Abstract
Background
Serological studies indicate that evidence of coeliac disease (CD) exists in about 1% of all children, but we lack estimates of current diagnostic patterns among children and how they vary by socioeconomic group.
Methods
We identified all children aged 0–18 years between 1993 and 2012 who were registered with general practices across the UK that contribute to a large population-based general practice database. The incidence of CD was evaluated in each quintile of the Townsend index of deprivation and stratified by age, sex, country and calendar year.
Results
Among 2 063 421 children, we identified 1247 CD diagnoses, corresponding to an overall CD incidence of 11.9 per 100 000 person-years, which was similar across the UK countries and higher in girls than in boys. We found a gradient of CD diagnosis across socioeconomic groups, with the rate of diagnosis being 80% higher in children from the least-deprived areas than in those from the most-deprived areas (incident rate ratio 1.80, 95% CI 1.45 to 2.22). This pattern held for both boys and girls and across all ages. Across all four countries of the UK, we found similar associations between CD and socioeconomic status. While CD incidence up to age 2 remained stable over the study period, diagnoses at older ages have almost tripled over the past 20 years.
Conclusions
Children living in less socioeconomically deprived areas in the UK are more likely to be diagnosed with CD. Increased implementation of diagnostic guidelines could result in better case identification in more-deprived areas.
Keywords: coeliac disease, incidence, children, socioeconomic status
What is already known on this topic?
Serological studies indicate that evidence of coeliac disease exists in about 1% of all children yet clinical diagnoses are much less prevalent.
Existing studies on the socio-demographic distribution of childhood clinically diagnosed coeliac disease are in small study populations and findings are contradictory.
What this study adds?
Clinical diagnoses of coeliac disease among children aged over 2 years have almost tripled over the last two decades.
Children from the most socioeconomically deprived areas are only half as likely to be diagnosed with coeliac disease as those from less-deprived areas.
Introduction
Screening studies among children in the USA and Western Europe report a seroprevalence of coeliac disease (CD) of around 1%.1–6 However, over the last two decades, several studies have reported an increased incidence of biopsy-detected CD in children,7 the reason for which is unclear. One explanation for the increase is that it represents improved ascertainment due to heightened clinical awareness underpinned by improved accuracy and availability of diagnostic tests8 and/or to screening programmes in people with associated diseases.9 If this increase in clinically diagnosed paediatric CD does only represent improved ascertainment, then one might expect it to be more marked in higher socioeconomic groups. This is because children of higher socioeconomic status are more likely to seek healthcare,10 and therefore opportunities for investigation in so-called ‘at-risk’ groups would be expected to occur more frequently among them, which may lead to more frequent testing for CD.11 The few studies that have investigated a possible socioeconomic gradient in CD have reported conflicting results.12–21 In view of this and the lack of population-based studies, assessing patterns of clinical diagnosis of CD among children in the UK, we examined the incidence of CD up to 18 years of age and its variation by socioeconomic group, taking into account age, sex, calendar period and country within the UK.
Methods
Data were obtained from The Health Improvement Network (THIN), a nationally representative UK database of primary care records, containing medical diagnoses, events and drug prescriptions.22 Our cohort was all children aged 0–18 years registered with a THIN general practice (GP) at any time from 1 January 1993 to 31 December 2012. We identified all incident diagnoses of CD based on the presence of one or more of the following Read codes recorded in children's GP records: J690.00-Coeliac disease, J690.13-Gluten enteropathy, J690z00-Coeliac disease NOS. For patients with more than one CD code, the earliest was considered as the date of disease diagnosis. Children with CD recorded before their entry date were considered to have prevalent disease and were thus excluded.
The incidence of CD per 100 000 person-years was calculated by dividing the number of children with CD by the total follow-up time contributed to the study period by all children. Incidence was stratified by age, sex, country of residence (England, Scotland, Wales and Northern Ireland), calendar year (grouped as quinquennia) and household socioeconomic quintile, measured by the Townsend index.23 Townsend index measures area-level (approximately 50 households) deprivation based on local unemployment, car ownership, overcrowding and home ownership from the 2001 Census; use of quintiles maintains anonymity.
Poisson regression was used to calculate unadjusted incidence rate ratios (IRRs) for CD by all factors, and potential interactions were assessed using the likelihood ratio test (LRT). Age was grouped into three age bands (0–2, 3–9, 10–18), similar to that in previous demographic studies of CD.17 24 25 Although prevalent CD cases had been excluded, children with a first diagnosis recorded soon after their GP registration may have had prevalent disease upon study entry (diagnosed before but recorded near the time of GP registration). Therefore, we conducted a sensitivity analysis excluding patients whose first CD diagnosis or gluten-free product prescription was recorded within 6 months of initial registration unless they were diagnosed at younger than age 2 years. This method of excluding potentially prevalent cases was based on Lewis et al's26 but tailored to children since diagnoses very early in life are likely to be incident regardless of proximity to registration. We conducted a second sensitivity analysis to exclude potential CD overestimation using a restricted CD definition where cases had to have at least one gluten-free product prescription associated with their CD diagnosis. In the UK, children with CD are eligible to receive free prescriptions from their GP to purchase gluten-free foods, funded by the public National Health Service. While patients with CD are not required to obtain these prescriptions, they would not be received unless a definitive CD diagnosis was made. Analyses were performed using Stata V.12.
Results
There were 2 063 421 children in our study population, contributing a total of 10 508 374 person-years. Their median follow-up was 3.8 years (IQR 1.5–7.9) and 1247 children were diagnosed with CD, corresponding to an overall incidence rate of 11.9 per 100 000 person-years.
Socio-demographic distribution of CD diagnoses
Table 1 shows variations in the rate of CD diagnosis by sex, age, country, calendar period and socioeconomic deprivation. The rate of CD in girls was 53% higher (IRR 1.53, 95% CI 1.37 to 1.72) than in boys. Incidence varied by age with the lowest CD in children younger than 1 after which it increased to 18.7 and 17.9 per 100 000 person-years at ages 1 and 2 years, respectively. Incidence then decreased, ranging between 8.4 and 15.1 per 100 000 person-years between ages 3 and 18 years. We did not find significant geographic variation across the UK, with a similar CD incidence observed in the four countries. Across the 20-year period studied, there was a clear increase with a CD diagnosis rate in the last five years (2008–2012) that was 75% higher than in 1993–1997 (IRR 1.75, 95% CI 1.31 to 2.34).
Table 1.
Incidence of coeliac disease (N=2 063 421)
| Number of coeliac disease cases | Person-years | Rate per 100 000 person-years (95% CI) | Unadjusted incidence rate ratios (95% CI) | |
|---|---|---|---|---|
| Overall | 1247 | 10 508 374 | 11.9 (11.2 to 12.5) | |
| Sex | ||||
| Male | 514 | 5 448 627 | 9.4 (8.6 to 10.3) | 1 |
| Female | 733 | 5 059 747 | 14.5 (13.5 to 15.6) | 1.53 (1.37 to 1.72) |
| Age (years) | ||||
| <1 | 24 | 599 728 | 4.0 (2.7 to 6.0) | |
| 1 | 125 | 668 994 | 18.7 (15.7 to 22.2) | |
| 2 | 119 | 662 631 | 17.9 (15.0 to 21.5) | |
| 3 | 91 | 653 575 | 13.9 (11.3 to 17.1) | |
| 4 | 97 | 643 093 | 15.1 (12.4 to 18.4) | |
| 5 | 67 | 629 754 | 10.6 (8.4 to 13.5) | |
| 6 | 81 | 618 239 | 13.1 (10.5 to 16.3) | |
| 7 | 74 | 608 210 | 12.1 (9.7 to 15.3) | |
| 8 | 66 | 597 770 | 11.0 (8.7 to 14.0) | |
| 9 | 64 | 587 151 | 10.9 (8.5 to 13.9) | |
| 10 | 62 | 576 655 | 10.7 (8.4 to 13.8) | |
| 11 | 55 | 566 426 | 9.7 (7.4 to 12.6) | |
| 12 | 56 | 554 291 | 10.1 (7.7 to 13.1) | |
| 13 | 58 | 539 042 | 10.7 (8.3 to 13.9) | |
| 14 | 65 | 521 484 | 12.4 (9.7 to 15.9) | |
| 15 | 43 | 507 872 | 8.4 (6.3 to 11.4) | |
| 16 | 43 | 495 529 | 8.6 (6.4 to 11.7) | |
| 17 | 57 | 477 930 | 11.9 (9.2 to 15.5) | |
| Country | ||||
| England | 1003 | 8 194 945 | 12.2 (11.5 to 13.0) | 1 |
| Scotland | 139 | 1 286 924 | 10.8 (9.1 to 12.7) | 0.88 (0.73 to 1.05) |
| Wales | 69 | 649 399 | 10.6 (8.4 to 13.4) | 0.87 (0.68 to 1.10) |
| Northern Ireland | 36 | 377 106 | 9.5 (6.9 to 13.2) | 0.77 (0.55 to 1.08) |
| Calendar period | ||||
| 1993–1997 | 50 | 603 213 | 8.3 (6.3 to 10.9) | 1 |
| 1998–2002 | 222 | 2 405 398 | 9.2 (8 to 1.10.5) | 1.11 (0.82 to 1.51) |
| 2003–2007 | 404 | 3 572 886 | 11.3 (10.2 to 12.5) | 1.36 (1.01 to 1.83) |
| 2008–2012 | 571 | 3 926 877 | 14.5 (13.4 to 15.8) | 1.75 (1.31 to 2.34) |
| Socioeconomic deprivation (quintile of Townsend index) | ||||
| 1 (least deprived) | 350 | 2 479 655 | 14.1 (12.7 to 15.7) | 1.80 (1.45 to 2.22) |
| 2 | 295 | 2 032 782 | 14.5 (12.9 to 16.2) | 1.85 (1.48 to 2.30) |
| 3 | 221 | 2 043 017 | 10.8 (9.5 to 12.3) | 1.38 (1.09 to 1.73) |
| 4 | 198 | 1 901 385 | 10.4 (9.0 to 11.9) | 1.33 (1.05 to 1.67) |
| 5 (most deprived) | 110 | 1 402 742 | 7.8 (6.5 to 9.4) | 1 |
| Missing | 73 | 648 793 | 11.2 (8.9 to 14.1) | 1.43 (1.06 to 1.92) |
CI, confidence interval.
Table 2 shows how the incidence of CD varied by sex, age and country over the study period. There was a 39% increase in boys (IRR 1.39, 95% CI 0.92 to 2.10) and a twofold increase in girls (IRR 2.09, 95% CI 1.39 to 3.13). However, there was no statistically significant interaction between sex and calendar period (LRT p value for interaction=0.4). Conversely, across the three age groups studied, there was a statistically significant interaction with calendar period (LRT p<0.001). Children aged 0–2 years had a roughly constant CD incidence over time, whereas children aged 3–18 years had threefold increase from 1993–1997 to 2008–2012 periods. CD incidence increased over time in England and Scotland, while we did not observe a statistically significantly increase in Wales and Northern Ireland.
Table 2.
Incidence of coeliac disease over time by sex and age and relative incidence in 2008–2012 compared with 1993–1997 (N=2 063 421)
| Calendar period | |||||
|---|---|---|---|---|---|
| Calendar year | 1993–1997* | 1998–2002* | 2003–2007* | 2008–2012* | Unadjusted IRR for calendar period† (95% CI) |
| Sex | |||||
| Male | 7.8 (5.3 to 11.6) | 7.7 (6.3 to 9.3) | 9.3 (7.9 to 10.8) | 10.9 (9.6 to 12.6) | 1.39 (0.92 to 2.10) |
| Female | 8.8 (5.9 to 12.9) | 10.9 (9.2 to 13.0) | 13.5 (11.8 to 15.3) | 18.3 (16.5 to 20.3) | 2.09 (1.39 to 3.13) |
| Age (years) | |||||
| 0–2 | 16.7 (11.5 to 24.1) | 14.2 (11.1 to 18.0) | 13.9 (11.3 to 17.3) | 12.9 (10.4 to 15.8) | 0.77 (0.50 to 1.18) |
| 3–9 | 5.7 (3.4 to 9.7) | 8.7 (7.1 to 10.7) | 12.1 (10.4 to 14.0) | 16.4 (14.5 to 18.6) | 2.85 (1.66 to 4.88) |
| 10–18 | 4.1 (2.1 to 8.8) | 7.2 (5.6 to 9.2) | 9.4 (7.9 to 11.1) | 13.5 (11.8 to 15.4) | 3.24 (1.60 to 6.56) |
| Country | |||||
| England | 8.9 (6.7 to 11.9) | 9.9 (8.6 to 11.4) | 11.7 (10.5 to 13.0) | 14.8 (13.5 to 16.2) | 1.65 (1.22 to 2.24) |
| Scotland | – | 5.1 (3.0 to 8.7) | 11.2 (8.5 to 14.8) | 14.3 (11.3 to 17.9) | 2.77 (1.56 to 4.90)‡ |
| Wales | 11.0 (3.5 to 34.2) | 9.2 (5.2 to 16.2) | 10.3 (6.8 to 15.5) | 11.5 (8.1 to 16.4) | 1.04 (0.32 to 3.42) |
| Northern Ireland | 5.9 (0.8 to 42.3) | 6.3 (2.8 to 14.0) | 5.3 (2.5 to 11.2) | 16.4 (10.8 to 24.9) | 2.76 (0.37 to 20.46) |
Interaction tests: sex and calendar year, LRT p value=0.4; age group and calendar year, LRT p value<0.001; country and calendar year, LRT p value=0.05.
*Incidence rates of coeliac diseases per 100 000 person-years (95% CI).
†Comparing the latest with the earliest calendar period.
‡Since there were no incident coeliac disease cases in the Scottish practices between 1993 and 1997, we used the period 1998–2002 as baseline.
CI, confidence interval; IRR, incidence rate ratio; LRT, likelihood ratio test.
Incident CD diagnoses across socioeconomic status
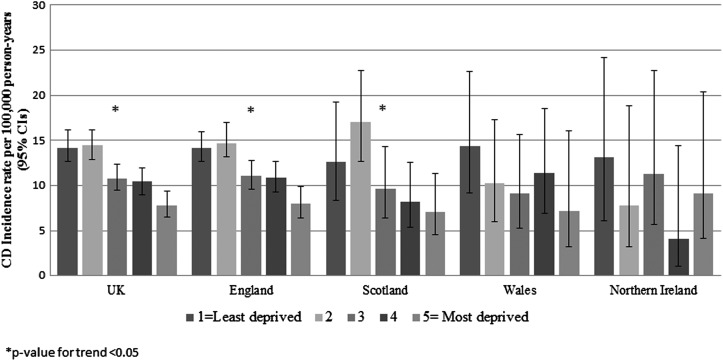
We found a gradient across socioeconomic groups in CD diagnoses, with the highest CD incidence in children from the two least socioeconomically deprived areas, followed by a progressive reduction of CD incidence in the latter three quintiles (table 1). The lowest incidence was in the most socioeconomically deprived areas. Tables 3 and 4 show, respectively, the absolute rate of CD and the adjusted IRRs for the association of CD with socioeconomic status, stratified by sex, age, calendar period and country. We observed a similar relationship between socioeconomic status and CD in both sexes, in all age groups, over time, and in all countries (all LRTs not statistically significant for interaction between these variables and socioeconomic status). There was a statistically significant socioeconomic gradient in each time period other than 1993–1997, when the highest incidence rate was observed in the third quintile. Moreover, although we did not find a statistically significant socioeconomic trend in CD in smaller populations from Wales and Northern Ireland, they also had the highest incidence rate of CD in children from the least socioeconomically deprived areas and the lowest CD incidence rate in children from more socioeconomically deprived areas, in the 5th and 4th quintiles, respectively (figure 1).
Table 3.
Incidence of coeliac disease across socioeconomic groups (N=2 063 421)
| Quintile of deprivation | 1=least deprived* | 2* | 3* | 4* | 5=most deprived* | Missing |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 11.4 (9.7 to 13.4) | 11.4 (9.5 to 13.6) | 9.3 (7.7 to 11.4) | 7.4 (5.9 to 9.3) | 6.4 (4.8 to 8.5) | 8.2 (5.6 to 11.9) |
| Female | 17.0 (14.8 to 19.6) | 17.9 (15.4 to 20.7) | 12.4 (10.4 to 14.8) | 13.6 (11.4 to 16.2) | 9.4 (7.3 to 12.0) | 14.4 (10.8 to 19.2) |
| Age (years) | ||||||
| 0–2 | 15.6 (12.3 to 19.8) | 18.5 (14.5 to 23.5) | 14.4 (11.0 to 18.8) | 10.4 (7.5 to 14.3) | 9.3 (6.3 to 13.8) | 13.1 (8.3 to 20.5) |
| 3–9 | 14.6 (12.4 to 17.1) | 15.7 (13.2 to 18.6) | 9.9 (8.0 to 12.3) | 11.9 (9.7 to 14.6) | 8.3 (6.2 to 11.0) | 11.9 (8.5 to 16.8) |
| 10–18 | 13.0 (10.9 to 15.4) | 11.6 (9.5 to 14.2) | 10.1 (8.1 to 12.5) | 8.9 (7.0 to 11.3) | 6.7 (4.9 to 9.2) | 9.2 (6.0 to 14.2) |
| Calendar period | ||||||
| 1993–1997 | 8.2 (4.5 to 14.9) | 5.3 (2.4 to 11.9) | 10.5 (5.9 to 18.5) | 8.1 (4.2 to 15.6) | 4.7 (1.7 to 12.6) | 16.4 (8.2 to 32.8) |
| 1998–2002 | 12.3 (9.7 to 15.6) | 10.0 (7.5 to 13.4) | 8.6 (6.3 to 11.7) | 8.2 (5.9 to 11.5) | 5.2 (3.2 to 8.4) | 8.2 (4.7 to 14.5) |
| 2003–2007 | 12.6 (10.4 to 15.2) | 14.8 (12.2 to 17.9) | 11.4 (9.1 to 14.2) | 9.3 (7.2 to 12.0) | 7.6 (5.5 to 10.6) | 8.5 (5.4 to 13.5) |
| 2008–2012 | 17.4 (14.9 to 20.4) | 18.4 (15.5 to 21.7) | 11.7 (9.5 to 14.3) | 13.0 (10.6 to 16.0) | 10.2 (7.8 to 13.3) | 14.4 (10.3 to 20.0) |
| Country | ||||||
| England | 14.2 (12.7 to 15.9) | 14.7 (12.9 to 16.7) | 11.1 (9.6 to 12.8) | 10.9 (9.3 to 12.7) | 8.0 (6.4 to 9.9) | 11.9 (9.2 to 15.4) |
| Scotland | 12.6 (8.2 to 19.1) | 17.0 (12.7 to 22.7) | 9.6 (6.4 to 14.5) | 8.2 (5.4 to 12.6) | 7.1 (4.5 to 11.3) | 9.2 (4.8 to 17.8) |
| Wales | 14.4 (9.2 to 22.6) | 10.2 (6.0 to 17.3) | 9.1 (5.3 to 15.6) | 11.4 (6.9 to 18.5) | 7.2 (3.2 to 16.0) | 7.3 (1.0 to 51.9) |
| Northern Ireland | 13.1 (6.2 to 24.2) | 7.8 (3.2 to 18.8) | 11.3 (5.7 to 22.7) | 4.1 (1.0 to 14.4) | 9.1 (4.1 to 20.4) | 9.6 (4.0 to 23.1) |
*Incidence rates of coeliac diseases per 100 000 person-years (95% CIs).
CI, confidence interval.
Table 4.
Adjusted incidence rate ratios for the association of coeliac disease with socioeconomic status, stratified by sex, age, calendar period and country (N=2 063 421)
| Quintile of deprivation | 1=least deprived* | 2* | 3* | 4* | 5=most deprived | p Value for trend** | Missing* |
|---|---|---|---|---|---|---|---|
| Sex‡ | |||||||
| Male | 1.76 (1.26 to 2.46) | 1.78 (1.26 to 2.50) | 1.45 (1.02 to 2.06) | 1.15 (0.79 to 1.66) | 1 | <0.001 | 1.26 (0.78 to 2.03) |
| Female | 1.79 (1.35 to 2.37) | 1.89 (1.42 to 2.52) | 1.30 (0.96 to 1.77) | 1.44 (1.06 to 1.94) | 1 | <0.001 | 1.51 (1.03 to 2.20) |
| Age (years)§ | |||||||
| 0–2 | 1.57 (0.99 to 2.48) | 1.92 (1.21 to 3.04) | 1.48 (0.92 to 2.38) | 1.08 (0.65 to 1.79) | 1 | 0.004 | 1.38 (0.76 to 2.51) |
| 3–9 | 1.70 (1.22 to 2.36) | 1.86 (1.33 to 2.59) | 1.17 (0.82 to 1.67) | 1.41 (0.99 to 2.01) | 1 | <0.001 | 1.43 (0.91 to 2.23) |
| 10–18 | 1.97 (1.37 to 2.83) | 1.74 (1.19 to 2.53) | 1.52 (1.03 to 2.23) | 1.35 (0.90 to 2.00) | 1 | <0.001 | 1.39 (0.82 to 2.37) |
| Calendar period† | |||||||
| 1993–1997 | 1.47 (0.49 to 4.63) | 1.00 (0.28 to 3.56) | 1.98 (0.63 to 6.15) | 1.58 (0.49 to 5.14) | 1 | 0.95 | 3.14 (0.94 to 10.50) |
| 1998–2002 | 2.22 (1.31 to 3.79) | 1.87 (1.10 to 3.25) | 1.59 (0.90 to 2.81) | 1.55 (0.87 to 2.76) | 1 | 0.001 | 1.54 (0.73 to 3.24) |
| 2003–2007 | 1.63 (1.11 to 2.38) | 1.92 (1.31 to 2.81) | 1.48 (1.00 to 2.20) | 1.21 (0.81 to 1.83) | 1 | <0.001 | 1.11 (0.63 to 1.97) |
| 2008–2012 | 1.71 (1.25 to 2.34) | 1.81 (1.31 to 2.48) | 1.15 (0.81 to 1.61) | 1.28 (0.92 to 1.80) | 1 | <0.001 | 1.39 (0.91 to 2.13) |
| Country¶ | |||||||
| England | 1.79 (1.04 to 2.30) | 1.86 (1.44 to 2.39) | 1.39 (1.07 to 1.82) | 1.37 (1.04 to 1.79) | 1 | <0.001 | 1.47 (1.05 to 2.06) |
| Scotland | 1.66 (0.89 to 3.10) | 2.23 (1.29 to 3.86) | 1.26 (0.68 to 2.34) | 1.11 (0.59 to 2.08) | 1 | 0.004 | 1.21 (0.54 to 2.71) |
| Wales | 2.02 (0.81 to 5.07) | 1.43 (0.55 to 3.73) | 1.26 (0.48 to 3.34) | 1.58 (0.62 to 4.05) | 1 | 0.188 | 0.95 (0.11 to 7.91) |
| Northern Ireland | 1.45 (0.53 to 4.00) | 0.85 (0.26 to 2.80) | 1.20 (0.41 to 3.46) | 0.44 (0.08 to 2.20) | 1 | 0.340 | 1.21 (0.36 to 4.06) |
Interaction tests: sex and socioeconomic status, LRT p value=0.67; age group and socioeconomic status, LRT p value 0.78; calendar years and socioeconomic status, LRT p value=0.42; countries and socioeconomic status, LRT p value=0.87.
*Incidence rate ratio compared with most deprived (5th quintile).
Adjusted for: ‡Age and calendar period and country;
§Sex and calendar period and country;
†Sex and age and country;
¶Sex and calendar period and age.
**Excluding missing data.
LRT, likelihood ratio test.
Figure 1.
Coeliac disease incidence across countries of the UK according to socioeconomic group. CD, coeliac disease; CI, confidence interval.
Sensitivity analyses
There were 230 children diagnosed with CD after 2 years of age with a first CD record or gluten-free prescription within 6 months of GP registration (18.4% potentially prevalent cases out of the total 1247 case population). After excluding these potentially prevalent cases, the overall incidence was 9.7 per 100 000 person-years. Variations between subgroups, however, remained similar to the main analyses (see online supplementary table S1). After restricting our CD cases to children with a gluten-free product prescription, there were 1007 children (80.8% of the total 1247 case population), resulting in an incidence of 9.6 per 100 000 person-years. Again, IRRs remained similar to main analyses, showing the same incidence patterns by sex, age, calendar time, country and socioeconomic status (see online supplementary table S2).
Discussion
Main findings
The overall CD incidence of 11.9 per 100 000 person-years was similar across the UK countries and higher in girls than in boys. While CD incidence up to age 2 remained stable over time, diagnoses in older children have almost tripled over the past 20 years. We found a strong socioeconomic gradient in CD diagnoses such that children living in less socioeconomically deprived areas were about twice as likely to be diagnosed as those in more-deprived areas. This pattern held for boys and girls and for all ages. Across all four countries, we found evidence of a similar socioeconomic gradient in CD diagnosis.
Strengths and limitations
This is the largest study of childhood CD in which the possible role of socio-demographic aspects on the rate of CD diagnosis has been examined. The demographics of our study population are comparable to those of children in the UK population,27 so our findings are likely to be representative of this population. We considered CD diagnosis by using a physician's report as recorded in GP as we did not have comprehensive information on serological or histological testing for each patient. While our pragmatic approach may have resulted in false-positive cases, the accuracy of CD recording in primary care was specifically validated against medical records28 in a small sample of patients, showing a good concordance between paper and electronic records. We used a single diagnostic code to maximise the sensitivity in the main analysis, but when we used a more specific case definition (including only children with at least one CD code and at least one prescription for gluten-free product), the incidence patterns across age, sex, calendar year and socioeconomic status remained very similar to our main analyses. We found that 80.8% of children with CD had a prescription for a gluten-free product that was similar to the finding reported by Hall et al.29 They conducted a questionnaire study of a sample of patients with CD identified by Read codes in northeast England, and while they confirmed that all patients did have CD disease, only 86.1% of their patients with CD obtained a gluten-free prescription. We believe therefore that it is unlikely that there has been any great amount of over-ascertainment of CD in our study.
Moving on from our outcome to consider our principal exposure measurement, the Townsend index is a validated measure of how socioeconomically deprived an area is within the UK based on standardised indicators. However, this score gives an overall deprivation index of the people living in a particular area of approximately 50 households, not taking into account individual variation in deprivation levels or the differences between urban and rural areas. The small number of CD cases registered in Wales and Northern Ireland reduced the statistical power for these particular analyses.
The relationship between CD diagnosis and socioeconomic grouping did not hold in the time period 1993–1997, which could be related to the differences in diagnosis tools and clinical presentation during that period compared with the following ones. Diagnostic biopsies at that time were frequently carried out by general paediatricians using Crosby or Watson capsules, rather than requiring referral to a paediatric gastroenterologist for endoscopic biopsy. Furthermore, only in the most recent decades has there been a shift towards older average age at diagnosis among children and altered clinical presentation30 (ie, less distension, failure to thrive and more subtle symptomatology).31 It is possible that these issues could have contributed to the different diagnosis rates among socioeconomic groups that we have observed. An alternative perspective is that the apparently older age at diagnosis observed in various studies has been driven by greater ascertainment and therefore earlier (younger) diagnosis of older (>10 years of age) children. Finally, we cannot exclude that the lack of a gradient seen in the period 1993–1997 was simply related to the lack of adequate sample size during these years.
Comparison with previous literature
Socioeconomic status has been proposed as a possible factor in the development of CD, although existing studies show contradictory results. As table 5 shows, these studies have several differences in study design, outcome, exposure definition, study population and setting. Most of them were conducted using groups of already-diagnosed patients with CD,12–19 and the only two population-based studies consider patients detected by serological screening rather than clinical diagnosis.20 21 Three studies have been conducted in different areas of the UK on children.14 15 18 Whyte et al14 reported a higher risk of CD diagnosis in children, aged <16 years, belonging to the least-deprived areas compared with those from the most-deprived areas in South Wales, which is similar to our results. This cross-sectional study used The Welsh Index of Multiple Deprivation as a measure of the socioeconomic status that is comparable to the Townsend index. Conversely, a small Scottish cohort study15 found no large difference between the Standard Index of Multiple Deprivation and urban/rural indices in children with CD and the general population in Scotland. In 2009, Robert et al18 reported that in an area of the South of England children from manual social classes IV and V had a fourfold increased risk of CD compared with those from professional social classes I and II. Finally, looking at all ages in the UK population, our recent population-based study, using the Clinical Practice Research Database,13 also described that CD occurred more commonly in areas with the least socioeconomic deprivation. Outside the UK, three Swedish studies16 17 19 have reported contradictory results on the association between socioeconomic status and CD (table 5). Similarly to our results, Burger et al12 have recently described, using registered pathology reports in the Netherlands, that patients diagnosed in childhood were more often from higher socioeconomic status compared with patients diagnosed later in life.
Table 5.
Previous literature on the association between socioeconomic status and coeliac disease
| Geographic area | Study population | Source of the outcome N=number of cases |
Source of socioeconomic status | Main findings | |
|---|---|---|---|---|---|
| Diagnosed CD (serology and/or biopsy-positive cases) within medical settings | |||||
| Burger et al12 | Netherlands | Subjects identified into the Dutch Pathology Registry, which covers all pathology labs in Netherlands 1995–2010 N= 6444 |
CD diagnosis according to biopsy reports N=4014 |
The socioeconomic status scores based on income, level of education and employment | Patients diagnosed with CD during childhood were more often from an area with a higher socioeconomic status compared with patients diagnosed later in life (p<0.001) |
| West et al13 | The UK | All ages UK population registered with the Clinical Practice Research Datalink—1990–2011 N=65 856 848 person-years |
People with Read codes representing CD (J690.00; J690.13; J690z00; J690100; J690.14; J690000) N=9087 |
Indices of Multiple Deprivation | The CD incidence was 27% lower in people from the most-deprived areas than in people from the least-deprived ones (IRR 0.83, 95% CI 0.77 to 0.89) |
| Whyte et al14 | Cardiff, Newport and Powys (South Wales) | The total paediatric population (age <16) in South Wales (UK national census 2008) N=298 530 children |
CD diagnosis according to ESPGHAN 1990 criteria in the same tertiary medical centre between 1995 and 2012 N=232 |
Welsh Index of Multiple Deprivation | The prevalence of CD in the lowest deprivation level was 1.16/1000 and 0.49/1000 in the highest deprivation level |
| White et al15 | Southeast Scotland | The total paediatric population (age <16) in Southeast Scotland—1990–2009 (Scotland census) N=∼225 000 children |
CD diagnosis according to ESPGHAN 1990 criteria. Data from hospital records (ICD codes of CD), paediatric pathology records, regional clinical database, regional serology database and the electronic hospital record N=266 |
The Scottish government data for the Standard Index of Multiple Deprivation and urban/rural indices | The median of the Standard Index of Multiple Deprivation score and urban–rural classification indices of patients with CD were comparable to the general population of southeastern Scotland |
| Olén et al16 | Sweden | Individuals aged 16–64 years using the Total Population Register 1969–2008 N=174 186 subjects |
CD diagnosis according to biopsy reports collected from all Swedish pathology departments N=29 096 |
European Socioeconomic Classification based on occupation. Data collected using The Swedish Occupational Register |
Individuals from the lowest social class were 11% less likely to be diagnosed with CD (OR 0.89, 95% CI 0.84 to 0.94) |
| Wingren et al17 | Sweden | Prospective evaluation of babies born in Sweden between 1987 and 1993 (follow-up 2 years) N=392 568 men and 372 112 women |
The Swedish National Hospital Discharge Registry according to ICD codes of CD N=845 in men and 1401 in women |
Information on the mothers’ pre-tax equalised household income and social allowance for the year before delivery (five classes) | Boys born to mothers in an overt low socioeconomic position had a higher risk of CD (OR 1.37, 95% CI 1.03 to 1.82) than those with mothers with high income and no social allowance |
| Robert et al18 | South East England | Babies born in the south east of England between 1970 and 1999 (mean follow-up duration 18 years) using the Oxford record linkage study database having linked maternity data in the same dataset N=248 521 |
Children with both a maternity record and a subsequent admission for CD (ICD codes of CD) in the Oxford record linkage study database N=90 |
Information collected from maternal records in the Oxford record linkage study database | Children from manual social classes IV and V had a 4.02 increased risk of coeliac disease (95% CI1.96 to 8.25) compared with those from professional social classes I and II |
| Ludvigsson19 | Sweden | Babies born in southeast Sweden between 1997 and 1999 (follow-up 15 years) N=15 875 single births |
Coeliac cases reported by eight paediatric departments A case was included if he had intestinal biopsy suggesting CD, no symptoms after the introduction of a gluten-free diet and/or no or only minor histopathological abnormalities consistent with CD at the control biopsy under treatment with gluten-free diet N=45 |
Information collected in questionnaire completed by the mothers shortly after childbirth on: place of living 1 year before conception, maternal employed during pregnancy, paternal employed the year before the conception, family crowed living | CD was less common in mothers who had worked <3 months during pregnancy (OR=0.29; 95% CI 0.09 to 0.94; p=0.039). The other socioeconomic factors were not associated |
| Screening detected CD in the general population | |||||
| Kondrashova et al20 | Finland and Russia | Schoolchildren Russia Karelia: age ranged 6.2–18.3 years (1997–2001) N=1988 children Northern Finland: age ranged 7–18 years (1994) N= 3654 children |
Serological screening by tTGA All subjects who were positive were offered an intestinal biopsy to confirm CD diagnosis. N=4 in Russia and 34 in Finland |
Comparison between two areas with opposite socioeconomic condition (poor Russia vs rich Finland). | 0.6% of the children (12/1988; CI 0.3% to 1.1%) in Russian Karelia tested positive for tTGA compared with 1.4% (52/3654; CI 1.1% to 1.9%) in the Finnish cohort. Biopsy-proven CD: N=4 in Russia and 34 in Finland (no biopsy in 13 subjects) |
| West et al21 | Cambridge | Participants, age 45–76 years registered with a general practice in Cambridge, England (1990–1995) N=7527 |
Serological screening by EMA N=87 |
Participant-reported occupation categorised as professional, skilled, unskilled/partly skilled | EMA positivity less common in partly skilled or unskilled workers, as compared to professionals (OR 0.51, 95% CI 0.18 to 1.43) |
CD, coeliac disease; EMA, antiendomisial antibody; ESPGHAN, European Society of Paediatric Gastroenterology, Hepatology and Nutrition; ICD, international classification of disease; IRR, incident rate ratio; tTGA, IgA antitransglutaminase.
Interpretation
It is not possible from this study nor from previous literature to conclusively explain the reasons for this gradient, which may indicate either that the ascertainment of disease varies among social classes or that there is a true variation in incidence by socioeconomic status. For example, individuals from more-deprived areas may be less likely to seek medical care or consultation in general and thus be potentially less likely to be tested for CD.10 32 33 Aside from ascertainment, however, it is also possible that people of different socioeconomic groups are exposed to different risk factors, which might indirectly contribute to CD development. Two studies of screening-detected CD (table 5), one in the UK showing a weak socioeconomic gradient21 and the other reporting a higher prevalence of detected CD in children from Finland than in those from a more socioeconomically deprived Russian area,20 may support this conjecture. The duration of exclusive breast feeding and the optimal timing of gluten introduction for infants in terms of their contribution to the risk of developing CD have been debated for several years. Previous studies have implied a window of time (4–6 months of age) during which the introduction of gluten might facilitate induction of tolerance (window of tolerance);34–38 however, evidence from two newly published clinical trials has not confirmed this.39 40 Vriezinga et al recruited HLA-DQ2 or HLADQ8-positive newborns with a first-degree relative affected by CD from eight countries and showed no difference in the risk of CD by 3 years of age between infants randomised to 100 mg of immunologically active gluten daily (combined with lactose) and those given a placebo (lactose only) between 4 and 6 months.39 Furthermore, they observed no association of breastfeeding duration, regardless of whether it was exclusive or with gluten introduction, with CD development. Lionetti et al40 conducted a trial in Italy of predisposed newborns (those with a first-degree relative with CD) and found that the introduction of gluten at 12 months vs 6 months slightly delayed the onset of CD, but CD prevalence was no different by 5 years of age. In addition, the authors did not detect any effect of breast feeding on the development of CD. These trials do not support the possibility that differences in time of gluten introduction or breastfeeding duration explain the socioeconomic gradient we observed in our population. Furthermore, an analysis of breast feeding in England showed a lower prevalence of exclusive breast feeding at 6–8 weeks postdelivery among women from the most-deprived areas than in the least-deprived areas.41 Since the higher incidence of CD in children from less-deprived groups that we observed is opposite to the relationship this would be expected to cause, this is further evidence that known differences in breast feeding are unlikely to explain the socioeconomic gradient of CD.
Another potential explanation of the observed socioeconomic gradient could be related to the so-called hygiene hypothesis.42 This hypothesises that a decreased antigenic exposure in childhood in less-deprived groups causes an immunological over-reaction at the time of a subsequent antigenic contact,42 43 that is, gluten in the case of CD. A greater exposure to childhood infection may also occur in children of lower socioeconomic groups,44 which could eventually protect them from later development of CD via this mechanism. This explanation, however, is inconsistent with previous evidence of early infections as a potential risk factor for CD.45 46 Lastly, little is known about dietary variation in gluten according to socioeconomic group,47 so we cannot speculate whether this may play a role.48
Conclusion
Based on the current evidence, the most plausible explanation for the socioeconomic gradient in the incidence of childhood CD whereby children from least-deprived areas have CD diagnosed more often than those from the most-deprived areas is that ascertainment of disease varies rather than the true occurrence of CD. Awareness campaigns and the implementation of diagnostic guidelines may help to implement strategies for case-finding in all children and reduce this inequality. Moreover, a greater use of the new paediatric guidelines49 50 with the possibility to diagnose symptomatic cases without biopsy might increase the access to diagnosis in children from the most-deprived areas. Future studies might continue to explore the possible association between exposures to different specific risk factors with the occurrence of CD across socioeconomic groups.
Supplementary Material
Footnotes
Contributors: FZ conceptualised and designed the study, drafted the initial manuscript, carried out the statistical analysis and approved the final manuscript as submitted. JW conceptualised and designed the study, obtained the funding, coordinated and supervised data analysis, reviewed and revised the manuscript, and approved the final manuscript as submitted. CJC, KMF and TRC reviewed and revised the manuscript, obtained the funding, and approved the final manuscript as submitted. CC reviewed and revised the manuscript and approved the final manuscript as submitted. LJT conceptualised and designed the study, obtained the funding, prepared the data and initial analysis, coordinated and supervised data analysis, critically reviewed the manuscript and approved the final manuscript as submitted.
Funding: Supported by CORE/Coeliac UK; JW is funded by a University of Nottingham/Nottingham University Hospitals NHS Trust Clinical Senior Research Fellowship, which also partly funds FZ's PhD.
Competing interests: None.
Ethics approval: THIN Scientific Review Committee (protocol 13-075).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med 2003;163:286 10.1001/archinte.163.3.286 [DOI] [PubMed] [Google Scholar]
- 2.Tommasini A, Not T, Kiren V, et al. Mass screening for coeliac disease using antihuman transglutaminase antibody assay. Arch Dis Child 2004;89:512–15. 10.1136/adc.2003.029603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mäki M, Mustalahti K, Kokkonen J, et al. Prevalence of celiac disease among children in Finland. N Engl J Med 2003;348:2517–24. 10.1056/NEJMoa021687 [DOI] [PubMed] [Google Scholar]
- 4.Mustalahti K, Catassi C, Reunanen A, et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med 2010;42:587–95. 10.3109/07853890.2010.505931 [DOI] [PubMed] [Google Scholar]
- 5.Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J gastroenterol 2012;107:1538–44. 10.1038/ajg.2012.219 [DOI] [PubMed] [Google Scholar]
- 6.Bingley PJ, Norcross AJ, Lock RJ, et al. Undiagnosed coeliac disease at age seven: population based prospective birth cohort study. BMJ 2004;328:322–3. 10.1136/bmj.328.7435.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang JY, Kang AH, Green A, et al. Systematic review: worldwide variation in the frequency of coeliac disease and changes over time. Aliment Pharmacol Ther 2013;38:226–45. 10.1111/apt.12373 [DOI] [PubMed] [Google Scholar]
- 8.Rostom A, Dubé C, Cranney A, et al. The diagnostic accuracy of serologic tests for celiac disease: a systematic review. Gastroenterology 2005;128:S38–46. 10.1053/j.gastro.2005.02.028 [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal S, Lebwohl B, Green PH. Screening for celiac disease in average-risk and high-risk populations. Therap Adv Gastroenterol 2012;5:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goddard M, Smith P. Equity of access to health care services: Theory and evidence from the UK. Soc Sci Med 2001;53:1149–62. 10.1016/S0277-9536(00)00415-9 [DOI] [PubMed] [Google Scholar]
- 11.Costa Font J, Hernández-Quevedo C, McGuire A. Persistence despite action? Measuring the patterns of health inequality in England (1997–2007). Health Policy 2011;103:149–59. 10.1016/j.healthpol.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 12.Burger JP, Roovers EA, Drenth JP, et al. Rising incidence of celiac disease in the Netherlands; an analysis of temporal trends from 1995 to 2010. Scand J Gastroenterol 2014:1–9. [DOI] [PubMed] [Google Scholar]
- 13.West J, Fleming KM, Tata LJ, et al. Incidence and prevalence of celiac disease and dermatitis herpetiformis in the UK over two decades: population-based study. Am J Gastroenterol 2014;109:757–68. 10.1038/ajg.2014.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whyte L, Kotecha S, Watkins W, et al. Coeliac disease is more common in children with high socio-economic status. Acta Paediatr 2014;103:289–94. 10.1111/apa.12494 [DOI] [PubMed] [Google Scholar]
- 15.White LE, Merrick VM, Bannerman E, et al. The rising incidence of celiac disease in Scotland. Pediatrics 2013;132:e924–31. 10.1542/peds.2013-0932 [DOI] [PubMed] [Google Scholar]
- 16.Olén O, Bihagen E, Rasmussen F, et al. Socioeconomic position and education in patients with coeliac disease. Dig Liver Dis 2012;44:471–6. 10.1016/j.dld.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 17.Wingren CJ, Björck S, Lynch KF, et al. Coeliac disease in children: a social epidemiological study in Sweden. Acta Paediatr 2012;101:185–91. 10.1111/j.1651-2227.2011.02434.x [DOI] [PubMed] [Google Scholar]
- 18.Roberts S, Williams J, Meddings D, et al. Perinatal risk factors and coeliac disease in children and young adults: a record linkage study. Aliment Pharmacol Ther 2009;29:222–31. 10.1111/j.1365-2036.2008.03871.x [DOI] [PubMed] [Google Scholar]
- 19.Ludvigsson J. Socio-economic characteristics in children with coeliac disease. Acta Paediatr 2005;94:107–13. 10.1080/08035250410018274 [DOI] [PubMed] [Google Scholar]
- 20.Kondrashova A, Mustalahti K, Kaukinen K, et al. Lower economic status and inferior hygienic environment may protect against celiac disease. Ann Med 2008;40:223–31. 10.1080/07853890701678689 [DOI] [PubMed] [Google Scholar]
- 21.West J, Logan R, Hill P, et al. Seroprevalence, correlates, and characteristics of undetected coeliac disease in England. Gut 2003;52:960–5. 10.1136/gut.52.7.960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis JD, Schinnar R, Bilker WB, et al. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf 2007;16:393–401. 10.1002/pds.1335 [DOI] [PubMed] [Google Scholar]
- 23.Townsend P. Deprivation. J Soc Policy 1987;16:125–46. 10.1017/S0047279400020341 [DOI] [Google Scholar]
- 24.Wingren CJ, Agardh D, Merlo J. Sex differences in coeliac disease risk: a Swedish sibling design study. Dig Liver Dis 2012;44:909–13. 10.1016/j.dld.2012.06.016 [DOI] [PubMed] [Google Scholar]
- 25.McGowan KE, Castiglione DA, Butzner JD. The changing face of childhood celiac disease in North America: impact of serological testing. Pediatrics 2009;124:1572–8. 10.1542/peds.2008-2373 [DOI] [PubMed] [Google Scholar]
- 26.Lewis JD, Bilker WB, Weinstein RB, et al. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf 2005;14:443–51. 10.1002/pds.1115 [DOI] [PubMed] [Google Scholar]
- 27.NHS. Information Centre: Final General Practice Registered Populations. 2011. http://www.ic.nhs.uk/statistics-and-data-collections/population-and-geography/gp-registered-populations/attribution-dataset-gp-registered-populationsscaled-to-ons-population-estimates-2011
- 28.West J. Studies of its frequency and consequence, in Division of Epidemiology and Public Health 2005, University of Nottingham.
- 29.Hall NJ, Rubin GP, Charnock A. Intentional and inadvertent non-adherence in adult coeliac disease. A cross-sectional survey. Appetite 2013;68:56–62. 10.1016/j.appet.2013.04.016 [DOI] [PubMed] [Google Scholar]
- 30.Kelly D, Phillips A, Elliott E, et al. Rise and fall of coeliac disease 1960–85. Arch Dis Child 1989;64:1157–60. 10.1136/adc.64.8.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steens RF, Csizmadia CG, George EK, et al. A national prospective study on childhood celiac disease in the Netherlands 1993–2000: an increasing recognition and a changing clinical picture. J Pediatr 2005;147:239–43. 10.1016/j.jpeds.2005.04.013 [DOI] [PubMed] [Google Scholar]
- 32.Kozyrskyj AL, Dahl ME, Chateau DG, et al. Evidence-based prescribing of antibiotics for children: role of socioeconomic status and physician characteristics. Can Med Assoc J 2004;171:139–45. 10.1503/cmaj.1031629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braveman P. Health disparities and health equity: concepts and measurement. Annu Rev Public Health 2006;27:167–94. 10.1146/annurev.publhealth.27.021405.102103 [DOI] [PubMed] [Google Scholar]
- 34.Ivarsson A, Persson L, Nyström L, et al. Epidemic of coeliac disease in Swedish children. Acta Paediatr 2000;89:165–71. 10.1111/j.1651-2227.2000.tb01210.x [DOI] [PubMed] [Google Scholar]
- 35.Ivarsson A, Myléus A, Norström F, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics 2013;131:e687–94. 10.1542/peds.2012-1015 [DOI] [PubMed] [Google Scholar]
- 36.Ivarsson A, Hernell O, Stenlund H, et al. Breast-feeding protects against celiac disease. Am J Clin Nutr 2002;75:914–21. [DOI] [PubMed] [Google Scholar]
- 37.Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA 2005;293:2343–51. 10.1001/jama.293.19.2343 [DOI] [PubMed] [Google Scholar]
- 38.Størdal K, White RA, Eggesbø M. Early feeding and risk of celiac disease in a prospective birth cohort. Pediatrics 2013;132:e1202–9. 10.1542/peds.2013-1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vriezinga SL, Auricchio R, Bravi E, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med 2014;371:1304–15. 10.1056/NEJMoa1404172 [DOI] [PubMed] [Google Scholar]
- 40.Lionetti E, Castellaneta S, Francavilla R, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med 2014;371:1295–303. 10.1056/NEJMoa1400697 [DOI] [PubMed] [Google Scholar]
- 41.Oakley LL, Renfrew MJ, Kurinczuk JJ, et al. Factors associated with breastfeeding in England: an analysis by primary care trust. BMJ Open 2013;3:pii: e002765 10.1136/bmjopen-2013-002765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown EM, Arrieta M-C, Finlay BB. A fresh look at the hygiene hypothesis: How intestinal microbial exposure drives immune effector responses in atopic disease. Semin Immunol 2013;25:378–87. 10.1016/j.smim.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 43.Rautava S, Isolauri E. The development of gut immune responses and gut microbiota: effects of probiotics in prevention and treatment of allergic disease. Curr Issues Intestinal Microbiol 2002;3:15–22. [PubMed] [Google Scholar]
- 44.Simonsen J, Frisch M, Ethelberg S. Socioeconomic risk factors for bacterial gastrointestinal infections. Epidemiology 2008;19:282–90. 10.1097/EDE.0b013e3181633c19 [DOI] [PubMed] [Google Scholar]
- 45.Welander A, Tjernberg AR, Montgomery SM, et al. Infectious disease and risk of later celiac disease in childhood. Pediatrics 2010;125:e530–6. 10.1542/peds.2009-1200 [DOI] [PubMed] [Google Scholar]
- 46.Myléus A, Hernell O, Gothefors L, et al. Early infections are associated with increased risk for celiac disease: an incident case-referent study. BMC Pediatr 2012;12:194 10.1186/1471-2431-12-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson M, Dick K, Holmes B. Food budget standards and dietary adequacy in low-income families. Proc Nutr Soc 2002;61:569–77. 10.1079/PNS2002193 [DOI] [PubMed] [Google Scholar]
- 48.Kasarda DD. Can an increase in celiac disease be attributed to an increase in the gluten content of wheat as a consequence of wheat breeding? J Agric Food Chem 2013;61:1155–9. 10.1021/jf305122s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Husby S, Koletzko S, Korponay-Szabo I, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136–60. 10.1097/MPG.0b013e31821a23d0 [DOI] [PubMed] [Google Scholar]
- 50.Murch S, Jenkins H, Auth M, et al. Joint BSPGHAN and Coeliac UK guidelines for the diagnosis and management of coeliac disease in children. Arch Dis Child 2013;98:806–11. 10.1136/archdischild-2013-303996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.