Abstract
The caudal intralaminar nuclei are a major source of glutamatergic afferents to the basal ganglia. Experiments in the 6-hydroxydopamine rat model have shown that the parafascicular nucleus is overactive and its lesion alleviates basal ganglia neurochemical abnormalities associated with dopamine depletion. Accordingly, removal of this excitatory innervation of the basal ganglia could have a beneficial value in the parkinsonian state. To test this hypothesis, unilateral kainate-induced chemical ablation of the centromedian thalamic nucleus (CM) has been performed in MPTP-treated monkeys. Successful lesions restricted to the CM boundaries (n = 2) without spreading over other neighboring thalamic nuclei showed an initial, short-lasting, and mild change in the parkinsonian motor scale but no effect against levodopa-induced dyskinesias. The lack of significant and persistent motor improvement leads us to conclude that unilateral selective lesion of the CM alone cannot be considered as a suitable surgical approach for the treatment of PD or levo-dopa-induced dyskinesias. The role of the caudal intralaminar nuclei in the pathophysiology of movement disorders of basal ganglia origin remains to be clarified.
Keywords: intralaminar thalamic nuclei, MPTP monkey model, kainic acid lesion, Parkinson’s disease
The centromedian-parafascicular thalamic complex (CM-Pf) is one major source of glutamatergic innervation of several basal ganglia nuclei, including the striatum,1-15 the subthalamic nucleus (STN),4,7,12-21 the globus pallidus, and the substantia nigra.15,21,22 Projections arising from the caudal intralaminar nuclei are excitatory and use glutamate as neurotransmitter.23-25
In recent years, a number of studies have suggested a possible role for the CM-Pf complex on the pathophysiology of movement disorders.26,27 In rats, Pf neurons that innervate the STN28,29 and the striatum25 are known to be hyperactive after unilateral midbrain dopaminergic lesion with 6-hydroxydopamine (6-OHDA). Indeed, the chemical ablation of Pf in rodents is highly effective in reversing the increases on STN metabolic activity typically observed after dopamine depletion.30 Interestingly, early observations considered the CM/Pf as a target by deep brain stimulation (DBS) for the treatment of levodopa-induced dyskinesias in Parkinson’s disease,31-33 and this has recently been proposed in combination with DBS of the globus pallidus pars interna (GPi).34 On the other hand, important cell loss has been described in the CM-Pf of PD patients35 as well as in rodents after nigrostriatal damage.25,36
To further assess the relevance of the CM/Pf as a potential therapeutic target for parkinsonism and levodopa-induced dyskinesias, we have performed chemical ablation of the caudal intralaminar nuclei in MPTP-treated monkeys and determined its impact on parkinsonian motor signs and dyskinesias.
SUBJECTS AND METHODS
A total number of five young adult male Macaca fascicularis primates (body weight ranging from 3.8 to 4.5 kg) were used in this study. Animals were handled at all times according to the European Council Directive 86/609/EEC as well as in agreement with the Society for Neuroscience Policy on the Use of Animals in Neuroscience Research. The experimental design was approved by the Ethical Committee for Animal Testing of the University of Navarra (ref: 037/2000).
MPTP Intoxication
The neurotoxin MPTP (Sigma Chemical, St. Louis, MO) was administrated intravenously though the saphenous vein as a 0.2% solution in saline (0.2 mg/kg, one weekly injection) until reaching nonreversible, stable parkinsonian symptoms. The severity of the parkinsonian syndrome was behaviorally evaluated using the rating scale proposed by Kurlan et al.37 This scale rates parkinsonian motor symptoms as facial expression (0–3), resting tremor (0–3), action or intention tremor (0–3), posture (0–2), gait (0–2), bradykinesia (0–4), balance coordination (0–2), gross motor skills of upper limb (0–3) and lower limb (0–3), and defense reaction (0–2) in an accumulating scale where the maximum score (i.e. highest severity) is 29. Once the parkinsonian syndrome was stabilized, treatment with levodopa or apomorphine (for monkeys who did not drink levodopa) was given once daily. Levodopa was orally administered at a dose of 25 mg/kg (Madopar levodopa/benserazide, 200:50; Roche, France). Apomorphine was administered subcutaneously at a dose of 0.5 mg/kg. Both drugs were given to assess motor improvement and to induce dyskinesias. The change in the score of Kurlan et al. scale from the “off” medication condition to the “on” state was monitored and the duration of the “on” response recorded. Levodopa (or apomorphine)-induced dyskinesias were rated as: 0, when absent; 1 (mild), when present only occasionally under stress or while performing delicate motor tasks; 2 (moderate) for dyskinesias present most of the “on” time but not interfering with voluntary movements; and 3 (severe) when dyskinesias were continuous, generalized, and violent, and perturb motor behavior. This scoring system coincides with the Obeso’s scale included in the Core Assessment Program for Intracerebral Transplantation38 subsequently modified and validated for clinical assessment of dyskinesias in PD patients.39
The CM lesion was made between 4 and 12 months after the last MPTP injection in all monkeys and after 1 to 8 months of initiating levodopa/apomorphine treatment (see later).
Anesthesia and Postsurgical Care
Preliminary anesthesia was induced by intramuscular injection of a solution of Imalgenet (1% of a solution of ketamine, 75 mg/kg). Surgical anesthesia was then achieved with a mixture of 5 mg/kg ketamine (Imalgene) and 0.5 mg/kg midazolam (Dormicum), resulting in deep anesthesia levels for 2 to 3 hours. Immediately prior to surgery, local anesthesia was induced with Xilonibsa Spray (10% solution of lidocain). An intramuscular injection of 1 mL of Finadyne (5 mg/kg) was given as analgesic once the surgery was completed. This injection was repeated 24 and 48 hours postsurgery. A similar schedule was also undertaken for the application of antibiotics (ampicilin, 0.5 mL/day). During survival time, animals were kept under constant control in single cages and fed with food and water “ad libitum.”
Stereotactic Surgery
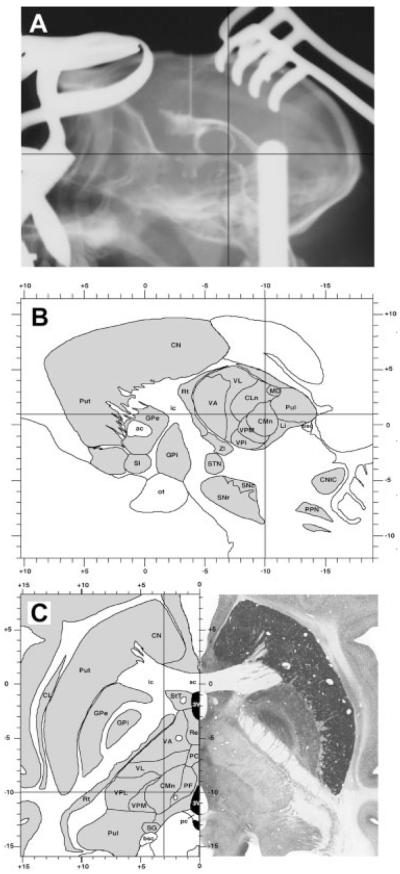
Coordinates were taken from a stereotaxic atlas of our own. Selected coordinates for targeting the CM are as follows: 9.5 to 10.5 mm caudal to the center of the anterior commissure (ac), 3.5 to 4.5 mm lateral to the midline, and 0.5 to 1 mm dorsal to ac–pc line. Target selection—assisted by ventriculography—is illustrated in Figure 1. Each ventriculography consisted on the delivery of 0.4 mL of Rx contrast (Omnigraph 300) into the frontal horn of the lateral ventricle to obtain a complete filling of the ventricular system. Once the Rx plate was developed, both the anterior (ac) and the posterior commissures (pc) were localized. The ac–pc line was then measured and used as a reference for positioning the head parallel to the horizontal plane, as well as for calibrating the stereotaxic atlas of reference.
FIG. 1.
Ventriculography-assisted stereotaxic surgery targeting the centromedian thalamic intralaminar nucleus (CM). (A) Sagittal projection from an Rx plate showing the complete filling of the ventricular system as well as the coordinates for approaching the CM nucleus. (B, C) Stereotaxic coordinates for the CM nucleus, calculated according to a stereotaxic atlas of our own. Selected coordinates are illustrated in sagittal (B) and horizontal (C) brain maps of the primate Macaca fascicularis.
Chemical Lesion of the CM
Using a Hamilton microsyringe, a total amount of 0.1 to 0.5 μL (50 mM) of kainic acid dissolved in saline was pressure-injected in the left CM nucleus unilaterally. Once the syringe was placed in the calculated target, and prior to start injecting the kainate, an additional ventriculography was performed to better assess the accurate placement of the needle. Injection was achieved in pulses of 0.05 μL every 2 minutes. Once completed, the syringe was left in place for 15 minutes before withdrawal, to minimize the reflux through the injection track.
Tissue Processing
Six weeks after surgery, the animals were anesthetized with an overdose of chloral hydrate and perfused transcardially. The perfusates consisted on a saline Ringer solution, immediately followed by 3,000 mL of cold fixative containing 4% paraformaldehyde and 0.1% glutaraldehyde in 0.125 M PB, pH 7.4. Next, the perfusion was continued using 1,000 mL of a cryoprotective solution containing 10% glycerin and 1% dimethylsulphoxide (DMSO) in 0.125 M PB, pH 7.4. After the perfusion, the skull was opened and the brain removed. Tissue blocks (15-mm-thick) were stored for 48 hours in a cryoprotective solution containing 20% glycerin and 2% DMSO in 0.125 M PB, pH 7.4. Finally, frozen coronal sections (40-μm-thick) were obtained in a sliding microtome and collected in 0.125 M PB, neutral pH. Sections were mounted in Superfrost slides, dried thoroughly, and then stained according to the Nissl method (1% of a solution of cresyl violet in distilled water). Once the staining was completed, sections were dehydrated in ascending series of ethanol, cleared in xylene, and finally coverslipped with Entellan (Merck).
RESULTS
Injection Sites and Lesion Extent
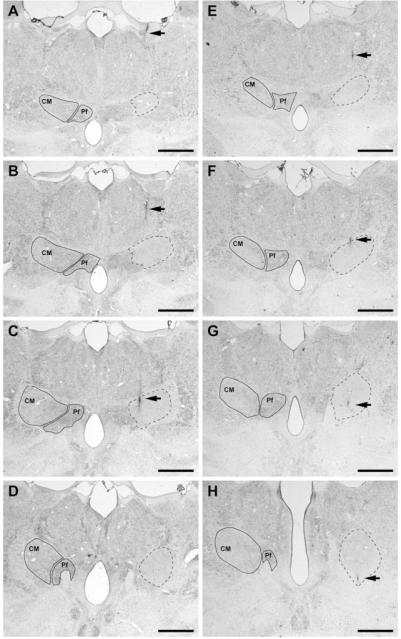
The CM was targeted in all animals (n = 5) but large variations in the size of the lesion area were noticed as a function of the amount of kainic acid delivered. Thus, kainate-injected volumes ranging between 0.2 and 0.5 μL (n = 3) resulted in a large extent of tissue lesioned, comprising not only CM, but spreading over the Pf, central lateral nucleus, paracentral nucleus, mediodorsal thalamic nucleus, and several subdivisions of the ventral posterior thalamic nucleus. This was associated with marked postural instability, prostration, and poor general health leading to early sacrifice. This precluded any formal evaluation and accordingly such animals were discarded for further analysis. In two monkeys (nos. 41 and 44) receiving a smaller amount of kainate (0.1 μL), the lesion was restricted and well located within the CM boundaries (see Fig. 2). Data presented here have been gathered only from these two monkeys in which the lesions were fairly limited to the CM nucleus.
FIG. 2.
Coronal sections through the primate thalamus (Nissl-stained) showing the extent and location of the lesioned area obtained after delivering 0.1 μL of kainic acid. (A–H) Coronal sections through four representative levels of either the primate thalamus no. 41 (A–D) or the primate no. 44 (E–H) comprising the full rostrocaudal extent of the CM nucleus (A–D as well as E–H from rostral to caudal). Within each section, the boundaries of the CM and the parafascicular nucleus (Pf) are delineated in the contralateral thalamus for reference purposes. On the left thalamus (shown on the right side of each microphotograph), the location and extent of the lesion is delineated by dashed lines. It could be easily appreciated that the kainate-induced lesion comprises the full extent of the nucleus, neighboring nuclei remaining largely unaffected. Arrows illustrate the needle track. Scale bar A–H: 2,500 μm.
Analysis of Motor Function
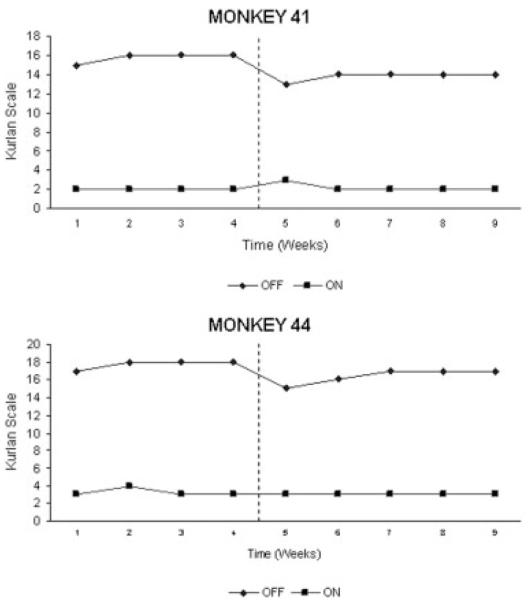
Monkeys (41 and 44) exhibited a full and stable parkinsonian syndrome before surgery (see Fig. 3). Levodopa induced a marked motor improvement (mean change in “off”/“on” score 86 and 82%) lasting for a mean of 60 and 100 minutes. Choreic dyskinesias, pre-dominating in the trunk and lower limbs bilaterally, were elicited after 2 to 4 weeks of levodopa treatment. The dyskinesias were of moderate severity (score 2/3).
FIG. 3.
Evolution of motor scores (Kurlan’s scale) and response to levodopa in the two monkeys with focal lesion of the CM nucleus. There is no significant change in the “off” and “on” medication states before and after surgery. Vertical dots indicate the time of surgery.
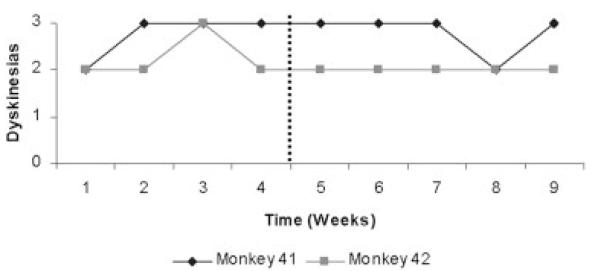
Lesion of the CM was not associated with a significant improvement in the parkinsonian syndrome (see Fig. 3). Immediately after surgery, both monkeys exhibited a short-lasting improvement in the motor score related with increased facial expression (no. 41) and trunk posture (no. 44). This was very modest and transitory. No other item of Kurlan et al.37 scale showed any change. The response to levodopa was maintained and there were no significant changes in the distribution and severity of levodopa-induced dyskinesias (see Fig. 4).
FIG. 4.
Evolution of the scores (0 absent; 3, generalized and severe) for levodopa-induced dyskinesias. There is not a significant change in the dyskinesia scores as a result of the restricted lesion within CM boundaries for monkeys no. 41 and no. 44. Vertical dots indicate the time of surgery.
DISCUSSION
Previous data in the 6-OHDA rat model25,28-30 and newer concepts about the functional organization of the basal ganglia40,41 led us to hypothesize that the CM could play a role in the pathophysiology of PD. Neuronal activity of the pedunculopontine and STN as well as the cortico-striatal afferents is known to be increased in the parkinsonian state.29 It was therefore conceivable that the CM glutamatergic interconnections with the basal ganglia could be engaged in a network self-perpetuating the hyperactive state. Blockade or lesion of the STN and PPN improves parkinsonian features in animal models.42-45 Lesion of the STN, in particular, induced a marked motor benefit that led to the current revitalization of surgery for PD. We took the effect previously observed with chemical subthalamotomy in MPTP monkeys44 as the reference gold standard for the current experience with lesion of the CM nucleus. Certainly, the effect observed in the CM lesion monkeys was not at all similar to what was encountered after STN lesion.
The failure to observe motor improvement in our MPTP monkeys cannot be attributed to wrong placement of the lesion. Indeed, the brain of the two selected monkeys showed a lesion perfectly well sited to affect most of the CM without significant extension to surrounding structures, including the Pf. It is also unlikely that the unilateral only topography of the CM lesion can be taken as explanation for the findings reported here. In the 6-OHDA rat, unilateral lesion of the Pf is associated with significant amelioration of basal ganglia metabolic abnormalities30 and unilateral surgery of the STN or GPi may portray a robust anti-parkinsonian action in both MPTP monkeys and patients with PD.46,47 Finally, it may be argued that the sample size is too small to reach any valuable conclusion. We, indeed, acknowledge that a number of two monkeys with accurate placement of the lesions is definitely limited. In this regard, it is worth indicating that a larger number of monkeys underwent surgery, but placement of a lesion strictly limited to the CM has proven to be very difficult. Nevertheless, past experiences showing a robust effect of STN or PPN lesions similarly used two monkeys.42,43,48 We are therefore obliged to conclude that selective lesion of the CM projection to the basal ganglia in MPTP-treated monkeys is unlikely capable of improving the parkinsonian state or eliminating levodopa-induced dyskinesias.
The negative findings presented here should not discard a putative role of the CM in movement control. The parkinsonian and dyskinetic states are relatively simple functional situations seen as opposite poles of abnormal motor behaviors.49 It may be that CM activity is related to other, more complex, components of movement control. For instance, in the intact monkey,50 the CM neurons fired particularly in relation with reward anticipation and decision, and in the 6-OHDA rat model, lesion of the CM improved performance in a motivational task that measured the latency to retrieve a reward.51 It may be, therefore, that in the primate brain the CM is important to modulate striatal and STN excitability in contextdependent tasks or reward related activities. Thus, the CM may play a role in the origin of other features of PD. Interestingly, the possibility of achieving an extra benefit in patients with PD by combining pallidal with CM + Pf DBS has been raised recently.34 DBS of the CM-Pf alone had not a major benefit against the cardinal features of PD or against LID, somehow mimicking the results reported here. On the other hand, there appears to be a marked improvement on freezing of gait, a feature that is frequently resistant to both dopaminergic drugs and basal ganglia surgery.
The possibility of improving tics and obsessive compulsive behavior by lesion of the thalamus including the intralaminar nuclei has been considered for many years and until recently.52-54 Although most authors claimed clear cut improvement, the lack of adequate clinical evaluations and reliability of the intended lesions preclude any conclusion.55 More recently, DBS has been applied to the treatment of Tourette’s syndrome. Most reports deal with pallidal DBS but Visser-Vandewalle et al.56 treated three patients by targeting the CM, substantia periventricularis, and nucleus ventrooralis internus. They described that tics, including vocal tics, disappeared after more than 1 year of follow-up in two cases. The same target was used in an isolated patient by Bawja et al.57 A few other instances of patients successfully treated with a combination of pallidal and thalamic (targeting the CM) DBS have been described in recent years.58,59 These results have to be judged cautiously as the general tendency is to report positive outcomes and silence negative results, particularly when dealing with surgical treatments. Nevertheless, they are encouraging regarding the treatment of severe patients with Tourette’s syndrome and in keeping with our suggestion that the CM/Pf complex may be more relevant when dealing with patients with associative and behavioral disorders.
In conclusion, we found no evidence for a direct influence of the CM in modulating basal ganglia activity in the MPTP monkey model. This negative finding serves to exert some caution when considering the CM nucleus as a potential anti-parkinsonian target as far as the cardinal motor features of PD are concerned. On the other hand, its putative value in combination with other targets cannot be dismissed as now. Moreover, a putative role for thalamic surgery in the management of Tourette’s syndrome and other neurobehavioral disorders may be worth further studies.
Acknowledgments
Supported by grants from the Michael J. Fox Foundation, Ministerio de Educación y Ciencia refs: BFU2006-06744 and SAF2005-08416-C02-01, FIS refs: PI051037 and 013/2003, Fundación de Investigación Médica Mutua Madrilenña, and by the agreement between FIMA and the “UTE project FIMA.” Expert technical assistance in tissue handling and preparation was provided by our technician Ms. Elvira Roda.
REFERENCES
- 1.Parent A, Mackey A, De Bellefeuille L. The subcortical afferents to caudate nucleus and putamen in primate: a fluorescent retrograde double labeling study. Neuroscience. 1993;10:1137–1150. doi: 10.1016/0306-4522(83)90104-5. [DOI] [PubMed] [Google Scholar]
- 2.Nakano K, Hasegawa Y, Tokushige A, Nakagawa S, Kayahara T, Mizuno N. Topographical projections from the thalamus, subthalamic nucleus and pedunculopontine tegmental nucleus to the striatum in japanese monkey, Macaca fuscata. Brain Res. 1990;437:54–68. doi: 10.1016/0006-8993(90)90339-d. [DOI] [PubMed] [Google Scholar]
- 3.Sadikot AF, Parent A, François C. The centre median and parafascicular thalamic nuclei project respectively to the sensorimotor and associative-limbic striatal territories in the squirrel monkey. Brain Res. 1990;510:161–165. doi: 10.1016/0006-8993(90)90746-x. [DOI] [PubMed] [Google Scholar]
- 4.Sadikot AF, Parent A, François C. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J Comp Neurol. 1992;315:137–159. doi: 10.1002/cne.903150203. [DOI] [PubMed] [Google Scholar]
- 5.Sadikot AF, Parent A, Smith Y, Bolam JP. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a light and electron microscopic study of the thalamostriatal projection in relation to striatal heterogeneity. J Comp Neurol. 1992;320:228–242. doi: 10.1002/cne.903200207. [DOI] [PubMed] [Google Scholar]
- 6.Fenelon G, François C, Percheron G, Yelnik J. Topographic distribution of the neurons of the central complex (centre-médian-parafascicular complex) and other thalamic neurons projecting to the striatum in macaques. Neuroscience. 1991;45:495–510. doi: 10.1016/0306-4522(91)90244-i. [DOI] [PubMed] [Google Scholar]
- 7.Féger J, Bevan M, Crossman AR. The projections from the parafascicular thalamic nucleus to the subthalamic nucleus and the striatum arise from separated neuronal populations: a comparison with the corticostriatal and corticosubthalamic efferents in a retrograde fluorescent double-labeling study. Neuroscience. 1994;60:125–132. doi: 10.1016/0306-4522(94)90208-9. [DOI] [PubMed] [Google Scholar]
- 8.Deschênes M, Bourassa J, Parent A. Two different types of thalamic fibers innervate the rat striatum. Brain Res. 1995;701:288–292. doi: 10.1016/0006-8993(95)01124-3. [DOI] [PubMed] [Google Scholar]
- 9.Sidibé M, Smith Y. Differential synaptic innervation of striatofugal neurons projecting to the internal or external segments of the globus pallidus by thalamic afferents in the squirrel monkey. J Comp Neurol. 1996;365:445–465. doi: 10.1002/(SICI)1096-9861(19960212)365:3<445::AID-CNE8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Rudkin TM, Sadikot AF. Thalamic input to parvalbumin-immunoreactive GABAergic interneurons: organization in normal striatum and effect of neonatal decortication. Neuroscience. 1999;88:1165–1175. doi: 10.1016/s0306-4522(98)00265-6. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalo N, Lanciego JL, Castle M, Vázquez A, Erro E, Obeso JA. The parafascicular thalamic complex and basal ganglia circuitry: further complexity to the basal ganglia model. Thalamus Relat Sys. 2002;1:341–348. [Google Scholar]
- 12.Vercelli A, Marini G, Tredici G. Anatomical organization of the telencephalic connections of the parafascicular nucleus in adult and developing rats. Eur J Neurosci. 2003;18:275–289. doi: 10.1046/j.1460-9568.2003.02743.x. [DOI] [PubMed] [Google Scholar]
- 13.Lanciego JL, Gonzalo N, Castle M, Sánchez-Escobar C, Aymerich MS, Obeso JA. Thalamic innervation of striatal and subthalamic neurons projecting to the rat entopeduncular nucleus. Eur J Neurosci. 2004;19:1267–1277. doi: 10.1111/j.1460-9568.2004.03244.x. [DOI] [PubMed] [Google Scholar]
- 14.Castle M, Aymerich MS, Sánchez-Escobar C, Gonzalo N, Obeso JA, Lanciego JL. Thalamic innervation of the direct and indirect basal ganglia pathways in the rat: ipsi- and contralateral projections. J Comp Neurol. 2005;483:143–153. doi: 10.1002/cne.20421. [DOI] [PubMed] [Google Scholar]
- 15.Tandé D, Féger J, Hirsch EC, François C. Parafascicular nucleus projection to the extrastriatal basal ganglia in monkeys. NeuroReport. 2006;17:277–280. doi: 10.1097/01.wnr.0000201508.46126.de. [DOI] [PubMed] [Google Scholar]
- 16.Sugimoto T, Hattori T. Confirmation of the thalamosubthalamic projection by electron microscope autoradiography. Brain Res. 1983;267:335–339. doi: 10.1016/0006-8993(83)90885-5. [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto T, Hattori T, Mizuno N, Itoh K, Sato M. Direct projections from the centromedian-parafascicular complex to the subthalamic nucleus in the cat and rat. J Comp Neurol. 1993;214:209–216. doi: 10.1002/cne.902140208. [DOI] [PubMed] [Google Scholar]
- 18.Royce GJ, Mourey RJ. Efferent connections of the centromedian and parafascicular thalamic nuclei: an autoradiographic investigation in cat. J Comp Neurol. 1985;235:277–300. doi: 10.1002/cne.902350302. [DOI] [PubMed] [Google Scholar]
- 19.Deschênes M, Bourassa J, Doan VD, Parent A. A single-cell study of the axonal projections arising from the posterior intralaminar thalamic nuclei in the rat. Eur J Neurosci. 1996;8:329–343. doi: 10.1111/j.1460-9568.1996.tb01217.x. [DOI] [PubMed] [Google Scholar]
- 20.Marini G, Pianca L, Tredici G. Descending projections arising from the parafascicular nucleus in rats: trajectory of fibers, projection pattern and mapping of terminations. Somatosens Mot Res. 1999;16:207–222. doi: 10.1080/08990229970465. [DOI] [PubMed] [Google Scholar]
- 21.Kincaid AE, Penney JB, Jr, Young AB, Newman SW. The globus pallidus receives a projection from the parafascicular nucleus in the rat. Brain Res. 1991;553:18–26. doi: 10.1016/0006-8993(91)90224-j. [DOI] [PubMed] [Google Scholar]
- 22.Yasukawa T, Kita T, Xue Y, Kita H. Rat intralaminar projections to the globus pallidus: a biotinylated dextran amine anterograde tracing study. J Comp Neurol. 2004;471:153–167. doi: 10.1002/cne.20029. [DOI] [PubMed] [Google Scholar]
- 23.Mouroux M, Féger J. Evidence that the parafascicular projection to the subthalamic nucleus is glutamatergic. NeuroReport. 1993;4:613–615. doi: 10.1097/00001756-199306000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Mouroux M, Hassani OK, Féger J. Electrophysiological study of the excitatory parafascicular projection to the subthalamic nucleus and evidence for ipsi- and contralateral controls. Neuroscience. 1995;67:399–407. doi: 10.1016/0306-4522(95)00032-e. [DOI] [PubMed] [Google Scholar]
- 25.Aymerich MS, Barroso-Chinea P, Pérez-Manso M, et al. Consequences of unilateral nigrostriatal denervation on the thalamostriatal pathway in rats. Eur J Neurosci. 2006;23:2099–2108. doi: 10.1111/j.1460-9568.2006.04741.x. [DOI] [PubMed] [Google Scholar]
- 26.Kerkerian-Le Goff L, Bacci JJ, Salin P, et al. Intralaminar thalamic nuclei are main regulators of basal ganglia possible involvement in the pathophysiology of Parkinson’s Disease. In: Bolam P, Ingham CA, Magill PJ, editors. The basal ganglia VIII (Advances in behavioral biology, Vol. 56) Springer; New York: 2005. pp. 331–340. [Google Scholar]
- 27.Lanciego JL, Castle M, Barroso-Chinea P, Aymerich MS. On the relationships between the caudal intralaminar nuclei of the thalamus and the basal ganglia: implications for the pathophysiology of Parkinson’s disease. In: Di Giovanni G, editor. The basal ganglia pathophysiology: recent advances. Transworld Research Network; Kerala, India: 2007. pp. 1–17. [Google Scholar]
- 28.Orieux G, François C, Féger J, et al. Metabolic activity of excitatory parafascicular and pedunculopontine inputs to the subthalamic nucleus in a rat model of Parkinson’s disease. Neuroscience. 2000;97:79–88. doi: 10.1016/s0306-4522(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch EC, Perier C, Orieux G, et al. Metabolic effects of nigrostriatal denervation in basal ganglia. Trends Neurosci. 2000;23:78–85. doi: 10.1016/s1471-1931(00)00021-5. [DOI] [PubMed] [Google Scholar]
- 30.Bacci JJ, Kachidian P, Kerkerian-Le Goff L, Salin P. Intralaminar thalamic nuclei lesions: widespread impact on dopamine denervation-mediated cellular defects in the rat basal ganglia. J Neuropathol Exp Neurol. 2004;63:20–31. doi: 10.1093/jnen/63.1.20. [DOI] [PubMed] [Google Scholar]
- 31.Caparros-Lefebvre D, Ruchoux MM, Blond S, Petit H, Percheron G. Long-term thalamic stimulation in Parkinson’s disease: postmortem anatomoclinical study. Neurology. 1994;44:1856–1860. doi: 10.1212/wnl.44.10.1856. [DOI] [PubMed] [Google Scholar]
- 32.Caparros-Lefebvre D, Blond S, Feltin MP, Pollak P, Benabid AL. Improvement of levodopa induced dyskinesias by thalamic deep brain stimulation is related to slight variations in electrode placement: possible involvement of the centre median parafascicularis complex. J Neurol Neurosurg Pshychiatr. 1999;67:308–314. doi: 10.1136/jnnp.67.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krauss JK, Pohle T, Weigel R, Burgunder JM. Deep brain stimulation of the centre median-parafascicular complex in patients with movement disorders. J Neurol Neurosurg Psychiatr. 2002;72:546–548. doi: 10.1136/jnnp.72.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzone P, Stocchi F, Galati S, et al. Bilateral implantation of centromedian-parafascicularis complex and GPi: a new combination of unconventional targets for deep brain stimulation in severe Parkinson disease. Neuromodulation. 2006;9:221–228. doi: 10.1111/j.1525-1403.2006.00063.x. [DOI] [PubMed] [Google Scholar]
- 35.Henderson JM, Carpenter K, Cartwright H, Halliday GM. Degeneration of the centre median-parafascicular complex in Parkinson’s disease. Ann Neurol. 2000;47:345–352. [PubMed] [Google Scholar]
- 36.Freyaldenhoven TE, Ali SF, Schmued LC. Systemic administration of MPTP induces thalamic degeneration in mice. Brain Res. 1997;759:9–17. doi: 10.1016/s0006-8993(97)00045-0. [DOI] [PubMed] [Google Scholar]
- 37.Kurlan R, Kim MH, Gash DM. Oral levodopa dose-response study in MPTP-induced hemiparkinsonian monkeys: assessment with a new rating scale for monkey parkinsonism. Mov Disord. 1991;6:111–118. doi: 10.1002/mds.870060205. [DOI] [PubMed] [Google Scholar]
- 38.Langston JW, Widner H, Goetz CG, et al. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 39.Goetz CG, Stebbins GT, Shale HM, Lang AE, Chernik DA. Utility of an objective dyskinesia rating scale for Parkinson’s disease: inter- and intrarater reliability assessment. Mov Disord. 1994;9:390–394. doi: 10.1002/mds.870090403. [DOI] [PubMed] [Google Scholar]
- 40.Obeso JA, Rodriguez-Oroz MC, Rodriguez M, et al. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci. 2000;23:S8–S19. doi: 10.1016/s1471-1931(00)00028-8. [DOI] [PubMed] [Google Scholar]
- 41.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 42.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 43.Aziz TZ, Peggs D, Sambrook MA, Crossman AR. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Disord. 1991;6:288–292. doi: 10.1002/mds.870060404. [DOI] [PubMed] [Google Scholar]
- 44.Guridi J, Herrero MT, Luquin MR, et al. Subthalamotomy in parkinsonian monkeys. Behavioural and biochemical analysis. Brain. 1996;119:1717–1727. doi: 10.1093/brain/119.5.1717. [DOI] [PubMed] [Google Scholar]
- 45.Stefani A, Lozano AM, Peppe A, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130:1596–1607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- 46.Alvarez L, Macías R, Guridi J, et al. Dorsal subthalamotomy for Parkinson’s disease. Mov Disord. 2001;16:72–78. doi: 10.1002/1531-8257(200101)16:1<72::aid-mds1019>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 47.Alvarez L, Macias R, Lopez G, et al. Bilateral subthalamotomy in Parkinson’s disease: initial and long-term response. Brain. 2005;128:570–583. doi: 10.1093/brain/awh397. [DOI] [PubMed] [Google Scholar]
- 48.Nandi D, Aziz TZ, Giladi N, Winter J, Stein JF. Reversal of akinesia in experimental parkinsonism by GABA antagonist microinjections in the pedunculopontine nucleus. Brain. 2002;125:2418–2430. doi: 10.1093/brain/awf259. [DOI] [PubMed] [Google Scholar]
- 49.Guridi J, Obeso JA. The subthalamic nucleus, hemiballismus and Parkinson’s disease: reappraisal of a neurosurgical dogma. Brain. 2001;124:5–19. doi: 10.1093/brain/124.1.5. [DOI] [PubMed] [Google Scholar]
- 50.Minamimoto T, Hori Y, Kimura M. Complementary process to response bias in the centromedian nucleus of the thalamus. Science. 2005;308:1798–1801. doi: 10.1126/science.1109154. [DOI] [PubMed] [Google Scholar]
- 51.Henderson JM, Schleimer SB, Allbutt H, et al. Behavioral effects of parafascicular thalamic lesions in animal models of parkinsonism. Behav Brain Res. 2005;162:222–232. doi: 10.1016/j.bbr.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 52.Hassler R, Dieckmann G. Stereotaxic treatment of tics and inarticulate cries or coprolalia considered as motor obsessional phenomena in Gilles de la Tourette’s disease. Rev Neurol (Paris) 1997;123:89–100. [PubMed] [Google Scholar]
- 53.Cappabianca P, Spaziante R, Carrabs G, de Divitiis E. Surgical stereotactic treatment for Gilles de la Tourette’s syndrome. Acta Neurol. 1987;9:273–280. [PubMed] [Google Scholar]
- 54.Babel TB, Warnke PC, Ostertag CB. Immediate and long term outcome after infrathalamic and thalamic lesioning for intractable Tourette’s syndrome. J Neurol Neurosurg Psychiatry. 2001;70:666–671. doi: 10.1136/jnnp.70.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Temel Y, Visser-Vandewalle V. Surgery in Tourette syndrome. Mov Disord. 2004;19:3–14. doi: 10.1002/mds.10649. [DOI] [PubMed] [Google Scholar]
- 56.Visser-Vandewalle V, Temel Y, Boon P, et al. Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. Report of three cases. J Neurosurg. 2003;99:1094–1100. doi: 10.3171/jns.2003.99.6.1094. [DOI] [PubMed] [Google Scholar]
- 57.Bajwa RJ, de Lotbiniere AJ, King RA, et al. Deep brain stimulation in Tourette’s syndrome. Mov Disord. 2007;22:1346–1350. doi: 10.1002/mds.21398. [DOI] [PubMed] [Google Scholar]
- 58.Houeto JL, Karachi C, Mallet L, et al. Tourette’s syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatr. 2005;76:992–995. doi: 10.1136/jnnp.2004.043273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ackermans L, Temel Y, Cath D, et al. Deep brain stimulation in Tourette’s syndrome: two targets? Mov Disord. 2006;21:709–713. doi: 10.1002/mds.20816. [DOI] [PubMed] [Google Scholar]