered in two separate treatment sessions utilizing a new, sharply-contoured vacuum applicator (CoolCurve+ applicator, Zeltiq Aesthetics, Inc.) in treating unwanted flank fat on 20 treatment sites in 10 subjects. Two cycles were delivered to each flank in a bilateral treatment protocol in a single office visit. Investigator measurements show the previous generation contoured applicator (CoolCurve) and the applicator evaluated in this study (CoolCurve+) treat approximately the same volume of tissue. While the cooling plate sizes are similar, the new applicator has a curved edge to better fit a patient's contour. The geometry of the applicator cup is also different. The new applicator cup has approximately 1.6 cm longer ears (lateral edges) and an approximately 6° increased angle to better accommodate contoured treatment areas, such as flanks.
Subjects
Twenty sites were treated in ten adult subjects. Subjects ranged from 33 to 56 years of age, averaging 42.2 years old and were all females. Subjects selected for the study had clearly visible fat on their flanks, and a body mass index (BMI) of up to 30. BMI was measured after measuring a subject's height and weight; these values were entered into an online BMI calculator (BMI Calculator, Tim O's Studios, LLC, Austin, TX). For the duration of the study, subjects were instructed to avoid implementing major diet or lifestyle changes in order to maintain their weight within 5 lbs of baseline measurement.
Cryolipolysis Treatment
Cryolipolysis treatment was delivered at a Cooling Intensity Factor 41.6, corresponding to an average energy extraction rate of 72.9 mW/cm2. Two cycles were administered in sequence to each flank, with 50% overlap. The flank fat was drawn by moderate vacuum suction between cooled plates in the applicator. At the conclusion of each 60 minute cycle, the treatment area was vigorously massaged by hand for 5 minutes [21]; then the remaining flank fat was treated using the same protocol. The two treatment cycles were positioned anterior to posterior with approximately 50% overlap. Thus, 4 cycles (2 per flank) were administered on the treatment day, treating both flanks with 2 cycles each, overlapping 50%.
Photographic Evaluation of Treatment Efficacy
Treatment efficacy was determined by blinded-expert analysis of clinical photographs viewed in pairs. At the baseline pre-treatment visit and 12-week follow-up visits, photographs were acquired using a standardized photography set-up using a professional digital camera with a 60 mm lens (D300 camera, Nikon Inc., Melville, NY) with floor-standing, external, bilaterally-symmetrical strobe lighting, with 1,000 W heads and soft-boxes (Dynalite, Inc., Union, NJ) to ensure consistency. Subjects were photographed with their feet separated at a fixed distance using a foot positioning guide and arms in a fixed, standard position. At the completion of the study, clinical photographs were reviewed by four blinded, independent physicians to choose which photographs were pre- versus post-treatment photographs. The pre- and post-treatment photograph pairs for each subject were randomized and presented to the blinded, independent reviewers, then the reviewers were asked to determine which image was captured prior to treatment. The degree of improvement was quantified by three of the independent physician reviewers who graded each pair of photographs from 0 (none) to 10 (complete) removal of flank fat. For any photographs that were incorrectly identified as baseline images by a reviewer, the corresponding 0–10 improvement score was assigned a negative value. For example, an incorrectly identified baseline image that was scored an improvement score of 3 was scored as −3 in the data analysis.
Side Effects
Side effects were assessed by the treating physician. All subjects were examined at the follow-up visit.
Results
One subject failed to return for her follow-up visit. The subjects' weights ranged from 119.3 to 172.7 lbs. (mean 142.1 lbs.) with their BMI ranging from 21.1 to 28.1 (mean 24.3). All subjects maintained their weights within 5 lbs. of their initial baseline weights by the end of the study period (Table1).
Table 1.
Subject Weight and BMI Data
| Treatment visit |
12-week follow-up visit |
|||
|---|---|---|---|---|
| Subject | Weight (lb) | BMI | Weight (lb) | Weight change (lb) |
| 01 | 158.0 | 24.7 | 154.2 | −3.8 |
| 02 | 129.8 | 22.3 | 128.9 | −0.9 |
| 03 | 161.5 | 27.7 | 161.2 | −0.3 |
| 04 | 148.8 | 28.1 | 147.7 | −1.1 |
| 05 | 132.4 | 22.7 | 130.5 | −1.9 |
| 06 | 119.3 | 23.3 | 115.3 | −4.0 |
| 08 | 143.7 | 26.3 | 142.1 | −1.6 |
| 09 | 127.9 | 21.3 | 130.5 | 2.6 |
| 10 | 126.6 | 21.1 | 124.5 | −2.1 |
At the treatment visit, all subjects had BMI <29 and agreed to maintain weight over the course of the study by avoiding diet and exercise changes. At 12-week follow-up, all subjects maintained weight within 5 lbs. of baseline.
The photographic review attempting to identify which photographs were taken before and after treatment demonstrated 94% correct identification of baseline images by four blinded, independent physician reviewers. Two reviewers correctly identified all baseline photographs and two reviewers each scored one incorrectly, thus 34 of 36 before and after pairs were correctly identified (Table2). The 0 (none) to 10 (complete) mean improvement score as rated by three blinded physician reviewers was 4.3 ± 1.4 (mean ± sem; Table2; Figs. 1 and 2).
Table 2.
Independent Photographic Review and Improvement Score Data
| Independent photo review (correct/incorrect) |
Improvement score (0–10) |
||||||
|---|---|---|---|---|---|---|---|
| Subject | Reviewer 1 | Reviewer 2 | Reviewer 3 | Reviewer 4 | Reviewer 2 | Reviewer 3 | Reviewer 4 |
| 01 | C | C | C | C | 6 | 5 | 3 |
| 02 | C | C | C | C | 3 | 4 | 5 |
| 03 | C | C | C | C | 4 | 2 | 2 |
| 04 | C | C | C | I | 3 | 4 | −2 |
| 05 | C | I | C | C | −3 | 5 | 5 |
| 06 | C | C | C | C | 7 | 8 | 6 |
| 08 | C | C | C | C | 7 | 5 | 6 |
| 09 | C | C | C | C | 6 | 5 | 5 |
| 10 | C | C | C | C | 5 | 4 | 5 |
Four blinded, independent physicians correctly identified 94% of baseline images; 34 of 36 correct. Three physicians assigned improvement scores from 0 (none) to 10 (complete), resulting in a mean improvement score of 4.3 + 1.4 (mean + sem).
Figure 1.
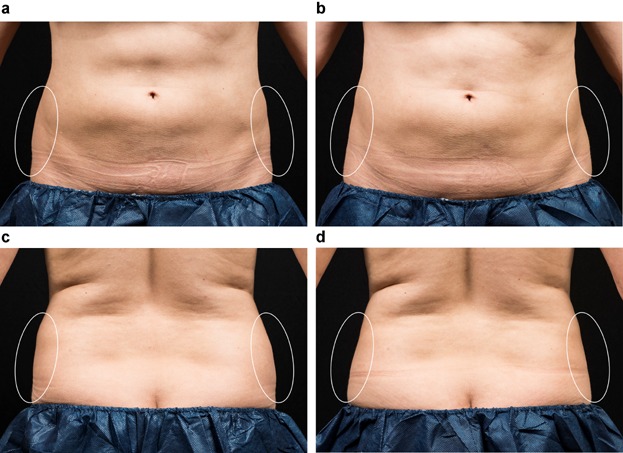
This 41 year-old female received two sequential cryolipolysis cycles to each flank. Clinical photographs show flank fat reduction between baseline (a, c) and 12 weeks post-treatment (b, d). This subject had a weight gain of only 2.6 lbs. from her initial visit until the end of the study.
Figure 2.
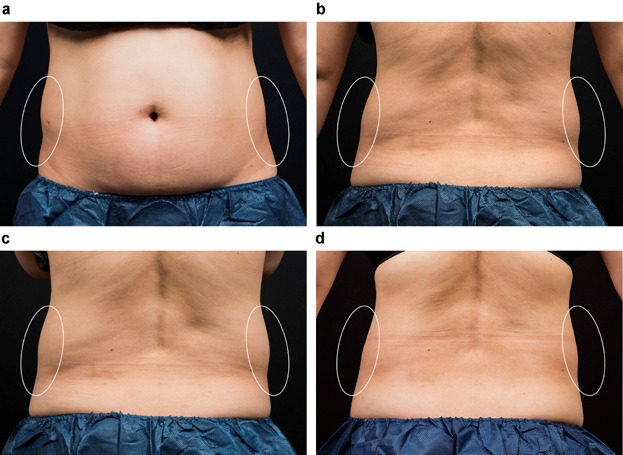
This 42 year-old female received two sequential cryolipolysis cycles to each flank. Clinical photographs show flank fat reduction between baseline (a, c) and 12 weeks post-treatment (b, d). This subject lost only 0.9 lbs. from her baseline visit to her final photographic evaluation, 12 weeks later.
Side-effects were mild and were limited to erythema, edema, bruising, numbness, and tingling at the treatment site. All of the side effects resolved without intervention prior to the 12-week follow-up visit.
Discussion
This study demonstrates that the new cryolipolysis applicator with a more sharply contoured vacuum cup and curved cooling panels is both safe and effective for administering cryolipolysis treatments to the flanks. This study assessed treatment efficacy at 3 months; whereas, other clinical studies evaluated patients at 4 [21] and 6 [9] months, thus the subjects presented in this study may attain some additional fat reduction with longer follow-up [9,21], or multiple treatment sessions as shown by Brightman and Geronemus [25].
The newly-designed applicator was developed to increase tissue draw and improve fit for curved surfaces like the flanks, and should allow treatment of patients who may otherwise not be able to be treated with the conventional applicator, due to the inability to gain sufficient suction and fit to keep the applicator in place. In addition, fit should be improved in most patients in the flank regain. Improvement after a single treatment incorporating 2 cycles per side was a score of 4.3 on a scale of 0 (none) to 10 (complete). The mean 94% correct identification of baseline images by four blinded, independent physicians for this study was similar to the results shown by Kaminer et al [23] in a much larger study, and greater than that shown by Garibyan [22]. Garibyan et al. treated 11 subjects unilaterally on one flank and reported that blinded evaluators correctly identified the treated side in 79% of subjects [22]. Kaminer et al. showed a 92% correct identification of baseline photographs by blinded, independent reviewers that evaluated fifty subjects treated on the flanks [23]. Areas aside from the flanks have shown similar statistically significant improvement. Mayoral et al. treated 20 subjects to their lower abdomens and demonstrated an 86% correct identification of pre-treatment images [24].
This study utilized conventional 2D photography to assess treatment efficacy, and was shown to produce visible improvement that was statistically significant when evaluated by blinded physician rating of digital images. Fat reduction can also be objectively quantified by methods such as ultrasound [7,8,12,13,15,17,19,21] and 3D Vectra imaging [11,25], and these methods may have more sensitivity to measure more subtle differences. In this current study, it was fortunate that the simpler, and less expensive, method of photography demonstrated statistically significant improvement. Future studies should employ multiple methods of evaluating fat reduction, to enable better comparison between different protocols. Efficacy assessment by clinical photographs may be affected by patient weight change, but this is unlikely for the current study since the subjects had an average 1.5 lb. weight loss at follow-up, well within the 5 lb. specification from baseline. The weight loss is likely due to increased awareness of diet and exercise while in the study, rather than fat reduction from cryolipolysis, since small volumes of fat are removed during cryolipolysis. This modest weight loss is not expected to affect the clinical efficacy as evaluated by blinded photographic review, and likely falls within the resolution limits of daily variations in weight and scale accuracy.
Cryolipolysis has also been shown to be effective in treating areas not on the abdomen as well. These different applications require the development of additional applicators to fit various body sites. Stevens et al. administered treatments to the lateral thigh in 40 subjects and showed 87% correct identification of pre-treatment photos [13]. Munavalli treated 18 male subjects for pseudogynecomastia and that study demonstrated an 80% correct identification of baseline photographs [19]. The significant efficacy of the sharply contoured vacuum cup applicator in the current study is consistent with other studies of cryolipolysis reported in the medical literature, as is the very low side-effect profile. Future studies incorporating a series of treatment sessions to the same area should further the amount of improvement seen in the current study. Treating even more sharply contoured areas such as extremities will require even more contoured vacuum cup applicators.
Conclusion
This study demonstrated the safety and efficacy of a new, sharply contoured vacuum cup applicator administered for a single treatment using two overlapping cycles on two flanks. Enhanced cryolipolysis clinical outcomes can be attained by selecting the appropriate applicator to maximize tissue draw for the intended treatment area and by delivering a sufficient number of cycles to adequately address the subcutaneous fat volume. The cryolipolysis treatment protocol should be customized to the individual in order to ensure high efficacy and patient satisfaction.
References
- 1.Collins HA, Stahlman M, Scott HW., Jr The occurrence of subcutaneous fat necrosis in an infant following induced hypothermia used as an adjuvant in cardiac surgery. Ann Surg. 1953;138:880–885. doi: 10.1097/00000658-195312000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rotman H. Cold panniculitis in children. Adiponecrosis E frigore of Haxthausen. Arch Dermatol. 1966;94:720–721. [PubMed] [Google Scholar]
- 3.Solomon LM, Beerman H. Cold panniculitis. Arch Dermatol. 1963;88:897–900. doi: 10.1001/archderm.1963.01590240221036. [DOI] [PubMed] [Google Scholar]
- 4.Epstein EH, Jr, Oren ME. Popsicle panniculitis. N Engl J Med. 1970;82:966–967. doi: 10.1056/NEJM197004232821709. [DOI] [PubMed] [Google Scholar]
- 5.Manstein D, Laubach H, Watanabe K, Farinelli W, Zurakowski D, Anderson RR. Selective cryolysis: A novel method of non-invasive fat removal. Lasers Surg Med. 2008;40:595–604. doi: 10.1002/lsm.20719. [DOI] [PubMed] [Google Scholar]
- 6.Zelickson B, Egbert BM, Preciado J, Allison J, Springer K, Rhoades RW, Manstein D. Cryolipolysis for noninvasive fat cell destruction: initial results from a pig model. Dermatol Surg. 2009;35:1462–1470. doi: 10.1111/j.1524-4725.2009.01259.x. [DOI] [PubMed] [Google Scholar]
- 7.Dover J, Burns J, Coleman S, Fitzpatrick R, Garden J, Goldberg D, Geronemus R, Kilmer S, Mayoral F, Weiss R, Zelickson B, Tanzi E. A prospective clinical study of noninvasive cryolipolysis for subcutaneous fat layer reduction—Interim report of available subject data. Lasers Surg Med. 2009;41(S21):43. [Google Scholar]
- 8.Burns AJ, Allison J, Bachelor E, Dover J, Coleman S, Fitzpatrick R, Garden J, Geronemus R, Goldberg D, Kilmer S, Kramer S, Levinson M, Tanzi E, Weiss R, Zelickson B, Mayoral F, Okamoto E, Riopelle J. Analysis of side effects of non-invasive cryolipolysis for subcutaneous fat layer reduction—Interim Report from Controlled Clinical Trials. Lasers Surg Med. 2010;42(S22):21. [Google Scholar]
- 9.Dover J, Kaminer M, Teahan M, Barrett L. Patient satisfaction at 2 and 6 months after a single non-invasive cryolipolysis treatment for subcutaneous fat layer reduction. Lasers Surg Med. 2011;43(S23):968. [Google Scholar]
- 10.Shek SY, Chan NP, Chan HH. Non-invasive cryolipolysis for body contouring in Chinese—A first commerial experience. Lasers Surg Med. 2011;44:125–130. doi: 10.1002/lsm.21145. [DOI] [PubMed] [Google Scholar]
- 11.Garibyan L, Sipprell 3rd WH, Jalian HR, Sakamoto FH, Avram M, Anderson RR. Three-dimensional volumetric quantification of fat loss following cryolipolysis. Lasers Surg Med. 2014;46:75–80. doi: 10.1002/lsm.22207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boey GE, Wasilenchuk JL. Fat reduction in the inner thigh using a prototype cryolipolysis applicator. Dermatol Surg. 2014 doi: 10.1097/01.DSS.0000452628.99209.4f. ; in press. [DOI] [PubMed] [Google Scholar]
- 13.Stevens WG, Bachelor EP. Cryolipolysis conformable surface applicator for non-surgical fat reduction in lateral thighs. Aesthet Surg J. 2014 doi: 10.1093/asj/sju024. ;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein KB, Zelickson B, Riopelle JG, Okamoto E, Bachelor EP, Harry RS, Preciado JA. Non-invasive cryolipolysis for subcutaneous fat reduction does not affect serum lipid levels or liver function tests. Lasers Surg Med. 2009;41:785–790. doi: 10.1002/lsm.20850. [DOI] [PubMed] [Google Scholar]
- 15.Coleman SR, Sachdeva K, Egbert BM, Preciado J, Allison J. Clinical efficacy of noninvasive cryolipolysis and its effects on peripheral nerves. Aesthetic Plast Surg. 2009;33:482–488. doi: 10.1007/s00266-008-9286-8. [DOI] [PubMed] [Google Scholar]
- 16.Stevens WG, Pietrzak LK, Spring MA. Broad overview of a clinical and commercial experience with coolsculpting. Aesthet Surg J. 2013;33:835–846. doi: 10.1177/1090820X13494757. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki GH, Abelev N, Tevez-Ortiz A. Noninvasive selective cryolipolysis and reperfusion recovery for localized natural fat reduction and contouring. Aesthet Surg J. 2014;34:420–431. doi: 10.1177/1090820X13520320. [DOI] [PubMed] [Google Scholar]
- 18.Dierickx CC, Mazer JM, Sand M, Koenig S, Arigon V. Safety, tolerance, and patient satisfaction with noninvasive cryolipolysis. Dermatol Surg. 2013;39:1209–1216. doi: 10.1111/dsu.12238. [DOI] [PubMed] [Google Scholar]
- 19.Munavalli G. Cryolipolysis for the treatment of male pseudogynecomastia. Lasers Surg Med. 2013;45(S25):16. [Google Scholar]
- 20.Bernstein EF. Longitudinal evaluation of cryolipolysis efficacy: Two case studies. J Cosmet Dermatol. 2013;12:149–152. doi: 10.1111/jocd.12036. [DOI] [PubMed] [Google Scholar]
- 21.Boey GE, Wasilenchuk JL. Enhanced clinical outcome with manual massage following cryolipolysis treatment: A 4-month study of safety and efficacy. Lasers Surg Med. 2014;46:20–26. doi: 10.1002/lsm.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garibyan L, Sipprell 3rd WH, Jalian HR, Sakamoto FH, Avram M, Anderson RR. Three-dimensional volumetric quantification of fat loss following cryolipolysis. Lasers Surg Med. 2014;46:75–80. doi: 10.1002/lsm.22207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaminer M, Weiss R, Newman J, Allison J . Visible Cosmetic Improvement with Cryolipolysis: Photographic Evidence. Presented at the Annual Meeting of the American Society for Dermatologic Surgery, Phoenix, AZ, 2009.
- 24.Mayoral F, Kaminer M, Kilmer S, Weiss R, Zelickson B. Effect of multiple cryolipolysis treatments on the abdomen. Lasers Surg Med. 2012;44(S24):15. [Google Scholar]
- 25.Brightman L, Geronemus R. Can second treatment enhance clinical results in cryolipolysis? Cos Derm. 2011;24:85–88. [Google Scholar]


