Abstract
Background
Intramuscular lipoma is a relatively common benign neoplasm that is occasionally described as an infiltrating lipoma. Typical benign tumors show a clear margin, however, the infiltrative growth pattern of this lipoma mimics that of a malignant tumor. Although its growth has an effect on muscle bundles and it is known to never metastasize, the mechanism of infiltrative growth is not well understood. Previously, little attention has been paid to pathogenic features of muscle fibers around an intramuscular lipoma.
Methods
In the present study, we focused on pathologic changes of the surrounding skeletal muscles especially to the degenerative features of involving muscular types, and evaluate the role of type-selective muscular degeneration for the infiltrative growth of intramuscular lipomas. Following a review of the medical records in our institute, 17 lesions containing muscle tissues in their specimens (15 infiltrating lipomas, 2 well-circumscribed lipomas) were analyzed immunohistochemically. The tumor from the most recent case was also subjected to ultrastructural analysis. Two cases of the traumatic muscle damage were also evaluated as the control experiments.
Results
These analyses revealed type-selective muscle involution in 11 of 17 intramuscular lipomas and in 10 of 11 of the infiltrative type, with an involving pattern that resembled that of a neurogenic or myogenic disorder. Immunoreactivity to cathepsin-D, a lysosomal catabolic enzyme, was increased in the involved muscle fibers. Subsarcolemmal vacuoles in the muscle fibers of the peripheral areas were also positive for cathepsin-D, while degenerative findings were not visually apparent in these areas. Ultrastructural analysis revealed degenerative changes in those fibers. Neither positive staining for cathepsin-D nor type-selective atrophy was detected in the sections of traumatic muscle damage.
Conclusions
Our findings suggest that type-selective muscular degeneration and endomysial fatty growth as a result of atrophy may modulate the infiltrating growth characteristic of intramuscular lipoma.
Background
An intramuscular lipoma is a relatively common benign neoplasm that causes concern for both clinicians and pathologists, because of its large size, deep location, and infiltrating growth, which has led to its description as an infiltrating lipoma. Typically benign tumors show a clear margin, however, the infiltrative growth pattern of this tumor mimics that of a malignant one. Further, its growth has an effect on the numbers of muscle bundles, though it never metastasizes. The mechanisms of their infiltrative growth are not fully understood, although it has been reported a possible role of aberrant high mobility group proteins (HMGs) during the development of lipomatous tumors [1,2]. Excessive endomysial fatty growth is not always induced by a true neoplastic condition, however, it could also develop as a result of muscle damage [3,4]. Previously, little attention has been paid to pathogenic features of muscle fibers around an intramuscular lipoma and the surrounding muscle changes have not been elucidated. In the present study, we focused on pathologic changes of the surrounding skeletal muscle fibers in intramuscular lipomas, especially to the degenerative features of involving muscular types. We evaluate the role of type-selective muscular degeneration for the infiltrative growth of intramuscular lipomas using immunohistological analyses.
Methods
Following a review of the medical records in our institute, surgically and histologically proven intramuscular lipomas were selected, and representative formalin-fixed and paraffin-embedded tissue sections from 22 cases were stained with hematoxylin-eosin (H-E). Two cases of the traumatic muscle damage were also evaluated as the control experiments.
Immunohistochemical analysis
Seventeen lesions containing muscle tissues in their specimens (15 infiltrating lipomas, 2 well-circumscribed lipomas) were used. Characters of lipomas were summarized in Table 1. Consecutive tissue sections were incubated with antibodies against troponin T (NCL-TROPT, 1:20; Novocastra Lab. Ltd., Newcastle, UK), sarcoplasmic or endoplasmic reticulum Ca2+ ATPases (SERCA) 1, (NCL-SERCA1, 1:100; Novocastra), SERCA2 (NCL-SERCA2, 1:100; Novocastra), cathepsin-D (rabbit antiserum, 1:100; DAKO, Glostrup, Denmark), and MAC387 (DSKO-MAC387, 1:100; DAKO), after which they were subjected to staining using a streptavidin-biotin-peroxidase complex method. Prior to troponin T- and MAC387-immunostaning, tissue sections were treated with 0.1% trypsin in 0.2% calcium chloride for 15 minutes at 37°C, following deparaffinization. NCL-TROPT antibody against to troponin T reacts with fast muscle troponin, but not slow muscle troponin T [5]. The SERCA are the ATP dependent calcium pumps in intracellular organelles that are responsible in part for the maintenance of low cytoplasmic free Ca2+ ion concentrations [6]. The SERCA1 gene is exclusively expressed in type II (fast) skeletal muscle, while SERCA2 isoform expressed mainly in type I (slow) skeletal muscle. NCL-SERCA1 and NCL-SERCA2 is useful in experiment measuring the time course of transition from the fast to the slow isoform of the Ca2+ ATPases. Accordingly, type-selective muscle staining was available using these antibodies.
Table 1.
Immunohistochemical analysis of intramuscular lipomas.
| Case | Age (year) | Gender | Location | Histological type | Atrophic muscle fiber* | Cathepsin-D |
| 1 | 57 | M | sternocleidomastoideus | infiltrative | slow-dominant atrophy | + |
| 2 | 65 | M | rectus femolis | infiltrative | slow-dominant atrophy | + |
| 3 | 48 | M | adductor longus | infiltrative | slow-dominant atrophy | + |
| 4 | 43 | M | vastus medialis | infiltrative | slow-dominant atrophy | + |
| 5 | 71 | M | trapezius | infiltrative | slow-dominant atrophy | + + |
| 6 | 64 | F | vastus lateralis | infiltrative | fast-dominant atrophy | + + |
| 7 | 71 | F | tensor fascia lata | infiltrative | fast-dominant atrophy | + |
| 8 | 37 | F | deltoideus | infiltrative | fast-dominant atrophy | + |
| 9 | 47 | F | latissimus dorsi | infiltrative | fast-dominant atrophy | + + |
| 10 | 66 | F | deltoideus | infiltrative | fast-dominant atrophy | + + |
| 11 | 48 | F | vastus medialis | infiltrative | non-dominant atrophy | + + |
| 12 | 59 | F | brachioradialis | infiltrative | non-dominant atrophy | + |
| 13 | 51 | F | vastus medialis | infiltrative | non-dominant atrophy | + |
| 14 | 43 | F | longissimus thoracics | infiltrative | non-dominant atrophy | + + |
| 15 | 49 | M | sartorius | infiltrative | non-dominant atrophy | + + |
| 16 | 15 | F | rectus femolis | well-circumscribed | slow-dominant atrophy | + |
| 17 | 54 | F | levator scapulae | well-circumscribed | non-dominant atrophy | + |
| 18 | 3 | M | paravertebral muscle | well-circumscribed | - | - |
| 19 | 57 | F | sartorius | well-circumscribed | - | - |
| 20 | 43 | F | rhomboideus | well-circumscribed | - | - |
| 21 | 21 | F | hypotener | well-circumscribed | - | - |
| 22 | 51 | M | rectus femolis | well-circumscribed | - | - |
*: Immunohistochemical evaluation using troponin T and SERCA -: Impossible specimens to evaluate immunohistochemically because of no muscle tissue +: Involving muscle tissues are positive to cathepsin-D. ++: Muscle tissues are stained with cathepsin-D around small fatty vacuoles or nuclear chain.
Ultramicroscopic analysis
The tumor from the most recent case, case 11 (Table 1), was also subjected to ultrastructural analysis. The specimen was fixed in 2.5% glutaraldehyde, post-fixed in 1% OsO4, and embedded in Epon 812. The sections were stained with uranyl acetate and lead citrate, and then examined with a JEM-1200EX II electron microscope (JEOL Ltd. Tokyo, Japan).
Results
No atypical feature was detected in any tumor cells of the specimens. Tumor tissues showed infiltration in 15 cases and lipocytes were admixed with skeletal muscle fibers in various ratios. In 2 out of the other 7 cases, the tumors were composed entirely of lipocytes and skeletal muscle tissue was seen in the surrounding area. Macroscopically, all of these 7 cases were well demarcated from their surrounding tissues. Accordingly, the former group of 15 tumors was classified as the infiltrative type, and the latter 7 as the well-circumscribed type, according to the classification of Fletcher and Martin-Bates [7]. In the infiltrative type, fatty cells had grown in the endomysial space, however, some of the muscle fibers in the tumor were spared, though they showed extensive damage. Various degrees of degenerative changes including sarcoplasmic mass and subsarcolemmal vacuolar changes were found in the muscle fibers, though focal necrosis or opaque fibers were not detected. A saw-tooth like appearance due to compression by the fatty cells was also detected. Further, markedly thin atrophic or involutional muscle fibers were seen, however, they were not always grouped, while split fibers were shown in some cases. Nuclear swelling, central nuclei, or nuclear chains were detected in the atrophic and/or degenerative fibers (Fig. 1).
Figure 1.
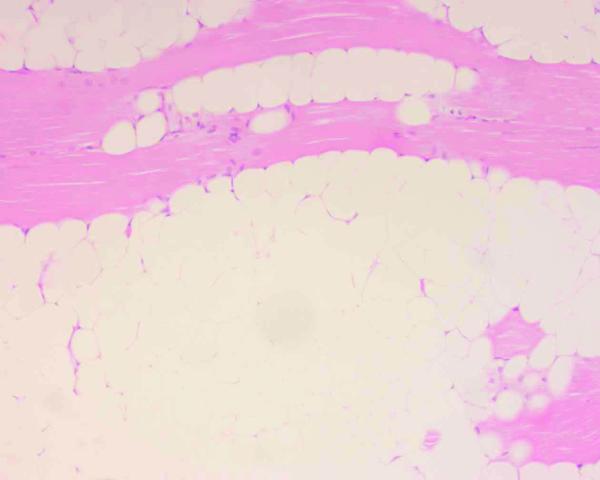
Intramuscular lipoma specimen. Various degrees of muscle atrophy, as well as pyknotic nuclei or nuclear swelling, shown in the specimen (hematoxylin-eosin stain, ×200).
Immunohistochemical analyses using SERCA and troponin T revealed type-selective muscle fiber atrophy or involution in 11 of 17 cases. Of these, 5 lesions had type II (fast) dominant atrophy and type I (slow) dominant was revealed in 6 cases, while in the remaining 6 cases, type-selective change was not found (Table 1). Type-selective atrophy was not confined to the areas with fatty cell growth, but was also found in peripheral areas where fatty cell growth was not prominent (Figs. 2A,2B,2C). Further, cathepsin-D was found in muscle fibers undergoing degenerative change and immunoreactivity for this lysosomal enzyme was diffusely distributed in some fibers, while focal subsarcolemmal staining was revealed in others. Subsarcolemmal vacuoles in the muscle fibers of the peripheral areas were positive for cathepsin-D (Fig. 3), while degenerative findings were not visually apparent in these areas. More severely degenerative findings like segmental necrosis or opaque fibers were not microscopically demonstrated. Monocytes/macrophages positive for Mac 387 were detected in 12 cases, though they were located in the perivascular spaces and myophagia change was not observed. Neither positive staining for cathepsin-D nor type-selective atrophy was detected in the sections of traumatic muscle damage (Figs. 4A,4B,4C).
Figure 2.
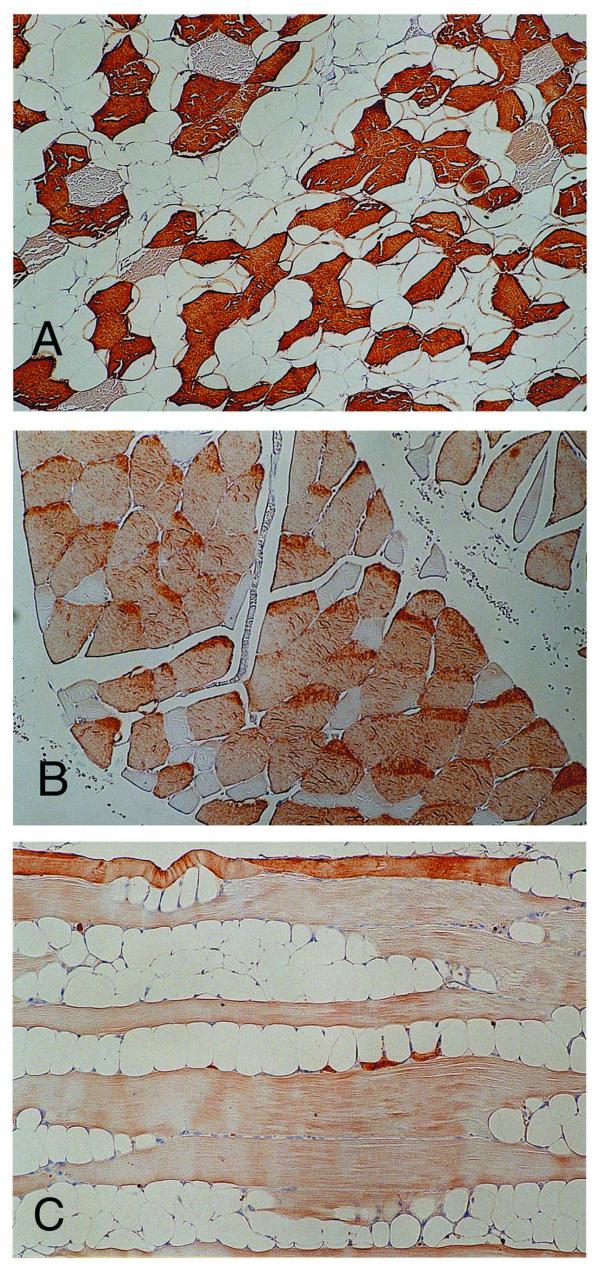
Immunohistochemical study for the involving muscle fibers. (A) SERCA1-positive fast muscle tissues are dominantly preserved, with the other type prominently decreased (×125). (B) Slow muscle fiber tissues reacting to SERCA2 are conserved, and SERCA2-negative fibers are smaller and angulated (×125). (C) Fast muscle tissues stained by troponin T are shown dominantly atrophic and replaced with fatty tissues (×125).
Figure 3.
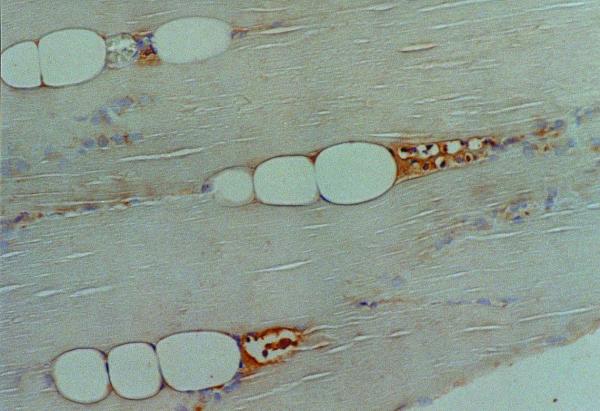
Cathepsin-D immunohistochemical study. Muscle regions developed sequentially from small subsarcolemmal vacuoles are positive for cathepsin-D (×250).
Figure 4.
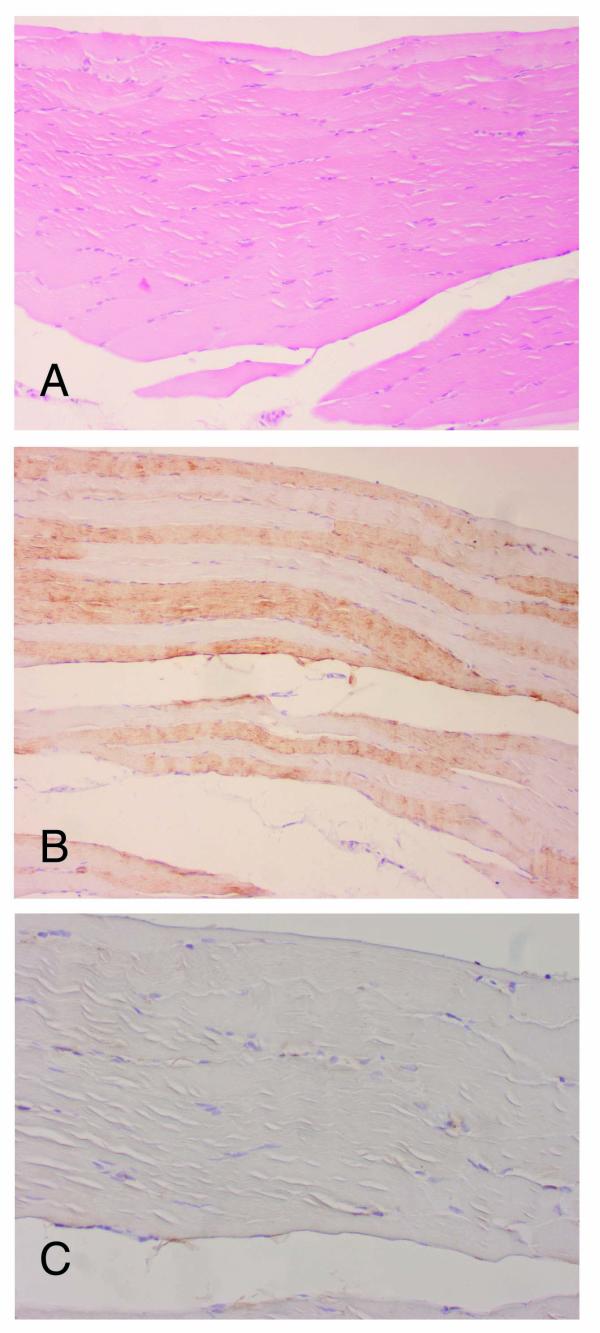
Histological analyses for the traumatic muscle damage. (A) No atypical finding was demonstrated in the specimen (hematoxylin-eosin stain, ×100). In the immunohistochemical study, neither type-selective atrophy in SERCA1 (×100) (B) nor positive staining for cathepsin-D (×200) (C) was detected in the specimens.
The ultrastructural analysis revealed endomysial fatty cell growth and an increase of mononuclear cells with small vacuoles in the endomysial portions. Mitochondria were occasionally accumulated in subsarcolemmal protein and lysosomes increased in the sarcoplasm (Fig. 5A), and their distribution was consistent with the immunoreactivity to anti-cathepsin-D. Further, Z-Disc loss and an increase of mitochondria were observed in some of the degenerative fibers, with glycogen abundantly accumulated (Fig. 5B) and satellite cells increased in these muscle fibers. However, focal contraction or necrosis was not observed. Unfortunately, nerve lesions were not evaluated, because there were no terminal motor nerve fibers or neuromuscular junctions included in the specimens.
Figure 5.
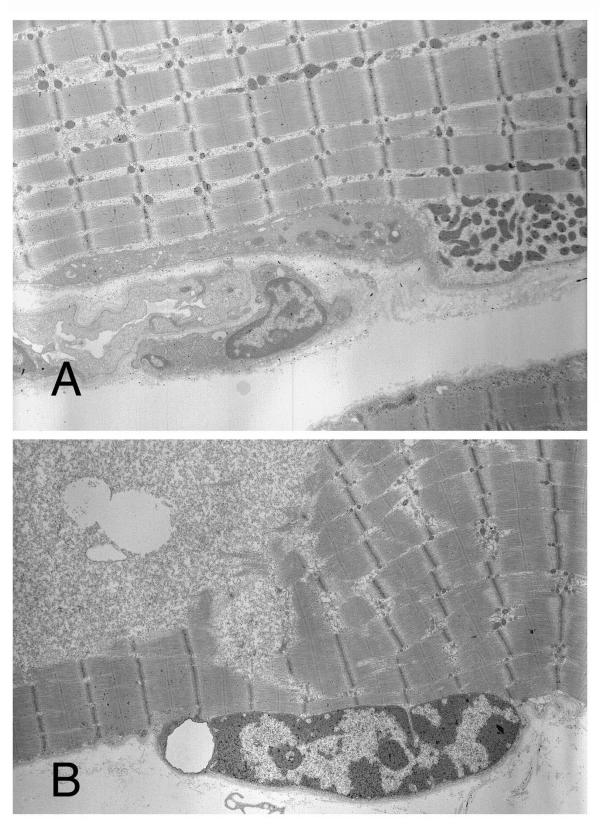
Ultrastructural study. (A) Mitochondria are shown increased in the sarcoplasm (×4000). (B) Glycogen is abundantly accumulated in degenerated muscle fibers (×4000).
Discussion
A lipoma is the most common type of soft tissue tumor and solitary lipomas have been classified into two types; cutaneous/superficial and deep-seated/subfascial. In spite of some descriptions about superficial lipomas [8], little is known about the etiology or histogenesis of intramuscular lipomas, which are the deep-seated type. Intramuscular and intermuscular lipomas are relatively rare as compared to superficial lipomas, and have been reported to comprise 1.8% and 0.3% of all fatty tumors, respectively [9]. Microscopically, an intramuscular lipoma is composed of mature lipocytes, the same as lipomas found in other areas. Macroscopically, intramuscular lipomas are not always as well circumscribed as the superficial type and such cases have been designated as infiltrating lipomas. Fletcher and Martin-Bates noted that an intramuscular lipoma could also be subdivided into infiltrative and well-circumscribed types [7], however, the factors contributing to these variations are not well understood.
Fat cell overgrowth due to muscular damage is not uncommon and has been reported in several orthopedic diseases such as muscular dystrophy [10] and rheumatoid arthritis [11], as well as other joint diseases and various neuropathies [3]. The present study was performed to investigate the association between lipomatous cell growth and surrounding muscular degeneration in intramuscular lipomas, and evaluate the contribution of focal muscular atrophy to the infiltrating growth of these tumors.
Interestingly, type-selective muscle fiber atrophy or degenerative change was found in 70% of the present tumors (11/17 cases), most of which (10/11 case) were the infiltrating type. Type-selective muscle involvement in these 11 cases strongly suggests that they have had any neurogenic or myogenic disorders, [4] at the focal lesions, and endomysial fat overgrowth could be explained as a result of muscle involution. Type-selectivity was not the same in these 11 cases and the fundamental disease mechanism seems to be heterogeneous. Further, increases of immunoreactivity to cathepsin-D, a lysosomal catabolic enzyme, was corresponded to the degree of degenerative or atrophic changes in these specimens, and ultrastructural analysis also revealed degeneration in the surrounding muscle fibers of the lesion.
The main factor determining the differences in muscle pathology between the various disorders is the time course, and chronic or longstanding disorders offer more opportunity various histological aspects than those that are rapidly progressive. The relatively big size and the longstanding of the intramuscular lipomas might be promoting disuse atrophy in the surrounding muscle fibers, and rarely muscle type-selective atrophy. An intramuscular lipoma itself may cause the surrounding muscle atrophy due to compressive growth, but such a case of muscle atrophy will often likely be presented without muscle types-selectivity. Neither cathepsin-D reactivity nor muscle types-selectivity was found in the control sections of our study, i.e., traumatic muscles. Our immunohistochemical analyses revealed type-selective muscular atrophy in a majority of the present intramuscular lipomas, suggesting any association of focally neurogenic or myogenic disorders in the lesions. Ultrastructural analysis revealed degenerative findings of the surrounding muscle, but could not demonstrate any specific findings involved with neurogenic or myogenic disorders. Patterns in the involved muscle fibers in the intramuscular lipomas could be divided into different groups and it was presumable that pathogenic mechanisms of the involved muscular degeneration were heterogeneous. As a results of these focally muscular atrophy, the endomysial fatty over growth in the involving muscles could modulate the infiltrating growth of an intramuscular lipoma into the surrounding muscular tissues. In the present study, surgically and histologically proven intramuscular lipomas were analyzed, and only one case was available to evaluate ultrastructurally. Unfortunately, neuromuscular junction or nerve bundles were not included in the ultrastructural specimens, and the involvement of neurogenic factors with the pathogenesis could not be estimated. In a future report, the primary cause of focal muscle involution should be made clearly.
Conclusions
The findings of the present study suggest that type-selective muscular degeneration and endomysial fatty growth as a result of focal atrophy may modulate the infiltrating growth characteristic of intramuscular lipoma.
Competing interests
No author has any other financial or non-financial interests, and declare them to this article.
Authors' contributions
Mori K and Chano T carried out histological analyses and preparation of the manuscript. Matsumoto K participated in its design and sample correction. Ishizawa M and Matsusue Y participated in sample correction. Okabe H participated in its design and coordination. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The authors thank Sawako Hirayama and Ai Fujisawa for their assistance with the histological experiments.
Contributor Information
Kanji Mori, Email: kanchi@belle.shiga-med.ac.jp.
Tokuhiro Chano, Email: chano@belle.shiga-med.ac.jp.
Keiji Matsumoto, Email: f-matsu8@f7.dion.ne.jp.
Michihito Ishizawa, Email: ishizawa47@msn.com.
Yoshitaka Matsusue, Email: matsusue@belle.shiga-med.ac.jp.
Hidetoshi Okabe, Email: okabe@belle.shiga-med.ac.jp.
References
- Tallini G, Dal Cin P, Rhoden KJ, Chiapetta G, Manfioletti G, Giancotti V, Fusco A, Van den Berghe H, Sciot R. Expression of HMGI-C and HMGI (Y) in ordinary lipoma and atypical lipomatous tumors: immunohistochemical reactivity correlates with karyotypic alterations. Am J Pathol. 1997;151:37–43. [PMC free article] [PubMed] [Google Scholar]
- Tkachenko A, Ashar HR, Meloni AM, Sandberg AA, Chada KK. Misexpression of disrupted HMGI architectural factors activates alternative pathways of tumorigenesis. Cancer Res. 1997;57:2276–2280. [PubMed] [Google Scholar]
- Cullen MJ, Mastaglia FL. Pathological reactions of skeletal muscle. In: Mastaglia FL, Walton SJ, editor. In Skeletal muscle pathology. New York: Churchill Livingstone; 1982. pp. 88–139. [Google Scholar]
- Dubowitz V. Definition of pathological changes seen in muscle biopsies. In: Dubowitz V, editor. In Muscle biopsy-a practical approach. England: Bailliere Tindall; 1985. pp. 82–128. [Google Scholar]
- Bird IM, Dhoot GK, Wilkinson JM. Identification of multiple variants of fast muscle troponin T in the chicken using monoclonal antibodies. Eur J Biochem. 1985;150:517–525. doi: 10.1111/j.1432-1033.1985.tb09052.x. [DOI] [PubMed] [Google Scholar]
- Molnar E, Seidler NW, Jona I, Martonosi AN. The binding of monoclonal and polyclonal antibodies to the Ca2+-ATPase of sacroplasmic reticulum: effects on interactions between ATPase molecules. Biochimica et Biophysica Acta. 1990;1023:147–167. doi: 10.1016/0005-2736(90)90410-P. [DOI] [PubMed] [Google Scholar]
- Fletcher CDM, Martin-Bates E. Intramuscular and intermuscular lipoma: neglected diagnosis. Histopathology. 1988;12:275–287. doi: 10.1111/j.1365-2559.1988.tb01942.x. [DOI] [PubMed] [Google Scholar]
- Enzinger FM, Weiss SW. Benign lipomatous tumors. In: Enzinger FM, Weiss SW, editor. In Soft Tissue Tumors. 4. St Louis: Mosby; 2001. pp. 571–639. [Google Scholar]
- Fletcher CDM, Akerman M, Dal Cin P, de Wever I, Mandahl N, Mitelman F, Rosai J, Rydholm A, Sciot R, Tallini G, van den Berghe H, van de Ven W, Vanni R, Willen H. Correlation between clinicopathological features and karyotype in lipomatous tumors. A report of 178 cases from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. Am J Pathol. 1996;148:623–630. [PMC free article] [PubMed] [Google Scholar]
- Emery EH. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- Touno M, Senda M, Nakago K, Yokoyama Y, Inoue H. Muscle fiber changes of the vastus medialis in rheumatoid patients. Acta Med Okayama. 1996;50:157–164. doi: 10.18926/AMO/30503. [DOI] [PubMed] [Google Scholar]


