Abstract
Interactions between the dual BCR/ABL and Src inhibitor bosutinib and the Chk1 inhibitor PF-00477736 were examined in BCR/ABL+ leukemia cells, particularly imatinib-resistant cells, including those with the T315I mutation. Bosutinib blocked PF-00477736-induced ERK1/2 activation and sharply increased apoptosis in association with Mcl-1 inhibition, p34(cdc2) dephosphorylation, BimEL up-regulation, and DNA damage in imatinib-resistant CML or Ph+ ALL cell lines. Inhibition of Src or MEK1 by shRNA singnificantly enhanced PF-0047736 lethality. Bosutinib/PF-00477736 co-treatment also potentiated cell death in CD34+ CML patient samples, including dasatinib-resistant blast crisis cells exhibiting both T315I and E355G mutations, but was minimally toxic to normal CD34+ cells. Finally, combined in vivo treatment significantly suppressed BaF3/T315I tumor growth and prolonged survival in an allogeneic mouse model. Together, these findings suggest that this targeted combination strategy warrants attention in IM-resistant CML or Ph+ALL.
Keywords: chronic myeloid leukemia, BCR/ABL, bosutinib, Chk1 inhibitor
Introduction
Chronic myelogenous leukemia (CML) and Ph+ acute lymphoplastic leukemia (ALL) treatment has been revolutionized by the BCR/ABL kinase inhibitor imatinib mesylate (IM) and second-generation dual Src-BCR/ABL inhibitors e.g., dasatinib and bosutinib [1;2]. Unfortunately, resistance to imatinib or other kinase inhibitors develops, often due to “gatekeeper” or contact region point mutations (e.g., T315I) [3]. While Ponatinib (AP24534, Iclusig®) targets the T315I mutation, vascular toxicities limit its usage [4]. Consequently, novel strategies are urgently needed in this setting. Bosutinib (SKI-606) is an oral, dual-competitive Src and ABL tyrosine kinase inhibitor (TKI) approved for adult CML patients resistant or intolerant to prior therapy.
Cell cycle checkpoints ensure cell-cycle arrest following DNA damage, permitting repair or triggering apoptosis if damage is irreparable, preserving genomic integrity [5;6]. Checkpoint responses are initiated by ATM (Ataxia telangiectasia mutated) and ATR (Ataxia telangiectasia and Rad3-related), which induce the checkpoint kinases Chk1 and Chk2 and phosphorylation of proteins that initiate cell-cycle arrest. Chk1 is activated by ATR phosphorylation at Ser345 and Ser317, and phosphorylates phosphatase cdc25A/C, targeting it for ubiquitin-mediated degradation [7] and preventing dephosphorylation/activation of cdk2 and cdk1, triggering cell cycle arrest. Chk1 inhibition itself induces DNA damage by disrupting DNA replication [8]. PF-00477736 is a selective small molecule Chk1 inhibitor which abrogates the intra-S and G2-M checkpoints, thereby sensitizing cells to DNA damage [9]. PF-00477736 potentiates genotoxic agent lethality in solid tumor cells and xenograft models, and is in phase 1 clinical trials combined with gemcitabine [10].
We reported that MEK1/2 inhibitors interacted synergistically with Chk1 inhibitors, including the multi-kinase inhibitor UCN-01 and the more specific Chk1 inhibitor AZD7762 in human myeloid leukemia and multiple myeloma cells [11-13]. Similar interactions were observed in human multiple myeloma cells exposed to UCN-01 and the dual Src/BCR-ABL inhibitor dasatinib in vitro and in vivo [14]. Such interactions reflect the ability of Src inhibitors to block cytoprotective ERK1/2 activation in response to Chk1 inactivation [15]. Here we assessed interactions between the Src/ABL inhibitor bosutinib and the clinically relevant and selective Chk1 inhibitor (PF-00477736) in BCR/ABL+ CML or ALL cells, focusing on highly IM-resistant models exhibiting kinase mutations. Our results demonstrate synergistic in vitro and in vivo interactions between bosutinib and PF-00477736 in imatinib-resistant CML and Ph+ ALL (but not normal) cells, and suggest that enhanced cell killing involves a BCR/ABL-independent mechanism.
Materials and Methods
Cell lines
BaF3/BCR-ABL/T315I (BaF3/T315I), K562 and LAMA cells were obtained as previously described [16]. Adult/T315I and BV173/E255K IM-resistant cells were generated as before [17]. All cells were cultured in RPMI1640 medium containing 10% fetal bovine serum (FBS).
Patient samples
Bone marrow or peripheral blood was obtained with informed consent from CML patients. CD34+ cells were separated and the BCR/ABL-ATP binding site sequenced as previously described [17]. Normal cord blood CD34+ cells were processed as above. All studies were approved by the VCU Investigational Review Board.
Reagents
Bosutinib and PF-00477736 (PF) were provided by Pfizer Pharmaceuticals (NY). Src knockdown (shSrc/pLKO.1) plasmids were purchased from Thermo Scientific (Waltham, MA). shBim/pSR, shCont (empty vector) and shMEK1/tDt plasmids were generated as before [18].
Transfection
shMEK1/tDt and shBim/pSR plasmids were transfected by Amaxa nucleofector (Lonza, Koeln, Germany) according to protocol (Lonza, Walkersville, MD) [17]. Src (shSrc-pLKO.1) knockdown followed the Addgene (Cambridge, MA) protocol as before [19].
Monitoring apoptosis
Apoptosis was evaluated by either annexin V-fluorescein isothiocyanate (FITC) staining (BD Biosciences) or 7-aminoactinomycin D (7-AAD, Sigma–Aldrich) by flow cytometry as described previously [19].
Immunoblot (Western blot)
Western blot analysis was performed as before [17]. Primary antibodies included: cleaved caspase-3, cleaved PARP, p44/42 MAPK (Erk1/2) (Thr202/Tyr204), p-Src (Tyr416), Bim, BclxL (Cell Signaling, Beverly, MA); antiphosphotyrosine (4G10), γ-H2A.X, (Upstate, Lake Placid, NY); Bcl-2, Abl, p-cdc2 (Y15), Cdc2 (Santa Cruz, CA); and actin and tubulin (Sigma-Aldrich). Blots were stripped and re-probed with actin or tubulin antibodies to ensure equal loading and transfer of proteins.
Statistical analysis
The significance of differences between experimental conditions was determined by Student's t-test. PASW statistics 18 software was used to evaluate survival rates (Kaplan Meier). Synergism in cells exposed to a range of PF-00477736 and bosutinib concentrations (fixed ratio) was determined by Median Dose Effect analysis [20] and the Calcusyn software program. Combination Index values <1.0 denote synergistic interactions.
Animal studies
Animal studies (athymic nude mice; Jackson Laboratories) were approved by Virginia Commonwealth University's institutional animal care committee. 2 × 106 BaF3/T315I cells in 100 μL phosphate-buffered saline (PBS) were injected into the right legs. For in vivo studies, bosutinib was dissolved in 0.5% methylcellulose and 0.4% polysorbate 80 (Tween 80) and orally administered. PF-00477736 was dissolved in 50 nM sodium acetate buffer and 4% dextrose (pH=4) and administered intraperitoneally (IP). Drugs were given 5 days/week. Mice were monitored for tumor growth every other day by caliper measurement. Tumor volumes were calculated using the formula (length × width2)/2. When tumor length or width reached 20 mm, mice were euthanized in accordance with institutional guidelines.
Results
PF-00477736 (PF) enhances bosutinib lethality in imatinib-resistant or sensitive cells
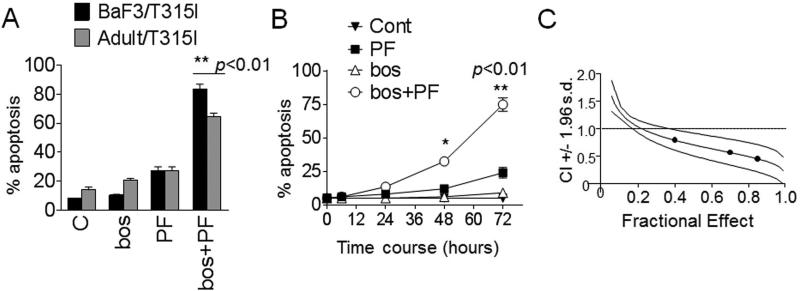
Exposure of highly IM-resistant Adult/T315I or BaF3/T315I cells (72 hr) to 0.3-0.4 mol/L PF or bosutinib 1.4 mol/L alone minimally induced cell death (i.e, less than 25%). However, combined PF/bosutinib treatment robustly induced apoptosis in both cell lines (~ 65-75%; Fig. 1A). Time-course analysis indicated that simultaneous exposure of BaF3/T315I to 0.4 mol/L PF and 1.4 mol/L bosutinib minimally induced apoptosis at relatively early time points (e.g. 24 hr), but triggered extensive cell death at later intervals (48-72 hr; Fig 1B). Median dose effect analysis of apoptosis, in which BaF3/T315I cells were exposed to a range of PF and bosutinib concentration alone and in combination at a fixed concentration ratio, yielded CI values substantially less than 1.0, indicating synergistic interactions (Fig 1C).
Figure 1. PF-00477736 enhances bosutinib lethality in imatinib-resistant cells.
(A) Adult/T315I and BaF3/T315I cells were exposed to PF-00477736 (PF) 0.4 mol/L alone or in combination with 1.4 mol/L bosutinib (bos) for 72 hr, after which the percentage of apoptotic cells was determined by monitoring 7-AAD+ by flow cytometry. (B) BaF3/T315I cells were treated with a range of PF and bosutinib concentrations administered at a fixed ratio. At the end of this period, the percentage of 7AAD+ cells was determined by flow cytometry. CI values were determined in relation to the fractional effect by using a commercially available software program as described in Materials and Methods. CI values less than 1.0 correspond to a synergistic interaction. (C) BaF3/T315I cells were treated with PF (0.4 M) or bosutinib (1.4 M) individually or in combination for the indicated intervals, after which the extent of cell death was determined. Values represent the means for three separate experiments performed in triplicate ± SD. * p < 0.05, ** p < 0.01, significantly greater than values for single agent treatment.
Similar interactions were observed in other IM-sensitive CML or Ph+ALL cell lines. Concomitant exposure of K562, LAMA, BV173/E255K cells to relatively low bosutinib concentrations (20-150 nmol/L) and minimally toxic PF concentrations (0.05-0.3 mol/L) significantly increased apoptosis compared to single agents in all cases (Fig 1S).
Bosutinib blocks PF–induced ERK1/2 activation and cleavage of caspase-3 and PARP but not BCR/ABL signaling
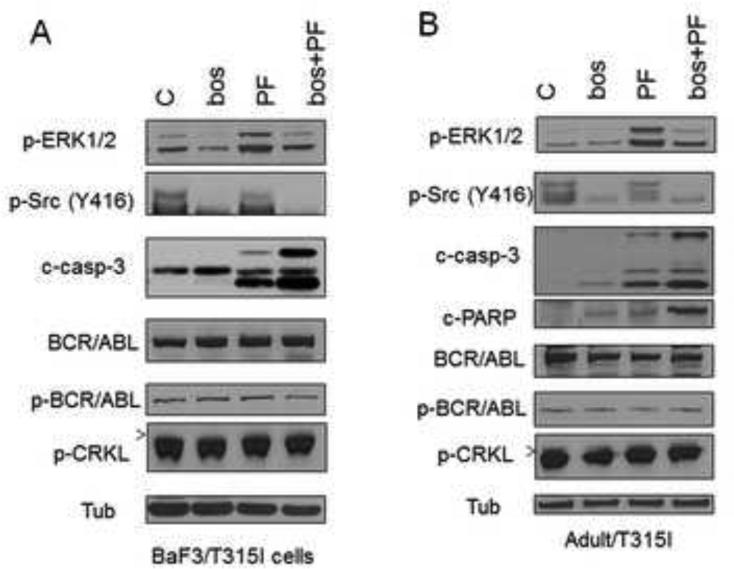
Exposure of Adult/T315I or BaF3/T315I cells to 0.4 mol/L PF and 1.4 mol/L bosutinib individually had little effect on procaspase-3 activation or PARP cleavage, whereas the effects of combined exposure of PF with bosutinib were pronounced (Fig 2).
Figure 2. Bosutinib blocks PF–induced ERK1/2 activation in association with induction of cleavage of caspase-3 and PARP.
(A) BaF3/T315I and (B) Adult/T315I cells were treated with bosutinib (1.4 μM) ± PF (0.4 μM) for 36 hr, after which cells were lysed and subjected to Western blot analysis. Expression of the indicated proteins was determined by Western blotting using the indicated antibodies. Each lane was loaded with 25 μg of protein; blots were stripped and re-probed with antibodies to tubulin to ensure equivalent loading and transfer. Results are representative of three independent experiments.
Next, the effects of combined treatment with PF and bosutinib on various signaling pathways, including those related to BCR/ABL, were then investigated. In highly IM-resistant BaF3/T315I and Adult/T315I cells, bosutinib alone or the PF/bosutinib combination did not inhibit BCR/ABL activation (phosphorylation) or that of its downstream signaling target, p-CRKL (Fig 2). Notably, exposure of BaF3/T315I and Adult/T315I cells to the Chk1 inhibitor PF (0.3-0.4 mol/L) resulted in ERK1/2 phosphorylation/activation as a possible cytoprotective response (Fig 2), consistent with previous findings involving UCN-01 [11;15;21;22]. Notably, bosutinib co-administration sharply attenuated PF–induced ERK1/2 phosphorylation in BaF3/T315I and Adult/T315I (Fig 2), analogous to the ability of the Src inhibitor dasatinib to block UCN-01-mediated ERK1/2 activation in multiple myeloma cells [14].
The PF/bosutinib regimen induces Bim expression, down-regulates Mcl-1, and promotes p34cdc2 activation and DNA damage
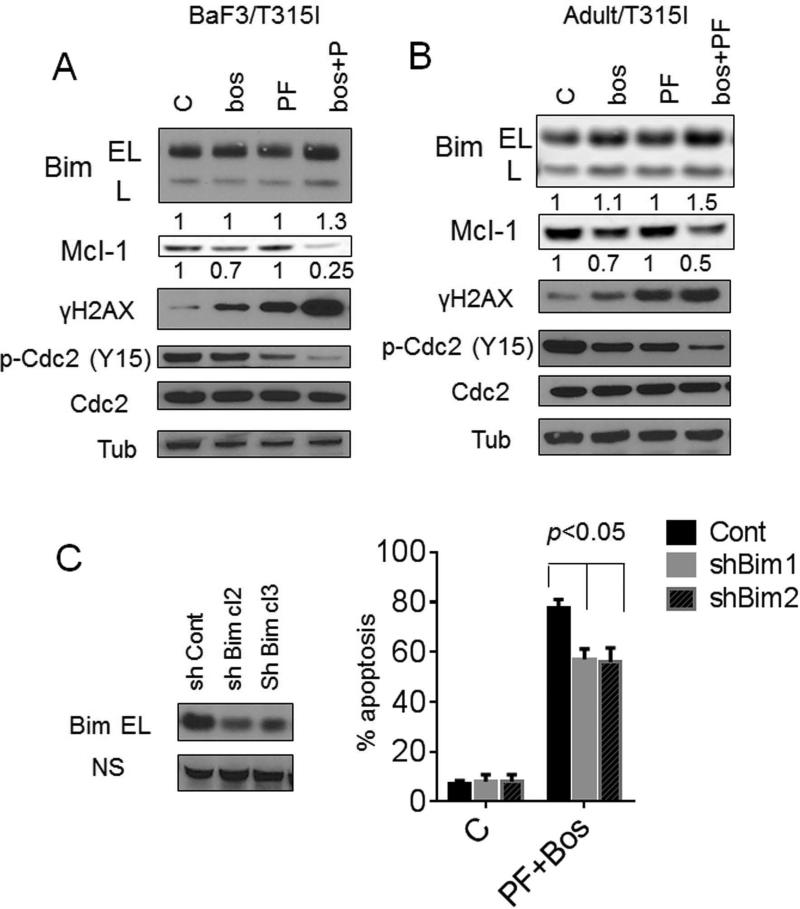
Consistent with our previous findings involving dasatinib in multiple myeloma cells [14], exposure of BaF3/T315I cells to PF or bosutinib alone or in combination did not modify Bcl-2 or Bcl-xL expression. However bosutinib/PF co-exposure modestly but discernibly up-regulated BimEL and Bim-L, accompanied by more pronounced down-regulation of Mcl-1 (Fig 3A). The latter event was minimally affected by a pan-caspase inhibitor (Boc-fmk; data not shown), arguing that Mcl-1 down-regulation does not simply reflect caspase-dependent degradation. Similar results were observed in Adult/T315I cells (Fig 3B). Bosutinib/PF co-exposure markedly potentiated dephosphorylation (activation) of p34cdc2 at inhibitory sites, and increased γH2A.X expression, an indicator of double-stranded DNA breaks (Fig 3A and B). To determine whether Bim contributed to PF/bosutinib lethalilty, BaF3/T315I cells were stably transfected with shRNA Bim/pSR or shCont/pSR., shBim-BaF3/T315I cells exhibited significantly reduced susceptibility to PF/bosutinib lethality compared to shCont-BaF3/T315I cells (p <0.05; Fig 3C), supporting a role for Bim in the lethality of this regimen.
Figure 3. Bosutinib/PF induces Bim up-regulation and Mcl-1 down-regulation while promoting p34cdc2 activation and DNA damage.
BaF3/T315I (A) and Adult/T315I (B) cells were treated with 1.4 μM bosutinib alone or with PF (0.4 μM) for 36 hr after that cells were lysed and applied to Western blot. Expression of the indicated proteins was determined by Western blotting using the indicated antibodies. Each lane was loaded with 25 μg of protein; blots were stripped and reprobed with antibodies to tubulin to ensure equivalent loading and transfer. Values below the blots indicate densitometric determinations relative to untreated controls. (C) BaF3/T315I cells were stably transfected with shRNA-Bim or shRNA-control constructs in a pSUPER.retro vector. Two clones displaying reduced endogenous expression of Bim were isolated and used in subsequent experiments. The cells were exposed to PF/bosutinib for 72 hr, after which the percentage of apoptotic cells was determined by 7-AAD+ (p < 0.05, significantly greater than values for single-agent treatment). All values represent the means ± SD for duplicate determinations performed on 3 separate occasions.
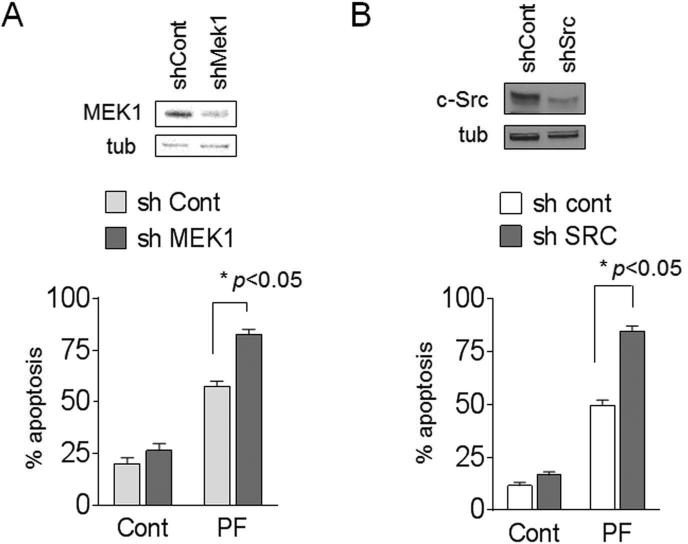
MEK1 or Src shRNA knockdown potentiates PF lethality
To elucidate the functional role of Src and MEK1 in PF responses, BaF3/T315I cells were transiently transfected with shRNAs targeting Src or MEK1. BaF3/T315I cells transfected with a construct encoding MEK1 shRNA exhibited marked down-regulation of MEK1 expression (Fig 4A). MEK1 knockdown significantly sensitized BaF3/T315I cells to PF lethality compared to controls (p<0.05). Similarly, Src shRNA knockdown in BaF3/T315I cells also significantly increased PF lethality compared to scrambled sequence controls (p<0.05; Fig 4B). These and the preceding findings argue that interruption of the Src/MEK/ERK signaling pathway e.g., by bosutinib increases PF lethality and suggest that regimen lethality does not depend upon BCR/ABL inactivation.
Figure 4. Inhibition of either MEK1 or Src by shRNA enhances PF lethality.
BaF3/T315I cells were transiently transfected with shCont or (A) shMEK1 or (B) sh-Src. Forty eight hours after transfection, cells were analyzed by WB and/or exposed to 0.4 M PF for 48 hr, after which the percentage of apoptotic cells was determined by 7-AAD+ (* p < 0.05 vs controls).
In vivo interactions between PF-00477736 and bosutinib against IM-resistant BaF3/T315I cells
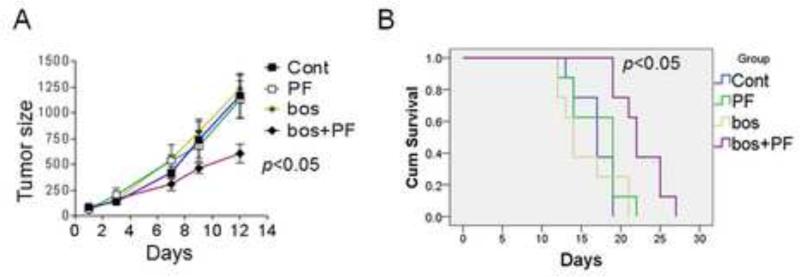
The in vivo activity of the bosutinib/PF against IM-resistant cells was then investigated. 2.5 × 106 BaF3/T315I cells were injected into nude mice in the right leg; after 3-4 days, when tumors were detectable, mice were grouped and treated with bosutinib 150 mg/kg/d p.o. with or without PF 11 mg/kg/d i.p. for 5 days/week. Notably, bosutinib/PF co-treatment significantly reduced tumor growth compared to control, bosutinib or PF treatment alone (p<0.05, Fig 5A). Mice treated with bosutinib/PF experienced a significant prolongation of median survival e.g., 21.5± 2.5 days compared to vehicle (16.8 ± 2.3 days), PF (17.3 ± 2.5 days) or bosutinib group (16 ± 3.5 days) (p<0.05 in all cases) (Fig 5B). Finally, treatment with agents alone or in combination did not induce significant weight loss (e.g., ≥ 10% compared to controls (data not shown).
Figure 5. PF potentiates bosutinib activity against IM-resistant leukemia in vivo.
(A) Mice inoculated with BaF3/T315I cells were treated with vehicle (blue), bosutinib (150 mg/kg p.o. once daily; green), PF (11 mg/kg i.p. daily (yellow), or the combination of PF+bosutinib (red). Drugs were administered 5 days per week. (B) Survival of mice treated as above was evaluated from the first day of treatment until death using Kaplan-Meier curves.
PF potentiates bosutinib lethality in a primary imatinib (and dasatinib)-resistant patient specimens, including those expressing the T315I mutation, but not in normal CD34+ cells
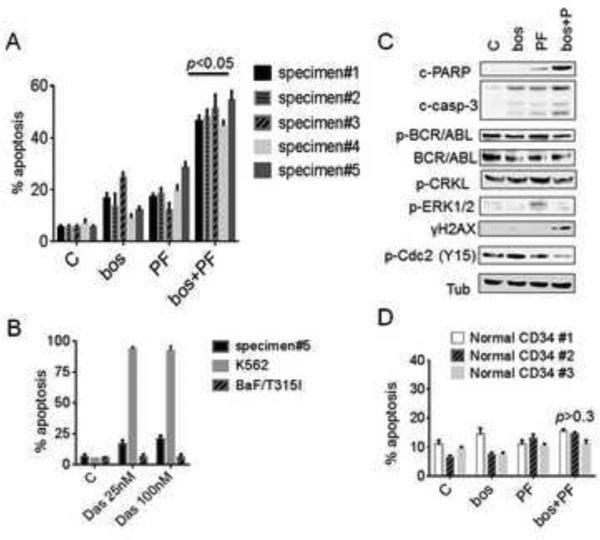
To extend findings to primary CML cells, parallel studies were carried out utilizing bone marrow- or peripheral blood-gated CD34+ cells from 5 patients with progressive chronic phase CML who had previously received IM, and one patient in CML-myeloid blast crisis. Exposure (48-72 hr) of CD34+ CML cells to 0.2–0.4 mol/L PF or 0.2-1.2 mol/L bosutinib individually induced minimal toxicity, whereas combined treatment triggered sharp increases in cell death (p<0.05). All samples underwent sequencing of the BCR/ABL kinase domain. Whereas specimens #1-4 did not display any mutations, BCR/ABL sequencing of cells obtained from a CML patient progressing to blast crisis while receiving dasatinib following imatinib failure (specimen #5) displayed both T315I and E355G mutations (Fig S2). Cells from specimen #5 exhibited marked in vitro resistance to dasatinib compared to un-mutated K562 cells, similar to results obtained with BaF3/T315I cells (Fig 6A). Notably, exposure of cells from specimen #5 to 0.4 mol/L PF or 1.2 mol/L bosutinib individually induce minimal lethality, whereas combined treatment produced significant increases in cell death (p<0.05, Fig 6B). Because a substantial number of blasts were available from this patient, Western blot analysis was performed. Whereas PF and bosutinib individually had little effect on procaspase-3 activation and PARP cleavage, the effects of combined PF/bosutinib exposure were pronounced (Fig. 6C). Consistent with cell line data, PF or bosutinib alone, or the combination of PF/bosutinib failed to inhibit BCR/ABL activation (phosphorylation) or that of its downstream target p-CRKL. Significantly, bosutinib co-administration substantially attenuated PF–induced ERK1/2 phosphorylation while enhancing DNA damage and dephosphorylation of p34cdc2, as in cell lines. Finally, the combination regimen exhibited only modest lethality toward three normal CD34+ cord blood samples compared to untreated controls (Fig 6D).
Figure 6. PF potentiates bosutinib lethality in primary patient specimens including the T315I mutation but not in normal CD34+ cells.
(A) CD34+ cells from 5 patients with CML were exposed to PF (0.2–0.4 mol/L) or 0.1-1.2 mol/L bosutinib individually or in combination for 48-72 hr, after which the percentage of apoptotic cells was determined by annexin V/PI using flow cytometry, (* p<0.05, significantly greater than values for single-agent treatment). All samples were sequenced in the BCR/ABL kinase domain. (B) Cells from specimen #5 (clinically dasatinib-resistant), K562 cells, amd BaF3/T315I cells were exposed to dasatinib 25 -100 nmol/L for 48 hr, after which the percentage of apoptotic cells was determined as above. (C) Cells from specimen #5 were treated with PF (0.4 mol/L), bosutinib (1.2 mol/L) or in combination for 30 hr, after which cells were lysed and subjected to Western blot analysis. (D) Normal cord blood CD34+ cells were isolated and exposed to PF 0.4 mol/L and/or bosutinib at a concentration of 1.2 mol/L for 48 hr, after which the percentage of apoptotic cells was determined by annexin V/PI positivity using flow cytometry. p > 0.3 = not significantly different compared to values for either agent alone.
Discussion
Bosutinib is an orally bioavailable Src/ABL TKI with activity against all phases of CML, including in some patients exhibiting resistance or intolerance to prior therapy e.g., IM [23]. However, TKIs such as imatinib, nilotinib, dasatinib and bosutinib are not recommended for patients with certain mutations in the ATP binding site, particularly T315I [24]. Furthermore, ponatinib and omacetaxine, which have been approved for use in patients with the T315I mutation, have vascular side effects which may limit their utility [25]. An alternative strategy to bypass this problem is to develop other approaches that act through BCR/ABL-independent mechanisms. Here, efforts were directed at circumventing resistance to the T315I mutation through a strategy combining bosutinib with the Chk1 inhibitor PF-00477736. The present results indicate that PF-00477736 sharply increases bosutinib lethality in BCR/ABL+ leukemia cells, and more specifically, in cells exhibiting marked resistance to TKIs through acquisition of the important, clinically relevant gatekeeper mutation (T315I). Notably, induction of cell death in these cells occurred independently of BCR/ABL inactivation, arguing that this process most likely involves engagement of cell death pathways which are not inhibited by the BCR/ABL pathway.
Chk1 inhibitors disable an important cell cycle checkpoint mechanism that allows cells to arrest and undergo DNA repair in order to maintain genomic integrity [7;9;26]. To date, Chk1 inhibitors have primarily been utilized to enhance the efficacy of genotoxic agents [22;27]. We previously reported that activation of the MEK1/2/ERK1/2 pathway represents an important compensatory, cytoprotective response to Chk1 inhibitors in human leukemia and multiple myeloma cells, and that interruption of MEK1/2 kinase function by either pharmacologic or genetic means blocked Chk1-inhibitor–induced ERK1/2 activation and potentiated apoptosis [28]. Subsequent studies demonstrated that disruption of Src, which acts upstream of the Ras/Raf/MEK/ERK pathway, by genetic means or by Src inhibitors such as dasatinib also enhanced Chk1 inhibitor lethality in multiple myeloma cells [14]. The present studies demonstrate that the dual BCR/ABL-Src inhibitor bosutinib may analogously potentiate Chk1 inhibitor lethality in BCR/ABL+ CML and ALL leukemia cells. Of note, in each case, interactions were associated with inactivation of ERK1/2 and up-regulation of the pro-apoptotic protein Bim, which is targeted for degradation by ERK1/2. The observations that shRNA knockdown of MEK1/2 or Src potentiated PF lethality, whereas shRNA knock-down of Bim significantly attenuated PF/bosutinib-induced apoptosis argue for functional contributions of these proteins to regimen lethality. It is also likely that down-regulation of Mcl-1, a protein important for the survival of myeloid leukemia cells [29], including CML cells [30], as well as dephosphorylation of cdc2, unscheduled activation of which has been associated with induction of cell death [31], may have contributed to the activity of this regimen.
A feature common to the present as well as previous studies performed in multiple myeloma cells is the marked increase in DNA damage accompanying simultaneous interference with the Src/MEK/ERK and Chk1 pathways [11]. It has been shown that Chk1 inhibition by itself triggers DNA damage [8], and the lethal consequences of this phenomenon may be amplified by MEK1/2/ERK1/2 pathway inhibition, which can trigger down-regulation of DNA repair proteins e.g. ERCC1 and XRCC1 [32]. Thus, simultaneous disruption of both checkpoint and repair pathways may amplify DNA damage and lethality. In this context, CML cells, including primitive progenitors, are characterized by enhanced susceptibility to DNA damage and genomic instability [33;34], which may render them particularly vulnerable to this approach.
It is noteworthy that the bosutinib/PF strategy was effective in an animal model of the T315I, IM-resistant BCR/ABL+ leukemia. Specifically, doses of bosutinib and PF that exerted essentially no effect on tumor growth or animal life-span significantly reduced tumor burden and prolonged animal survival. Such findings suggest that interactions observed in vitro are not restricted to this setting, but may also be observed in vivo. Importantly, the combination regimen exhibited little toxicity in treated animals, which may reflect, at least in part, the intrinsic vulnerability of neoplastic versus normal cells to checkpoint disruption [35].
Importantly, activity of the bosutinib/PF regimen was not limited to IM-resistant cell lines, but was also observed in multiple primary CD34+ CML cell samples obtained from patients who had progressed following IM therapy. The failure to detect mutations in the BCR/ABL kinase domain in 4 of these samples indicates that alternative resistance mechanisms may have been operative, and that such mechanisms were also vulnerable to the present strategy. Significantly, cells from a patient who entered blast crisis while receiving dasatinib displayed complex mutations i.e., both T315I and E355G, and exhibited in vitro resistance to dasatinib and bosutinib individually. However, combined treatment with bosutinib/PF resulted in a significant increase in apoptosis in these cells, accompanied by multiple events observed in IM-resistant cell lines e.g., inactivation of ERK1/2, increased expression of γH2A.X, and activation/dephosphorylation of cdc2. Notably, as also observed in cell lines, the regimen failed to induce inactivation of BCR/ABL or its downstream target, CRKL, consistent with the postulated BCR/ABL-independence of this strategy. Collectively, these findings suggest that interactions between PF and bosutinib observed in IM-resistant cell lines can be recapitulated in at least some primary BCR/ABL+ leukemia cells expressing the T315I mutation. Finally, the relative lack of toxicity of this regimen in normal CD34+ hematopoietic cells is consistent with intact checkpoints in and lack of dependence on the Src/MEK/ERK pathway for the survival of non-transformed cells [36;37].
In summary, the present studies indicate that the Chk1 inhibitor PF interacts synergistically with the dual ABL/Src inhibitor bosutinib in highly IM-resistant BCR/ABL+ CML and ALL cells through a BCR/ABL-independent process that may involve multiple mechanisms, including inactivation of ERK1/2 and Src, up-regulation of Bim, down-regulation of Mcl-1, activation of p34cdc2, and induction of DNA damage. The combination is active against BCR/ABL mutant cells (e.g T315I), despite the fact that it did not diminish activation of BCR/ABL or its downstream target CRKL. This finding argues that the regimen does not act by restoring the ability of bosutinib to inhibit BCR/ABL in mutant cells; instead, it acts through independent mechanisms to induce cell death. The present findings also demonstrate that this strategy preferentially kills leukemia cells, is active in vivo, and is effective in primary BCR/ABL+ leukemia cells, including those expressing the T315I mutation. Collectively, these observations argue that a regimen combining PF with bosutinib warrants attention in the setting of BCR/ABL+ leukemias unresponsive to alternative therapies. Accordingly, efforts to explore this possibility are currently underway.
Supplementary Material
Highlights.
Bosutinib/PF co-treatment synergically induces apoptosis in imatinib-resistant cells.
Bosutinib blocks PF-induced ERK1/2 activation and sharply increases apoptosis.
Bosutinib/PF suppresses BaF3/T315I tumor growth and prolongs survival.
Bosutinib/PF potentiates apoptosis in CML patient samples but not normal cells.
Acknowledgement
This study is supported by award CA100866 from the NIH (S. Grant), and award 6238-12 from the Leukemia and Lymphoma Society of America (T. Nguyen and S. Grant).
Abbreviations
- CML
chronic myeloid leukemia
- ALL
Acute lymphoblastic leukemia
- ERK
extracellular-signal-regulated kinases
- MEK
mitogen-activated protein kinase kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Contributions:
Conception and design: Tri Nguyen and Steven Grant
Performed the research: Tri Nguyen, Elisa Hawkins, Akhil Kolluri, Maciej Kmieciak, Haeseong Park, Hui Lin
Analyzed the data: Tri Nguyen
Wrote the paper: Tri Nguyen and Steven Grant
Study supervision: Steven Grant
Conflict of Interest Statement: The authors whose names are listed above certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Reference List
- 1.Quintas-Cardama A, Kantarjian H, Cortes J. Imatinib and beyond--exploring the full potential of targeted therapy for CML. Nat Rev Clin Oncol. 2009;6(9):535–43. doi: 10.1038/nrclinonc.2009.112. [DOI] [PubMed] [Google Scholar]
- 2.Quintas-Cardama A, Kantarjian H, Cortes J. Flying under the radar: the new wave of BCR-ABL inhibitors. Nat Rev Drug Discov. 2007;6(10):834–48. doi: 10.1038/nrd2324. [DOI] [PubMed] [Google Scholar]
- 3.O'Hare T, Eide CA, Deininger MW. Bcr-Abl kinase domain mutations, drug resistance and the road to a cure of chronic myeloid leukemia. Blood. 2007 doi: 10.1182/blood-2007-03-066936. [DOI] [PubMed] [Google Scholar]
- 4.Prasad V, Mailankody S. The accelerated approval of oncologic drugs: lessons from ponatinib. JAMA. 2014;311(4):353–4. doi: 10.1001/jama.2013.284531. [DOI] [PubMed] [Google Scholar]
- 5.Branzei D, Foiani M. Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol. 2010;11(3):208–19. doi: 10.1038/nrm2852. [DOI] [PubMed] [Google Scholar]
- 6.Lobrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer. 2007;7(11):861–9. doi: 10.1038/nrc2248. [DOI] [PubMed] [Google Scholar]
- 7.Dai Y, Grant S. New insights into Chk kinase 1 in the DNA damage repair (DDR) signaling network: rationale for employing Chk1 inhibitors in cancer chemotherapy. Clinical Cancer Research. 2010;16:376–83. doi: 10.1158/1078-0432.CCR-09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syljuasen RG, Sorensen CS, Hansen LT, Fugger K, Lundin C, Johansson F, et al. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol. 2005;25(9):3553–62. doi: 10.1128/MCB.25.9.3553-3562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blasina A, Hallin J, Chen E, Arango ME, Kraynov E, Register J, et al. Breaching the DNA damage checkpoint via PF-00477736, a novel small-molecule inhibitor of checkpoint kinase 1. Mol Cancer Ther. 2008;7(8):2394–404. doi: 10.1158/1535-7163.MCT-07-2391. [DOI] [PubMed] [Google Scholar]
- 10.Fang DD, Cao J, Jani JP, Tsaparikos K, Blasina A, Kornmann J, et al. Combined gemcitabine and CHK1 inhibitor treatment induces apoptosis resistance in cancer stem cell-like cells enriched with tumor spheroids from a non-small cell lung cancer cell line. Front Med. 2013;7(4):462–76. doi: 10.1007/s11684-013-0270-6. [DOI] [PubMed] [Google Scholar]
- 11.Dai Y, Landowski TH, Rosen ST, Dent P, Grant S. Combined treatment with the checkpoint abrogator UCN-01 and MEK1/2 inhibitors potently induces apoptosis in drug- sensitive and -resistant myeloma cells through an IL-6-independent mechanism. Blood. 2002;100(9):3333–43. doi: 10.1182/blood-2002-03-0940. [DOI] [PubMed] [Google Scholar]
- 12.Dai Y, Yu C, Singh V, Tang L, Wang Z, McInistry R, et al. Pharmacological inhibitors of the mitogen-activated protein kinase (MAPK) kinase/MAPK cascade interact synergistically with UCN-01 to induce mitochondrial dysfunction and apoptosis in human leukemia cells. Cancer Res. 2001;61(13):5106–15. [PubMed] [Google Scholar]
- 13.Pei XY, Dai Y, Youssefian LE, Chen S, Bodie WW, Takabatake Y, et al. Cytokinetically quiescent (G0/G1) human multiple myeloma cells are susceptible to simultaneous inhibition of Chk1 and MEK1/2. Blood. 2011;118(19):5189–200. doi: 10.1182/blood-2011-02-339432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai Y, Chen S, Shah R, Pei XY, Wang L, Almenara JA, et al. Disruption of Src function potentiates Chk1-inhibitor-induced apoptosis in human multiple myeloma cells in vitro and in vivo. Blood. 2011;117(6):1947–57. doi: 10.1182/blood-2010-06-291146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai Y, Chen S, Pei XY, Almenara JA, Kramer LB, Venditti CA, et al. Interruption of the Ras/MEK/ERK signaling cascade enhances Chk1 inhibitor-induced-DNA damage in vitro and in vivo in human multiple myeloma cells. Blood. 2008;112(6):2439–49. doi: 10.1182/blood-2008-05-159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen TK, Rahmani M, Harada H, Dent P, Grant S. MEK1/2 inhibitors sensitize Bcr/Abl+ human leukemia cells to the dual Abl/Src inhibitor BMS-354/825. Blood. 2007;109(9):4006–15. doi: 10.1182/blood-2006-09-045039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen T, Dai Y, Attkisson E, Kramer L, Jordan N, Nguyen N, et al. HDAC inhibitors potentiate the activity of the BCR/ABL kinase inhibitor KW-2449 in imatinib-sensitive or -resistant BCR/ABL+ leukemia cells in vitro and in vivo. Clin Cancer Res. 2011;17(10):3219–32. doi: 10.1158/1078-0432.CCR-11-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen TK, Jordan N, Friedberg J, Fisher RI, Dent P, Grant S. Inhibition of MEK/ERK1/2 sensitizes lymphoma cells to sorafenib-induced apoptosis. Leuk Res. 2010;34(3):379–86. doi: 10.1016/j.leukres.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen TK, Grant S. Dinaciclib (SCH727965) inhibits the unfolded protein response through a. Mol Cancer Ther. 2014;13(3):662–74. doi: 10.1158/1535-7163.MCT-13-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou TC. Drug combination studies and their synergy quantification using the Chou- Talalay method. Cancer Res. 2010;70(2):440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 21.Yu C, Dai Y, Dent P, Grant S. Coadministration of UCN-01 with MEK1/2 inhibitors potently induces apoptosis in BCR/ABL+ leukemia cells sensitive and resistant to ST1571. Cancer Biol Ther. 2002;1(6):674–82. doi: 10.4161/cbt.319. [DOI] [PubMed] [Google Scholar]
- 22.Ma CX, Ellis MJ, Petroni GR, Guo Z, Cai SR, Ryan CE, et al. A phase II study of UCN-01 in combination with irinotecan in patients with metastatic triple negative breast cancer. Breast Cancer Res Treat. 2013;137(2):483–92. doi: 10.1007/s10549-012-2378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill BG, Kota VK, Khoury HJ. Bosutinib: a third generation tyrosine kinase inhibitor for the treatment of chronic myeloid leukemia. Expert Rev Anticancer Ther. 2014:1–6. doi: 10.1586/14737140.2014.924400. [DOI] [PubMed] [Google Scholar]
- 24.Syed YY, McCormack PL, Plosker GL. Bosutinib: a review of its use in patients with Philadelphia chromosome-positive chronic myelogenous leukemia. BioDrugs. 2014;28(1):107–20. doi: 10.1007/s40259-013-0082-x. [DOI] [PubMed] [Google Scholar]
- 25.Hoy SM. Ponatinib: a review of its use in adults with chronic myeloid leukaemia or Philadelphia chromosome-positive acute lymphoblastic leukaemia. Drugs. 2014;74(7):793–806. doi: 10.1007/s40265-014-0216-6. [DOI] [PubMed] [Google Scholar]
- 26.Patil M, Pabla N, Dong Z. Checkpoint kinase 1 in DNA damage response and cell cycle regulation. Cell Mol Life Sci. 2013;70(21):4009–21. doi: 10.1007/s00018-013-1307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sausville E, Lorusso P, Carducci M, Carter J, Quinn MF, Malburg L, et al. Phase I dose- escalation study of AZD7762, a checkpoint kinase inhibitor, in combination with gemcitabine in US patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;73(3):539–49. doi: 10.1007/s00280-014-2380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pei XY, Dai Y, Tenorio S, Lu J, Harada H, Dent P, et al. MEK1/2 inhibitors potentiate UCN-01 lethality in human multiple myeloma cells through a Bim-dependent mechanism. Blood. 2007;110(6):2092–101. doi: 10.1182/blood-2007-04-083204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moulding DA, Quayle JA, Hart CA, Edwards SW. Mcl-1 expression in human neutrophils: regulation by cytokines and correlation with cell survival. Blood. 1998;92(7):2495–502. [PubMed] [Google Scholar]
- 30.Aichberger KJ, Mayerhofer M, Krauth MT, Skvara H, Florian S, Sonneck K, et al. Identification of mcl-1 as a BCR/ABL-dependent target in chronic myeloid leukemia (CML): evidence for cooperative antileukemic effects of imatinib and mcl-1 antisense oligonucleotides. Blood. 2005;105(8):3303–11. doi: 10.1182/blood-2004-02-0749. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu T, O'Connor PM, Kohn KW, Pommier Y. Unscheduled activation of cyclin B1/Cdc2 kinase in human promyelocytic leukemia cell line HL60 cells undergoing apoptosis induced by DNA damage. Cancer Res. 1995;55(2):228–31. [PubMed] [Google Scholar]
- 32.Li W, Melton DW. Cisplatin regulates the MAPK kinase pathway to induce increased expression of DNA repair gene ERCC1 and increase melanoma chemoresistance. Oncogene. 2012;31(19):2412–22. doi: 10.1038/onc.2011.426. [DOI] [PubMed] [Google Scholar]
- 33.Chakraborty S, Stark JM, Sun CL, Modi H, Chen W, O'Connor TR, et al. Chronic myelogenous leukemia stem and progenitor cells demonstrate chromosomal instability related to repeated breakage-fusion-bridge cycles mediated by increased nonhomologous end joining. Blood. 2012;119(26):6187–97. doi: 10.1182/blood-2011-05-352252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takagi M, Sato M, Piao J, Miyamoto S, Isoda T, Kitagawa M, et al. ATM-dependent DNA damage-response pathway as a determinant in chronic myelogenous leukemia. DNA Repair (Amst) 2013;12(7):500–7. doi: 10.1016/j.dnarep.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432(7015):316–23. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 36.Hubner A, Jaeschke A, Davis RJ. Oncogene addiction: role of signal attenuation. Dev Cell. 2006;11(6):752–4. doi: 10.1016/j.devcel.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Sharma SV, Gajowniczek P, Way IP, Lee DY, Jiang J, Yuza Y, et al. A common signaling cascade may underlie “addiction” to the Src, BCR-ABL, and EGF receptor oncogenes. Cancer Cell. 2006;10(5):425–35. doi: 10.1016/j.ccr.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.