Abstract
BACKGROUND
Androgens control homeostasis of the normal prostate and growth of prostate cancer (PCa) through the androgen receptor (AR) by regulating gene networks involving in cell proliferation, differentiation, and survival. We demonstrated previously that expression of Skp2, a key protein regulating cell entry into the S phase, is inhibited by androgens in an AR-dependent manner (Oncogene, 23(12): 2161–76, 2004). However, the underlying mechanism of this regulation is unknown.
METHODS
Using the LNCaP PCa cell line as a working model, the effect of androgens on the expression of Skp2 was examined by Western and Northern blot analyses. Cell cycle was measured by fluorescence-activated cell sorting (FACS). Gene transfection was performed by electroporation to manipulate the expression levels of proteins studied.
RESULTS
At physiological levels androgens markedly repressed Skp2 expression but slightly induced Skp2 expression at subphysiological levels. Androgens modestly decreased the stability of the Skp2 protein. Androgenic repression of Skp2 expression was completely abolished by E1A-mediated inactivation of pocket proteins including RB, p130 and p107. Moreover, ectopic expression of p107 inhibited Skp2 expression, and silencing of p107 partially blocked androgenic repression of Skp2.
CONCLUSIONS
Our data indicate that androgens repress Skp2 expression via p107-dependent and -independent pathways in PCa cells. These regulatory mechanisms may be targeted for the development of new therapeutics of androgen-refractory PCa.
INTRODUCTION
PCa is the most commonly diagnosed malignancy and the second leading cause of cancer death in men in the United States. PCa cells depend on androgens for proliferation and survival (1–3). Androgen deprivation therapy (ADT) by surgical castration, administration of luteinizing hormone-releasing hormone (LHRH) agonists or blockade of the AR by antiandrogens is the standard systemic treatment for advanced/metastatic PCa (4). Although the majority of patients exhibit clinical improvement, most patients invariably relapse with a more aggressive form of disease that has been termed castration-resistant, androgen-refractory or androgen-independent PCa. It is believed that while androgen deprivation blocks the major signaling pathways that drive PCa cell growth and survival, androgen withdrawal may also result in an increase in expression of a set of genes favoring PCa progression. Indeed, a number of oncogenic proteins, including Bcl-2, E2F1, EZH2 and c-Met, are overexpressed in castration-resistant PCa after ADT (3,5–10).
The F-box protein Skp2 is another oncoprotein that is overexpressed in androgen-independent PCa (11). Skp2 was originally cloned as a gene that is highly expressed in many cancer cell lines (12). It primarily functions as an ubiquitin E3 ligase by forming a Skp1/Culin1/F-box protein Skp2 (SCF) complex. A number of tumor suppressor proteins, including p27KIP1, p130, p57KIP2 and FOXO1, are targeted and degraded by the SCFSkp2 complex and the proteasome pathway (13–16). Expression of Skp2 protein is elevated in prostate cancers and correlated with prostate cancer progression (17,18). Transgenic expression of Skp2 induces hyperplasia, dysplasia and low-grade carcinoma in the mouse prostate (19). Moreover, knockout of Skp2 in PTEN-negative tumors significantly reduces prostate tumor mass (20). Thus, findings from both human and mouse PCa studies suggest that Skp2 is a PCa relevant gene.
Previous studies from our laboratories and others have demonstrated that androgens repress expression of Skp2 protein in an AR-dependent manner in PC-3 and LNCaP PCa cell lines, although the underlying mechanism is unclear (3,21). In the present study, we demonstrate that androgens at physiological concentrations modestly decrease the stability of Skp2 protein, but markedly repress Skp2 expression at the mRNA level. We provide further evidence that androgenic repression of Skp2 expression is mediated through p107-dependent and-independent pathways.
MATERIALS AND METHODS
Cell lines and cell culture
LNCaP cells were purchased from American Type Culture Collection and cultured in RPMI 1640 containing 10% fetal bovine serum (FBS), 100 μg/ml streptomycin, 100 units/ml penicillin, and 0.25 μg/ml amphotericin B. For androgen dose and time course studies, cells were plated and the next day the media was changed to medium with 10% CSS. After 24 h, a synthetic androgen, methyltrieolone (R1881), antiandrogen bicalutamide (Zeneca Pharmaceuticals) or EtOH was added. For protein and RNA synthesis inhibition, LNCaP cells were treated with cycloheximide (CHX; 20 μg/ml) and actinomycin D (4 μM), respectively.
Plasmids and antibodies
Expression vectors for wild-type adenoviral E1A (12S) and the mutant RG2 were kindly provided by Dr. R. F. Kelm (22). Expression vectors for p107 and p130 were gifts from Dr. B. Dynlacht. Antibodies against Skp2 (H-435), p27KIP1 (F-8), AR (N-20), Erk2 (D-2), p107 (C-18), E2F1 (C-20) were purchased from Santa Cruz Biotechnology. The antibody against PSA (M0750) was obtained from DakoCytomation.
Cell transfection
LNCaP cells were transfected with plasmid DNA by electroporation. Cells were mixed with DNA and electroporated using a BTX T830 spare wave electroporator. SMARTpools of siRNA specific for p107 and nonspecific control siRNA were purchased from Dharmacon. Cells were transfected with 200 nM of the siRNA as described above. Cells were harvested 48–72 h after transfection. Approximately 75–90% transfection efficiencies were routinely achieved (23).
Western blot analysis
Protein samples were prepared by lysing cells in modified RIPA buffer (1x PBS, 1% NP-40, 0.1% SDS, and protease inhibitor cocktail (Sigma-Aldrich)). Lysates (50–100 μg) were separated on SDS-PAGE gels and transferred to a nitrocellulose membrane. The membrane was probed with the specific primary antibody and HRP-conjugated secondary antibody and then visualized by chemiluminescence.
Northern blot analysis
Total cellular RNA (10~15 μg) was applied to and run on 1.2% denaturing formaldehyde-agarose gels and transferred onto positively charged nylon membranes. Filters were hybridized first with [32P]dCTP-labeled full-length cDNA for human Skp2 gene and, after stripping, re-hybridized with [32P]dCTP-labeled GAPDH as a loading control.
Fluorescence-activated cell sorting (FACS) analysis of the cell cycle
LNCaP cells treated with different doses of R1881 were collected and fixed in freshly prepared ice-cold 70% ethanol for 30 min. After washing with 1× PBS, the cells were stained with 10 μg/ml propidium iodide and 50 μg/ml RNase A. Cells (1 × 106) were incubated for 30 min. Cell cycle distributions were determined by flow cytometry analysis using a FACSCalibur flow cytometer (Becton Dickinson). Cell population at the S phase of the cell cycle was determined by ModFit II software.
Cell growth assay
Cell growth was monitored by absorbance using the MTS assay according to manufacturer’s instructions (Promega). Briefly, LNCaP cells were plated in charcoal-stripped medium in 96-well plates at a density of 5,000 cells per well. At 48 h after plating, cells were treated with different concentrations of R1881 for 48 h. 20 μl of CellTiter 96R AQueous Solution Reagent (Promega) was added to cells and incubated at 37 C in the cell incubator. Cell growth was measured in a microplate reader at 490 nm.
RESULTS
Biphasic regulation of Skp2 by androgens
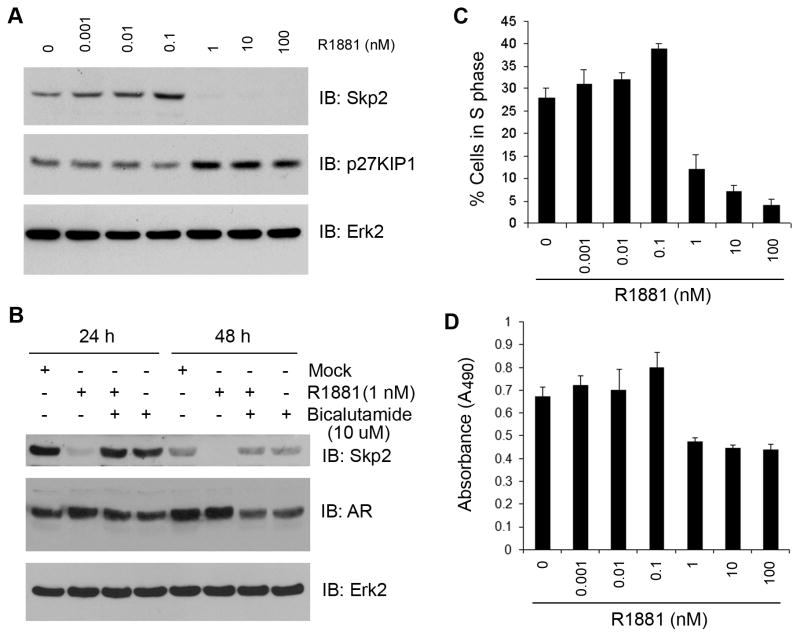
To explore the molecular mechanism underlying androgen regulation of Skp2 in PCa cells, we treated LNCaP cells with different doses of R1881, a synthetic androgen. As demonstrated in Fig. 1A, physiological or higher concentrations of R1881 completely inhibited expression of Skp2 in LNCaP cells. In contrast, treatment of LNCaP cells with sub-physiological concentrations (0.001~0.1 nM) of R1881 resulted in a modest increase in Skp2 protein (Fig. 1A), which is consistent with a recent report (24). In agreement with the finding that p27KIP1 is a well-studied ubiquitin degradation target of Skp2 (13), the levels of p27KIP1 proteins were inversely correlated with Skp2 levels in LNCaP cells treated with either low or high doses of R1881 (Fig. 1A). Consistent with our previous report that androgens repress Skp2 expression in a manner dependent on the AR (3), androgen-induced inhibition of Skp2 was completely blocked by treatment of LNCaP cells with antiandrogen bicalutamide (Fig. 1B). The effectiveness of bicalutamide was evident by the decreased expression of AR proteins in these cells (Fig. 1B). Skp2 plays an important role in the entry of cells into the S phase of the cell cycle. In correlation with the levels of Skp2 protein, the percentage of S-phase cells slightly increased in cells treated with low doses of R1881; whereas treatment of cells with high doses of R1881 markedly decreased the percentage of cells in S phase (Fig. 1C). In agreement with this observation, low doses of R1881 (e.g. 0.1 nM versus mock treatment, P < 0.001, Student’s t-test) stimulated LNCaP cell growth whereas high doses of R1881 (1 nM or higher) inhibited cell growth (Fig. 1D). These data indicate that expression of Skp2 protein is biphasically regulated by androgens in LNCaP PCa cells.
Fig. 1.
Effect of androgen treatment on Skp2 expression and cell proliferation. A: LNCaP cells were grown in 10% CSS for 24 h and then incubated for an additional 48 h with the given concentrations of R1881. Western blots were performed with antibodies to Skp2, p27KIP1 and Erk2 (loading control). B: LNCaP cells were grown in 10% CSS for 24 h and then either treated with vehicle (mock) or R1881 (1 nM) in the presence or absence of bicalutamide (10 μM). At indicated time points, cells were harvested and lysed for western blot analysis with antibodies indicated. C: LNCaP cells were treated as in (A) and harvested for FACS analysis. The percentages of cells in S phase shown are the average of three independent experiments. D: Growth of LNCaP cells treated with different concentrations of R1881 was measured by the MTS assay as described in Materials and Methods. Data are means + s.d. from experiments with five replicates (n = 5).
The protein stability of Skp2 is modestly affected by androgens in LNCaP cells
Given that expression of Skp2 is drastically inhibited by physiological or higher concentrations of androgens (Fig. 1A), we focused our efforts on understanding the molecular basis of androgenic repression of Skp2. After treatment with 5 nM of R1881 for 48 h, LNCaP cells were treated with the de novo protein synthesis inhibitor cycloheximide (CHX) and protein levels of Skp2 were measured by Western blots at different time points. Consistent with the data shown in Fig. 1A, the overall levels of Skp2 protein were much lower in androgen-treated than untreated cells (Fig. 2A). In contrast, the levels of p27KIP1, the degradation target of Skp2 was higher in androgen-treated than untreated cells (Fig. 2A). Quantitative analysis indicated that the stability of Skp2 slightly increased following androgen treatment (Fig. 2B). Thus, these data suggest that androgens have a small, but slight effect on Skp2 protein stability in LNCaP cells.
Fig. 2.
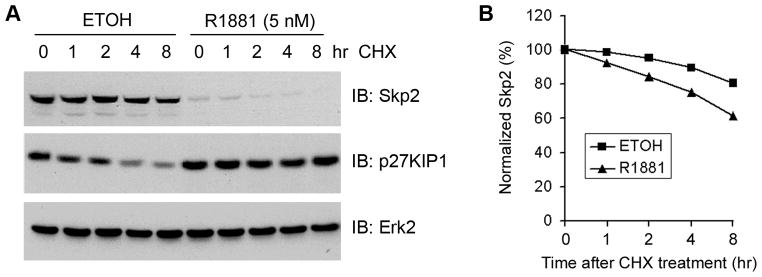
Effect of androgen treatment on Skp2 protein stability. A: LNCaP cells were treated with 5 nM of R1881 for 48 h and then treated with cycloheximide (20 μg/ml). At the time points indicated cells were harvested and lysed for Western blot analysis with antibodies as indicated. B: Quantification of the Skp2 protein signal intensity was obtained from exposures in which the signal was not saturated during the entire time course. Signal intensities were normalized to the signal intensity obtained at the time zero.
Expression of Skp2 is suppressed at the transcriptional level by high doses of androgens
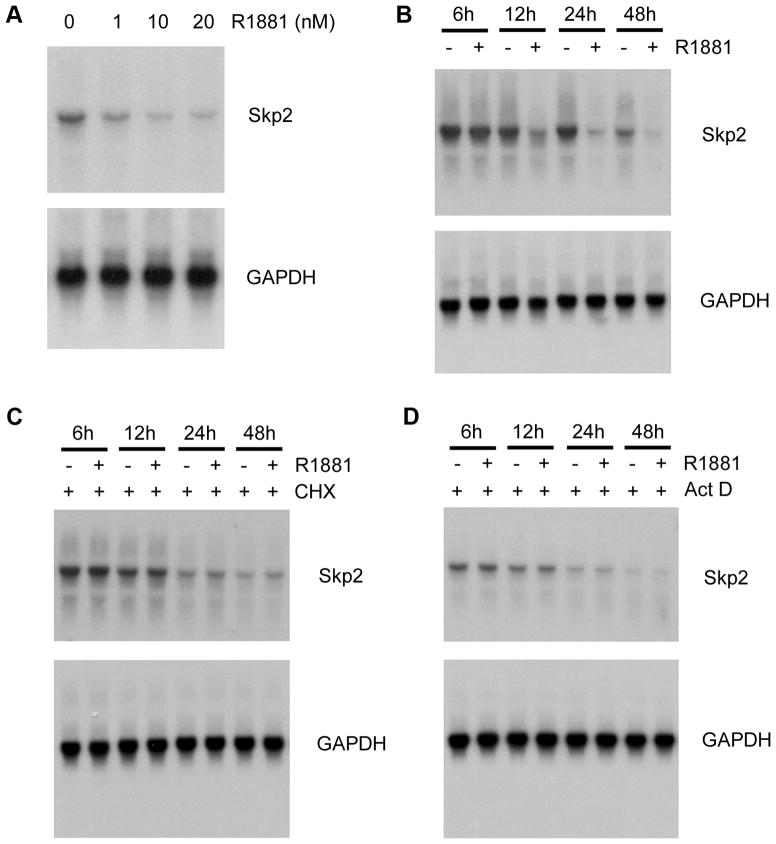
As demonstrated by Northern blot analysis, treatment of LNCaP cells with 1 nM or higher concentrations of R1881 inhibited expression of Skp2 mRNA in a dose-dependent manner (Fig. 3A). Time-course studies demonstrated that expression of Skp2 mRNA begins to decrease at 12 h after androgen treatment (Fig. 3B), suggesting that androgenic regulation of Skp2 expression could be mediated by an indirect mechanism. To further test this hypothesis, LNCaP cells were pretreated with CHX for 30 min and then treated with or without 5 nM of R1881. As demonstrated in Fig. 3C, pretreatment of cells with CHX completely abrogated androgen-induced inhibition of Skp2 expression, indicating that androgenic regulation of Skp2 requires new protein synthesis. Next, we sought to determine whether androgen-induced downregulation of Skp2 is due to a decrease in the rate of Skp2 mRNA synthesis or increased stability. LNCaP cells were pretreated with the mRNA synthesis inhibitor actinomycin D (Act D) 30 min before androgen treatment. As demonstrated in Fig. 3D, Act D treatment completely abolished androgen-induced inhibition of Skp2 expression. Together, these data suggest that androgens repress Skp2 expression at the transcription level.
Fig. 3.
Effect of androgen treatment on Skp2 mRNA expression. A: LNCaP cells were treated with different doses of R1881 as indicated for 48 h. Total RNA (15 μg) was applied for Northern blot analysis and hybridized with Skp2 and GAPDH cDNAs as probes. B: Time-course study on the androgenic effect on Skp2 mRNA expression. LNCaP Cells were treated with 5 nM of R1881 for varying lengths of time, from 6–48 h, and Skp2 mRNA expression was examined by Northern blot analysis. C: Skp2 repression by androgens is blocked by the protein synthesis inhibition. LNCaP cells were pretreated with CHX (20 μg/ml) for 30 min and then treated with or without 5 nM of R1881. At the time points indicated, cells were harvested and RNAs were isolated and subject to Northern blot analysis. D: Effect of ActD on Skp2 repression by androgens. LNCaP cells were pretreated with 4 μM ActD for 30 min and then treated with or without 5 nM of R1881. At the time points indicated, cells were harvested and RNAs were isolated and subject to Northern blot analysis. GAPDH cDNA was used as a control for the normalization of RNA loaded in these experiments.
Inactivation of pocket proteins by the adenoviral protein E1A blocks androgenic repression of Skp2 expression
A previous study suggested that the Skp2 gene promoter contains a functional E2F response element and that ectopic expression of E2F1 induces expression of the endogenous Skp2 gene in human fibroblasts (25). We demonstrate previously that androgens at physiological concentrations inhibit expression of E2F1 (3). We reasoned that androgens may repress Skp2 expression by downregulating E2F1. To test this hypothesis, we first determined whether Skp2 is regulated by E2F1 in PCa cells. LNCaP cells were transfected with E2F1, and levels of Skp2 were examined by Western blots. As demonstrated in Fig. 4A, transfection of E2F1 resulted in a marked increase in the level of E2F1 protein. However, a modest increase in the level of Skp2 protein was detected in E2F1-transfected cells (Fig. 4A), suggesting that E2F1 and its cognate inhibitor RB may not be the major regulators of Skp2 expression in LNCaP cells. To further verify this observation, we transfected LNCaP cells with wild-type and mutated adenoviral protein E1A (RG2), which interacts with and inactivates all the pocket proteins including RB, p107 and p130 (3,22). As expected, androgen treatment of mock-transfected LNCaP cells decreased expression of Skp2 (Fig. 4B). Expression of prostate-specific antigen (PSA), included as a positive control, increased in response to androgen treatment (Fig. 4B). Surprisingly, androgen-induced inhibition of Skp2 expression, but not PSA induction, was completely blocked by transfection of both wild-type and mutated E1A (Fig. 4B). Together, these data suggest that androgens may repress Skp2 expression via pocket proteins other than RB.
Fig. 4.
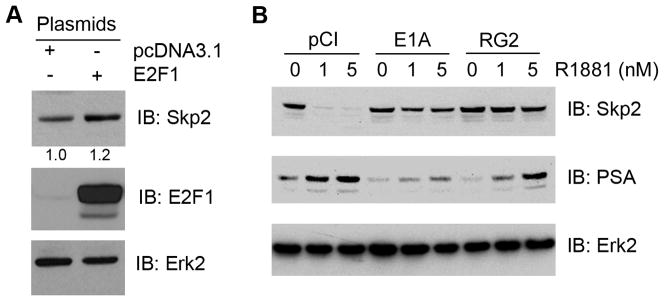
Potential role of E2F1 and pocket proteins in regulation of Skp2 expression. A: Effect of E2F1 overexpression on Skp2 expression in PCa cells. LNCaP cells were transfected with the empty vector pcDNA3.1 or E2F1. At 36 h after transfection, cells were harvested and lysed for Western blot analysis antibodies as indicated. B: Potential role of pocket proteins in androgenic repression of Skp2 expression in PCa cells. LNCaP cells were transfected the empty vector pCI, wild-type and mutated E1A (RG2). At 24 h after transfection, the media was changed to CSS and then R1881 or vehicle was added. After 48 h cells were harvested and protein expression of Skp2, E1A, PSA and Erk2 (loading control) was determined by Western blot.
Androgenic repression of Skp2 expression is partially blocked by p107 silencing
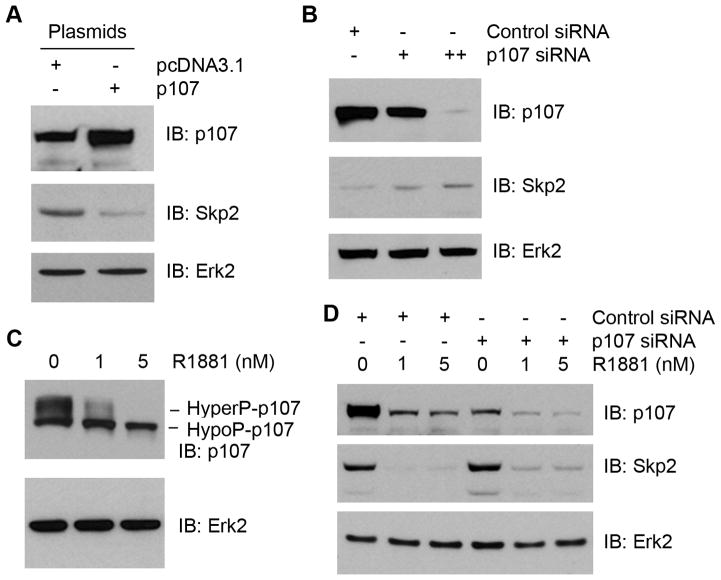
Given that E2F1 overexpression resulted in a minimal increase in Skp2 expression (Fig 4A), we next focused our efforts on the regulation of Skp2 by the pocket proteins p130 and p107 rather than RB. LNCaP cells were transfected with expression vectors for p130 and p107, and the effect of overexpression of p130 or p107 on Skp2 levels was examined. Ectopic expression of p130 failed to inhibit Skp2 expression in LNCaP cells (data not shown), suggesting that p130 may not be involved in the Skp2 regulation in these cells. In contrast, forced expression of p107 decreased the level of Skp2 protein in LNCaP cells (Fig. 5A). Moreover, small interference RNA (siRNA)-mediated silencing of p107 resulted in an increase in Skp2 expression in a dose-dependent manner (Fig. 5B). By monitoring the band shift of p107 protein, we demonstrated that androgen treatment resulted in a shift of p107 protein from the hyperphosphorylated state to the hypophosphorylated state (Fig. 5C), suggesting that androgen treatment activates p107 by decreasing its phosphorylation. Finally, we determined whether knockdown of p107 blocks androgen-induced repression of Skp2. As demonstrated in Fig. 5D, endogenous p107 was effectively knocked down by p107-specific siRNA. As expected, androgen treatment resulted in a dramatic decrease in the level of Skp2 protein in LNCaP cells treated with control siRNA (Fig. 5D). Importantly, androgen-induced repression of Skp2 was partially abrogated by p107 knockdown (Fig. 5D). It is worth noting that androgen treatment resulted in a decrease in the density of p107 protein bands in both control and p107 siRNA-treated cells (Fig. 5D, top panel). This phenomenon is most likely due to androgenic induction of p107 phosphorylation (data not shown). Thus, these data indicate that androgen-induced repression of Skp2 is mediated by the signaling pathways both dependent on and independent of p107 in LNCaP PCa cells.
Fig. 5.
Role of RB related protein p107 in androgen regulation of Skp2. A: Effect of p107 overexpression on Skp2 expression in PCa cells. LNCaP cells were transfected with the given plasmids and harvested after 48 h. Protein levels for Skp2, p107 and Erk2 (loading control) were analyzed by Western blot. B: Effect of p107 knockdown on Skp2 expression in PCa cells. LNCaP cells were treated with the indicated siRNA pools. At 48 h after transfection, protein levels for Skp2, p107 and Erk2 (loading control) were analyzed by Western blot. C: Androgen regulation of p107 phosphorylation. LNCaP cells were treated with different doses of R1881 as indicated. At 48 h after treatment cells were harvested and lysed. Proteins were separated by 6% SDS-PAGE under reducing conditions and immunoblotted with anti-p107 antibody. D: Effect of p107 knockdown on androgen repression of Skp2 expression in PCa cells. LNCaP cells were treated with the indicated siRNA pools. At 36 h after transfection R1881 or vehicle was added and incubated an additional 36 h. Western blots were performed to analyze protein expression of Skp2, p107 and Erk2 (loading control).
DISCUSSION
Previous studies have shown that overexpression of Skp2 correlates with malignancy in the prostate (17,18). This is supported by mouse studies where transgenic expression of Skp2 induces dysplasia and low-grade carcinoma in the mouse prostate (19). In contrast, deletion of both alleles of Skp2 significantly reduces prostate tumor mass induced by deletion of the PTEN tumor suppressor gene (20). Importantly, overexpression of Skp2 is associated with metastasis and androgen-independent progression of PCa (11). We and others have demonstrated that treatment of PCa cells with physiological concentrations of androgens decreases Skp2 expression (3,21). In the present study we demonstrated that expression of Skp2 is regulated by androgens in a biphasic manner. In contrast, androgens at sub-physiological levels (0.001~0.1 nM) slightly increase expression of Skp2 in LNCaP cells, which is consistent with a recent report (24). Although levels of circulating androgens drops following androgen deprivation therapy, residual levels of androgens remain in PCa cells (26). These levels of androgens may therefore favor PCa growth and progression by enhancing the expression of Skp2 in recurrent prostate cancers. Thus, the response of Skp2 expression to androgen manipulation in PCa cells represents an important target for intervention of PCa progression.
While increasing evidence indicates that Skp2 is an androgen regulated gene, the underlying regulatory mechanism is unclear. As demonstrated in fibroblasts, Skp2 is a transcription target of E2F1 (25). However, E2F1 may not play a major role in regulating Skp2 expression in LNCaP cells since our data show that overexpression of E2F1 resulted in a minimal increase in Skp2 expression. Instead, we provide evidence that overexpression of the pocket protein p107 markedly decreases expression of Skp2 protein in LNCaP cells. Moreover, knockdown of p107 by siRNA increases Skp2 expression. Importantly, androgen-induced repression of Skp2 is partially abolished by p107 knockdown. Thus, our data identify both p107-dependent and -independent pathways that are responsible for androgenic repression of Skp2 expression in LNCaP PCa cells.
It has been shown previously that physiological concentrations of androgens increase expression of cyclin-dependent kinase inhibitors including p21WAF1, p27KIP1 and p15INK4B (3,21,27,28) but decrease the activity of cyclin-dependent kinases (CDKs) (21,28). In contrast, subphysiological doses of androgens (e.g. 0.1 nM of dihydrotestosterone, DHT) reduce p27KIP1 expression but enhance CDK activity (29). Consistent with findings that the pocket proteins RB, p130 and p107 are phosphorylated and inactivated by CDKs, phosphorylation of RB and p130 proteins decreases in PCa cells treated with physiological concentrations of androgens, but increases in PCa cells treated with subphysiological concentrations of androgens (3,8,29). Here we provide evidence that phosphorylation of p107 is reduced in LNCaP cells treated with physiological concentrations of androgens. Thus, it can be speculated that high concentrations of androgens induce expression of CDK inhibitors (e.g. p21WAF1, p27KIP1 and p15INK4B). Increased expression of these proteins promotes inactivation of CDKs and decreased phosphorylation and activation of p107, thereby repressing Skp2 expression.
Taken together, our data demonstrate that androgens regulate Skp2 expression primarily at the mRNA level. We identify the pocket protein p107 as an important molecule that mediates androgenic repression of Skp2 in LNCaP PCa cells. Since silencing of p107 only partially reverses the androgenic repression of Skp2, a p107-independent mechanism is likely involved in the androgen-induced inhibition of Skp2. Further studies are therefore warranted to fully understand how Skp2 expression is regulated by androgens in PCa cells. Given the significant role of Skp2 in the development and progression of PCa, understanding of the molecular mechanisms underlying the androgen regulation of Skp2 could provide opportunities for the development of new therapeutics for this disease.
Acknowledgments
We would like to thank R. F. Kelm and B. Dynlacht for plasmids. This work was supported in part by funds from the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, IRT: 0849 to X.Z.), the National Institutes of Health (CA121277, CA125747 and CA091956 to D.J.T., CA130908 and CA134514 to H.H.) and Department of Defense (W81XWH-09-1-622 to H.H.).
References
- 1.Isaacs JT. Antagonistic effect of androgen on prostatic cell death. Prostate. 1984;5:545–557. doi: 10.1002/pros.2990050510. [DOI] [PubMed] [Google Scholar]
- 2.Kyprianou N, Isaacs JT. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology. 1988;122:552–562. doi: 10.1210/endo-122-2-552. [DOI] [PubMed] [Google Scholar]
- 3.Huang H, Zegarra-Moro OL, Benson D, Tindall DJ. Androgens repress Bcl-2 expression via activation of the retinoblastoma (RB) protein in prostate cancer cells. Oncogene. 2004;23:2161–2176. doi: 10.1038/sj.onc.1207326. [DOI] [PubMed] [Google Scholar]
- 4.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 5.McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LW, Hsieh JT, Tu SM, Campbell ML. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992;52:6940–6944. [PubMed] [Google Scholar]
- 6.Agus DB, Cordon-Cardo C, Fox W, Drobnjak M, Koff A, Golde DW, Scher HI. Prostate cancer cell cycle regulators: response to androgen withdrawal and development of androgen independence. J Natl Cancer Inst. 1999;91:1869–1876. doi: 10.1093/jnci/91.21.1869. [DOI] [PubMed] [Google Scholar]
- 7.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 8.Bohrer LR, Chen S, Hallstrom TC, Huang H. Androgens suppress EZH2 expression via retinoblastoma (RB) and p130-dependent pathways: a potential mechanism of androgen-refractory progression of prostate cancer. Endocrinology. 2010;151:5136–5145. doi: 10.1210/en.2010-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Bohrer LR, Rai AN, Pan Y, Gan L, Zhou X, Bagchi A, Simon JA, Huang H. Cyclin-dependent kinases regulate epigenetic gene silencing through phosphorylation of EZH2. Nat Cell Biol. 2010;12:1108–1114. doi: 10.1038/ncb2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verras M, Lee J, Xue H, Li TH, Wang Y, Sun Z. The androgen receptor negatively regulates the expression of c-Met: implications for a novel mechanism of prostate cancer progression. Cancer Res. 2007;67:967–975. doi: 10.1158/0008-5472.CAN-06-3552. [DOI] [PubMed] [Google Scholar]
- 11.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Kobayashi R, Galaktionov K, Beach D. p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell. 1995;82:915–925. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 13.Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 14.Tedesco D, Lukas J, Reed SI. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCF(Skp2) Genes Dev. 2002;16:2946–2957. doi: 10.1101/gad.1011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamura T, Hara T, Kotoshiba S, Yada M, Ishida N, Imaki H, Hatakeyama S, Nakayama K, Nakayama KI. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci USA. 2003;100:10231–10236. doi: 10.1073/pnas.1831009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H, Regan KM, Wang F, Wang D, Smith DI, van Deursen JM, Tindall DJ. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci USA. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang G, Ayala G, De Marzo A, Tian W, Frolov A, Wheeler TM, Thompson TC, Harper JW. Elevated Skp2 protein expression in human prostate cancer: association with loss of the cyclin-dependent kinase inhibitor p27 and PTEN and with reduced recurrence-free survival. Clin Cancer Res. 2002;8:3419–3426. [PubMed] [Google Scholar]
- 18.Ben-Izhak O, Lahav-Baratz S, Meretyk S, Ben-Eliezer S, Sabo E, Dirnfeld M, Cohen S, Ciechanover A. Inverse relationship between Skp2 ubiquitin ligase and the cyclin dependent kinase inhibitor p27Kip1 in prostate cancer. J Urol. 2003;170:241–245. doi: 10.1097/01.ju.0000072113.34524.a7. [DOI] [PubMed] [Google Scholar]
- 19.Shim EH, Johnson L, Noh HL, Kim YJ, Sun H, Zeiss C, Zhang H. Expression of the F-box protein SKP2 induces hyperplasia, dysplasia, and low-grade carcinoma in the mouse prostate. Cancer Res. 2003;63:1583–1588. [PubMed] [Google Scholar]
- 20.Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH, Yang WL, Wang J, Egia A, Nakayama KI, Cordon-Cardo C, Teruya-Feldstein J, Pandolfi PP. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464:374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu L, Schulz H, Wolf DA. The F-box protein SKP2 mediates androgen control of p27 stability in LNCaP human prostate cancer cells. BMC Cell Biol. 2002;3:22. doi: 10.1186/1471-2121-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu SL, Rand A, Kelm RJ, Jr, Getz MJ. The retinoblastoma gene family members pRB and p107 coactivate the AP-1-dependent mouse tissue factor promoter in fibroblasts. Oncogene. 2000;19:3352–3362. doi: 10.1038/sj.onc.1203675. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Cheville JC, Pan Y, Roche PC, Schmidt LJ, Tindall DJ. PTEN induces chemosensitivity in PTEN-mutated prostate cancer cells by suppression of Bcl-2 expression. J Biol Chem. 2001;276:38830–38836. doi: 10.1074/jbc.M103632200. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Sun D, Ji P, Mohler J, Zhu L. An AR-Skp2 pathway for proliferation of androgen-dependent prostate-cancer cells. J Cell Sci. 2008;121:2578–2587. doi: 10.1242/jcs.030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Wang C. F-box protein Skp2: a novel transcriptional target of E2F. Oncogene. 2006;25:2615–2627. doi: 10.1038/sj.onc.1209286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 27.Lu S, Liu M, Epner DE, Tsai SY, Tsai MJ. Androgen regulation of the cyclin-dependent kinase inhibitor p21 gene through an androgen response element in the proximal promoter. Mol Endocrinol. 1999;13:376–384. doi: 10.1210/mend.13.3.0254. [DOI] [PubMed] [Google Scholar]
- 28.Tsihlias J, Zhang W, Bhattacharya N, Flanagan M, Klotz L, Slingerland J. Involvement of p27Kip1 in G1 arrest by high dose 5 alpha-dihydrotestosterone in LNCaP human prostate cancer cells. Oncogene. 2000;19:670–679. doi: 10.1038/sj.onc.1203369. [DOI] [PubMed] [Google Scholar]
- 29.Knudsen KE, Arden KC, Cavenee WK. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J Biol Chem. 1998;273:20213–20222. doi: 10.1074/jbc.273.32.20213. [DOI] [PubMed] [Google Scholar]