Abstract
Oxidant stress drives nuclear factor κB (NF-κB) activation and NF-κB-dependent proinflammatory gene expression in endothelial cells during several pathological conditions, including ischemia/reperfusion injury. We showed that the Nck family of adaptor proteins linked tyrosine kinase signaling to oxidant stress-induced activation of NF-κB through the classic IκB kinase (IKK)-dependent pathway. Depletion of Nck prevented oxidant stress induced by exogenous peroxide or hypoxia/reoxygenation injury from triggering the activation of NF-κB in endothelial cells, increases in the abundance of the pro-inflammatory molecules ICAM-1 (intracellular adhesion molecule 1) and VCAM-1 (vascular cell adhesion molecule 1), and leukocyte recruitment. Nck depletion also attenuated endothelial cell expression of genes encoding proinflammatory factors, but not those encoding antioxidants. We further showed that Nck promoted oxidant stress-induced activation of NF-κB by coupling the tyrosine phosphorylation of platelet-endothelial cell adhesion molecule-1 (PECAM-1) to the activation of p21 activated kinase, which mediates oxidant stress-induced NF-κB signaling. Consistent with this model, treatment of mice subjected to ischemia/reperfusion injury in the cremaster muscle with a Nck inhibitory peptide inhibited leukocyte adhesion and emigration and the accompanying vascular leak. Together, these data identify Nck as an important mediator of oxidant stress-induced inflammation and a potential therapeutic target for ischemia/reperfusion injury.
Introduction
Oxidant stress contributes to inflammation in various cardiovascular pathologies including ischemia/reperfusion injury, diabetic complications, and atherosclerosis (1, 2). In endothelial cells, oxidant stress promotes increased endothelial permeability and expression of mRNAs encoding proinflammatory adhesion molecules (for example, intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1)) that mediate leukocyte homing (3, 4). The NF-κB family of redox-sensitive transcription factors classically mediate proinflammatory gene expression (5). The best-characterized NF-κB isoform consists of a p65 subunit (hereafter NF-κB) either as a homodimer or a heterodimer with a p50 subunit (5). Typically, proinflammatory stimuli activate the IκB kinase (IKK) complex to stimulate serine phosphorylation, ubiquitination, and degradation of inhibitory IκB proteins thereby allowing nuclear localization of NF-κB. IKK also phosphorylates NF-κB on Ser536 in the transactivation domain, enhancing its transcriptional activity (6).
Oxidant stress may activate NF-κB through both IKK-dependent and IKK-independent mechanisms (3, 4). Tyrosine phosphorylation mediates various oxidative stress-induced signaling responses, since oxidation of critical cysteine residues in the catalytic domain of tyrosine phosphatases inactivates the phosphatase domain thereby enhancing tyrosine phosphorylation (7). The tyrosine kinase inhibitor herbimycin A blunts NF-κB activation following hypoxia-reoxygenation or addition of exogenous H2O2 (8, 9); however, the role of tyrosine phosphorylation in oxidant stress-induced NF-κB activation remains unclear. Early work found that H2O2 stimulates IKK-independent NF-κB activation through direct IκB tyrosine phosphorylation in T cells (10, 11). Although high amounts of oxidant stress (300–500 μM H2O2) can induce tyrosine phosphorylation of IκB in endothelial cells (9, 12, 13), moderate oxidant stress activates canonical IKK-dependent NF-κB activation in certain cell types (3). Consistent with the latter model, endothelial responses to LPS, angiotensin II, and hemodynamic shear stress all require oxidant stress for canonical IKK-dependent activation of NF-κB, albeit through largely unknown mechanisms (14–16).
The Nck family of SH2 and SH3 domain-containing adaptor proteins (Nck1 and Nck2; Nck1/2) classically couple tyrosine kinase signaling to cytoskeletal remodeling responses during cell migration (17, 18). Nck1 and Nck2 share 68% amino acid identity, are present in nearly all cell types, and show both distinct and conserved downstream signaling partners (18). Nck1/2 recruitment to tyrosine phosphorylated proteins at the plasma membrane drives activation of the serine/threonine kinase p21-activated kinase (PAK) (19), and we have demonstrated a critical role for PAK in oxidant stress-dependent canonical NF-κB activation by shear stress (20). In the current work, we tested the hypothesis that Nck critically coupled oxidant stress-induced tyrosine phosphorylation to activation of the PAK and NF-κB pathways to drive proinflammatory responses.
Results
Oxidant stress activates Nck-dependent canonical NF-κB signaling
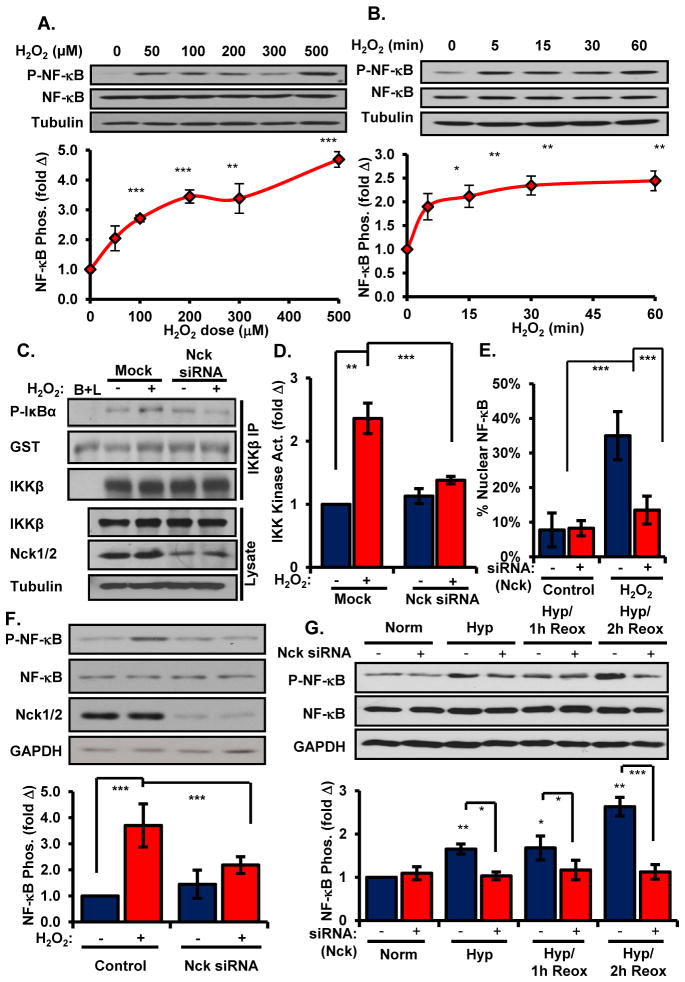
To determine how oxidant stress regulates endothelial NF-κB activation, we first examined the dose response and time course for H2O2-induced NF-κB activation in human aortic endothelial cells (HAECs). Treatment with a low dose of H2O2 (100 μM) was sufficient to induce phosphorylation of Ser536 in NF-κB (Figure 1A), indicative of IKK-dependent NF-κB activation, which was maximal by 5–15 minutes and sustained for at least 60 minutes (Figure 1B). In contrast, this dose of H2O2 did not increase tyrosine phosphorylation of IκB, as shown by Western blotting and immunoprecipitation with the anti-phosphotyrosine antibody 4G10 (21, 22) (Supplemental Figure 1A/B). Consistent with these phosphorylation patterns, low dose H2O2 treatment resulted in enhanced IKK activation within 15 minutes (Figure 1C/D), suggesting that low amounts of oxidant stress promote canonical NF-κB activation in endothelial cells. To determine whether Nck facilitates oxidant stress-induced NF-κB activation, we tested H2O2-induced NF-κB activation following siRNA-mediated knockdown of Nck1 and Nck2 (~75% knockdown, Supplemental Figure 2A). Treatment with Nck siRNA completely blunted oxidant stress-induced IKK kinase activity (Figure 1C/D). Consistent with Nck-dependent NF-κB activation, Nck siRNA also blunted H2O2-induced nuclear accumulation (Figure 1E, Supplemental Figure 2B) and phosphorylation (Figure 1F) of NF-κB.
Figure 1. Oxidant stress requires Nck for canonical NF-κB activation in endothelial cells.
(A) Phosphorylation of NF-κB in HAECs treated with increasing doses of H2O2 was determined by Western blotting. Phospho-NF-κB was normalized to total NF-κB and conveyed as a fold change compared to untreated conditions. (B) Phosphorylation of NF-κB in HAECs treated with H2O2 for the indicated times was determined as in (A). (C/D) IKK activity was determined in HAECs transfected with Nck siRNA and treated with H2O2. (E) HAECs were treated as in (C) and nuclear translocation of NF-κB activation was determined by immunofluorescence staining (fig. S2B). At least 100 cells per condition were scored for the presence or absence of nuclear NF-κB staining for each experiment. (F) Phosphorylation of NF-κB in HAECs treated as in (C) was determined by Western blotting. (G) Phosphorylation of NF-κB in HAECs treated with Nck siRNA and exposed to hypoxia followed by reoxygenation (Hyp./reox.) was determined by Western blotting. n=4–5 independent experiments in all panels. * p<0.05, ** p<0.01, *** p<0.001.
Reperfusion of ischemic tissues stimulates oxidant stress-dependent NF-κB activation (3, 4). We next sought to determine whether Nck mediated inflammation in the hypoxia/reoxygenation model of endogenous oxidant stress. HAECs were exposed to short term hypoxia (5% O2, 1 hr) followed by reoxygenation (21% O2, 2 hr), conditions that increase ICAM-1 and VCAM-1 abundance in oxidant stress-dependent manner (23). While reoxygenation enhanced NF-κB activation, Nck siRNA completely inhibited NF-κB activation in response to hypoxia/reoxygenation injury (Figure 1G).
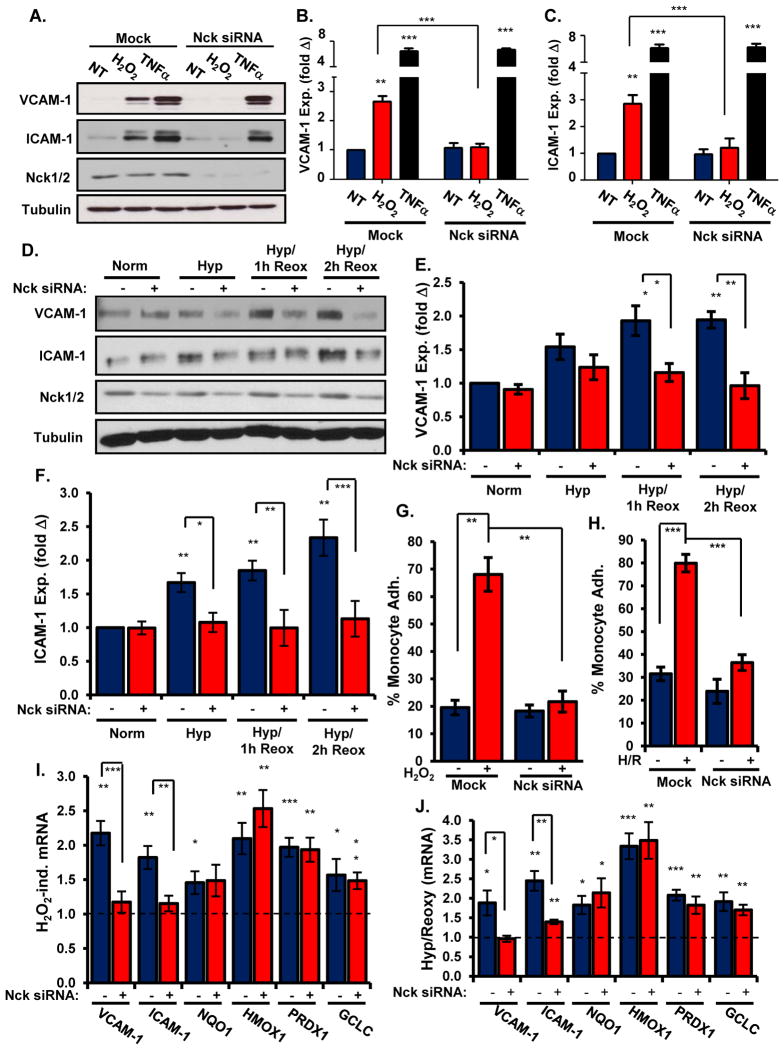
NF-κB activation drives proinflammatory gene expression (3, 4), and Nck siRNA effectively blocked increases in ICAM-1 and VCAM-1 abundance induced by H2O2 but not by the proinflammatory cytokine tumor necrosis factor-α (TNFα) (Figure 2A–C). Consistent with these results, addition of a membrane permeable peptide containing the Nck-binding sequence of PAK (Nck blocking peptide), but not a control peptide lacking critical proline residues (24), suppressed H2O2-induced increases in VCAM-1 abundance (Supplemental Figure 2C). Furthermore, Nck siRNA similarly blunted increases in both VCAM-1 (Figure 2D/E) and ICAM-1 (Figure 2D/F) quantity in cells exposed to hypoxia/reoxygenation injury. Multiple proinflammatory stimuli promote leukocyte-endothelial cell interactions through oxidant stress-induced increases in VCAM-1 and ICAM-1 abundance (3, 4). Whereas treating endothelial cells with either H2O2 (Figure 2G) or hypoxia/reoxygenation injury (Figure 2H) enhanced THP-1 monocyte adhesion, Nck depletion blunted adhesion of these cells following either stimulus (Figure 2G/H, Supplemental Figure 3A/B). Together, these data demonstrate that the Nck adaptor proteins couple oxidant stress to activation of the NF-κB pathway and proinflammatory gene expression in endothelial cells.
Figure 2. Depleting Nck expression blunts oxidant stress-induced endothelial activation and leukocyte recruitment.
(A–C) The abundance of VCAM-1 (A, B) or ICAM-1 (A, C) in HAECs transfected with Nck1/2 siRNA and treated with H2O2 or TNFα was determined by Western blotting. (D–F) The abundance of VCAM-1 (D, E) or ICAM-1 (D, F) in HAECs transfected with Nck siRNA and exposed to hypoxia/reoxygenation was determined by Western blotting. (G/H) HAECs transfected with Nck1/2 siRNA were exposed to H2O2 (G) or hypoxia/reoxygenation injury (H). The adhesion of Cell Tracker Green-labeled THP-1 monocytes was determined in static adhesion assays (fig. S3) and conveyed as percent of monocytes adhering under each condition. (I/J) HAECs transfected with Nck1/2 siRNA were exposed to H2O2 (I) or hypoxia/reoxygenation (J). Changes in the expression of genes encoding proinflammatory and antioxidant factors were determined by qRT-PCR. Results show the fold change in gene expression compared to untreated conditions. n=5 independent experiments in all panels. * p<0.05, ** p<0.01, *** p<0.001.
Nck siRNA does not affect expression of anti-oxidant genes following oxidant stress
In addition to inflammation, oxidative stress drives the expression of components in protective anti-oxidant response systems to alleviate the pathological consequences of future oxidant stress (25). The redox-sensitive transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2) promotes the expression of multiple antioxidant genes, such as NAD(P)H dehydrogenase quinone 1 (NQO1), heme oxygenase-1 (HMOX1), peroxyredoxin-1 (PRDX1), and the glutamate-cysteine ligase catalytic subunit (GCLC) (25, 26). Since oxidative stress activates the Keap1-Nrf2 signaling pathway directly through oxidative modification of critical cysteine residues in Keap1 (26), the Nck adaptors should not contribute to Nrf2 activation. Therefore, we next tested whether Nck depletion differentially affects the inflammatory and adaptive response to oxidant stress. H2O2 enhanced endothelial cell mRNA expression of both proinflammatory (VCAM-1, ICAM-1) and antioxidant genes (NQO1, HMOX1, PRDX1, GCLC) (Figure 2I). However, only proinflammatory genes showed significantly reduced expression following siRNA-mediated Nck depletion (Figure 2I). Similarly, Nck siRNA blunted inflammatory gene expression but not antioxidant gene expression following hypoxia-reoxygenation injury (Figure 2J). Together, these data show that the adaptive response to oxidant stress remains intact following Nck depletion.
Oxidant stress activates Nck signaling through tyrosine phosphorylation of PECAM-1
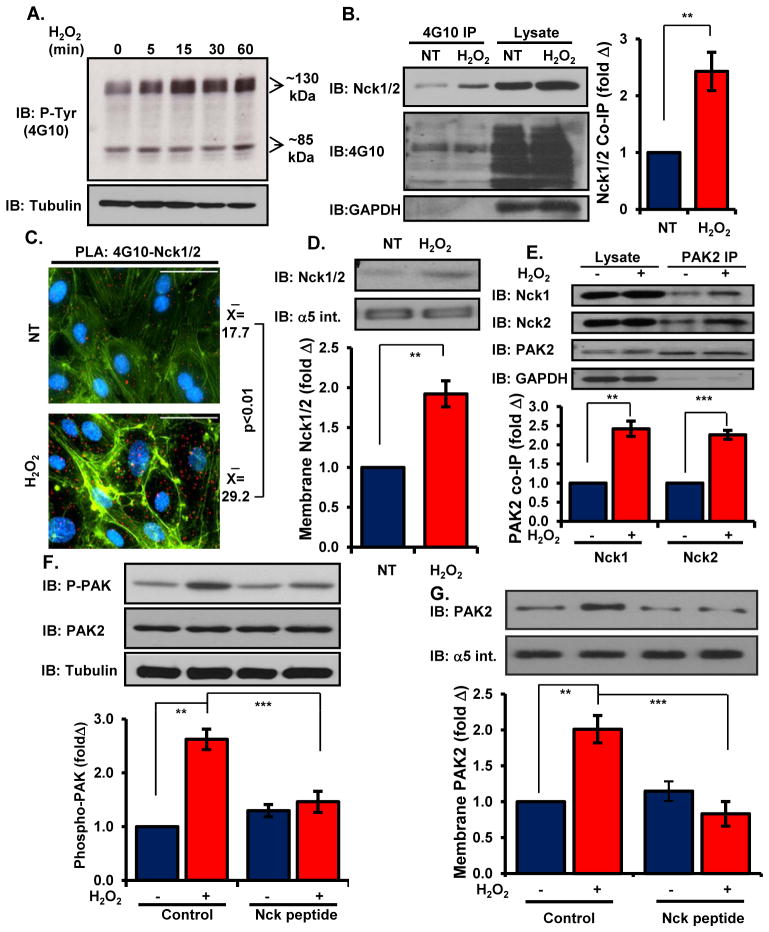
Lack of IκB tyrosine phosphorylation at low amounts of H2O2 did not indicate a reduced capacity for oxidant stress-induced tyrosine phosphorylation. Western blotting with the phospho-tyrosine-specific antibody 4G10 demonstrated that low-dose H2O2 treatment induced a 2-fold increase in total cellular tyrosine phosphorylation content by 15 minutes that continued to increase for 60 minutes (Figure 3A, Supplemental Figure 4A). This increase in tyrosine phosphorylation was accompanied by an increase in Nck co-immunoprecipitation with tyrosine phosphorylated proteins (Figure 3B). Proximity ligation assays, used to assay protein-protein interactions, similarly showed a significant increase in Nck interaction with tyrosine phosphorylated proteins following H2O2 treatment (Figure 3C). This enhancement of Nck-phospho-tyrosine interactions correlated with enhanced recruitment of Nck to the cell membrane fraction (Figure 3D).
Figure 3. Nck couples oxidant stress-induced tyrosine phosphorylation to activation of PAK2.
(A) Total cellular tyrosine phosphorylation was determined in cells treated with H2O2 for the indicated times. Representative blots are shown. (B) Nck co-immunoprecipitation with tyrosine phosphorylated proteins was determined in cells treated with H2O2. (C) HAECs were treated as in (B) and Nck/phosphotyrosine interactions were analyzed in situ by proximity ligation assays. Average proximal ligations per cell are shown. (D) Cells were treated as in (B), and Nck recruitment to the membrane fraction was determined by Western blotting and normalized to the integral membrane protein α5 integrin. (E) HAECs were treated as in (B) and PAK2 co-immunoprecipitation with Nck1 and Nck2 was analyzed. (F) Activation of PAK2 was determined by Western blotting in cells pretreated with Nck-blocking peptide before treatment with H2O2. (G) Cells were treated as in (F), and PAK2 recruitment to the membrane fraction was determined by Western blotting and normalization to the integral membrane protein α5 integrin. n=5 independent experiments for each panel. * p<0.05, ** p<0.01, *** p<0.001.
We have shown that PAK signaling enhances oxidant stress-dependent canonical NF-κB activation in endothelial cells exposed to shear stress (20). Since Nck can promote PAK membrane translocation and activation (19, 27), we next characterized whether H2O2 activates PAK in a Nck-dependent fashion. Treatment with exogenous H2O2 enhanced the interaction between PAK2, the primary isoform in endothelial cells (27), and both Nck1 and Nck2, as determined by co-immunoprecipitation (Figure 3E), suggesting that these isoforms may be functionally redundant. Nck siRNA inhibited the increase in the phosphorylation of PAK2 induced by both exogenous H2O2 (Supplemental Figure 4B/C) and hypoxia/reoxygenation injury (Supplemental Figure 4D), consistent with a critical role for Nck in oxidant stress-induced proinflammatory signaling. Similarly, treatment with the Nck blocking peptide completely suppressed both the phosphorylation (Figure 3F) and translocation of PAK2 to the membrane fraction (Figure 3G) following oxidant stress.
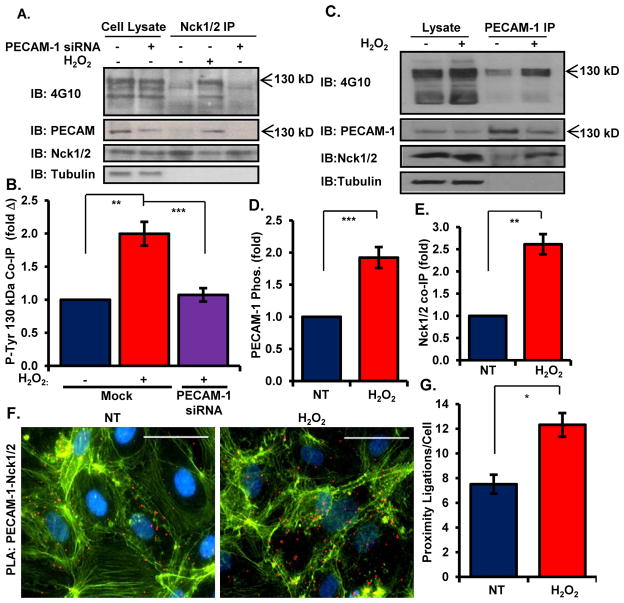
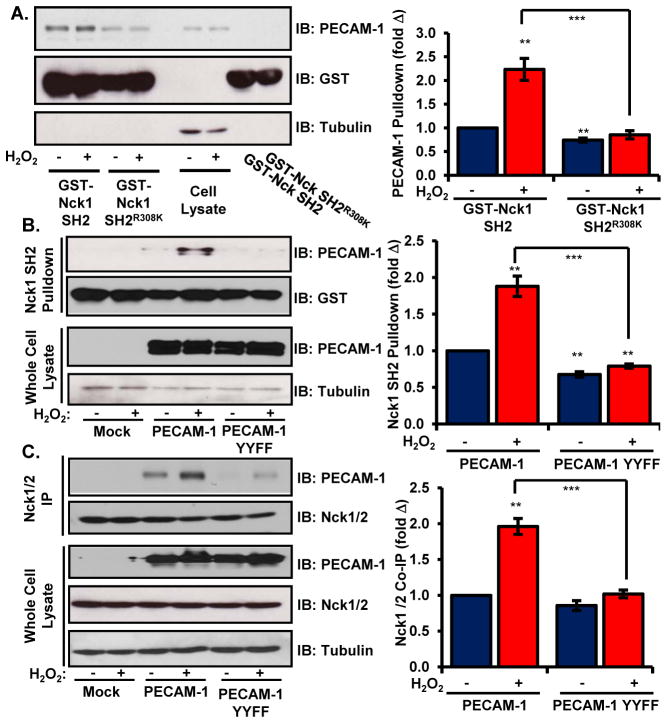
While multiple tyrosine phosphorylated proteins may recruit Nck to the membrane to initiate proinflammatory signaling, Nck co-immunoprecipitations showed a prominent phospho-tyrosine band at ~130 kDa (Figure 4A/B). Therefore, we next sought to identify this protein based on size, abundance, and known tyrosine phosphorylated proteins. We identified the 130 kDa protein as platelet-endothelial cell adhesion molecule-1 (PECAM-1, CD31), as PECAM-1 siRNA caused the disappearance this phosphotyrosine band from Nck immunoprecipitates (Figure 4A/B). PECAM-1 showed increased tyrosine phosphorylation following H2O2 treatment (Figure 4C/D), and H2O2 promoted co-immunoprecipitation of PECAM-1 and Nck in endothelial cells (Figure 4C/E, Supplemental Figure 5A). Immunocytochemistry indicated that PECAM-1, phosphotyrosine, and Nck1/2 colocalized following H2O2 treatment (Supplemental Figure 5C). Consistent with PECAM-1-driven Nck recruitment, proximity ligation assays demonstrated enhanced interactions between PECAM-1 and Nck1/2 following H2O2 treatment (Figure 4F/4G). Oxidant stress similarly stimulated the interaction of PECAM-1 with PAK2 (Supplemental Figure 5B). Consistent with our model for Nck recruitment to tyrosine phosphorylated PECAM-1, endothelial PECAM-1 showed increased precipitation with the recombinant GST-Nck1 SH2 domain following H2O2 treatment, while SH2 domains containing a point mutation reducing its affinity for phosphotyrosine (R308K) demonstrated diminished PECAM-1 binding both under basal and H2O2-stimulated conditions (Figure 5A). Similarly, wild-type PECAM-1 expressed in HEK293 cells showed enhanced precipitation with GST-Nck1 SH2 domains following H2O2 treatment, whereas these SH2 domains failed to precipitate a phosphorylation-deficient PECAM-1 (YYFF, mutation to Phe at Tyr663 and Tyr686) (Figure 5B). The PECAM-1 YYFF mutant also showed reduced co-immunoprecipitation with endogenous Nck1/2, suggesting that PECAM-1 phosphorylation mediates its interaction with the Nck adaptor proteins (Figure 5C).
Figure 4. Oxidant stress-induced PECAM-1 phosphorylation stimulates interactions with NcK.
(A/B) Nck was immunoprecipitated from HAECs treated with H2O2, and Nck-interacting tyrosine phosphorylated proteins were identified by Western blotting with the 4G10 antibody. Enhanced interaction with a 130kDa tyrosine phosphorylated protein was blunted by PECAM-1 siRNA. Representative Western blots are shown. (C–E) PECAM-1 was immunoprecipitated from HAECs treated with H2O2. Changes in PECAM-1 phosphorylation (C/D) and Nck coimmunoprecipitation (C/E) were analyzed by Western blotting and normalized to total PECAM-1 in the immunoprecipitates. Representative Western blots are shown. (F) HAECs were treated as in (A) and PECAM-1/Nck interactions were analyzed in situ by proximity ligation assays. Average proximal ligations per cell is shown. n=5 independent experiments for A–E and n=4 independent experiments for (F). * p<0.05, ** p<0.01.
Figure 5. PECAM-1 phosphorylation is required for its interaction with the Nck SH2 domain.
(A) Pulldown of PECAM-1 with GST-tagged Nck1 SH2 domains following H2O2 treatment in HAECs was determined by Western blotting and normalized to PECAM-1 abundance in the cell lysates. The GST-tagged R308K mutant SH2 domain served as a negative control for phosphotyrosine-dependent interactions. Representative Western blots are shown. (B/C) HEK293 cells expressing wild-type PECAM-1 or the phosphorylation deficient PECAM-1 YYFF mutant were lysed and precipitation with either (B) the GST-Nck1 SH2 domain or (C) endogenous Nck1/2 was determined by Western blotting. Precipitated PECAM-1 was normalized to either total PECAM-1 in the lysates (A) or total Nck1/2 abundance in the immunoprecipitates (B). Representative Western blots are shown. n=5 independent experiments for each panel. ** p < 0.01, *** p < 0.001.
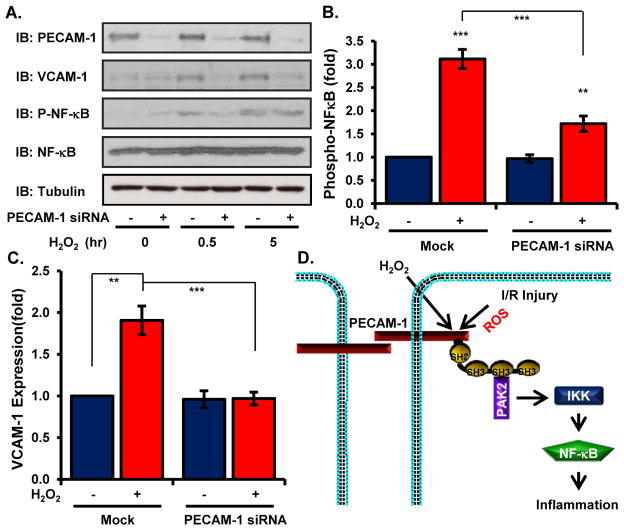
To assess the functional importance of PECAM-1-Nck interactions in the response to oxidative stress, we analyzed oxidative stress-induced proinflammatory responses following PECAM-1 knockdown using siRNA. PECAM-1 knockdown significantly blunted both NF-κB activation (Figure 6A/B) and increases in VCAM-1 abundance (Figure 6A/C), suggesting that oxidative stress phosphorylates PECAM-1 resulting in Nck recruitment and Nck-dependent proinflammatory signaling through PAK2 and NF-κB (Figure 6D).
Figure 6. PECAM-1 is required for oxidant stress-induced proinflammatory responses.
(A/B) HAECs transfected with PECAM-1 siRNA were treated with H2O2 for the indicated times and analyzed for phosphorylated NF-κB (A, B) and VCAM-1 abundance (A, C) by Western blotting. Representative Western blots are shown. n=5 independent experiments. *** p<0.001, **** p < 0.0001. (D) Model of oxidant stress-induced activation of NF-κB activation through PECAM-1-dependent recruitment of Nck.
Nck inhibitors blunt oxidant stress-induced leukocyte-endothelial interactions during ischemia/reperfusion injury in vivo
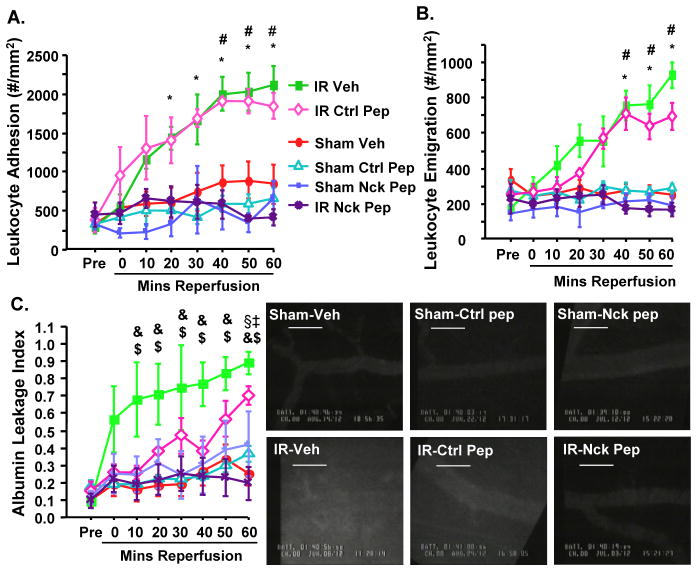
To test Nck’s role in oxidant stress-driven inflammation in vivo, we utilized intravital microscopy to visualize leukocyte recruitment in the cremaster muscle model of ischemia/reperfusion injury. Since endothelial PAK signaling also regulates vascular permeability in multiple systems (28–30), FITC-BSA was also used to measure endothelial barrier integrity. Ischemia/reperfusion injury induced both leukocyte adhesion (Figure 7A) and emigration (Figure 7B) over the course of the reperfusion period as compared to sham-operated controls. Mice receiving the Nck blocking peptide, but not the control peptide, showed significantly attenuated ischemia/reperfusion-induced leukocyte adhesion and emigration to amounts comparable to those seen in sham-operated controls (Figure 7A/B). Similar to other models (28–30), treatment with the Nck blocking peptide also significantly blunted vascular permeability following ischemia/reperfusion injury (Figure 7C/D).
Figure 7. The Nck blocking peptide blunts inflammation and permeability during ischemia/reperfusion injury in vivo.
Mice were pretreated with vehicle, control peptide (Ctrl Pep) or the Nck blocking peptide (Nck-Pep) and subjected to sham or ischemia/reperfusion (I/R) injury. Intravital microscopy was performed on cremasteric postcapillary venules. (A) Leukocyte adhesion within the venules (conveyed as the number of leukocytes adhering to the vessel wall per mm2), (B) leukocyte emigration out of the vessel (number of emigrated cells per mm2 of interstitum), and (C) leakage of FITC-albumin (as a measure of vessel permeability) were measured before ischemia (pre) and throughout reperfusion. (D) Representative images of FITC-albumin leakage at 60 min reperfusion are shown. n=4–8 mice per group. Statistical comparisons (Bonferroni, P≤0.0033):* comparing Vehicle I/R and Control Peptide-I/R to pretreatment values (p < 0.001), # comparing Vehicle-I/R and Control Peptide-I/R to all other groups (p < 0.001), & comparing Vehicle-I/R group to pretreatment (p < 0.001), ‡ comparing Control Peptide-I/R to pretreatment (p < 001), $ comparing Vehicle-I/R to Nck Peptide-I/R (p < 0.01). § comparing Control Peptide-I/R to Nck Peptide-I/R (p < 0.01).
Together, these data suggest that blunting Nck signaling either by reduced expression or by competitive inhibition abrogates oxidant stress-induced inflammation following ischemia/reperfusion injury.
Discussion
Oxidative stress in ischemia/reperfusion injury can induce both direct tissue damage and indirect damage by stimulating local inflammation. We characterized a role for the Nck adaptor proteins in critically coupling oxidant stress-induced tyrosine phosphorylation to endothelial NF-κB activation and proinflammatory gene expression. Nck inhibition with either siRNA or a membrane-permeable peptide significantly blunted oxidant stress-induced proinflammatory signaling through PAK2 and NF-κB, proinflammatory adhesion molecule expression (ICAM-1, VCAM-1), and leukocyte binding. Nck regulated proinflammatory responses to both exogenous oxidant stress (H2O2) and endogenous oxidant stress (hypoxia/reoxygenation injury), whereas Nck did not affect antioxidant gene expression or TNFα-mediated cell adhesion molecule expression, suggesting that Nck selectively couples oxidant stress to proinflammatory responses. Nck promoted these proinflammatory effects by coupling the tyrosine phosphorylation of PECAM-1 to the activation of PAK2, a Ser/Thr kinase that mediates oxidant stress-induced NF-κB activation. Consistent with the results obtained in these in vitro systems, treatment with a Nck inhibitory peptide completely ablates leukocyte recruitment and vascular permeability following ischemia/reperfusion injury in the cremaster muscle. Taken together, these data reveal that the Nck family of adaptor proteins couple oxidant stress to proinflammatory responses in endothelial cells and may serve as a therapeutic target to limit oxidant stress-induced inflammation.
Multiple studies demonstrate an important role for Nck in mediating inflammatory responses, primarily through Nck’s role in actin dynamics. Interaction between Nck and the T cell receptor (TCR) stimulates PAK-dependent actin polymerization that is important for the formation of the immunological synapse (31, 32), and mice deficient in both Nck1 and Nck2 show reduced thymic selection and reduced TCR sensitivity (33, 34). Similarly, Nck modulates actin remodeling during phagocytosis in neutrophils and macrophages (35). Work from our group shows an important role for Nck in mediating endothelial activation (27); however the mechanisms of action appear to be independent of actin remodeling responses. Nck is required for PAK2 membrane recruitment in endothelial cells exposed to hemodynamic shear stress, and inhibiting PAK2 signaling blunts shear stress-induced activation of NF-κB (20, 27). PAK2 drives NF-κB activation through an NF-κB-inducing kinase and IKK-dependent pathway (20), and endothelial cells expressing a PAK2 construct showing enhanced Nck interactions demonstrate enhanced basal NF-κB signaling (27). We demonstrate here that oxidant stress drives PAK2 activation by stimulating Nck-dependent membrane targeting. While membrane targeting can promote PAK activation (19), activation of PAK typically requires interaction with the small GTPases Rac and Cdc42 (36). While a role for Nck in oxidant stress-induced Rac or cdc42 signaling has yet to be described, Nck can directly interact with the Rac guanine nucleotide exchange factor ELMO1 (37), suggesting Nck could localize membrane targeted PAK to sites of increased Rac activation. The interplay between these pathways should provide an interesting direction for future research.
Although PECAM-1 has been ascribed both proinflammatory and anti-inflammatory functions (38, 39), inhibiting PECAM-1 reduces ischemia/reperfusion injury in rodent models (40). PECAM-1 is a sensor for oxidative stress, showing enhanced phosphorylation and SHP-2 recruitment following a rapid increase in cellular H2O2 production (41). Increased PECAM-1 tyrosine phosphorylation correlated with Nck coimmunoprecipitation and Nck translocation to the plasma membrane, and PECAM-1 depletion by siRNA prevented oxidative stress-induced NF-κB activation and increases in VCAM-1 abundance. The amino acid sequences surrounding both Tyr663 and Tyr686 in the PECAM-1 cytoplasmic tail show similarities to Nck SH2 domain-binding sequences(42), and mutation of these two Tyr to Phe residues blunted the interaction between PECAM-1 and the Nck SH2 domain (Figure 5). Nck can interact with several tyrosine phosphorylated proteins, including PECAM-1, and localizes to multiple cellular sites, including cell-cell junctions and cell-matrix adhesions. PECAM-1 appeared to be critical for the proinflammatory signaling downstream of Nck recruitment, and we speculate that PECAM-1 may preferentially couple Nck to other important signaling partners or may alter the local substrates for PAK2. Consistent with this proinflammatory role, PECAM-1 deficient mice show reduced leukocyte transmigration following IL-1β stimulation (43, 44). Given Nck’s ability to stimulate cytoskeletal remodeling, PECAM-1-mediated interactions between leukocytes and endothelial cells may utilize Nck-dependent signaling to enhance cytoskeletal remodeling during leukocyte diapedesis.
Modular adaptor proteins, such as Nck, facilitate highly specific protein-protein interactions to couple environmental stimuli to distinct intracellular signals and effector responses (45). We show that H2O2 treatment significantly enhances the interaction between Nck and PAK2 (Figure 4E), an interaction that involves the second SH3 domain of Nck. This domain can interact in an intramolecular fashion with a (K/R)x(K/R)RxxS sequence within the linker region between the 1st and 2nd SH3 domain, an interaction that lowers the affinity of the second SH3 domain for its binding partners, indicative of an inactive confirmation (46). While direct oxidation of Nck has not been described, Nck can be phosphorylated on serine, threonine, and tyrosine residues (47), and the presence of a Ser residue in the linker sequence suggest that inducible phosphorylation at this site could enhance Nck’s affinity for binding partners. PDGF stimulates phosphorylation of Nck on both Ser and Tyr residues within this linker region between the first and second SH3 domain (48), though the function of these phosphorylation events is unknown. The effect of oxidative stress on Nck post-translational modification and inducible recruitment of signaling intermediates should provide an interesting direction for future research.
Although cell culture and animal models implicate oxidant stress in cardiovascular inflammation, treating cardiovascular disease with exogenous antioxidants has not revealed therapeutic benefit in human trials (49). While multiple factors may account for this deficiency, discrete protein-protein interactions and signaling processes that couple oxidant stress to inflammatory gene expression may provide a more stable therapeutic target and may provide a greater therapeutic benefit by leaving the oxidant stress-induced activation of negative feedback pathways intact. Since Nck inhibition does not blunt Nrf2-driven antioxidant genes, Nck-based therapeutics may prevent inflammation without reducing the preconditioning effect to subsequent ischemia/reperfusion injury. Furthermore, blocking PAK-Nck interactions reduces endothelial cell permeability to multiple stimuli (28, 30), suggesting that targeting Nck will provide an additional benefit compared to blocking NF-κB directly by reducing tissue edema. However, long term Nck inhibition may also be detrimental, as Nck inhibitors blunt endothelial cell migration and angiogenic remodeling (24, 50). Therefore, acute, reversible Nck inhibitors may provide the most therapeutic benefit for targeting acute inflammation while allowing preconditioning without blocking later tissue remodeling responses in chronically ischemic tissue.
Materials and Methods
Cell culture
Human aortic endothelial cells (HAECs) were cultured in MCDB131 containing 10% fetal bovine serum (FBS), 60 μg/ml heparin, 24 μg/ml bovine brain extract, 2 mM L-glutamine, and antibiotics (Gibco), and used between passage 6 to 10. Nck siRNA (SMARTPool, Dharmacon) transfections were performed using Lipofectamine2000 per manufacturer’s instructions. HAEC were treated with various doses of H2O2 or hypoxia under low serum conditions (0.5 % FBS). After initial dose requirements were determined, subsequent experiments utilized 100 μM H2O2. For hypoxia/reoxygenation experiments, HAECs were exposed to 5% O2 in a hypoxia chamber (Coy Laboratory Products) for 60 minutes prior reoxygenation at 21% O2 for up to 2 hours. HEK293 cells, grown in DMEM containing 10% FBS and antibiotics, were transfected with wildtype PECAM-1 or PECAM-1 contain Tyr to Phe mutations in Tyr663 and Tyr686 (YYFF mutation; gift of Dr. Deborah Newman, Medical College of Wisconsin) (41). Membrane permeable TAT-tagged peptides derived from the Nck-binding sequence in PAK1 (Nck peptide) were used at 20 μg/ml (24).
Immunoblotting
Western blotting was performed as previously described (20, 28). Primary antibodies included anti-PAK2, phospho-NF-κB (p65 subunit, Ser536), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ICAM-1 (Cell Signaling Technology), phosphotyrosine (4G10), phospho-PAK1/2/3 (Ser141; Invitrogen), Nck1/2, p65, VCAM-1, α5 integrin, and ERK1/2 (Santa Cruz Biotechnology). Nck1 and Nck2 specific antibodies were kindly provided by Dr. Louise Larose (McGill University) (51). Densitometry was performed using ImageJ software.
Immunocytochemistry
Cells were fixed in PBS containing 4% formaldehyde, permeabilized in 0.1% Triton X-100, and blocked in 1% BSA containing 10% goat serum. Primary antibodies (incubated overnight) included rabbit anti- NF-κB p65 (Santa Cruz), mouse anti-phospho-tyrosine (4G10, Millipore), rabbit anti-Nck1/2 (Millipore), mouse anti-Nck (Abcam), goat anti-PECAM-1 (Santa Cruz), mouse anti-PECAM-1 (Cell Signaling Technology). Staining was visualized with Alexa-conjugated secondary antibodies (Invitrogen) and viewed on a Nikon Eclipse Ti inverted fluorescent microscope. Images were taken by using the Photometrics Coolsnap120 ES2 camera and analyzed by the NIS Elements BR 3.00, SP5 imaging software. Greater than 100 cells were counted for nuclear NF-κB for each condition of individual experiments. Proximity ligation assays were performed using manufacturer instructions, counterstained with DAPI and phalloidin, and quantified using NIS Elements software.
Immunoprecipitation
Cells were lysed for immunoprecipitation as previously described (27). Lysates were pre-cleared for 15 min with γ-bind G beads and centrifuged. Cleared lysates were incubated for 3 hours with rabbit anti-Nck (Millipore), mouse anti-phosphotyrosine (4G10, Millipore), mouse anti-PECAM-1 (Cell Signaling Technology), or rabbit anti-IKKβ. Lysates were incubated with γ-bind G sepharose beads for 2 hours, and the beads were washed three times in lysis buffer. Immunoprecipitated proteins were dissociated from the beads by the addition of 2X sample buffer followed by boiling at 95 °C for 2 minutes. For IKK activity assays, IKKβ immunoprecipitates were washed twice in kinase buffer (25 mM Tris, 5 mM β-glycerophosphate, 2 mM dithiothreitol, 0.1 mM Na3VO4, 10 mM MgCl2, pH 7.5) and incubated for 30 min at 30°C with 1 μg GST-IκBα in kinase buffer containing 200 μM ATP. Kinase activity was measured by Western blotting for phosphorylated IκBα and normalized to IKKβ abundance in the immunoprecipitates. SH2 pulldown assays utilized the GST-tagged Nck1 SH2 domain or the R308K mutant SH2 domain with reduced affinity for phospho-tyrosine (gift of Dr. Louise Larose, McGill University) (52). For this experiment, lysates were incubated for 2 hours with glutathione sepharose beads preconjugated to GST-SH2 domain or GST-SH2 R308K, and subsequent interactions were analyzed by Western blotting as previously described.
Cytosol/membrane fractionation
Cells plated on 60 mm dishes were washed in ice-cold phosphate-buffered saline (PBS) and lysed in 200 μl of cytosol buffer (20 mM Tris, pH 7.5, 2 mM 2-mercaptoethanol, 5 mM ethylene glycol tetra-acetic acid (EGTA), 2 mM EDTA, and 2 mM Na3VO4, 1× protease inhibitor cocktail (RPI, Mount Prospect, IL)). Cell lysates were collected and centrifuged for 30 min at 15,000 rpm at 4°C. The supernatant was then collected as the cytosolic fraction. The remaining pellet was resuspended in 200 μl of cytosol buffer and centrifuged again for 30 min at 15,000 rpm at 4°C to completely remove any residual cytosolic proteins. The remaining pellet was resuspended in Membrane buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40, 10 mM NaF, 2 mM Na3VO4, and 1× protease inhibitor) and spun for 30 min at 15,000 rpm at 4°C. The supernatant was collected as the membrane fraction and analyzed by Western blot for PAK2 and Nck. The Membrane protein α5 integrin and the cytosol protein GAPDH were used to test the isolation efficiency.
Monocyte adhesion assay
Human THP1 monocytes labeled with Cell Tracker Green (Invitrogen) according to manufacturer’s protocol were resuspended in Hank’s balanced salt solution containing calcium and magnesium. Monocytes (5.0× 106) were added to confluent HAEC cultures and allowed to attach for 15 min at 37°C. The unbound monocytes in the supernatant and two washes were collected. Bound monocytes were visualized by epifluorecence microscopy, and both bound and nonbound monocytes were lysed in 200 mM NaOH Fluorescence in the individual lysates was measured in a FLUOStar fluorospectrometer, and monocyte adhesion was calculated as percentage change compared with untreated cells by normalizing to the unbound fraction.
Quantitative Real-Time PCR
Cells were lysed in TRIzol reagent (Invitrogen), and mRNA was extracted per manufacturer’s instructions. Following cDNA synthesis (iScript cDNA Synthesis Kit, Biorad), qRT-PCR was performed in a Biorad iCycler using Sybr Green Master Mix (Biorad). All primers utilized for qRT-PCR (Supplemental Table 1) were validated by melt curve analysis, gel electrophoresis of the PCR product, and sequencing of the PCR product. Expression of target genes were normalized to the housekeeping gene β2-microglobulin and conveyed as a fold change compared to the untreated condition using the 2−ΔΔCt method.
Intravital Microscopy
All animal protocols were approved by the LSU Health Sciences Center-Shreveport Animal Care and Use Committee and followed the National Institute of Health guidelines for the care and use of laboratory animals. The cremaster muscle was prepared for light and fluorescence intravital microscopy as described previously (53). Briefly, 8-week old, male C57Bl/6J mice were anesthetized with ketamine hydrochloride (150 mg/kg, IP) and xylazine (7.5 mg/kg, IP), and the jugular vein was canulated. At this time, mice received saline (vehicle), control peptide (100 μg, ~4 mg/kg) or PAK-Nck peptide (100 μg, ~4 mg/kg) via the canula. The cremaster muscle was then isolated, spread across a viewing pedestal and superfused with warm bicarbonate buffered saline at 1 ml/min. The tissue was transilluminated and red cell velocity (VRBC) was measured using an optical Doppler velocimeter. Postcapillary venules with a diameter between 20 μm and 40 μm were monitored and, after a 20–30 min stabilization period, a section of the least inflamed venule with a wall shear rate ≥500 s−1 was chosen for study. Background fluorescence was recorded. The mouse then received 25 mg/kg FITC-albumin in 0.1 ml saline via the jugular vein canula. This was allowed to circulate before a 1 min recording was taken of the venule using light microscopy (to assess leukocyte recruitment), followed by a recording of the fluorescent image (for measurement of albumin leakage). Thereafter a vascular clamp was used on the whole pedicle to induce ischemia for 30 min. The clamp was then released and recordings of the venule using light and fluorescence microscopy were taken at 10 min. intervals up to 60 min. reperfusion. Leukocyte adhesion and emigration were determined by off-line analysis. Leukocytes were considered adherent if they stopped for at least 30 s (conveyed as #/mm2 vessel wall), and emigrated leukocytes were leukocytes identified in the interstitium (conveyed as # cells/mm2 tissue). The index of vascular albumin leakage (permeability index) was determined from the ratio: (interstitial intensity – background)/(venular intensity – background) as previously reported (54) and measured using ImageJ 1.46 software from the NIH.
Statistical analysis
Data was tested for normality (Kolmogorov-Smirnov test) and significance using GraphPad Prism software. Data that passed the normality assumption was analyzed using using Student’s T-test and one-way or two-way ANOVA with Bonferroni post-tests where indicated. Data that failed the normality assumption were analyzed using the non-parametric Mann-Whitney U test with post hoc analysis. Data are shown as mean values ± standard error of the mean (SEM). Differences were considered statistically significant at a value of P < 0.05.
Supplementary Material
Fig. S1. H2O2 does not stimulate Tyr phosphorylation of IκBα at concentrations that activate NF-κB in endothelial cells.
Fig. S2. Nck siRNA reduces H2O2-indcued NF-κB activation and increase in VCAM-1 abundance.
Fig. S3. Micrographs of monocyte adhesion to oxidant stress-activated endothelial cells.
Fig. S4. Oxidant stress-induced tyrosine phosphorylation activates Nck-dependent PAK2 signaling.
Fig. S5. H2O2 stimulates Nck and PAK recruitment to PECAM-1.
Table S1. Quantitative real-time PCR primers.
Acknowledgments
The authors would like to thank Dr. Louise Larose (McGill University) for providing the Nck1 and Nck2 antibodies and the GST-Nck1 SH2 constructs, Dr. Deborah Newman (Medical College of Wisconsin) for providing the PECAM-1 constructs, and Dr. Sushil Jain (LSU Health Sciences Center – Shreveport) for providing the THP-1 monocytes.
Funding: This work was supported by the National Institute of Health [R01 HL098435 to A.W.O, R01 HL113303 to C.G.K., & P20-GM103433 from the National Institute of General Medical Sciences to K.Y.S.], by the Louisiana Board of Regents Superior Toxicology Fellowship [LEQSF (2008–13)-FG-20 to A.Y.J.], and by an American Heart Association Post-doctoral Fellowship [12POST12030375 to J.C.] and Pre-doctoral Fellowship [14PRE18660003 to A.Y.J.].
Footnotes
Author contributions: Experiments were performed by J.C., I.L.L., A.Y., and A.W.O., and data analysis was carried out by J.C., A.Y., B.T., K.Y.S., and A.W.O.. Consultation on experimental design and setup was provided by I.L.L, C.G.K., and K.Y.S., and J.C., A.Y., C.G.K., K.Y.S., and A.W.O, contributed to the preparation of the manuscript.
Competing interests: The authors declare that they have no competing interests.
References and Notes
- 1.Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med. 2011;51:1271–1288. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugamura K, Keaney JF., Jr Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Napetschnig J, Wu H. Molecular basis of NF-kappaB signaling. Annu Rev Biophys. 2013;42:443–468. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solt LA, May MJ. The IkappaB kinase complex: master regulator of NF-kappaB signaling. Immunol Res. 2008;42:3–18. doi: 10.1007/s12026-008-8025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:pe1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 8.Barchowsky A, Munro SR, Morana SJ, Vincenti MP, Treadwell M. Oxidant-sensitive and phosphorylation-dependent activation of NF-kappa B and AP-1 in endothelial cells. Am J Physiol. 1995;269:L829–836. doi: 10.1152/ajplung.1995.269.6.L829. [DOI] [PubMed] [Google Scholar]
- 9.Natarajan R, Fisher BJ, Jones DG, Fowler AA., 3rd Atypical mechanism of NF-kappaB activation during reoxygenation stress in microvascular endothelium: a role for tyrosine kinases. Free Radic Biol Med. 2002;33:962. doi: 10.1016/s0891-5849(02)00990-5. [DOI] [PubMed] [Google Scholar]
- 10.Schoonbroodt S, Ferreira V, Best-Belpomme M, Boelaert JR, Legrand-Poels S, Korner M, Piette J. Crucial role of the amino-terminal tyrosine residue 42 and the carboxyl-terminal PEST domain of I kappa B alpha in NF-kappa B activation by an oxidative stress. J Immunol. 2000;164:4292–4300. doi: 10.4049/jimmunol.164.8.4292. [DOI] [PubMed] [Google Scholar]
- 11.Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem. 2003;278:24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 12.Canty TG, Jr, Boyle EM, Jr, Farr A, Morgan EN, Verrier ED, Pohlman TH. Oxidative stress induces NF-kappaB nuclear translocation without degradation of IkappaBalpha. Circulation. 1999;100:II361–364. doi: 10.1161/01.cir.100.suppl_2.ii-361. [DOI] [PubMed] [Google Scholar]
- 13.Natarajan R, Fisher BJ, Jones DG, Ghosh S, Fowler AA., 3rd Reoxygenating microvascular endothelium exhibits temporal dissociation of NF-kappaB and AP-1 activation. Free Radic Biol Med. 2002;32:1033–1045. doi: 10.1016/s0891-5849(02)00813-4. [DOI] [PubMed] [Google Scholar]
- 14.Mohan S, Koyoma K, Thangasamy A, Nakano H, Glickman RD, Mohan N. Low shear stress preferentially enhances IKK activity through selective sources of ROS for persistent activation of NF-kappaB in endothelial cells. Am J Physiol Cell Physiol. 2007;292:C362–371. doi: 10.1152/ajpcell.00535.2005. [DOI] [PubMed] [Google Scholar]
- 15.Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–651. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 16.Menden H, Tate E, Hogg N, Sampath V. LPS-mediated endothelial activation in pulmonary endothelial cells: role of Nox2-dependent IKK-beta phosphorylation. Am J Physiol Lung Cell Mol Physiol. 2013;304:L445–455. doi: 10.1152/ajplung.00261.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaki SP, Rivera GM. Integration of signaling and cytoskeletal remodeling by Nck in directional cell migration. Bioarchitecture. 2013;3:57–63. doi: 10.4161/bioa.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lettau M, Pieper J, Janssen O. Nck adapter proteins: functional versatility in T cells. Cell Commun Signal. 2009;7:1. doi: 10.1186/1478-811X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu W, Katz S, Gupta R, Mayer BJ. Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr Biol. 1997;7:85–94. doi: 10.1016/s0960-9822(06)00052-2. [DOI] [PubMed] [Google Scholar]
- 20.Orr AW, Hahn C, Blackman BR, Schwartz MA. p21-activated kinase signaling regulates oxidant-dependent NF-kappa B activation by flow. Circ Res. 2008;103:671–679. doi: 10.1161/CIRCRESAHA.108.182097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Druker BJ, Mamon HJ, Roberts TM. Oncogenes, growth factors, and signal transduction. N Engl J Med. 1989;321:1383–1391. doi: 10.1056/NEJM198911163212007. [DOI] [PubMed] [Google Scholar]
- 22.Ley SC, Davies AA, Druker B, Crumpton MJ. The T cell receptor/CD3 complex and CD2 stimulate the tyrosine phosphorylation of indistinguishable patterns of polypeptides in the human T leukemic cell line Jurkat. Eur J Immunol. 1991;21:2203–2209. doi: 10.1002/eji.1830210931. [DOI] [PubMed] [Google Scholar]
- 23.Ziegelstein RC, He C, Hu Q. Hypoxia/reoxygenation stimulates Ca2+-dependent ICAM-1 mRNA expression in human aortic endothelial cells. Biochem Biophys Res Commun. 2004;322:68–73. doi: 10.1016/j.bbrc.2004.07.080. [DOI] [PubMed] [Google Scholar]
- 24.Kiosses WB, Hood J, Yang S, Gerritsen ME, Cheresh DA, Alderson N, Schwartz MA. A dominant-negative p65 PAK peptide inhibits angiogenesis. Circ Res. 2002;90:697–702. doi: 10.1161/01.res.0000014227.76102.5d. [DOI] [PubMed] [Google Scholar]
- 25.Bocci V. How a calculated oxidative stress can yield multiple therapeutic effects. Free Radic Res. 2012;46:1068–1075. doi: 10.3109/10715762.2012.693609. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Vrishni S, Singh BK, Rahman I, Kakkar P. Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic Res. 2010;44:1267–1288. doi: 10.3109/10715762.2010.507670. [DOI] [PubMed] [Google Scholar]
- 27.Yurdagul A, Jr, Chen J, Funk SD, Albert P, Kevil CG, Orr AW. Altered nitric oxide production mediates matrix-specific PAK2 and NF-kappaB activation by flow. Mol Biol Cell. 2013;24:398–408. doi: 10.1091/mbc.E12-07-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orr AW, Stockton R, Simmers MB, Sanders JM, Sarembock IJ, Blackman BR, Schwartz MA. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J Cell Biol. 2007;176:719–727. doi: 10.1083/jcb.200609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reutershan J, Stockton R, Zarbock A, Sullivan GW, Chang D, Scott D, Schwartz MA, Ley K. Blocking p21-activated kinase reduces lipopolysaccharide-induced acute lung injury by preventing polymorphonuclear leukocyte infiltration. Am J Respir Crit Care Med. 2007;175:1027–1035. doi: 10.1164/rccm.200612-1822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stockton R, Reutershan J, Scott D, Sanders J, Ley K, Schwartz MA. Induction of vascular permeability: beta PIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by PAK. Mol Biol Cell. 2007;18:2346–2355. doi: 10.1091/mbc.E06-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bubeck Wardenburg J, Pappu R, Bu JY, Mayer B, Chernoff J, Straus D, Chan AC. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity. 1998;9:607–616. doi: 10.1016/s1074-7613(00)80658-5. [DOI] [PubMed] [Google Scholar]
- 32.Gil D, Schamel WW, Montoya M, Sanchez-Madrid F, Alarcon B. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109:901–912. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 33.Roy E, Togbe D, Holdorf A, Trubetskoy D, Nabti S, Kublbeck G, Schmitt S, Kopp-Schneider A, Leithauser F, Moller P, Bladt F, Hammerling GJ, Arnold B, Pawson T, Tafuri A. Fine tuning of the threshold of T cell selection by the Nck adapters. J Immunol. 2010;185:7518–7526. doi: 10.4049/jimmunol.1000008. [DOI] [PubMed] [Google Scholar]
- 34.Roy E, Togbe D, Holdorf AD, Trubetskoy D, Nabti S, Kublbeck G, Klevenz A, Kopp-Schneider A, Leithauser F, Moller P, Bladt F, Hammerling G, Arnold B, Pawson T, Tafuri A. Nck adaptors are positive regulators of the size and sensitivity of the T-cell repertoire. Proc Natl Acad Sci U S A. 2010;107:15529–15534. doi: 10.1073/pnas.1009743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppolino MG, Krause M, Hagendorff P, Monner DA, Trimble W, Grinstein S, Wehland J, Sechi AS. Evidence for a molecular complex consisting of Fyb/SLAP, SLP-76, Nck, VASP and WASP that links the actin cytoskeleton to Fcgamma receptor signalling during phagocytosis. J Cell Sci. 2001;114:4307–4318. doi: 10.1242/jcs.114.23.4307. [DOI] [PubMed] [Google Scholar]
- 36.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 37.Zhang G, Chen X, Qiu F, Zhu F, Lei W, Nie J. A novel interaction between SH2 domain of signaling adaptor protein Nck-1 and the upstream regulator of the Rho family GTPase Rac1-engulfment and cell motility 1 (ELMO1) promotes Rac1 activation and cell motility. J Biol Chem. 2014 doi: 10.1074/jbc.M114.549550. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Privratsky JR, Newman DK, Newman PJ. PECAM-1: conflicts of interest in inflammation. Life Sci. 2010;87:69–82. doi: 10.1016/j.lfs.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27:2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 40.Turegun M, Gudemez E, Newman P, Zins J, Siemionow M. Blockade of platelet endothelial cell adhesion molecule-1 (PECAM-1) protects against ischemia-reperfusion injury in muscle flaps at microcirculatory level. Plast Reconstr Surg. 1999;104:1033–1040. doi: 10.1097/00006534-199909020-00021. [DOI] [PubMed] [Google Scholar]
- 41.Maas M, Wang R, Paddock C, Kotamraju S, Kalyanaraman B, Newman PJ, Newman DK. Reactive oxygen species induce reversible PECAM-1 tyrosine phosphorylation and SHP-2 binding. Am J Physiol Heart Circ Physiol. 2003;285:H2336–2344. doi: 10.1152/ajpheart.00509.2003. [DOI] [PubMed] [Google Scholar]
- 42.Frese S, Schubert WD, Findeis AC, Marquardt T, Roske YS, Stradal TE, Heinz DW. The phosphotyrosine peptide binding specificity of Nck1 and Nck2 Src homology 2 domains. J Biol Chem. 2006;281:18236–18245. doi: 10.1074/jbc.M512917200. [DOI] [PubMed] [Google Scholar]
- 43.Bixel MG, Li H, Petri B, Khandoga AG, Khandoga A, Zarbock A, Wolburg-Buchholz K, Wolburg H, Sorokin L, Zeuschner D, Maerz S, Butz S, Krombach F, Vestweber D. CD99 and CD99L2 act at the same site as, but independently of, PECAM-1 during leukocyte diapedesis. Blood. 2010;116:1172–1184. doi: 10.1182/blood-2009-12-256388. [DOI] [PubMed] [Google Scholar]
- 44.Thompson RD, Noble KE, Larbi KY, Dewar A, Duncan GS, Mak TW, Nourshargh S. Platelet-endothelial cell adhesion molecule-1 (PECAM-1)-deficient mice demonstrate a transient and cytokine-specific role for PECAM-1 in leukocyte migration through the perivascular basement membrane. Blood. 2001;97:1854–1860. doi: 10.1182/blood.v97.6.1854. [DOI] [PubMed] [Google Scholar]
- 45.Pawson T. Dynamic control of signaling by modular adaptor proteins. Curr Opin Cell Biol. 2007;19:112–116. doi: 10.1016/j.ceb.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Takeuchi K, Sun ZY, Park S, Wagner G. Autoinhibitory interaction in the multidomain adaptor protein Nck: possible roles in improving specificity and functional diversity. Biochemistry. 2010;49:5634–5641. doi: 10.1021/bi100322m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park D, Rhee SG. Phosphorylation of Nck in response to a variety of receptors, phorbol myristate acetate and cyclic AMP. Mol Cell Biol. 1992;12:5816–5823. doi: 10.1128/mcb.12.12.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meisenhelder J, Hunter T. The SH2/SH3 domain-containing protein Nck is recognized by certain anti-phospholipase C-gamma 1 monoclonal antibodies, and its phosphorylation on tyrosine is stimulated by platelet-derived growth factor and epidermal growth factor treatment. Mol Cell Biol. 1992;12:5843–5856. doi: 10.1128/mcb.12.12.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol. 2008;101:14D–19D. doi: 10.1016/j.amjcard.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Chaki SP, Barhoumi R, Berginski ME, Sreenivasappa H, Trache A, Gomez SM, Rivera GM. Nck enables directional cell migration through the coordination of polarized membrane protrusion with adhesion dynamics. J Cell Sci. 2013;126:1637–1649. doi: 10.1242/jcs.119610. [DOI] [PubMed] [Google Scholar]
- 51.Latreille M, Laberge MK, Bourret G, Yamani L, Larose L. Deletion of Nck1 attenuates hepatic ER stress signaling and improves glucose tolerance and insulin signaling in liver of obese mice. Am J Physiol Endocrinol Metab. 2011;300:E423–434. doi: 10.1152/ajpendo.00088.2010. [DOI] [PubMed] [Google Scholar]
- 52.Lussier G, Larose L. A casein kinase I activity is constitutively associated with Nck. J Biol Chem. 1997;272:2688–2694. doi: 10.1074/jbc.272.5.2688. [DOI] [PubMed] [Google Scholar]
- 53.Mori N, Horie Y, Gerritsen ME, Granger DN. Ischemia-reperfusion induced microvascular responses in LDL-receptor −/− mice. Am J Physiol. 1999;276:H1647–1654. doi: 10.1152/ajpheart.1999.276.5.H1647. [DOI] [PubMed] [Google Scholar]
- 54.Kanwar S, Hickey MJ, Kubes P. Postischemic inflammation: a role for mast cells in intestine but not in skeletal muscle. Am J Physiol. 1998;275:G212–218. doi: 10.1152/ajpgi.1998.275.2.G212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. H2O2 does not stimulate Tyr phosphorylation of IκBα at concentrations that activate NF-κB in endothelial cells.
Fig. S2. Nck siRNA reduces H2O2-indcued NF-κB activation and increase in VCAM-1 abundance.
Fig. S3. Micrographs of monocyte adhesion to oxidant stress-activated endothelial cells.
Fig. S4. Oxidant stress-induced tyrosine phosphorylation activates Nck-dependent PAK2 signaling.
Fig. S5. H2O2 stimulates Nck and PAK recruitment to PECAM-1.
Table S1. Quantitative real-time PCR primers.