Abstract
Background
The HEART Pathway is a decision aid designed to identify emergency department patients with acute chest pain for early discharge. No randomized trials have compared the HEART Pathway with usual care.
Methods and Results
Adult emergency department patients with symptoms related to acute coronary syndrome without ST-elevation on ECG (n=282) were randomized to the HEART Pathway or usual care. In the HEART Pathway arm, emergency department providers used the HEART score, a validated decision aid, and troponin measures at 0 and 3 hours to identify patients for early discharge. Usual care was based on American College of Cardiology/American Heart Association guidelines. The primary outcome, objective cardiac testing (stress testing or angiography), and secondary outcomes, index length of stay, early discharge, and major adverse cardiac events (death, myocardial infarction, or coronary revascularization), were assessed at 30 days by phone interview and record review. Participants had a mean age of 53 years, 16% had previous myocardial infarction, and 6% (95% confidence interval, 3.6%–9.5%) had major adverse cardiac events within 30 days of randomization. Compared with usual care, use of the HEART Pathway decreased objective cardiac testing at 30 days by 12.1% (68.8% versus 56.7%; P=0.048) and length of stay by 12 hours (9.9 versus 21.9 hours; P=0.013) and increased early discharges by 21.3% (39.7% versus 18.4%; P<0.001). No patients identified for early discharge had major adverse cardiac events within 30 days.
Conclusions
The HEART Pathway reduces objective cardiac testing during 30 days, shortens length of stay, and increases early discharges. These important efficiency gains occurred without any patients identified for early discharge suffering MACE at 30 days.
Keywords: acute coronary syndrome, chest pain, clinical trial, decision support techniques
Each year, 8 to 10 million patients complaining of chest pain present to an emergency department (ED) in the United States.1 When caring for these patients, emergency physicians use liberal testing strategies to prevent missing an acute coronary syndrome (ACS). This pervasive overtriage results in >50% of ED patients with acute chest pain receiving a comprehensive cardiac evaluation (serial cardiac biomarkers and stress testing or angiography) at a cost of $10 to 13 billion annually,2–6 yet <10% of these patients are ultimately diagnosed with ACS.6–10
American College of Cardiology/American Heart Association guidelines recommend that low-risk patients with acute chest pain should receive serial cardiac markers followed by objective cardiac testing (stress testing or cardiac imaging).11 However, guideline-adherent care among low-risk patients fails to accurately focus health system resources on those likely to beneft. Among low-risk patients, who have ACS rates <2%, objective cardiac testing is associated with a substantial number of false-positive and nondiagnostic tests, which often lead to invasive testing.12 Consensus is building within the US healthcare system about the need to more eff-ciently evaluate patients with acute chest pain.13
The HEART Pathway,14,15 which combines the HEART score,16–19 with 0- and 3-hour cardiac troponin tests, is a recently developed decision aid designed to identify ED patients who are safe for early discharge. Observational studies have demonstrated that the HEART Pathway can classify >20% of patients with acute chest pain for early discharge while maintaining a negative predictive value (NPV) for a major adverse cardiac event (MACE) rate of >99% at 30 days.13,14 However, the realtime use of the HEART Pathway has yet to be compared with usual care. Therefore, we have designed a randomized controlled trial to evaluate the efficacy of the HEART Pathway to guide providers’ testing and disposition decisions for patients with acute chest pain. We seek to determine whether the HEART Pathway can meaningfully reduce objective cardiac testing, increase early discharges, and reduce index hospital length of stay (LOS) compared with usual care while maintaining high sensitivity and NPV (>99%) for MACE.
Methods
Study Design
We conducted a randomized controlled single-center clinical trial funded by the American Heart Association from 9/2012-2/2014. All participants provided witnessed written informed consent and were randomized to the HEART Pathway or usual care strategies. In the HEART Pathway arm, ED attending physicians used the HEART Pathway to guide testing and disposition decisions. In the usual care arm, providers were encouraged to follow American College of Cardiology guidelines.11,20,21 This trial was approved by the Internal Review Board of the sponsoring organization and was registered with clinicaltrials.gov (clinical trial number, NCT01665521) before enrollment.
Setting
Participants were recruited from the ED (of institution name withheld for review). The study institution is a tertiary care academic medical center located in the Piedmont Triad area of North Carolina, serving urban, suburban, and rural populations. The ED is staffed by board-certified or board-eligible emergency physicians 24 hours per day, 7 days a week who directly provide care and oversee care provided by residents, physician assistants, and nurse practitioners. ED patient volume in 2013 consisted of ≈104000 patient encounters. Cardiac testing modalities routinely available to study participants included exercise stress echocardiogram, dobutamine stress echocardiogram, coronary computed tomographic angiography, stress nuclear imaging, stress cardiac magnetic resonance imaging, or invasive coronary angiography. Serum troponin measurements were performed using the ADVIA Centaur platform TnI-Ultra™ assay (Siemens, Munich, Germany), which has a 99th percentile of the upper reference limit and 10% coefficient of variation at 0.04 mg/L.
Participants
Patients ≥21 years old presenting with symptoms suggestive of ACS were screened during enrollment hours (6 days excluding Saturday, 80 hours per week). Eligibility criteria included the provider ordering an ECG and troponin for the evaluation of ACS. Patients were determined ineligible for the following reasons: new ST-segment elevation ≥1 mm, hypotension, life expectancy <1 year, a noncardiac medical, surgical, or psychiatric illness determined by the provider to require admission, previous enrollment, non-English speaking, and incapacity or unwillingness to consent.
Randomization
Trial participants were stratified by the presence of known coronary disease (including previous revascularization) and randomized within strata to 1 of the 2 treatment arms with equal probability using random permuted block randomization. The randomization sequence was generated using nQuery Advisor 6.0 (Statistical Solutions, Saugus, MA) and integrated into a secure electronic database, Research Electronic Data Capture,22 which was used by the study coordinators to register participants and obtain study group assignments. Study investigators and staff were blinded to the randomization sequence.
Randomization Arms
HEART Pathway
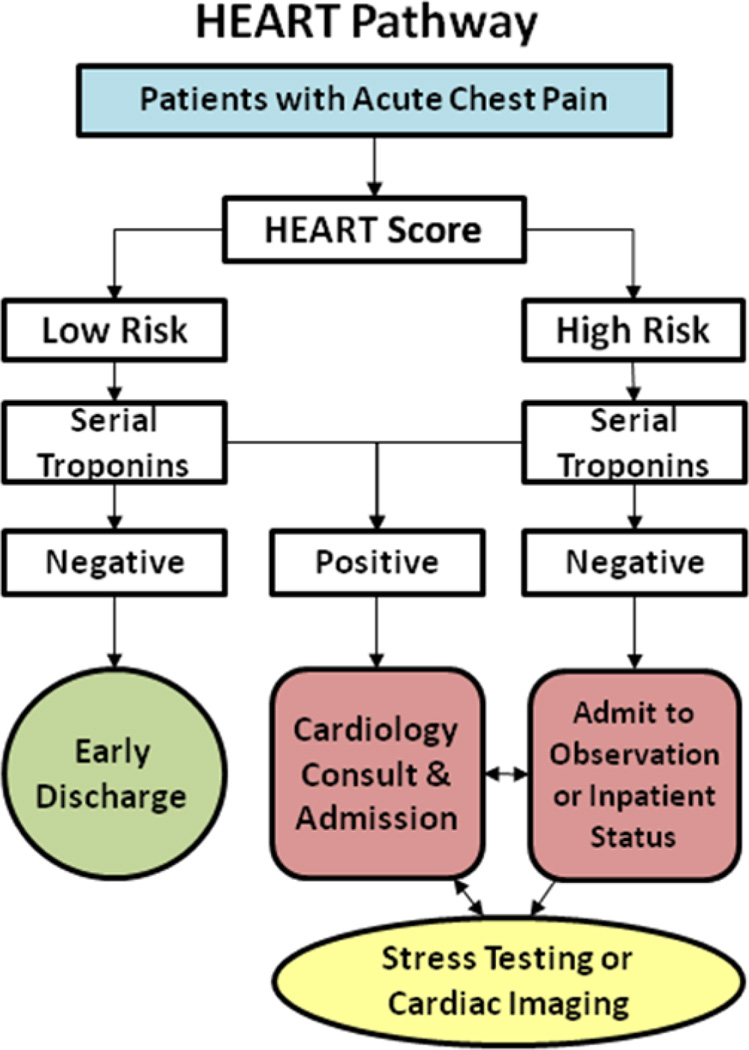
Participants were randomized to the HEART Pathway or usual care arms. Within the HEART Pathway arm, participants were risk stratified by attending ED providers using a validated clinical decision aid, the HEART score,16–19 and serial troponin measures at 0 and 3 hours after ED presentation. The HEART score consists of 5 components: history, ECG, age, risk factors, and troponin (Appendix 1). To calculate a HEART score, first each component is assessed (on a scale of 0–2), and then component scores are summed to produce the final score. A HEART score of 0 to 3 is consistent with a low-risk assessment, whereas a score of ≥4 is consistent with a high-risk assessment. To facilitate HEART score completion, study staff provided the physician with the participant’s ECG and a worksheet (Appendix 1) to complete at the bedside for each patient. On the basis of the HEART score and serial troponin results, the attending physicians received care recommendations according to the HEART pathway (Figure 1). For patients with low-risk HEART scores (HEART score of 0–3) and negative troponin results, the HEART pathway recommends discharge from the ED without further testing. These patients were encouraged to follow up with their primary care provider. In patients with a high-risk HEART score (HEART score of ≥4) or troponin above the 99th percentile threshold, the HEART Pathway recommends further evaluation (objective cardiac testing) in the hospital or observation unit (OU). For patients with an elevated troponin measurement or inducible ischemia on objective cardiac testing, the HEART pathway recommended cardiology consultation and admission to the hospital.
Figure 1.
HEART Pathway algorithm.
Usual Care
Care delivery in the usual care arm was at the discretion of the care providers and not determined by the trial protocol. However, providers were encouraged to follow American College of Cardiology/American Heart Association guidelines,11,20,21 which recommend serial cardiac biomarkers and objective cardiac testing before discharge from the OU or inpatient ward for patients with symptoms suggestive of ACS.14,23
HEART Pathway Adherence
Care delivered in both randomization arms was ultimately determined by provider discretion and not mandated by the trial protocol. The HEART Pathway was used by providers, in a manner consistent with its intent, as a decision aid rather than a substitute for clinical judgment. Therefore, some nonadherence to the care delivery described in Figure 1 was anticipated. To quantify and examine the effect of nonadherence on our outcomes, the number of patients in the HEART Pathway arm receiving adherent or nonadherent care was determined.
HEART Score Interobserver Agreement
Patients randomized to the HEART Pathway received a second HEART score assessment by an attending physician study investigator blinded to the initial assessment by the patient’s attending physician. Based on our Institutional Review Board recommendations, if a disagreement occurred in which the attending provider determined the patient to be low-risk, but the study investigator found the patient to be high-risk, the attending provider was made aware of this discrepancy.
Data Collection and Processing
Our trial was conducted in accordance with standards of good clinical practice, standardized reporting guidelines,24 and key data elements and definitions.25 A detailed source of data map was created before study initiation. Electronic medical records were used as the source for data elements reliably contained in the medical record. Research Electronic Data Capture data collection templates were used to prospectively collect and store data from patients and care providers for data elements not reliably present in the electronic medical records.
Follow-up was conducted during the index visit using structured record review. At 30 days, a structured record review was followed by a telephone interview using a validated scripted follow-up dialogue26 to further clarify events since discharge, identify events occurring at other care facilities, and to determine healthcare utilization since discharge. Outcome events reported at other healthcare facilities were confirmed using a structured review of those medical records. Incomplete follow-up at 30 days was handled using the following algorithm: participants with ongoing visits in the electronic medical records were considered to have complete information and were classified on the basis of data available in the medical record; participants with no ongoing visits were considered lost to follow up at the point of last contact. The Social Security Death Master File was used to search for participants unable to be contacted. In the event of discrepancy between a participant’s self-reported event and the medical record, the medical record was considered correct.
Outcomes
Healthcare Utilization
Our primary outcome was the rate of objective cardiac testing within 30 days of presentation, defined as the proportion of patients receiving any stress testing modality, coronary computed tomographic angiography, or invasive coronary angiography at the index visit or within 30 days. Secondary outcomes included early discharge rate, index LOS, and cardiac-related recurrent ED visits and nonindex hospitalization at 30 days. Early discharge was defined as discharge from the ED without objective cardiac testing. Hospitalization was defined as bedding a patient to an OU or inpatient ward in observation or inpatient status. LOS was recorded from the electronic medical records and represented the time from patient placement into an ED bed to hospital discharge. A cardiac-related recurrent ED visit was defined as any patient revisiting the ED with chest pain or other symptoms suggestive of ACS within the 30-day follow-up period. Thirty-day nonindex hospitalization was defined as an inpatient or OU evaluation for ACS within 30 days.
Safety Events
All participants were monitored for MACE, defined by a composite end point of all-cause mortality, myocardial infarction, or coronary revascularization within the 30-day follow-up period. Myocardial infarction was defined on the basis of Universal Definition of Myocardial Infarction.27 Coronary revascularization was defined as angioplasty with or without stent placement or coronary artery bypass surgery. MACE occurring in patients discharged without objective cardiac testing was considered a missed MACE. All safety events were reviewed by the Institutional Data Safety Monitoring Board.
End Point Adjudication
A consensus of 2 reviewers (C.D.M. and B.C.H.), blinded to treatment arm assignment, adjudicated the elements required to measure the occurrence of MACE and to determine cardiac-relatedness of recurrent ED visits and nonindex hospitalizations. To make these assessments, reviewers were provided participant’s index and discharge records, follow-up call information, records obtained from follow-up, and study definitions. Any disagreements were settled by consensus between the 2 reviewers or the involvement of a third reviewer.
Statistical Analysis
The proportion of patients receiving objective cardiac testing within 30 days, early discharge, and cardiac-related ED visits and nonindex hospitalizations were estimated for the HEART Pathway and usual care groups, and a 95% confidence interval for the differences between the 2 groups was calculated using exact calculations. Unadjusted differences between groups in these outcomes at index and 30 days were assessed using the Fisher exact test. LOS was calculated for each participant and summarized using median and interquartile ranges for each treatment arm. LOS had a non-normal (right-skewed) distribution, so treatment arms were compared using Mann–Whitney U tests. With an expected rate of 83% in the usual care arm, this study was powered to detect a 15% reduction in objective cardiac testing within 30 days with 90% power at the 5% 2-sided level of significance with an expected loss to follow-up rate of 10%.
Sensitivity, specificity, positive predictive value and NPV, and their exact 95% confidence intervals for MACE during the 30-day follow-up period were calculated for each treatment arm. In addition, to determine the incremental value of the HEART Pathway to serial troponin testing, the sensitivity, specificity, positive predictive value, and NPV of serial troponin results at 0 and 3 hours used alone (without the HEART score) were calculated. Missed MACE rates were estimated for the HEART Pathway and usual care groups, and an exact 95% confidence interval for the differences between the 2 groups was calculated. Unadjusted differences between groups in these outcomes at index and 30 days were assessed using the Fisher exact test. Patients with incomplete follow-up were considered to be free of 30-day MACE. Interobserver agreement for the HEART Pathway risk assessment was tested using a κ-statistic. Acceptable agreement was defined, a priori, as a κ of >0.60. To assess differences in hospital LOS by randomization arm (and to compare usual care with the high- and low-risk HEART Pathway groups), the Kaplan-Meier method was used. All outcomes were analyzed using intention-to-treat. Statistical analysis was performed using SAS 9.3 (Cary, NC).
Results
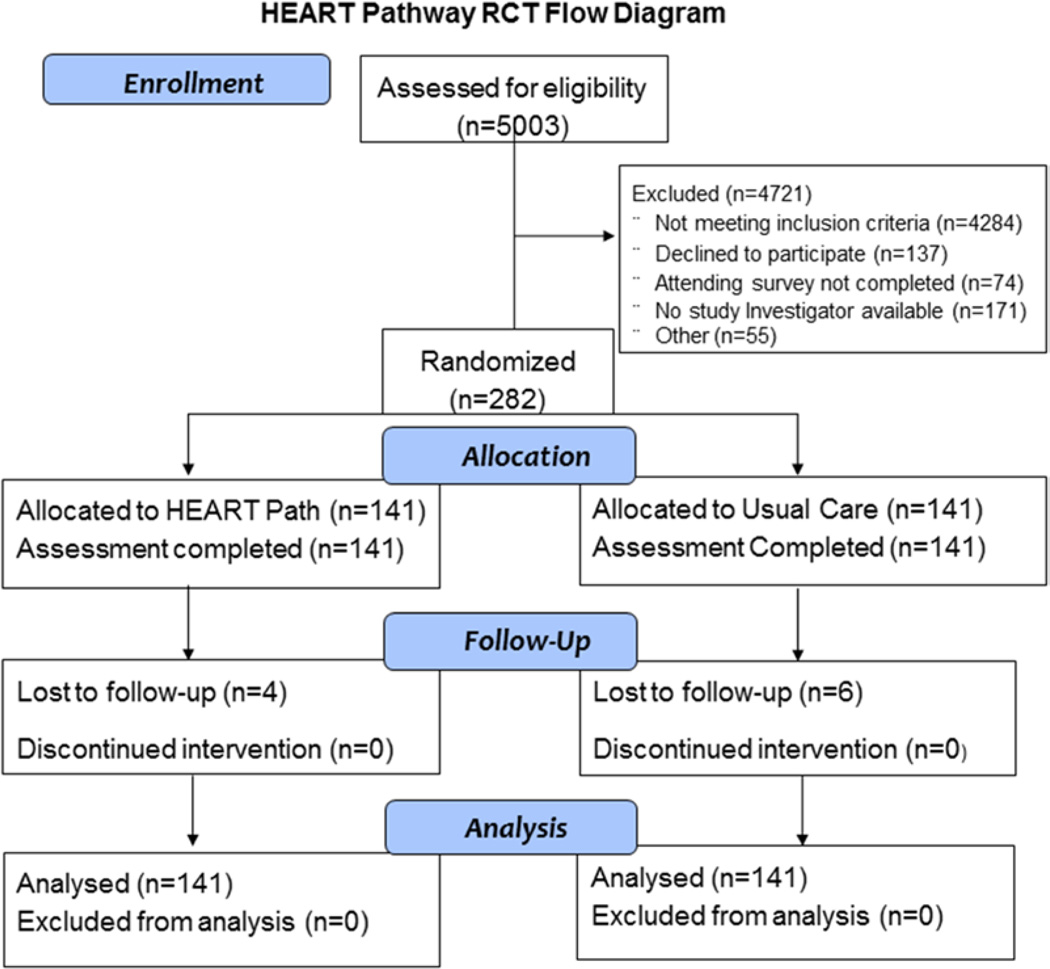
From 9/2012-2/2014, 282 patients with symptoms suggestive of ACS were enrolled, with 141 randomized to each arm. No participants were removed from the study after randomization. Assessment for 30-day events was complete on 96% (272/282) of participants (Figure 2), with their characteristics summarized in Table 1. Of the 10 patients lost to follow up, none appeared in the Social Security Death Master File. Among the 141 patients randomized to the HEART Pathway, 46.8% (66/141) were risk stratified into a low-risk group and 53.2% (75/141) into a high-risk group. Interobserver agreement was acceptable (κ=0.63). The frequency of HEART Pathway determinants is summarized in Table 2.
Figure 2.
Enrollment fow diagram.
Table 1.
HEART Pathway Randomized Controlled Trial Patient Characteristics
| HEART Pathway |
Usual Care |
|||
|---|---|---|---|---|
| Patient Characteristics | Number, n=141 | Percent | Number, n=141 | Percent |
| Age, y, mean±SD | 53.4±12.0 | … | 53.1±12.2 | … |
| Sex | ||||
| Female | 81 | 57.4 | 81 | 57.4 |
| Race | ||||
| White | 90 | 63.8 | 93 | 66.0 |
| Black | 48 | 34.0 | 46 | 32.6 |
| Asian | 1 | 0.7 | 0 | 0 |
| Native American | 1 | 0.7 | 1 | 0.7 |
| Others | 1 | 0.7 | 1 | 0.7 |
| Ethnicity | ||||
| Hispanic | 1 | 0.7 | 4 | 2.8 |
| Non-Hispanic | 140 | 99.3 | 137 | 97.2 |
| Risk factors | ||||
| Current smoking | 42 | 29.8 | 34 | 24.1 |
| Recent cocaine (last 90 days) | 3 | 2.1 | 3 | 2.1 |
| Hypertension | 75 | 53.2 | 82 | 58.2 |
| Hyperlipidemia | 61 | 43.3 | 60 | 42.6 |
| Diabetes mellitus | 31 | 22.0 | 27 | 19.2 |
| Family history of coronary disease | 44 | 31.4 | 58 | 41.4 |
| BMI, >30 kg/m2 | 71 | 50.4 | 81 | 57.5 |
| TIMI risk score, >1 | 60 | 42.6 | 63 | 44.7 |
| Previous coronary disease | 28 | 19.9 | 29 | 20.6 |
| Previous MI | 21 | 14.9 | 24 | 17 |
| Previous PCI | 14 | 9.9 | 19 | 13.5 |
| Previous CABG | 7 | 5.0 | 3 | 2.1 |
| Previous cerebral vascular disease | 3 | 2.1 | 9 | 6.4 |
| Previous peripheral vascular disease | 4 | 2 8 | 4 | 2.8 |
| Insurance status | ||||
| Insured | 105 | 74.5 | 106 | 76.3 |
| Private | 71 | 50.4 | 68 | 48.9 |
| Medicare | 21 | 14.9 | 21 | 15.1 |
| Medicaid | 13 | 9.2 | 17 | 12.2 |
| Uninsured | 36 | 25.5 | 33 | 23.7 |
BMI indicates body mass index; CABG, coronary artery bypass graft; MI, myocardial infarction; PCI, percutaneous coronary intervention; and TIMI, thrombolysis in myocardial infarction.
Table 2.
Frequency of HEART Pathway Determinants
| Risk stratification Measure | Number, n=141 | Percent |
|---|---|---|
| HEART score history | ||
| Slightly suspicious (0 points) | 52 | 36.9 |
| Moderately suspicious (1 point) | 54 | 38.3 |
| Highly suspicious (2 points) | 35 | 24.8 |
| Age | ||
| <45 (0 points) | 38 | 27 |
| 45–65 (1 point) | 80 | 56.7 |
| >65 (2 points) | 23 | 16.3 |
| ECG | ||
| Normal (0 points) | 79 | 56 |
| Nonspecifc changes (1 point) | 60 | 42.6 |
| Changes consistent with ACS (2 points) | 2 | 1.4 |
| Number of risk factors | ||
| 0 (0 points) | 16 | 11.4 |
| 1–2 (1 point) | 58 | 41.1 |
| ≥3 (2 points) | 67 | 47.5 |
| Troponin (initial) | ||
| Negative (0 points) | 133 | 94.3 |
| 1–3× normal limit (1 point) | 4 | 2.8 |
| >3× normal limit (2 points) | 4 | 2.8 |
| Total HEART score | ||
| 0 | 3 | 2.1 |
| 1 | 9 | 6.4 |
| 2 | 28 | 19.9 |
| 3 | 27 | 19.1 |
| 4 | 31 | 22 |
| 5 | 21 | 14.9 |
| ≥6 | 22 | 15.6 |
| Serial troponin at 3 h | ||
| Negative | 131 | 92.9 |
| Positive | 9 | 6.4 |
| Missing | 1 | 0.7 |
| HEART Pathway | ||
| Low risk (HEART score <3 and negative troponins at 0 and 3 h) | 66 | 46.8 |
| High risk (HEART score >3 or positive troponin at 0 or 3 h) | 75 | 53.2 |
ACS indicates acute coronary syndrome.
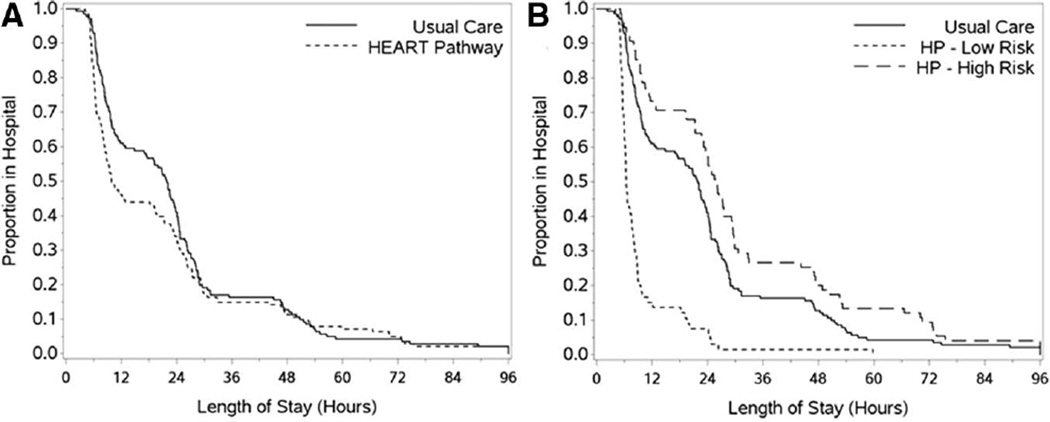
Patients randomized to the HEART Pathway had a 30-day objective cardiac testing rate of 56.7% (80/141) compared with a rate of 68.8% (97/141) in the usual care group: an absolute reduction of 12.1% (P=0.048). Early discharge occurred in 39.7% (56/141) of patients in the HEART Pathway arm compared with 18.4% (26/141): an absolute increase of 21.3% (P<0.001). Patients in the HEART Pathway group had a median LOS of 9.9 hours compared with 21.9 hours in the usual care group (Figure 3): a median reduction in LOS of 12 hours (P=0.013).
Figure 3.
Kaplan–Meier curves. A, Hospital length of stay by randomization arm. B, Hospital length of stay for HEART Pathway high- and low-risk groups versus usual care.
Within the HEART Pathway arm, 2.8% (4/141) had cardiac-related repeat ED visits compared with 4.3% (6/141) in the usual care arm (P=0.75). Cardiac-related nonindex hospitalizations occurred in 3.6% (5/141) of patients in the HEART Pathway arm compared with 2.8% (4/141) in the usual care arm (P>0.999).
MACE occurred in 17 of 282 patients, with all events occurring during their index visit.
No Patients identified for early discharge had missed MACE in either group during the 30-day follow-up period. No patients identified as low-risk by the HEART Pathway had an index or nonindex MACE. Index MACE occurred in 5.7% (8/141) patients in the HEART Pathway arm compared with 6.4% (9/141) in the usual care arm (P=1). Primary and secondary outcomes are summarized in Tables 3 and 4, respectively. The test characteristics of the HEART Pathway and serial troponins alone are summarized in Table 5. Nonadherence to the HEART Pathway occurred in 29% (19/66) of low-risk patients and 13% of (9/75) high-risk patients. None of the 19 low-risk patients had MACE at index or 30 days. Perfect adherence among high- and low-risk patients would have increased the early discharge rate to 46.8% (66/141).
Table 3.
Objective Cardiac Testing at 30 Days
| HEART Pathway |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low-Risk Patients |
High-Risk Patients |
Total |
Usual Care |
||||||
| Outcomes | Number, n=66 | Percent | Number, n=75 | Percent | Number, n=141 | Percent | Number, n=141 | Percent | P Value* |
| Objective cardiac testing at 30 days | 21 | 31.8 | 59 | 78.7 | 80 | 56.7 | 97 | 68.0 | 0.048 |
| Positive | 2 | 3.0 | 11 | 14.7 | 13 | 9.2 | 10 | 7.1 | 0.66 |
| Negative | 19 | 28.8 | 48 | 64.0 | 67 | 47.5 | 87 | 61.7 | 0.023 |
| Type of objective cardiac testing | |||||||||
| CCTA | 1 | 1.5 | 1 | 1.3 | 2 | 1.4 | 5 | 3.5 | 0.45 |
| ≥50% coronary stenosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | >0.999 |
| <50% coronary stenosis | 0 | 0 | 1 | 1.3 | 1 | 0.7 | 0 | >0.999 | |
| No coronary stenosis | 1 | 1.5 | 0 | 0 | 1 | 0.7 | 5 | 3.5 | 0.21 |
| Indeterminate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | … |
| Nuclear imaging | 0 | 0 | 2 | 2.7 | 2 | 1.4 | 2 | 1.4 | >0.999 |
| Positive | 0 | 0 | 2 | 2.7 | 2 | 1.4 | 1 | 0.7 | >0.999 |
| Negative | 0 | 0 | 0 | 0 | 2 | 1.4 | 1 | 0.7 | >0.999 |
| Exercise stress echocardiogram | 18 | 27.3 | 25 | 33.3 | 43 | 30.5 | 56 | 39.7 | 0.13 |
| Positive | 2 | 3.0 | 2 | 2.7 | 4 | 2.8 | 2 | 1.4 | 0.68 |
| Negative | 16 | 24.2 | 22 | 29.3 | 38 | 27.0 | 54 | 38.3 | 0.057 |
| Nondiagnostic | 0 | 0 | 1 | 1.3 | 1 | 0.7 | 0 | 0 | >0.999 |
| Dobutamine stress echocardiogram | 2 | 3.0 | 13 | 17.3 | 15 | 10.6 | 23 | 16.3 | 0.22 |
| Positive | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.7 | >0.999 |
| Negative | 2 | 3.0 | 13 | 17.3 | 15 | 10.6 | 21 | 14.9 | 0.37 |
| Nondiagnostic | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.7 | >0.999 |
| CMR | 0 | 0 | 7 | 9.3 | 7 | 9.3 | 2 | 1.4 | 0.17 |
| Positive | 0 | 0 | 1 | 1.3 | 1 | 1.3 | 0 | 0 | >0.999 |
| Negative | 0 | 0 | 6 | 8.0 | 6 | 8.0 | 2 | 1.4 | 0.28 |
| Angiography | 0 | 0 | 15 | 20.0 | 15 | 10.7 | 11 | 7.8 | 0.54 |
| Coronary stenosis present, ≥70% | 0 | 0 | 9 | 12.0 | 9 | 6.4 | 7 | 5.0 | 0.80 |
| Coronary stenosis present, <70% | 0 | 0 | 4 | 5.3 | 4 | 2.8 | 3 | 2.1 | >0.999 |
| No coronary stenosis | 0 | 0 | 2 | 2.7 | 2 | 1.4 | 1 | 0.7 | >0.999 |
CCTA indicates coronary computed tomographic angiography; and CMR, cardiac magnetic resonance.
P value for comparison of the HEART Pathway total vs usual care.
Table 4.
Safety Events and Healthcare Utilization Outcomes
| HEART Pathway |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low-Risk Patients |
High-Risk Patients |
Total |
Usual Care |
||||||
| Outcomes | Number, n=66 | Percent | Number n=75 | Percent | Number, n=141 | Percent. | Number, n=141 | Percent | P Value* |
| Index length of stay, h; median (IQR) | 6.4 (5.6–8.8) | … | 25.9 (11.4–46.7) | … | 9.9 (6.3–26.4) | … | 21.9 (8.4–28.2) | … | 0.013 |
| Index visit disposition | |||||||||
| Hospitalization | 19 | 28.8 | 66 | 88.0 | 85 | 60.3 | 110 | 78.1 | 0.002 |
| Observation unit | 18 | 27.3 | 25 | 33.3 | 43 | 30.5 | 62 | 44.0 | 0.31 |
| Inpatient ward (admission) | 1 | 1.5 | 41 | 54.7 | 42 | 29.8 | 48 | 34.0 | 0.52 |
| Discharge | 47 | 71.2 | 8 | 10.7 | 55 | 39.0 | 31 | 22.0 | 0.003 |
| AMA | 0 | 0 | 1 | 1.3 | 1 | 0.7 | 0 | 0 | >0.999 |
| Early discharge | 47 | 71.2 | 9 | 12.0 | 56 | 39.7 | 26 | 18.4 | 0.0001 |
| Recurrent hospital care at 30 days | |||||||||
| Repeat ED visit | 2 | 3.0 | 8 | 10.7 | 10 | 7.1 | 18 | 12.8 | 0.16 |
| Cardiac related | 0 | 0 | 4 | 5.3 | 4 | 2.8 | 6 | 4.3 | 0.75 |
| Nonindex hospitalization | 1 | 1.5 | 8 | 10.7 | 9 | 6.4 | 9 | 6.4 | >0.999 |
| Cardiac related | 0 | 0 | 5 | 6.7 | 5 | 3.6 | 4 | 2.8 | >0.999 |
| MACE at 30 days | |||||||||
| Cardiovascular death | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | … |
| MI | 0 | 0 | 7 | 9.3 | 7 | 50 | 9 | 6.4 | 0.80 |
| With revascularization | 0 | 0 | 1 | 1.3 | 1 | 0.7 | 5 | 3.6 | 0.21 |
| PCI | 0 | 0 | 1 | 1.3 | 1 | 0.7 | 4 | 2.8 | 0.37 |
| CABG | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.7 | >0.999 |
| Without revascularization | 0 | 0 | 1 | 1.3 | 1 | 0.7 | 0 | 0 | >0.999 |
| PCI | 0 | 0 | 1 | 1.3 | 1 | 0.7 | 0 | 0 | >0.999 |
| CABG | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | … |
AMA indicates against medical advice; CABG, coronary artery bypass graft; ED, emergency department; IQR, interquartile range; MACE, major adverse cardiac event; MI, myocardial infarction; and PCI, percutaneous coronary intervention.
Table 5.
Test Characteristics of the HEART Pathway and Serial Troponins
| Risk Stratification Strategy | Early Discharge (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|
| Serial troponins | 92.2% (87.8–96.6) | 87.5% (47.4–99.6) | 97.0% (92.5–99.2) | 63.6% (30.8–89.1) | 99.2% (95.8–100) |
| HEART Pathway | 39.7% (31.6–48.3) | 100% (63.1–100) | 49.6% (40.8–58.4) | 10.7% (4.7–19.9) | 100% (94.6–100) |
NPV indicates negative predictive values; and PPV, negative predictive values.
Discussion
Results of this trial demonstrate that the HEART Pathway substantively reduces healthcare utilization (objective cardiac testing, hospitalization, and hospital LOS) among patients with symptoms related to ACS. Among patients with acute chest pain, the HEART Pathway produced a meaningful reduction in objective cardiac testing, doubled the ED rate of early discharge, and reduced the hospital LOS by half a day. Furthermore, these reductions in utilization outcomes were accomplished without missing adverse cardiac events or increasing cardiac-related ED visits or nonindex hospitalizations.
This trial is the first to test the efficacy of the HEART Pathway or HEART score with real-time use. Previous studies have been observational or retrospective and assumed perfect provider adherence and application. Our trial adds greatly to our understanding of the performance of the HEART Pathway and HEART score by closely approximating their real-world use. Care delivered in both treatment arms was determined by the care provider’s discretion and not mandated by the trial protocol. Thus, the HEART Pathway was used, consistent with its intent, as a decision aid rather than a substitute for clinical judgment. Provider nonadherence to the HEART Pathway occurred in 29% (19/66) of low-risk patients, which is similar to nonadherence rates reported in other clinical decision aid studies.28 It is worth noting that none of these 19 patients had MACE at index or 30 days, and adherence among these patients would have increased the early discharge rate to 47%. Despite suboptimal adherence, real-world use of the HEART Pathway significantly reduced healthcare utilization outcomes relative to usual care.
When the results of this trial are considered in the context of previous HEART Pathway, HEART score, and other chest pain risk stratification decision aid studies, there is now strong evidence to support structured implementation of the HEART Pathway. The HEART score has been examined in >6000 patients and has demonstrated a high NPV for MACE at 6 weeks exceeding 98%.17–19 The HEART Pathway (which adds serial troponin measurements at 0 and 3 hours to the HEART score) has a higher sensitivity and NPV for adverse cardiac events than the HEART score alone.14 Previous studies of the HEART Pathway among patients identified for chest pain OU care demonstrated 100% sensitivity and NPV for MACE at 30 days and an early discharge rate of 82% in a low-risk cohort.14 Among 1005 patients in the Myeloperoxidase In the Diagnosis of Acute Coronary Syndromes Study (MIDAS),29 a multicenter cohort of patients with suspected ACS and planned objective cardiac testing, the HEART Pathway was 99% sensitive for ACS (cardiac death, myocardial infarction, or unstable angina) within 30 days with a NPV of >99% and an early discharge rate of 20%. Lower early discharge rates in MIDAS can be explained by the high prevalence of ACS events in the MIDAS cohort (22% incidence of ACS).15
There is also evidence that the HEART Pathway compares favorably with other methods of chest pain risk stratification. In the MIDAS cohort, the HEART Pathway had superior risk stratification performance than serial troponin results alone, an unstructured clinician assessment combined with serial troponin measures, and a competing chest pain decision aid (the North American Chest Pain Rule).15 Although the HEART Pathway has not been directly compared with the ADAPT 2-hour accelerated diagnostic protocol (ADAPT), recent evidence suggests that the HEART Pathway is likely to increase the early discharge rate relative to ADAPT without increasing missed MACE. A recent randomized controlled trial, enrolling a patient population similar to our trial (similar inclusion and exclusion criteria and MACE rates), demonstrated that ADAPT increased early discharge by only 8.3% (absolute) compared with usual care.28
Although the HEART Pathway decreased objective cardiac testing during 30 days, 12% (8/66) of the low-risk patients had objective testing completed as an outpatient during the 30-day follow-up period. We suspect that the main driver of outpatient objective testing among low-risk patients was a lack of comfort with risk stratification without objective cardiac testing among primary care physicians. This study did not include any formal outreach or HEART Pathway education to primary care providers. It is possible that outreach and education could have facilitated a greater decrease in outpatient objective testing among low-risk patients. Of the low-risk patients who received stress testing during the index visit or 30-day follow-up, 2 had reported inducible ischemia on stress echocardiography (1 during the index visit and 1 during follow-up). One of these patients went on to have a cardiac catheterization that demonstrated no coronary artery disease and the other was presumed to have a false-positive test by her cardiologist. Neither patient had MACE within the 30-day follow-up period.
Our trial has several limitations. Small sample size and enrollment from a single academic medical center may limit generalizability. This study was not powered to detect differences in MACE. However, given prior studies of the HEART score demonstrating high sensitivity for MACE, we feel it is unlikely that the safety of the 2 approaches differs. In addition, incomplete follow-up on 10 patients (4% of participants) may have caused misclassification and underestimation of MACE. However, none of these patients appeared in the Social Security Death Master File. Furthermore, given that all known MACE occurred during the index visit, the likelihood of MACE occurring shortly after discharge among these patients seems low. Nonadherence decreased the effect size of the HEART Pathway on healthcare utilization outcomes. However, by allowing provider nonadherence, this study provides a more accurate determination of the expected effect of HEART Pathway if implemented into clinical practice. Although interobserver agreement of the HEART Pathway was acceptable, some disagreements occurred. On the basis of our Institutional Review Board recommendations, if a disagreement occurred in which the attending provider determined the patient to be low-risk, but the study investigator found the patient to be high-risk, the attending provider was made aware of this discrepancy. Although this scenario was rare, unblinding in these cases may have influenced the study outcomes. In addition, the open-label nature of this trial may have resulted in contamination bias between randomization arms. Finally, more sensitive troponin assays are on the horizon than the 1 used in this analysis, but these assays have yet to be approved for clinical use in the United States. In spite of this limitation, the combination of serial troponins and clinical decision rules achieved high sensitivity for detection of MACE at 30 days. The performance of treatment arms combined with the highest sensitivity troponin assays is unclear. The HEART Pathway would be expected to maintain a high sensitivity for ACS, but the effect on specificity and early discharge rates is unknown.
Conclusions
Use of the HEART Pathway significantly decreased objective cardiac testing, resulted in an early discharge rate of ≈40%, and cut median LOS by 12 hours. No patients identified for early discharge had MACE at 30 days, and the HEART Pathway was not associated with increased cardiac-related return ED visits or nonindex hospitalizations. These important reductions in healthcare utilization outcomes were achieved despite suboptimal adherence to the HEART Pathway. The results of this small single-center trial require additional validation. However, when our results are considered in the context of previous HEART Pathway and HEART score analyses, there is strong evidence to support a multicenter trial of structured HEART Pathway implementation.
Supplementary Material
WHAT IS KNOWN
Current care patterns for low-risk patients with acute chest pain are ineffcient and expensive; they result in high hospitalization and stress testing rates while identifying few patients with acute coronary syndrome.
Prospective observational and retrospective studies suggest that the HEART Pathway can safely identify low-risk patients with acute chest pain for early discharge from the emergency department without stress testing or coronary angiography.
WHAT THE STUDY ADDS
This is the first clinical trial to examine the real-time use of the HEART Pathway to guide chest pain risk stratification and disposition decisions.
Use of the HEART Pathway at the Wake Forest Baptist Medical Center compared with usual care among patients with acute chest pain produced significant reductions in objective cardiac testing during 30 days, hospitalizations, and index hospital length of stay.
None of the patients identified for early discharge from the emergency department with the HEART Pathway had an adverse cardiac event at 30 days.
Acknowledgments
Sources of Funding
This study was funded by the American Heart Association (AHA) Clinical Research Program. The AHA had no role in the design, analysis, interpretation, or writing of this report. The corresponding author had complete access to all study data and final responsibility for article submission. Use of Research Electronic Data Capture was supported by the Wake Forest Translational Science Institute via a grant from National Center for Catalysis Research (M01 RR007122).
Footnotes
Disclosures
None.
References
- 1.Owens PL, Barrett ML, Gibson TB, Andrews RM, Weinick RM, Mutter RL. Emergency department care in the United States: a profile of national data sources. Ann Emerg Med. 2010;56:150–165. doi: 10.1016/j.annemergmed.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 2.Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, Leaming JM, Gavin LJ, Pacella CB, Hollander JE. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366:1393–1403. doi: 10.1056/NEJMoa1201163. [DOI] [PubMed] [Google Scholar]
- 3.Heller GV, Stowers SA, Hendel RC, Herman SD, Daher E, Ahlberg AW, Baron JM, Mendes de Leon CF, Rizzo JA, Wackers FJ. Clinical value of acute rest technetium-99m tetrofosmin tomographic myocardial perfusion imaging in patients with acute chest pain and nondiagnostic electrocardiograms. J Am Coll Cardiol. 1998;31:1011–1017. doi: 10.1016/s0735-1097(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 4.Roberts R, Kleiman NS. Earlier diagnosis and treatment of acute myocardial infarction necessitates the need for a ‘new diagnostic mind-set’. Circulation. 1994;89:872–881. doi: 10.1161/01.cir.89.2.872. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann U, Nagurney JT, Moselewski F, Pena A, Ferencik M, Chae CU, Cury RC, Butler J, Abbara S, Brown DF, Manini A, Nichols JH, Achenbach S, Brady TJ. Coronary multidetector computed tomography in the assessment of patients with acute chest pain. Circulation. 2006;114:2251–2260. doi: 10.1161/CIRCULATIONAHA.106.634808. [DOI] [PubMed] [Google Scholar]
- 6.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, Griffith JL, Selker HP. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342:1163–1170. doi: 10.1056/NEJM200004203421603. [DOI] [PubMed] [Google Scholar]
- 8.Pines JM, Isserman JA, Szyld D, Dean AJ, McCusker CM, Hollander JE. The effect of physician risk tolerance and the presence of an observation unit on decision making for ED patients with chest pain. Am J Emerg Med. 2010;28:771–779. doi: 10.1016/j.ajem.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Fleischmann KE, Goldman L, Johnson PA, Krasuski RA, Bohan JS, Hartley LH, Lee TH. Critical pathways for patients with acute chest pain at low risk. J Thromb Thrombolysis. 2002;13:89–96. doi: 10.1023/a:1016246814235. [DOI] [PubMed] [Google Scholar]
- 10.Gomez MA, Anderson JL, Karagounis LA, Muhlestein JB, Mooers FB. An emergency department-based protocol for rapidly ruling out myocardial ischemia reduces hospital time and expense: results of a randomized study (ROMIO) J Am Coll Cardiol. 1996;28:25–33. doi: 10.1016/0735-1097(96)00093-9. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction); American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; American Association of Cardiovascular and Pulmonary Rehabilitation; Society for Academic Emergency Medicine ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 2002 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Hermann LK, Weingart SD, Duvall WL, Henzlova MJ. The limited utility of routine cardiac stress testing in emergency department chest pain patients younger than 40 years. Ann Emerg Med. 2009;54:12–16. doi: 10.1016/j.annemergmed.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Redberg RF. Coronary CT angiography for acute chest pain. N Engl J Med. 2012;367:375–376. doi: 10.1056/NEJMe1206040. [DOI] [PubMed] [Google Scholar]
- 14.Mahler SA, Hiestand BC, Goff DC, Jr, Hoekstra JW, Miller CD. Can the HEART score safely reduce stress testing and cardiac imaging in patients at low risk for major adverse cardiac events? Crit Pathw Cardiol. 2011;10:128–133. doi: 10.1097/HPC.0b013e3182315a85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahler SA, Miller CD, Hollander JE, Nagurney JT, Birkhahn R, Singer AJ, Shapiro NI, Glynn T, Nowak R, Safdar B, Peberdy M, Counselman FL, Chandra A, Kosowsky J, Neuenschwander J, Schrock JW, Plantholt S, Diercks DB, Peacock WF. Identifying patients for early discharge: performance of decision rules among patients with acute chest pain. Int J Cardiol. 2013;168:795–802. doi: 10.1016/j.ijcard.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191–196. doi: 10.1007/BF03086144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backus BE, Six AJ, Kelder JC, Mast TP, van den Akker F, Mast EG, Monnink SH, van Tooren RM, Doevendans PA. Chest pain in the emergency room: a multicenter validation of the HEART score. Crit Pathw Cardiol. 2010;9:164–169. doi: 10.1097/HPC.0b013e3181ec36d8. [DOI] [PubMed] [Google Scholar]
- 18.Backus BE, Six AJ, Kelder JC, Bosschaert MA, Mast EG, Mosterd A, Veldkamp RF, Wardeh AJ, Tio R, Braam R, Monnink SH, van Tooren R, Mast TP, van den Akker F, Cramer MJ, Poldervaart JM, Hoes AW, Doevendans PA. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168:2153–2158. doi: 10.1016/j.ijcard.2013.01.255. [DOI] [PubMed] [Google Scholar]
- 19.Six AJ, Cullen L, Backus BE, Greenslade J, Parsonage W, Aldous S, Doevendans PA, Than M. The HEART score for the assessment of patients with chest pain in the emergency department: a multinational validation study. Crit Pathw Cardiol. 2013;12:121–126. doi: 10.1097/HPC.0b013e31828b327e. [DOI] [PubMed] [Google Scholar]
- 20.Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Jacobs AK. 2011 ACCF/ AHA focused update of the guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:2022–2060. doi: 10.1161/CIR.0b013e31820f2f3e. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor RE, Bossaert L, Arntz HR, Brooks SC, Diercks D, Feitosa-Filho G, Nolan JP, Vanden Hoek TL, Walters DL, Wong A, Welsford M, Woolfrey K. Acute Coronary Syndrome Chapter Collaborators. Part 9: acute coronary syndromes: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122(16 suppl 2):S422–S465. doi: 10.1161/CIRCULATIONAHA.110.985549. [DOI] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller CD, Hwang W, Case D, Hoekstra JW, Lefebvre C, Blumstein H, Hamilton CA, Harper EN, Hundley WG. Stress CMR imaging observation unit in the emergency department reduces 1-year medical care costs in patients with acute chest pain: a randomized study for comparison with inpatient care. JACC Cardiovasc Imaging. 2011;4:862–870. doi: 10.1016/j.jcmg.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollander JE, Blomkalns AL, Brogan GX, Diercks DB, Field JM, Garvey JL, Gibler WB, Henry TD, Hoekstra JW, Holroyd BR, Hong Y, Kirk JD, O’Neil BJ, Jackson RE, Aufderheide T, Blomkalns AL, Brogan GX, Christenson J, Collins S, Diercks DB, Fesmire FM, Garvey JL, Green GB, Lindsell CJ, Peacock WF, Pollack CV, Zalenski R. Multidisciplinary Standardized Reporting Criteria Task Force; Standardized Reporting Criteria Working Group of Emergency Medicine Cardiac Research and Education Group-International Standardized reporting guidelines for studies evaluating risk stratification of emergency department patients with potential acute coronary syndromes. Ann Emerg Med. 2004;44:589–598. doi: 10.1016/S0196064404012806. [DOI] [PubMed] [Google Scholar]
- 25.Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, Flaherty JT, Harrington RA, Krumholz HM, Simoons ML, Van De Werf FJ, IWeintraub WS, Mitchell KR, Morrisson SL, Brindis RG, Anderson HV, Cannom DS, Chitwood WR, Cigarroa JE, Collins-Nakai RL, Ellis SG, Gibbons RJ, Grover FL, Heidenreich PA, Khandheria BK, Knoebel SB, Krumholz HL, Malenka DJ, Mark DB, Mckay CR, Passamani ER, Radford MJ, Riner RN, Schwartz JB, Shaw RE, Shemin RJ, Van Fossen DB, Verrier ED, Watkins MW, Phoubandith DR, Furnelli T. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) J Am Coll Cardiol. 2001;38:2114–2130. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 26.Kline JA, Mitchell AM, Runyon MS, Jones AE, Webb WB. Electronic medical record review as a surrogate to telephone follow-up to establish outcome for diagnostic research studies in the emergency department. Acad Emerg Med. 2005;12:1127–1133. doi: 10.1197/j.aem.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernández-Avilés F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Joint ESC/ACCF/AHA/ WHF Task Force for the Redefinition of Myocardial Infarction Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 28.Than M, Aldous S, Lord SJ, Goodacre S, Frampton CM, Troughton R, George P, Florkowski CM, Ardagh M, Smyth D, Jardine DL, Peacock WF, Young J, Hamilton G, Deely JM, Cullen L, Richards AM. A 2-hour diagnostic protocol for possible cardiac chest pain in the emergency department: a randomized clinical trial. JAMA Intern Med. 2014;174:51–58. doi: 10.1001/jamainternmed.2013.11362. [DOI] [PubMed] [Google Scholar]
- 29.Diercks DB, Peacock WF, 4th, Hollander JE, Singer AJ, Birkhahn R, Shapiro N, Glynn T, Nowack R, Safdar B, Miller CD, Lewandrowski E, Nagurney JT. Diagnostic accuracy of a point-of-care troponin I assay for acute myocardial infarction within 3 hours after presentation in early presenters to the emergency department with chest pain. Am Heart J. 2012;163:74–80. doi: 10.1016/j.ahj.2011.09.028. e4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.