Abstract
Background
Heart rate variability (HRV) is known to be reduced in depression; however, is unclear whether this is a consequence of the disorder or due to antidepressant medication.
Methods
We analysed data on 4750 participants from the first wave of The Irish Longitudinal Study on Ageing (TILDA). Time [standard deviation of normal to normal intervals (SDNN ms2)] and frequency domain [low frequency (LF) and high frequency (HF)] measures of HRV were derived from 3-lead surface electrocardiogram records obtained during 10 min of supine rest. Depression was assessed using the Center for Epidemiologic Studies – Depression scale.
Results
Participants on antidepressants [with (n = 80) or without depression (n = 185)] differed significantly from controls (not depressed and not taking antidepressants n = 4107) on all measures of HRV. Depressed participants not taking antidepressants (n = 317) did not differ from controls on any measures of HRV. In linear regression analysis adjusted for relevant factors all antidepressants were associated with lower measures HRV. Participants on selective serotonin reuptake inhibitors (SSRIs) had higher measures of HRV relative to participants on tricyclic antidepressants or serotonin–norepinephrine reuptake inhibitors respectively.
Conclusions
Our results suggest that reductions in HRV observed among depressed older adults are driven by the effects of antidepressant medications. SSRIs have less impact on HRV than other antidepressants but they are still associated with lower measures of HRV. Study limitations include the use of a self-report measure of depression and floor effects of age on HRV could have limited our ability to detect an association between HRV and depression.
Key words: Antidepressants, depression, heart rate variability, older adults
Introduction
Depression is a known risk factor for the development of cardiovascular disease (CVD) and an independent predictor of poor prognosis following a cardiac event (Lett et al. 2004). Alterations in the autonomic nervous system (ANS) including a reduction in heart rate variability (HRV) may partly explain the increased risk of CVD, since low HRV is a known risk factor for myocardial infarction, arrhythmias, and cardiac mortality (Tsuji et al. 1994; Dekker et al. 2000; Carney & Freedland, 2009).
Although there is strong evidence that HRV is reduced in depression, it remains unclear whether these reductions are due to the effects of antidepressant medication or the disease per se. Rottenberg (2007) summarized 13 studies (312 depressed patients and 374 controls) and found significantly reduced HRV in depression. Subsequently, a review by Kemp et al. (2010b) on 302 depressed patients who were free from CVD (424 controls) also demonstrated reductions in HRV among individuals with depression. By contrast, a large study by Licht et al. (2008) concluded that reductions in HRV among depressed participants were mainly driven by the effects of antidepressants. Importantly, they found that major depressive disorder (MDD) patients without antidepressant use (n = 1018) did not differ consistently from controls (n = 515) on measures of HRV. Moreover, longitudinal follow-up from this study confirmed that MDD was not associated with HRV and showed that MDD patients using antidepressant medication, particularly tricyclic antidepressants (TCAs) and serotonergic noradrenergic reuptake inhibitors (SNRIs) had significantly lower HRV than controls (Licht et al. 2010). It is notable that the sample size in Licht's study was far larger than the total number of participants in the previous meta-analyses. However, some methodological issues have been highlighted that hamper definitive conclusions (Kemp et al. 2010a). Adding to the debate, Brunoni et al. (2013) have suggested that decreased HRV may be a trait marker for depression and argue that the pathophysiological features of MDD, rather than pharmacotherapy drive the reported reductions in HRV. Their hypothesis is based on evidence from a study examining the effect of transcranial direct current stimulation (tDCS) and sertraline [a selective serotonin reuptake inhibitor (SSRI)] on HRV. Overall, depressed subjects were found to have lower HRV than controls; however, despite resolution of depressive symptoms, neither treatment was associated with changes in HRV.
Antidepressant treatment clearly impacts on HRV, although a precise picture has yet to emerge. A meta-analysis by Kemp et al. (2010b) found evidence that TCAs significantly reduce HRV but all other antidepressants had a benign effect on HRV. The influence of physical illness and lifestyle factors such as smoking, alcohol use, high body mass index (BMI), and low physical activity also needs to be considered given that these factors occur more frequently in depression and are associated with decreased HRV (Rosenwinkel et al. 2001; Friedman, 2007). Moreover, the need to examine the role of co-morbid anxiety in reducing HRV was recently highlighted by Kemp et al. (2012) who found that MDD patients with generalized anxiety disorder (GAD) had greater reductions in HRV compared to MDD patients without co-morbid anxiety and controls. GAD is the most prevalent anxiety disorder among older adults (Schoevers et al. 2003) and a high prevalence of co-morbid depression and anxiety is commonly observed in this age group (Lenze et al. 2000).
To date, most of the research on depression and HRV has been conducted on young and middle-aged patients with depression. Although valuable, it has been suggested that research on older adults who have a greater risk of heart disease could have more clinical relevance (Jindal et al. 2008). HRV is known to decrease across the lifespan (Jennings & Mack, 1984; Yeragani et al. 1997; Agelink et al. 2001) and consequently the impact of depression on HRV is also likely to vary across age cohorts. Age-related decline may lower HRV to levels associated with increased risk of mortality therefore distinguishing low HRV due to depression from that due to normal ageing is challenging. It is possible that depression in old age may further reduce age-related declines in HRV and exacerbate the risk of cardiovascular morbidity (Gehi et al. 2005). Moreover, depression that first presents in late life is believed to have a different aetiopathogenesis from earlier-onset depression and this difference in pathophysiology may have specific consequences for autonomic function in older adults with depression.
Of the few studies that have investigated HRV in depressed older adults the results are conflicting; with some studies providing evidence of reduced HRV in depression (Carney & Freedland, 2009; Dauphinot et al. 2012) and others reporting no association (Gehi et al. 2005; Jindal et al. 2008). Notably, none of these studies have investigated the role of individual antidepressant classes on HRV; therefore, in older adults their effects on HRV are largely unknown. The aim of this study is to examine whether depression is associated with reduced HRV in older adults and investigate the extent to which any associations observed are confounded by lifestyle, co-morbid anxiety or the effect of antidepressant medication.
Methods
Study design and participants
We analysed data from the first wave of The Irish Longitudinal Study on Ageing (TILDA) collected between October 2009 and February 2011. Full details of the sampling procedure and response rate have been described elsewhere (Kearney et al. 2011a). In brief, TILDA is a nationally representative study of people aged ⩾50 years resident in Ireland. People with known or suspected dementia were ineligible at baseline for participation in TILDA.
Participants completed a computer-assisted personal interview (CAPI) in their own homes administered by trained professional interviewers. The TILDA questionnaire includes detailed questions on health, social and financial circumstances. Each participant was then invited to travel to one of two health centres for a comprehensive health assessment. Participants who were unable or unwilling to attend a health centre were offered a modified assessment in their own home. All health assessments were carried out by trained nurses. The study was approved by the Faculty of Health Sciences Research Ethics Committee at Trinity College Dublin, and participants were required to provide written informed consent prior to participation in the study. The measures specific to the current analysis are described in detail below.
Ethics
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Psychiatric assessment
Depression was assessed using the Center for Epidemiologic Studies – Depression scale (CES-D). The CES-D generates a total score with a range between 0 and 60 with higher scores indicating greater depressive symptoms. A cut-off score of 16 has been shown to have a sensitivity of 100% and specificity of 88% for MDD in an elderly population (Beekman et al. 1997).
Anxiety was assessed using the Hospital Anxiety Depression Scale – Anxiety subscale (HADS-A). Scores from this 7-item scale range from 0 to 21 with higher scores indicating greater anxiety symptoms. A cut-off score of ⩾11 has been used to classify participants with clinically significant anxiety (Zigmond & Snaith, 1983).
Measurement of HRV
HRV was only assessed during the health centre assessment. A continuous 10 min supine resting surface 3-lead electrocardiogram (ECG) was digitally recorded using the Medilog AR12 system (Schiller, Switzerland). Each recording was conducted in a comfortably lit, quiet room at ambient temperature (21–23°C). Subjects were instructed to breath spontaneously for the first 5 min period, and to control their breathing (paced) during the second 5 min period according to a pre-recorded set of auditory instructions (set at a rate of 12 breaths/min). Paced breathing experimentally controlled for the effect of respiratory rate on spectral HRV indices (Sandercock et al. 2008). The acquired ECG was sampled at 4 kHz, band-pass filtered and a proprietary algorithm was used to detect the R peak of each heart beat recorded on the ECG signal (Pardey & Jouravleva, 2004). Supra-ventricular ectopic beats and noise were excluded from the signal using linear interpolation. All recordings were screened for atrial fibrillation (AF) using criteria from the European Society of Cardiology (Camm et al. 2010), and those identified with AF were subsequently excluded from analysis. Mean resting heart rate (HR) was calculated over 5 min of spontaneous breathing.
Time domain measures derived from each 5 min epoch included the standard deviation of normal to normal intervals (SDNN ms2). Frequency domain (FD) features were calculated from spectral estimates derived using an autoregressive (Burg transform) parametric algorithm. FD features were derived by integrating the power spectral density across two frequency bands: low frequency power (LF, 0.04–0.15 Hz, ms2) and high frequency power (HF, 0.15–0.4 Hz, ms2). HF measures are thought to reflect parasympathetic activity while LF measures are thought to reflect both sympathetic and parasympathetic activity.
Measurement of covariates
Sociodemographic characteristics included age, sex, and highest level of educational attainment [primary (<8 years), secondary (8–12 years), tertiary (⩾12)]. In addition, the following health indicators were considered as covariates as these have been linked with both depression status and HRV. Objective measures of weight (one measure using SECA electronic floor scales) and height (one measure using SECA 240 wall-mounted measuring rod) were used to calculate BMI. Physical activity was assessed using the International Physical Activity Questionnaire – Short Form (Craig et al. 2003). Participants were classified into three groups representing low, medium and high levels of exercise. Participants self-reported how many standard alcoholic drinks they consumed in a week as well as whether they were a non-smoker, former smoker or current smoker. Self-reports were used to ascertain the presence of a doctors diagnosis of heart disease and other chronic conditions. Blood pressure (BP) was recorded using an automated oscillometric BP monitor (Omron model M10-IT). Participants were classified as hypertensive if the mean of their two seated systolic blood pressure measurements was ⩾140 mmHg and/or if the mean of their two seated diastolic blood pressure measurements was ⩾90 mmHg (Mancia et al. 2007).
Medication use was determined by recording medication names from the medicine bottles in the participant's home. Medications were classified using the World Health Organization Anatomical Therapeutic Chemical (ATC) system (WHO, 2011). A dichotomous variable for cardioactive medication was computed (1=yes, 0=no) with the following ATC codes: antihypertensive drugs, C02; diuretic drugs, C03; peripheral vasodilator drugs, C04; vasoprotective drugs, C05; β-blocking agents, C07; and calcium-channel blockers, C08. Dichotomous variables (1=yes, 0=no) for various classes of antidepressant medications were computed with the following ATC codes: SSRIs, N06AB; TCAs, N06AA; monoamine oxidase inhibitors (MAOIs), N06AF; SNRIs, NO6AX (16, 17, 23, 21); serotonin antagonist + reuptake inhibitors, N06AX (05, 06). We also distinguished benzodiazepine drugs (ATC codes N03AE, N05BA, N05CD, N05CF) and other non-depression-related psychoactive medication such as anaesthetic drugs, ATC code N01; analgesic drugs, ATC code N02; antiepileptic drugs, ATC code N03; anti-Parkinson disease drugs, ATC code N04; psycholeptic drugs, ATC code N05; psychostimulants, ATC code N06B; antidementia drugs, ATC code N06D; and other nervous system drugs, ATC code N07.
Statistical analysis
Statistical analysis was performed using SPSS version 20 (IBM Corp, USA). Distribution of continuous variables was assessed using Q-Q plots and histograms. Non-normally distributed measures of HRV (SDNN, LF, HF) were log-transformed for graphs and analysis (values were back-transformed for presentation of regression analysis and between group comparisons of adjusted means but not for graphs). Normally distributed variables were described as means and standard errors (s.e.), and were compared using independent t tests and categorical variables were compared using χ2 tests.
Baseline clinical and demographic characteristics among groups were assessed using ANOVA or the χ2 statistics. Linear regression was used to calculate mean values of HR, SDNN, LF and HF adjusted for age, sex, education, BMI, smoking, alcohol use, physical activity, heart disease, cardioactive medications and number of chronic diseases for each depression group. In an attempt to understand the individual effects of various types of psychoactive medication on HRV, we distinguished between participants on various types of psychoactive medication (exclusively) and compared their mean HR and HRV (raw values) with unmedicated controls.
Subsequently, the effect of anxiety and psychoactive medication on the relationship between depression and HRV was examined by entering information on these variables into a number of regression models with adjustment for potential confounders including age, sex, education, physical activity, alcohol use, number of chronic conditions (diabetes, arthritis, cancer, chronic lung disease, liver disease, stomach ulcers, substance abuse), history of CVD (angina, stroke, myocardial infarction), smoking (0=non/previous smoker, 1=current smoker), BMI and antihypertensive drugs as covariates. Unstandardized regression coefficients with 95% confidence intervals (CIs) and significance levels are presented here. Differences with p < 0.05 (two-tailed) were considered statistically significant.
Results
At baseline 8175 participants were recruited to the study. The household response rate was 62%. In total, 5034 (61%) participants who completed a health centre assessment were eligible for inclusion. Of these 96% successfully completed the CES-D and measurements of HRV, giving the 4750 included in the current analysis. The mean age was 61.6 years (s.d. = 8.3 years) and 55% of participants were female.
To test whether measures of HRV differed across persons with and without depression and according to antidepressant use, four distinct depression groups were created for the present study: 4107 controls without depression (score <16 on CES-D scale) or antidepressant use; 185 individuals without depression (score <16 on CES-D scale) but currently on antidepressants which we labelled as the ‘remitted’ group; 317 individuals with depression but not currently on antidepressants; and 80 individuals with depression and currently on antidepressants. Table 1 presents demographic characteristics, disease status, lifestyle behaviours, and medication use according to depression groups. Mean CES-D score was similarly high in both groups with depression (22 in participants not taking antidepressants and 25 in those on antidepressants). Relative to controls, participants with depression (current or remitted) were more likely to be female, had less education, were less physically active, were more likely to smoke and had more heart and other chronic diseases. Additionally, they had much higher levels of anxiety and medication use other than antidepressants (benzodiazepines, heart and blood pressure medications use and other psychotropic medications).
Table 1.
Sample characteristics by depression group
| Non-depressed controls (<16 on CES-D & no antidepressants) | Remitted depression (<16 on CES-D & on antidepressants) | Current depression (⩾16 on CES-D & no antidepressants) | Current depression (⩾16 on CES-D & on antidepressants) | p value | |
|---|---|---|---|---|---|
| (N = 4107) | (N = 185) | (N = 317) | (N = 80) | ||
| Age, mean (s.e.) | 61.7 (0.13) | 62.6 (0.62) | 60.4 (0.43) | 59.4 (0.75) | 0.001 |
| Female sex | 2179 (53.1) | 124 (67.0) | 212 (66.9) | 58 (72.5) | <0.001 |
| Education | |||||
| Primary | 828 (20.2) | 46 (24.9) | 91 (28.7) | 27 (33.8) | <0.001 |
| Secondary | 1743 (42.5) | 80 (43.2) | 117 (36.9) | 33 (41.3) | 0.279 |
| Tertiary | 1534 (37.4) | 59 (31.9) | 109 (34.4) | 20 (25.0) | 0.045 |
| BMIa, mean (s.e.) | 28.5 (0.08) | 28.8 (0.41) | 28.6 (0.29) | 29.3 (73) | 0.415 |
| Physical activityb | |||||
| Low | 1045 (25.6) | 68 (37.0) | 123 (39.0) | 34 (42.5) | <0.001 |
| Medium | 1501 (36.8) | 63 (34.2) | 106 (33.7) | 26 (32.5) | 0.522 |
| High | 1529 (37.5) | 53 (28.8) | 86 (27.3) | 20 (25.0) | <0.001 |
| Smoking status | |||||
| Never | 1948 (47.4) | 72 (38.9) | 119 (37.5) | 24 (30.0) | <0.001 |
| Former | 1600 (39.0) | 75 (40.5) | 110 (34.7) | 35 (43.8) | 0.341 |
| Current | 559 (13.6) | 38 (20.5) | 88 (27.8) | 21 (26.3) | <0.001 |
| Alcoholc | 5.98 (0.15) | 6.10 (0.78) | 5.25 (0.55) | 6.95 (2.2) | 0.493 |
| Heart diseased | 443 (10.8) | 30 (16.2) | 45 (14.2) | 16 (20.0) | 0.003 |
| No chronic conditionse | 0.45 (0.01) | 0.84 (0.06) | 0.68 (0.04) | 0.98 (0.10) | <0.001 |
| Anxietyf, mean (s.e.) | 5 (0.07) | 6 (0.38) | 9 (0.34) | 11 (0.75) | <0.001 |
| Severity of depression (mean CES-D score) | 4 (0.08) | 6 (0.43) | 22 (0.52) | 25 (1.3) | <0.001 |
| Medication use | |||||
| Selective serotonin reuptake inhibitors | 102 (55.1) | 49 (61.2) | 0.463 | ||
| Serotonin–norepinephrine reuptake inhibitors | 36 (19.5) | 17 (21.2) | 0.919 | ||
| Serotonin antagonist + reuptake inhibitors | 1 (0.5) | 1 (1.3) | 0.540 | ||
| Tricyclic antidepressants | 50 (27.0) | 21 (26.3) | 0.896 | ||
| Monoamine oxidase inhibitors | 1 (0.8) | 0 | 0.510 | ||
| Benzodiazepines | 115 (2.8) | 43 (23.2) | 44 (13.9) | 26 (32.5) | <0.001 |
| Other psychotropic medication | 244 (5.9) | 42 (22.7) | 59 (18.6) | 20 (25.0) | <0.001 |
| Heart or blood pressure medication | 1303 (31.7) | 77 (41.6) | 97 (30.6) | 34 (42.5) | 0.007 |
CES-D, Center for Epidemiologic Studies – Depression scale; BMI, body mass index.
Weight in kilograms divided by height in metres squared.
Exercise in three groups reported according the International Physical Activity Questionnaire (short form) scoring protocol.
Standard number of drinks a week (standard drinks a day by weekly frequency).
Angina or myocardial infarction or heart failure or abnormal heart rhythm.
Number of chronic conditions (diabetes, stroke, arthritis, cancer, stomach ulcers, substance abuse or liver disease.
Mean score from 7-item Hospital Anxiety and Depression Scale – anxiety.
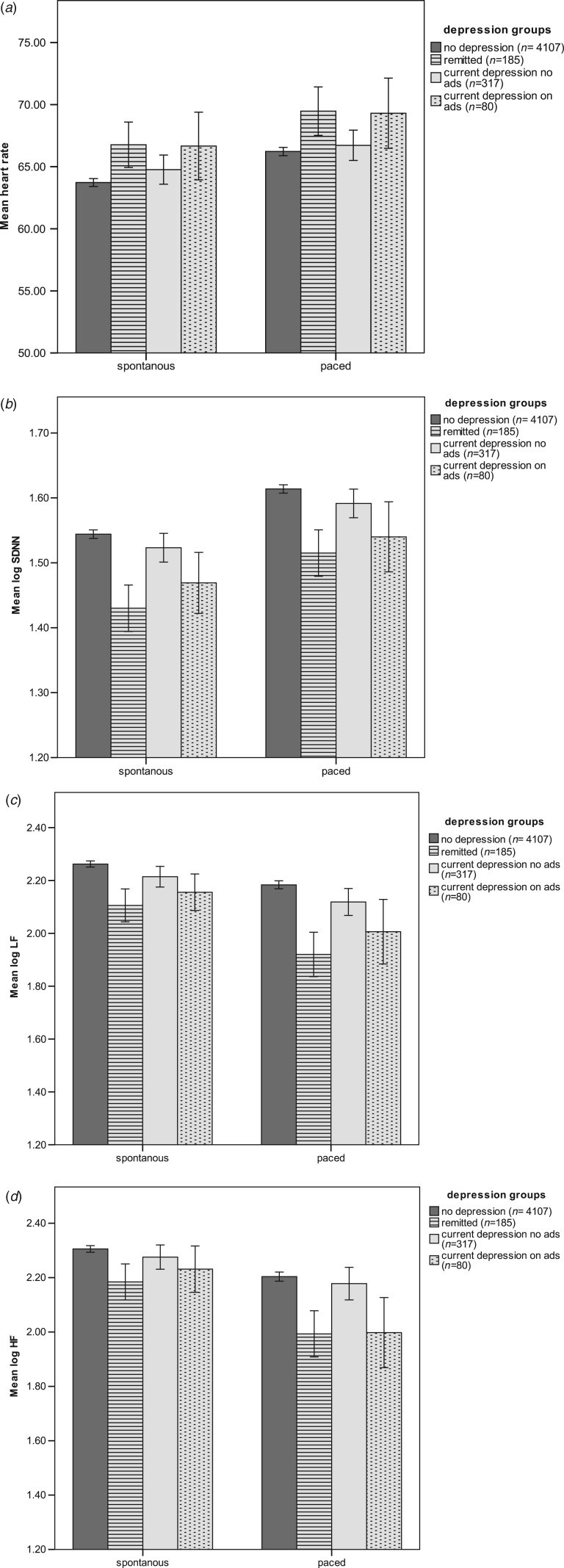
Fig. 1 shows mean raw log values of HR, SDNN, LF and HF during spontaneous and paced breathing conditions for the four depression groups. As illustrated, HR is increased in participants taking antidepressants [with (paced mean=69.3) or without (paced mean=69.5) depression] compared to controls (paced mean=66.2) or participants with depression who were not taking antidepressants (paced mean=66.7). A similar pattern emerges for measures of SDNN, LF and HF whereby depressed participants not taking antidepressants (e.g. paced mean SDNN = 1.59) have similar measures of HRV to controls (e.g. paced mean SDNN = 1.61); and participants taking antidepressants [with (e.g. paced mean SDNN = 1.54) or without depression (e.g. paced mean SDNN = 1.52)] have significantly lower measures of HRV than controls. For conciseness, only measures of HRV obtained from the paced breathing condition (5 min) are presented in the subsequent analysis, since the pattern of findings was similar in both breathing conditions.
Fig. 1.
Mean values for spontaneous and paced breathing conditions across depression groups (raw data) for (a) heart rate, (b) log standard deviation of normal to normal intervals (SDNN), (c) log low frequency (LF), (d) log high frequency (HF). Error bars: 95% confidence intervals.
Table 2 presents unadjusted and adjusted means (back transformed) for HR, SDNN, LF and HF across depression groups. Depressed participants who were not currently taking antidepressants did not differ significantly from controls on adjusted measures of SDNN, LF or HF. Participants who were on antidepressants (with or without depression) differed significantly from controls on all measures of HRV. In general, the ‘remitted’ group had the lowest measures of HRV observed in our study.
Table 2.
HR, SDNN intervals, LF, and HF by depression group during the paced breathing condition
| Non-depressed controlsa (<16 on CES-D & no antidepressants) | Remitted depressionb (<16 on CES-D & on antidepressants) | Current depressionc (⩾16 on CES-D & no antidepressants) | Current depressiond (⩾16 on CES-D & on antidepressants) | Controls v. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (N = 4107) | (N = 185) | (N = 317) | (N = 80) | Remitted (<16 on CES-D and on antidepressants) | Current depression (no antidepressants) | Current depression (on antidepressants) | ||||
| Mean (s.e.) | Mean (s.e.) | Mean (s.e.) | Mean (s.e.) | Effect sizeb | p value | Effect sizeb | p value | Effect sizeb | p value | |
| HR (beats/min) | ||||||||||
| Unadjusted | 66.2 (0.17) | 69.4 (0.98) | 66.7 (0.61) | 69.3 (1.4) | −3.2 | 0.00** | −0.79 | 0.42 | −2.4 | 0.01** |
| Adjusteda | 66.3 (0.03) | 66.7 (0.16) | 67.1 (0.13) | 67.3 (0.26) | 2.0 | 0.04* | 3.9 | 0.00** | 2.7 | 0.01** |
| SDNNc | ||||||||||
| Unadjusted | 40.7 (1.0) | 32.3 (1.0) | 38.9 (1.0) | 34.6 (1.1) | 4.5 | 0.00** | 2.2 | 0.06 | 2.2 | 02* |
| Adjusteda | 40.7 (1.0) | 38.9 (1.0) | 39.8 (1.0) | 39.7 (1.0) | −6.11 | 0.00** | −1.1 | 0.25 | −2.0 | 0.04* |
| LFd (ms2) | ||||||||||
| Unadjusted | 151.3 (1.0) | 83.1 (1.1) | 128.8 (1.1) | 102.3 (1.1) | 5.0 | 0.00** | 1.7 | 0.02* | 3.4 | 0.00** |
| Adjusteda | 147.9 (1.0) | 128.8 (1.0) | 144.5 (1.0) | 134.8 (1.1) | −8.5 | 0.00** | −1.3 | 0.18 | −2.5 | 0.01** |
| HFe (ms2) | ||||||||||
| Unadjusted | 158.4 (1.0) | 97.7 (1.1) | 147.9 (1.1) | 97.7 (1.2) | 2.4 | 00** | 1.0 | 0.41 | 2.4 | 0.01** |
| Adjusteda | 158.8 (1.0) | 147.2 (1.0) | 155.0 (1.0) | 144.8 (1.1) | −4.8 | 0.00** | 1.0 | 0.31 | 2.3 | 0.02* |
HR, Heart rate; SDNN, standard deviation of normal to normal intervals (SDNN); LF, low frequency; HF, high frequency; CES-D, Center for Epidemiologic Studies – Depression scale.
Adjusted for age, sex, education, body mass index, physical activity, smoking, alcohol use, heart disease, chronic disease, heart medication, anxiety.
t test used for unadjusted means, z test used for adjusted means.
Denotes significance at 0.05.
Denotes significance at 0.01.
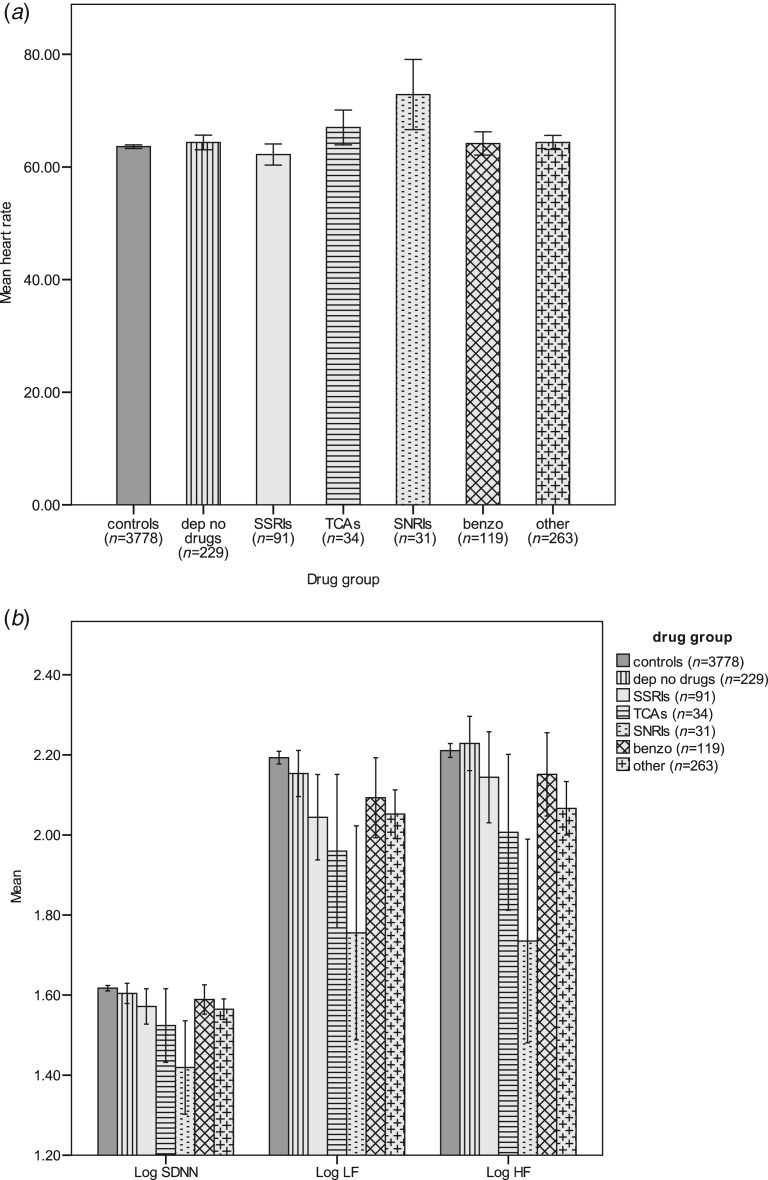
Six mutually exclusive medications groups were created to examine the individual effects of various types of psychoactive medication on measures of HRV: 3778 controls who were not taking antidepressants; 91 individuals who were only taking SSRI antidepressants; 34 individuals who were only taking TCAs; 31 individuals who were only taking SNRIs; 119 individuals who were only taking benzodiazepines and 263 participants who were taking medications collapsed into the group labelled ‘other psychotropic medications’.
Fig. 2a, b shows raw log values of HR, SDNN, LF and HF for each of these groups. Relative to controls, mean heart rates are significantly higher (p < 0.001) in participants on SNRIs and TCAs; whereas mean heart rates in participants on SSRIs are similar to controls. Once more, a similar pattern emerged for the effect of antidepressant medication on indices of HRV; whereby a graded decrease in HRV, dependent on antidepressant type was observed among participants. Almost all measures of HRV were significantly lower in participants on antidepressants. Participants on SNRIs had the lowest measures of HRV observed in our study. Among participants on antidepressant medication, SSRIs were associated with the highest measures of HRV. Participants on benzodiazepines did not differ from controls on measures of HRV; however, participants using ‘other psychoactive medication’ had significantly lower measures of HRV relative to controls.
Fig. 2.
Mean values by depression and medication group for (a) heart rate, (b) log standard deviation of normal to normal intervals (SDNN), low frequency (LF) and high frequency (HF) (raw data). Error bars: 95% confidence intervals.
Table 3 presents linear regression models examining the relationship between depression and HRV and the role of anxiety and psychoactive medication. In univariate analysis (model A) anxiety and all antidepressants are associated with increased HR; however, associations persist only for TCAs and SNRIs in the fully adjusted model (model C). Depression and all antidepressants are associated with reduced SDNN in univariate analysis; however, in the fully adjusted model only antidepressants (SSRIs, TCAs, SNRIs) and ‘other psychotropic’ medications are associated with significantly lower measures of SDNN. Finally, in fully adjusted analyses, SNRIs are associated with significantly lower measures of LF and both TCAs and SNRIs are associated with significantly lower measures of HF.
Table 3.
Linear regression models of depression, anxiety and medications on HR and measures of HRV
| Model Aa | Model Bb | Model Cc | |
|---|---|---|---|
| b (95% CI) | b (95% CI) | b (95% CI) | |
| HR | |||
| Depression | 0.886 (−0.246 to 2.01) | 0.034 (−1.26 to 1.33) | 0.310 (−1.66 to 1.04) |
| Anxiety | 1.33* (0.175 to 2.50) | 0.736 (−0.495 to 2.02) | 0.666 (−0.627 to 1.96) |
| SSRIs | −0.373 (−2.17 to 1.43) | −0.405 (−2.42 to 1.61) | −0.717 (−2.82 to 1.39) |
| TCAs | 5.04** (2.39 to 7.70) | 3.78** (0.918 to 6.64) | 3.54** (0.546 to 6.53) |
| SNRIs | 10.07** (7.10 to 13.0) | 11.1** (8.01 to 14.2) | 11.5** (8.35 to 14.7) |
| Benzodiazepines | 1.29 (−0.170 to 2.75) | 0.419 (−1.25 to 2.09) | 0.525 (−1.20 to 2.26) |
| Other psychotics | 1.04 (−0.137 to 2.22) | 0.574 (−0.710 to 1.85) | 0.368 (−0.991 to 1.72) |
| Log SDNN | |||
| Depression | −1.06** (−1.12 to −1.01) | −1.04 (−0.1.11 to 1.01) | −1.03 (−1.09 to 1.02) |
| Anxiety | 1.00 (−1.04 to 1.06) | 1.04 (−1.00 to 1.10) | 1.01 (−1.03 to 1.07) |
| SSRIs | −1.16** (−1.26 to −1.07) | −1.12** (−1.23 to −1.03) | −1.09* (−1.20 to −1.00) |
| TCAs | −1.03** (−1.42 to −1.12) | −1.17** (−1.33 to −1.03) | −1.18* (−1.35 to −1.03) |
| SNRIs | −1.42** (−1.62 to −1.24) | −1.44** (−1.66 to −1.25) | −1.51** (−1.73 to −1.58) |
| Benzodiazepines | −1.10** (−1.17 to −1.03) | −1.00 (−1.08 to 1.06) | −1.01 (−1.05 to 1.09) |
| Other psychotics | −1.15** (−1.21 to −1.09) | −1.11** (−1.18 to −1.05) | −1.09** (−1.16 to −1.03) |
| Log LF | |||
| Depression | −1.19** (−1.34 to −1.05) | −1.14* (−1.30 to −1.00) | −1.11 (−1.28 to 1.02) |
| Anxiety | 1.03 (−1.08 to 1.17) | 1.19 (1.05 to 1.36) | 1.09 (−1.04 to 1.24) |
| SSRIs | −1.50**(−1.81 to −1.24) | −1.35**(−1.67 to −1.10) | −1.22 (−1.51 to 1.10) |
| TCAs | −1.71** (−2.25 to −1.29) | −1.38* (−1.87 to −1.03) | −1.30 (−1.76 to 1.03) |
| SNRIs | −2.50** (−3.41 to −1.83) | −2.67** (−3.71 to −1.93) | −2.86** (−3.95 to −2.07) |
| Benzodiazepines | −1.33** (−1.55 to −1.14) | −1.03 (−1.56 to −1.19) | 1.08 (−1.09 to 1.29) |
| Other psychotics | −1.47** (−1.67 to −1.30) | −1.36** (−1.36 to −1.19) | −1.29** (−1.48 to −1.12) |
| Log HF | |||
| Depression | −1.12 (−1.28 to 1.00) | −1.04 (−1.20 to 1.11) | −1.05 (−1.23 to 1.09) |
| Anxiety | 1.05 (−1.20 to 1.08) | 1.04 (−1.10 to 1.20) | −1.12 (−1.30 to 1.02) |
| SSRIs | −1.29* (−1.59 to −1.05) | −1.19 (−1.50 to 1.05) | −1.13 (−1.43 to 1.11) |
| TCAs | −1.67** (−2.26 to −1.23) | −1.36 (−1.89 to 1.02) | −1.42* (−1.69 to −1.01) |
| SNRIs | −2.79** (−3.92 to −1.69) | −3.01** (−4.31 to −2.10) | −3.22** (−4.06 to −2.25) |
| Benzodiazepines | −1.19* (−1.41 to −1.01) | 1.03 (−1.17 to 1.25) | 1.10 (−1.09 to 1.34) |
| Other psychotics | −1.45** (−1.66 to −1.27) | −1.37** (−1.59 to −5.62) | −1.37** (−1.59 to −1.18) |
HR, Heart rate; HRV, heart rate variability; SDNN, standard deviation of normal to normal intervals; LF, low frequency; HF, high frequency; CI, confidence interval; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants; SNRIs, serotonin–norepinephrine reuptake inhibitors.
Univariate analysis of depression, anxiety and medications on HR and measures of HRV.
Adjusted for depression, anxiety and antidepressants.
Model B additionally adjusted for age, sex, education, body mass index, physical activity, smoking, alcohol use, heart disease, number of chronic conditions, heart medications.
Discussion
HRV in depression is now an important issue since both depression and decreased HRV have been shown to be predictors of cardiac morbidity and mortality. As a result, there has been substantial interest in the relationship between HRV and depression and the role, if any, of antidepressant medications. Our study has shown that antidepressants, rather than depression, have a significant impact on HR and measures of HRV. In older adults there exists a paucity of studies examining the effects of antidepressants on HRV, despite the high prevalence of CVD and increased prescribing of antidepressants in this age group.
Co-morbid anxiety or lifestyle factors did not explain our results. Overall, participants taking antidepressants (with or without current depression) had significantly lower measures of HRV than controls; whereas depressed individuals not taking antidepressants did not differ significantly from controls on all measures of HRV. This suggests that antidepressants rather than depression are responsible for the lower measures of HRV observed in our participants. If depression was responsible, we might have expected the remitted group to have superior measures of HRV compared to participants who were currently depressed but not taking antidepressants, particularly given some evidence that suggests antidepressants may reverse reductions in HRV associated with depression (Lavretsky et al. 1998; Touyz, 2005). This is not what we observed, in fact; the remitted group had the lowest measures of HRV observed in our study. Of course it must be acknowledged that some participants in our remitted group may have been taking antidepressants for conditions other than depression (anxiety, sleep problems, pain) and this could have impacted our findings, particularly the absence of an independent effect depression on HRV. Nevertheless, our results imply that antidepressants are associated with lower HRV in older adults. It has recently been hypothesised that low HRV may be a ‘trait’ rather that ‘state’ marker of depression (Brunoni et al. 2013). Our findings do not provide strong support for this hypothesis in older adults, since participants with depression who were not taking antidepressants had measures of HR and HRV that were in most cases similar to controls and in regression analysis depression was not associated with significantly lower HRV once antidepressants were considered in the model.
In our study, we compared measures of HRV between controls and participants who were only taking one class of psychoactive medication. This allowed us to examine the effect of individual medication classes on HRV. Overall, we found that participants on antidepressants (SSRIs, TCAs, SNRIs) had lower measures of HRV relative to controls; and those exclusively on SNRIs had the lowest measures of HRV recorded in our study. Although participants on SSRIs have better indices of HRV compared to participants on other antidepressants (TCAs and SNRIs), their measures are still significantly lower that controls on SDNN (p = 0.051) and LF (p = 0.007). The difference was non-significant (p = 0.251) for the HF (parasympathetic) measure of HRV. In SSRI users, low HRV was not accompanied by increased HR, providing support for the proposition (Licht et al. 2010) that SSRI's may have a beneficial effects on sympathetic activity that could counter their negative effects on parasympathetic activity and protect against CVD (Licht et al. 2010).
Overall, our results imply that HRV is not reduced in older adults with depression. In univariate analysis, depression was associated with reduced HRV; however, the association lost significance once antidepressant medications were included in the model. All antidepressants were associated with reduced measures of SDNN – our overall measure of HRV. SNRIs and TCAs were associated with reduced HF (parasympathetic) while only SNRIs were associated with significantly reduced LF (predominantly sympathetic). As a result, the hypothesis that reductions in HRV observed in depression are driven by the effects of antidepressants (Licht et al. 2008) is supported by our findings. HRV functioning is strongly age dependent, so our inferences must be constrained to older adults since the impact of depression on HRV may vary across age cohorts. In light of this, the findings of Kemp et al. (2010b, 2012) although in conflict with the findings of our study may also be true and reflect a differential relationship between depression and HRV in younger aged adults. In addition, floor effects of age on HRV could have limited our ability to detect reduced HRV due to depression from that due to normal aging and in this context our significant findings relating to antidepressants and HRV do appear robust.
The mechanisms through which antidepressants exert their effects on sympathetic/parasympathetic control over the heart remain incompletely understood. Increases in HR and decreases in HRV are believed to be due, at least in part, to the degree of anticholinergic activity associated with different antidepressant medications leading to diminished cardiac vagal tone (Lavretsky et al. 1998). At the brainstem level, serotonin reuptake inhibition may influence various relay nuclei of the parasympathetic nervous system (Raul, 2003; Ramage & Villalon, 2008). The antivagal effects of TCAs and SNRIs are believed to occur largely in the heart itself. Both types of antidepressants inhibit the reuptake of norepinephrine, causing a major increase in norepinephrine in the synaptic cleft (Esler et al. 1985).
Although our findings suggest that depression is not associated with impaired HRV we must acknowledge that our measure of depression was not a formal diagnosis of MDD and this may have influenced our findings. A formal classification of MDD may have revealed an association between depression and HRV which persisted after controlling for antidepressant use. Previous evidence of a negative association between depression severity and HRV, such that the more severe the depression, the lower the HRV provides support for this possibility (Kemp et al. 2010a, b). Accordingly, our analyses might have missed important depression effects if the cut-off score did not discriminate correctly. We therefore repeated the regression analysis using the CES-D as a continuous measure and found a similar pattern of findings to those using a cut-off score. Subsequent waves of the TILDA study include the DSM-IV based Composite International Diagnostic Interview – short form which will allow us to test the validity of our findings compared with a gold standard measure of depression.
We must also acknowledge that antidepressant use could be a marker of disease severity; therefore depression and not the antidepressants could have caused the reductions in HRV observed in our study. However, depression is known to be under-diagnosed and under-treated in older adults and estimates suggest that less than 20% of cases are detected or treated in the community (Cole & Dendukuri, 2003). We therefore would expect the group representing participants who were classified as depressed by the CES-D but who were not taking antidepressants to have included individuals with undiagnosed MDD. If the disorder and not the antidepressants were responsible for impaired HRV we might have expected to observe lower measures of HRV in this group. However, this was not the case; depressed participants not taking antidepressants had measures of HRV that were similar to controls. A further consideration is that our analysis assumes that depressed individuals not taking antidepressants are similar to depressed individuals who take antidepressants and this may not be correct. Prior to treatment, depression severity could have been greater in those taking antidepressants, so longitudinal data are required to disentangle whether the observed effects are due to some intrinsic effects of severe depression or an independent antidepressant effect. HRV was only assessed in participants who attended for a centre based assessment. Non-responders to the health centre assessment were older than health centre attendees; but importantly, were not more severely depressed. Although, mean CES-D score was slightly higher in participants who did not have a health assessment (6.2) and those who opted for a home assessment (6.8) compared to those who attended a health centre (5.6), these differences were not statistically significant (p = 0.084).
The main strengths of this study are the large population representative sample and comprehensive health assessment. Our paced breathing condition controlled for respiration rate, indicating that the relationships we observed are independent of variations in respiratory sinus arrhythmia driven by respiration. Limitations include that the data was analysed cross-sectionally and thus causal inferences cannot be made. Although many relevant risk factors were assessed and statistically controlled for in the analysis, the possibility of residual confounding exists. Participants were not randomly allocated to our groups so we acknowledge that attempts to statistically correct for group differences (as in Table 2) are contentious. However, our primary conclusions are drawn from the regression analyses presented in Table 3 and supported by the patterns observed in the graphs of raw data along with the comparisons of adjusted means from Table 2. Participants were not requested to refrain from eating, smoking, alcohol, caffeine, exercise, or medications prior to assessment, and time of day for assessment was not restricted based on practicality of delivering health assessments to participants from all over the country (Kearney et al. 2011b). These factors may affect reproducibility of results; however, time of day and food ingestion did not influence orthostatic blood pressure behaviour (another marker of autonomic function) in a previous TILDA pilot study (Fan et al. 2012).
In summary, this study provides an important clarification of the impact of depression and antidepressant medication on HRV. Our findings indicate that while SSRIs have less impact on HRV than other antidepressants with anticholinergic effects; they are still associated with lower measures of HRV. In older adults, the use of SNRIs poses a particular risk to the cardiovascular system since they are associated with the lowest measures of HRV observed in our study. Given that antidepressants such as SSRIs are now prescribed not only for depression, but also for a wide range of conditions; this issue has relevance to the general population. Longitudinal evidence is required to determine whether observed effects of antidepressants on HRV translate into CVD morbidity and mortality in depressed older adults.
Acknowledgements
The authors acknowledge the contribution of the participants in the study, members of the TILDA research team, study nurses and administrators. Claire O'Regan has had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Funding for the TILDA study is provided by The Atlantic Philanthropies, Irish Life plc and the Irish Government. The Irish government inputted some questions in the design of the study. The funders had no part in the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Declaration of Interest
None.
References
- Agelink MW, Majewski T, Wurthmann C, Postert T, Linka T, Rotterdam S, Klieser E (2001). Autonomic neurocardiac function in patients with major depression and effects of antidepressive treatment with nefazodone. Journal of Affective Disorders 62, 187–198. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W (1997). Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychological Medicine 27, 231–235. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Kemp AH, Dantas EM, Goulart AC, Nunes MA, Boggio PS, Mill JG, Lotufo PA, Fregni F, Bensenor IM (2013). Heart rate variability is a trait marker of major depressive disorder: evidence from the sertraline vs. electric current therapy to treat depression clinical study. International Journal of Neuropsychopharmacology 16, 1937–1949. [DOI] [PubMed] [Google Scholar]
- Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B (2010). Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 12, 1360–1420. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE (2009). Depression and heart rate variability in patients with coronary heart disease. Cleveland Clinical Journal of Medicine 76(Suppl. 2), S13–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MG, Dendukuri N (2003). Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. American Journal of Psychiatry 160, 1147–1156. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P (2003). International physical activity questionnaire: 12-country reliability and validity. Medicine and Scence in Sports and Exercise 35, 1381–1395. [DOI] [PubMed] [Google Scholar]
- Dauphinot V, Rouch I, Kossovsky MP, Pichot V, Dorey JM, Krolak-Salmon P, Laurent B, Roche F, Barthelemy JC (2012). Depressive symptoms and autonomic nervous system dysfunction in an elderly population-based study: the PROOF study. Journal of Affective Disorders 143, 153–159. [DOI] [PubMed] [Google Scholar]
- Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG (2000). Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis risk in communities. Circulation 102, 1239–1244. [DOI] [PubMed] [Google Scholar]
- Esler MD, Hasking GJ, Willett IR, Leonard PW, Jennings GL (1985). Noradrenaline release and sympathetic nervous system activity. Journal of Hypertension 3, 117–129. [DOI] [PubMed] [Google Scholar]
- Fan CW, Savva GM, Finucane C, Cronin H, O'Regan C, Kenny RA (2012). Factors affecting continuous beat-to-beat orthostatic blood pressure response in community-dwelling older adults. Blood Pressure Monitoring 17, 160–163. [DOI] [PubMed] [Google Scholar]
- Friedman BH (2007). An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology 74, 185–199. [DOI] [PubMed] [Google Scholar]
- Gehi A, Mangano D, Pipkin S, Browner WS, Whooley MA (2005). Depression and heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Archives of General Psychiatry 62, 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Mack ME (1984). Does aging differentially reduce heart rate variability related to respiration? Experimental Aging Research 10, 19–23. [DOI] [PubMed] [Google Scholar]
- Jindal RD, Vasko RC, Jennings JR, Fasiczka AL, Thase ME, Reynolds CF (2008). Heart rate variability in depressed elderly. American Journal of Geriatriac Psychiatry 16, 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney PM, Cronin H, O'Regan C, Kamiya Y, Savva GM, Whelan B, Kenny R (2011a) Cohort profile: the Irish Longitudinal Study on Ageing. International Journal of Epidemiology 40, 877–884. [DOI] [PubMed] [Google Scholar]
- Kearney PM, O'Regan C, Cronin H, Kenny RA (2011b). Effect of fasting on participation in clinical research among older people. European Geriatric Medicine 2, 187–189. [Google Scholar]
- Kemp AH, Quintana DS, Felmingham KL, Matthews S, Jelinek HF (2012). Depression, comorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: implications for cardiovascular risk. PloS ONE 7, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray M (2010a). Is heart rate variability reduced in depression without cardiovascular disease? [Letter to the Editor]. Biological Psychiatry 69, e3–e4. [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM (2010b). Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biological Psychiatry 67, 1067–1074. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Lesser IM, Wohl M, Miller BL (1998). Relationship of age, age at onset, and sex to depression in older adults. American Journal Geriatric Psychiatry 6, 248–256. [PubMed] [Google Scholar]
- Lenze EJ, Mulsant BH, Shear MK, Schulberg HC, Dew MA, Begley AE, Pollock BG, Reynolds CF (2000). Comorbid anxiety disorders in depressed elderly patients. American Journal Psychiatry 157, 722–728. [DOI] [PubMed] [Google Scholar]
- Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, Newman MF (2004). Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosomatic Medicine 66, 305–315. [DOI] [PubMed] [Google Scholar]
- Licht CM, de Geus EJ, van Dyck R, Penninx BW (2010). Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability. Biological Psychiatry 68, 861–868. [DOI] [PubMed] [Google Scholar]
- Licht CM, de Geus EJ, Zitman FG, Hoogendijk WJ, van Dyck R, Penninx BW (2008). Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA). Archives of General Psychiatry 65, 1358–1367. [DOI] [PubMed] [Google Scholar]
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B (2007). Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Journal of Hypertension 25, 1105–1187. [DOI] [PubMed] [Google Scholar]
- Pardey J, Jouravleva S (2004). The next-generation holter revolution: from analyse-edit-print to analyse-print. Computers in Cardiology 31, 373–376. [Google Scholar]
- Ramage AG, Villalon CM (2008). 5-hydroxytryptamine and cardiovascular regulation. Trends in Pharmacological Sciences 29, 472–481. [DOI] [PubMed] [Google Scholar]
- Raul L (2003). Serotonin2 receptors in the nucleus tractus solitarius: characterization and role in the baroreceptor reflex arc. Cellular and Molecular Neurobiology 23, 709–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwinkel ET, Bloomfield DM, Arwady MA, Goldsmith RL (2001). Exercise and autonomic function in health and cardiovascular disease. Cardiology Clinics 19, 369–387. [DOI] [PubMed] [Google Scholar]
- Rottenberg J (2007). Cardiac vagal control in depression: a critical analysis. Biological Psychology. 74, 200–211. [DOI] [PubMed] [Google Scholar]
- Sandercock G, Gladwell V, Dawson S, Nunan D, Brodie D, Beneke R (2008). Association between RR interval and high-frequency heart rate variability acquired during short-term, resting recordings with free and paced breathing. Physiological Measures 29, 795–802. [DOI] [PubMed] [Google Scholar]
- Schoevers RA, Beekman AT, Deeg DJ, Jonker C, van Tilburg W (2003). Comorbidity and risk-patterns of depression, generalised anxiety disorder and mixed anxiety-depression in later life: results from the AMSTEL study. International Journal of Geriatric Psychiatry 18, 994–1001. [DOI] [PubMed] [Google Scholar]
- Touyz RM (2005). Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Experimental Physiology 90, 449–455. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Venditti FJ Jr., Manders ES, Evans JC, Larson MG, Feldman CL, Levy D (1994) Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation 90, 878–883. [DOI] [PubMed] [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology (2011). Guidelines for ATC classification and DDD assignment 2012, Oslo.
- Yeragani VK, Sobolewski E, Kay J, Jampala VC, Igel G (1997). Effect of age on long-term heart rate variability. Cardiovascular Research 35, 35–42. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 67, 361–370. [DOI] [PubMed] [Google Scholar]