Abstract
Bile acid diarrhea (BAD) is usually seen in patients with ileal Crohn’s disease or ileal resection. However, 25% to 50% of patients with functional diarrhea or diarrhea-predominant irritable bowel syndrome (IBS-D) also have evidence of BAD. It is estimated that 1% of the population may have BAD. The causes of BAD include a deficiency in fibroblast growth factor 19 (FGF-19), a hormone produced in enterocytes that regulates hepatic bile acid (BA) synthesis. Other potential causes include genetic variations that affect the proteins involved in BA enterohepatic circulation and synthesis or in the TGR5 receptor that mediates the actions of BA in colonic secretion and motility. BAs enhance mucosal permeability, induce water and electrolyte secretion, and accelerate colonic transit partly by stimulating propulsive high-amplitude colonic contractions. There is an increased proportion of primary BAs in the stool of patients with IBS-D, and some changes in the fecal microbiome have been described. There are several methods of diagnosing BAD, such as 75selenium homotaurocholic acid test retention, serum C4, FGF-19, and fecal BA measurement; presently, therapeutic trials with BA sequestrants are most commonly used for diagnosis. Management involves the use of BA sequestrants including cholestyramine, colestipol, and colesevelam. FXR agonists such as obeticholic acid constitute a promising new approach to treating BAD.
Keywords: Malabsorption, FGF-19, FXR, C4, CYP7A1, Klotho β
INTRODUCTION: BILE ACIDS, FAT ABSORPTION, AND THE ENTEROHEPATIC CIRCULATION
Bile acids (BAs) are detergent molecules1 that are synthesized in the liver and are responsible for solubilization of fatty acids and monoglycerides (the lipolysis products of triglycerides), facilitating digestion and lipid absorption in the small intestine. The different BA molecules are differentiated by hydroxylation and conjugation. Chenodeoxycholic acid (CDCA) and cholic acid (CA) are primary BAs synthesized from cholesterol and conjugated with taurine or glycine in the liver; in the colon, bacteria deconjugate and dehydroxylate the BAs to form, respectively, lithocholic acid and deoxycholic acid (DCA).2
Taurine or glycine conjugation of the BAs permits the ionization of BAs which increases their solubility and their impermeability to cell membranes, allowing BAs to reach the critical micellar concentration for spontaneous formation of micelles. In the micelles, the polar BAs surround the insoluble, hydrophobic fatty acids and monoglycerides and present the hydrophobic fat molecules to the enterocyte brush border membrane of the small intestine for digestion and absorption.
The apical Na+-dependent bile salt transporter (ASBT) (also called ileal BA transporter or SLC10A2 [solute carrier family 10, member two]) is responsible for the active reuptake of BAs in the terminal ileum. This reabsorbs approximately 95% of BAs in the terminal ileum and results in a functional enterohepatic circulation of BA,3 transporting the BAs back to the liver. Several molecular mechanisms are involved in the enterohepatic circulation: farnesoid X receptor (FXR) is expressed in ileal enterocytes and hepatocytes; BAs are agonists of the FXR; sensing of the enterocyte BA pool by FXR affects the liver by way of the endocrine factor, fibroblast growth factor 19 (FGF-19); FGF-19 is released from enterocytes into the portal circulation and activates FGF receptor 4 (FGF-R4) in hepatocytes in a process that involves interaction with klothoβ on the hepatocyte membrane, resulting in downregulation of cholesterol 7α-hydroxylase (CYP7A1) and therefore inhibition of the BA synthesis. Cholerheic or BA diarrhea is thought to result predominantly from the interruption of the enterohepatic circulation.3
CLASSIFICATION OF BILE ACID MALABSORPTION/DIARRHEAS
The causes of BA diarrhea (BAD) are based on the original classification of BA malabsorption (BAM):
Type 1: Ileal dysfunction and impaired reabsorption, e.g., Crohn’s disease
Type 2: Primary, or idiopathic, BAD produces a similar picture of increased fecal BAs, watery diarrhea, and response to BA sequestrants in the absence of ileal or other obvious gastrointestinal disease
Type 3: Other gastrointestinal disorders which affect absorption, such as small intestinal bacterial overgrowth, celiac disease, or chronic pancreatitis
A fourth category of BAD may result from excessive hepatic BA synthesis; for example, the oral hypoglycemic drug, metformin, is associated with increased hepatic BA synthesis.4–6
WHAT’S NEW IN UNDERSTANDING THE ETIOPATHOGENESIS OF IDIOPATHIC BILE ACID DIARRHEA?
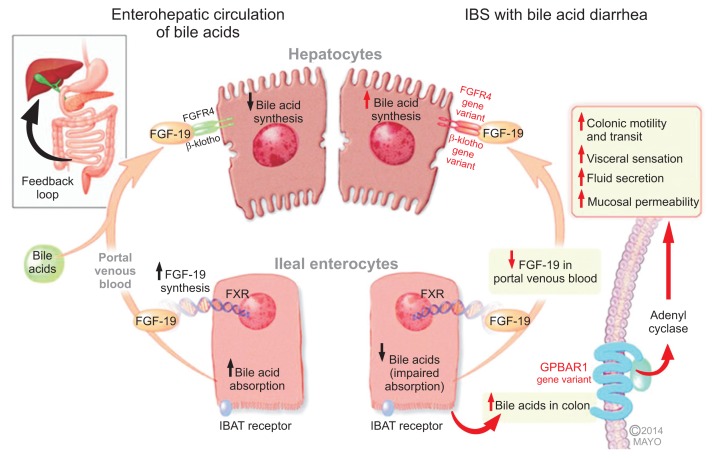
Recent literature has identified several novel potential mechanisms in the development of idiopathic BAD (Fig. 1).7
Fig. 1.
Mechanisms of bile acid (BA)-related bowel dysfunction in irritable bowel syndrome with diarrhea (IBS-D) or idiopathic BA diarrhea (Adapted from Camilleri M. J Physiol 2014;592(Pt 14):2967–2980).7 Enterohepatic circulation of bile acids: Ileal enterocytes absorb bile acid through a receptor-mediated process (ileal bile acid transporter [IBAT]). Intracellular bile acids activate the farnesoid-γ receptor to increase fibroblast growth factor 19 (FGF-19) synthesis. FGF-19 in the portal circulation downregulates hepatocyte bile acid synthesis. Disorders of FGF-19 synthesis by ileal enterocytes or genetic variations of FGFR4 or β-klotho lead to excess bile acid concentration in the colon, resulting in activation of the G protein-coupled bile acid receptor 1 (GPBAR1, or TGR5) with enteroendocrine cell stimulation (e.g., release of 5-hydroxytryptamine) and stimulation of colonic motility with acceleration of colonic transit, activation of visceral sensation and fluid secretion (through increased intracellular cAMP, increased mucosal permeability or chloride ion secretion). Genetic variation in GPBAR1 (TGR5) is associated with increased colonic transit in IBS-D.
1. Defective feedback inhibition of bile acid biosynthesis by FGF-19
FGF-19 produced in the ileum in response to BA absorption regulates hepatic BA synthesis.8 In a landmark article, Walters et al.9 reported lower serum FGF-19 in patients with BAM and an inverse relationship between FGF-19 and serum C4 (a surrogate of the rate of hepatic BA synthesis). These results were replicated by others.10,11
2. Genetic mutations in the apical sodium-bile acid transporter
Genetic mutations in the apical sodium-bile acid transporter (ASBT) are extremely rare.12,13 In addition, defective BA uptake into ileal mucosal biopsies was excluded by Bajor et al.14
3. Accelerated small bowel transit bypassing active bile acid transport in the ileum
Accelerated small bowel transit bypassing active BA transport in the ileum has been hypothesized as a cause of BAM in idiopathic15 and postradiation cases.16,17 While this is theoretically possible, it seems unlikely given the ASBT’s affinity for BA, and it is unclear whether the accelerated small bowel transit is a cause or an effect of the BAM.
4. Genetic variations in the proteins involved in feedback regulation of bile acid synthesis, specifically KlothoB gene and fibroblast growth factor 4 gene
The role of these genetic variants is based on significant associations of SNP rs17618244 in the KlothoB (KLB) gene with colonic transit in diarrhea-predominant irritable bowel syndrome (IBS-D).18 Pharmacogenetic studies of the influence of KLB (rs17618244) on the dose-response effects of administered chenodeoxycholate on the emptying rate of the ascending colon11 suggest that KLB variation may influence colonic response to BAM, and exome DNA sequencing studies showed KLB (rs1015450, downstream) association with fecal BAs and FGF-R4 (rs1966265, nonsynonymous) with colonic transit.19
5. Upregulation of the membrane bound bile acid receptor, TGR5 or GPBAR1
TGR5, or GPBAR1, is a member of the G protein-coupled receptor superfamily that functions as a cell surface receptor for BA,20 including colonic epithelial cells,21 regulating basal and cholinergic-induced secretion in rat colon22 and colonic transit.23 We have recently shown that genetic variation in GPBAR1 predisposes to quantitative changes in colonic transit and BA excretion.24
CELLULAR MECHANISMS OF BILE ACID DIARRHEA
BA chemistry determines effects on colonic mucosa; in general, the surface active properties that lead to increased colonic mucosal permeability and electrolyte and water secretion are associated with two hydroxyl groups at the 3,7 (CDCA) or 3,12 (DCA) positions in the α-configuration. BAs regulate many cell types in the gut wall and beyond by activating nuclear and plasma membrane receptors. Of these, the G protein-coupled receptor, TGR5, has emerged as a key mediator of the nongenomic actions of BAs. TGR5 is a cell-surface receptor that couples to Gαs, formation of cAMP, activation of protein kinase A and extracellular signal-regulated kinases, and inhibition of inflammatory signaling pathways.25
The mechanisms of diarrhea include increased mucosal permeability;26 water secretion through activation of CFTR via adenylate cyclase27,28 and inhibition of apical Cl/OH exchange;29 lubrication by increased mucus secretion (a direct effect on goblet cells);30,31 and acceleration of colonic motility, likely via TGR5 stimulation of myenteric ganglionic neurons.23 BAs induce colonic high amplitude propagated contractions.32
PREVALENCE OF BILE ACID DIARRHEA
Type 1 BAD is caused by ileal disease or resection, typically due to Crohn’s disease or radiation ileitis. The classical papers of Hofmann and Poley33–35 described the association of ileal disease of <100 cm length with diarrhea; when the extent of involvement was over 100 cm, there was associated steatorrhea as a result of BA deficiency.
Type 2 BAD is currently considered diarrhea without morphological abnormalities. Several studies have documented BAM in one-third to one-half of patients with chronic diarrhea or IBS-D, as summarized in a systematic review.36 Overall, the systematic review found that BAM was reported in 32% of patients with symptoms consistent with IBS-D, and there was a dose-response relationship to treatment with BA binders, based on severity of BAM assessed by 75selenium homotaurocholic acid test (75SeHCAT) retention at 7 days. Similar results were found in recent studies of patients presenting to an outpatient gastroenterology clinic in the United Kingdom37 and in a prospective research study at Mayo Clinic of local patients with IBS-D.10,38 In fact, the IBS-D patients had evidence of increased fecal BA excretion and increased hepatic BA synthesis.
It has been estimated that 1% of the population of Western countries suffers from BAD.39
INTERACTIONS OF THE MICROBIOME OF THE COLON AND BILE ACIDS
The colonic microbiome is responsible for the dehydroxylation of cholic and chenodeoxycholic acids to the secondary BAs, deoxycholic and lithocholic acids. Gut microbiota also regulate expression of fibroblast growth factor 15 in the mouse ileum and cholesterol CYP7A1 in the liver by FXR-dependent mechanisms.40 The microbiome influences the generation of BAs and other organic acids in the murine colon.41 In humans, BA pool size and composition appear to be major regulators of microbiome structure, which, in turn, appears to be an important regulator of BA pool size and composition.42 Ongoing research seeks to unravel the contributions of the microbiome and BA composition to diverse conditions including colorectal cancer,43 inflammatory bowel disease,44 and irritable bowel syndrome.45
CHANGES IN THE PROFILE OF FECAL BILE ACIDS IN IRRITABLE BOWEL SYNDROME
Several studies have now reported the profile of fecal BAs in patients with IBS-D. Duboc et al.45 reported that the percentage of fecal primary BA was significantly higher in IBS-D patients than in healthy controls, and it was significantly correlated with stool consistency and frequency. They also reported a significant increase of Escherichia coli and a significant decrease of leptum and bifidobacterium in IBS-D patients. Shin et al.38 confirmed that fecal levels of primary BAs (cholic and chenodeoxycholic [CDCA] acids) were higher in 31 subjects with IBS-D, compared with 30 healthy controls, and also reported that the proportions of fecal secretory BAs (chenodeoxycholic and deoxycholic [DCA] acids) were lower in 30 IBS-C patients compared with controls. An extension study of the latter cohort involving 64 patients with IBS-D confirmed the differences in the proportions of primary and secondary BAs in feces of patients with IBS-D. In addition, the phenotypes of patients with IBS-D and increased total fecal excretion of >2,337 μmol per 48 hours differed from that of IBS-D patients with normal fecal BA excretion, including higher body mass index, increased fecal fat excretion, higher proportion of primary BAs (CA and CDCA) in stool, and a trend to faster colonic transit.46
DIAGNOSIS OF BILE ACID MALABSORPTION
Table 1 summarizes the diagnostic tests for BAM and their pros and cons.47
Table 1.
Diagnostic Tests for Bile Acid Malabsorption and Their Pros and Cons
| BAM diagnosis | Advantages | Disadvantages |
|---|---|---|
| Therapeutic trial with BA sequestrant | Clinically applicable, widely used | Not definitive diagnosis of BAM; nonspecific amelioration of diarrhea due to other causes; poor compliance with some BA sequestrants |
| 14C glycocholate | May identify small bowel bacterial overgrowth | Radiation exposure, β emission, long t1/2 Varying normal values Positive breath excretion at 2–4 hr does not differentiate BAM from small bowel bacterial overgrowth Laborious test method (stool collection) |
| 75SeHCAT | γ Emission, short t1/2, with decreased radiation to extra-abdominal organs | Not available in United States |
| Well-defined normal values; level of isotope retention predicts response to BA sequestrant | Radiation exposure | |
| Simple test method: 2 patient visits | ||
| Serum C4 | No radiation | Fasting sample, diurnal variation |
| Normal values reported in adults | Requires further validation | |
| Not dependent on age, gender or cholesterol | False-positive in liver disease, treatment with statins and altered circadian rhythm | |
| Simple blood test: 1 patient visit | ||
| Serum FGF-19 | No radiation; commercial ELISA assay | Moderate sensitivity and specificity; requires further validation |
| Fecal BA | No radiation | Variable daily fecal BA excretion, requires at least 48 hr sample collection |
| Measures total and individual BAs | Cumbersome method (stool collection) | |
| Urine 2-propanol and acetamide | No radiation; urine sample | Special technology required: Field Asymmetric Ion Mobility Spectrometer; requires replication and validation |
Updated from Vijayvargiya P, et al. Clin Gastroenterol Hepatol 2013;11:1232–1239.47
BAM, bile acid malabsorption; BA, bile acid; 75SeHCAT, 75selenium homotaurocholic acid test; FGF-19, fibroblast growth factor 19; ELISA, enzyme-linked immunosorbent assay.
1. Direct measurements of bile acids
14C-glycocholate breath and stool test, 75selenium homotauro-cholic acid test (SeHCAT), 7α-hydroxy-4-cholesten-3-one (C4), and fecal BAs are direct measurements of BAs or surrogates for the rate of hepatic synthesis of BAs, which is proportional to BAM.
The 14C-glycocholate (14C-BA) breath and stool test is based on the principles48 that bacterial overgrowth in the small intestine enzymatically degrades the 14C-BA, releasing 14C-glycine which is absorbed into the portal circulation, is rapidly metabolized in the liver, and is exhaled into the breath as an early peak (typically <60 minutes from ingestion) of 14CO2. If 14C-BA is not reabsorbed in the terminal ileum and enters the large intestine, the 14C-BA is deconjugated by colonic bacteria and, if not absorbed by passive diffusion in the colon, is excreted in stool. This test is no longer widely utilized since development of less complex tests that have no radiation exposure.
The 75SeHCAT utilizes a synthetic 75selenium homotaurocholic BA that is resistant to bacterial degradation49 and passive diffusion.50 Like a natural BA, it is either actively absorbed in the terminal ileum or excreted into stool, and unaltered by its passage through the colon. The test involves the patient ingesting a capsule containing 75SeHCAT; retention of the isotope in the body at 7 days is measured noninvasively by whole body gamma counter and expressed as a percentage of administered dose. BA may undergo five enterohepatic circulations per day with ~5% loss in the stool with each circulation; retention rates of 5%, 10%, and 15% are used to estimate the relative severity of BAM.
Serum 7 α-hydroxy-4-cholesten-3-one (C4) measures BA synthesis, 90% of which is regulated by the rate-limiting enzyme, cholesterol CYP7A1. C4 is a downstream product of CY-P7A1. Serum C4 is a simple blood test, but it requires standardized specimen collection time because of diurnal variability.51 Accurate method for measurement uses liquid chromatography-tandem mass spectrometry.52 The clinical performance of the C4 assay demonstrated a sensitivity of 90%, specificity of 79%, negative predictive value of 98%, and positive predictive value of 74% when compared to the 75SeHCAT test. The high negative predictive value makes the assay attractive as a screening test to rule out BAM.53 C4 was unrelated to age, gender or serum cholesterol when analyzed against potential covariates.53 When compared to elevated 48-hour fecal BA excretion, elevated serum C4 did not identify phenotype differences (such as increased fecal fat and colonic transit) among patients with IBS-D, other than documenting the increased fecal BAs among those with elevated serum C4, defined as >47.1 ng/mL.46 In summary, serum C4 test is applicable to a majority of patients, but requires further clinical validation including responsiveness to BA sequestrants therapy or FXR agonists in patients with BAM.
Fecal measurements to quantify total and individual fecal BAs are technically cumbersome and not widely available.10,38,46 These Mayo Clinic studies showed that IBS-D is associated with higher serum C4, higher total fecal BA, and increased secretory BAs (e.g., CDCA, DCA). In addition, high fecal BA excretion was associated with a more significant IBS-D phenotype, characterized by higher fecal fat and a trend toward accelerated colonic transit.46 Indeed, fecal BA excretion and colonic transit were validated as biomarkers that identified mechanisms that could be targets of treatment in patients with IBS-D.54 Excretion of >2,337 μmol per 48 hours (upper limit of normal) is used as an index of BAM.46,54
An enzymatic 3α-steroid dehydrogenase assay indirectly measures fecal BA. 3α-Steroid dehydrogenase is used to oxidize deconjugated BAs and produces NADH, which is then measured biochemically. This method requires proper stereotactic alignment of enzyme and substrate and with a variety of conjugations (sulfonation, glucuronidation) of BAs while they are in the small intestine. This method would lack precision if it was used to measure concentrations of BAs in small bowel fluid or ileostomy effluent. In addition, because it does not assess BAs with hydroxyl groups in the β-configuration, it tends to underestimate total BAs.
2. Indirect measurements of bile acids
Serum FGF-19 is a useful screening test for BAD,39 given the inverse relationship between C4 and FGF-19 originally described by Walters et al.9 It has been validated in studies using 75SeHCAT retention as the gold standard55 and by comparisons with serum C4.56 In the study of Pattni et al.56 of 258 patients, sensitivity and specificity of FGF 19 at 145 pg/mL for detecting a C4 level >28 ng/mL were 58% and 79%, respectively, and for C4 >60 ng/mL (denoting high BA synthesis), the sensitivity and specificity of FGF-19 were 74% and 72%, respectively.55 The attraction of this diagnostic method is the ease of the enzyme-linked immunosorbent assay and the measurements based on a morning, fasting serum sample. Further validation studies, including responsiveness to therapy of BAM, are eagerly awaited.
Urine 2-propanol and acetamide57 are volatile organic compounds produced by the gut bacterial cleavage of BAs. This method uses an electronic nose (that mimics the biological olfactory system)58 and a Field Asymmetric Ion Mobility Spectrometer that separates ionized molecules based on their different mobilities in a high electric field.59 These volatile organic compounds were detected in urine of 23 patients with BAD (confirmed by 75SeHCAT), in contrast to 42 patients with ulcerative colitis and 45 healthy controls. Further studies are awaited.
MANAGEMENT OF BILE ACID DIARRHEA
1. Intraluminal bile acid binders
Cholestyramine and colestipol are generally considered first-line treatment for BAD; however, poor palatability results in low patient compliance.60 Several open label studies have recently demonstrated efficacy of these BA sequestrants in patients with IBS-D, especially those with evidence of BAM.61,62 For example, colestipol treatment improved IBS symptoms (IBS severity scoring system 220±109 vs 277±106; p<0.01), and 15 of 27 patients also fulfilled criteria for treatment response (adequate relief ≥50% of weeks 5 to 8), suggesting benefit both in bowel symptoms and global symptoms.
Alternatives are being tested, even though there are no large clinical trials specifically for the indication of BAD. Thus, patients may prefer colesevelam at a dose of up to 1.875 g, twice a day. In a pharmacodynamics study of 24 unselected patients with IBS-D,63 emptying of the ascending colon took an average of 4 hours longer in patients given colesevelam (1.875 g, twice a day) compared with placebo, treatment effect was significantly associated with baseline serum C4 levels, and colesevelam caused greater ease of stool passage and somewhat firmer stool consistency. In an unpublished open-label study (Camilleri 2014, unpublished) of the same dose of colesevelam in 12 IBS-D patients with elevated fecal BA excretion, we have also shown that colesevelam sequestered BAs and resulted in significantly firmer stool consistency.
2. Experimental agents inhibiting bile acid diarrhea by cellular mechanisms
FGF-19 production is stimulated by the FXR agonist, obeticholic acid,64 which may potentially reverse the FGF-19 deficiency postulated in BAM that leads to excessive hepatocyte BA synthesis. This treatment has been associated with improved stool frequency and consistency in a preliminary study of patients with BAD.65 Another FXR agonist, GW4064, attenuated Cl− secretory responses to both Ca2+ and cAMP-dependent agonists, and may be efficacious in the treatment of BAD through antisecretory actions on the colonic epithelium.66
CONCLUSION AND FUTURE DIRECTION
The pioneering work conducted 40 years ago by giants in this field (Drs. Alan Hofmann, Donald Small, Hans Fromm, and Vinton Chadwick) is finally going to have an impact beyond the patients with ileal resection or ileal Crohn’s disease. BA diarrhea is finally appreciated as a significant cause of functional, otherwise unexplained, chronic diarrhea in about one-third of such patients. The availability of simple diagnostic stool tests (fecal BA excretion performed at the time of fecal fat measurement) and, even more applicable, serum or urine tests will enhance the ability of physicians to diagnose this eminently treatable disorder.
In the future, BA sequestration with tablet formulations that are associated with higher compliance or Farnesoid X receptor agonists will impact the care of patients and likely reduce overall healthcare costs by reducing the need for expensive tests like colonoscopy and biopsies or treatments like biologic agents in patients with Crohn’s disease.
ACKNOWLEDGEMENTS
Dr. Camilleri is supported by grant R01-DK92179 from National Institutes of Health.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Hofmann AF, Small DM. Detergent properties of bile salts: correlation with physiological function. Annu Rev Med. 1967;18:333–376. doi: 10.1146/annurev.me.18.020167.002001. [DOI] [PubMed] [Google Scholar]
- 2.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann AF. The syndrome of ileal disease and the broken enterohepatic circulation: cholerhetic enteropathy. Gastroenterology. 1967;52:752–757. [PubMed] [Google Scholar]
- 4.Caspary WF, Zavada I, Reimold W, Deuticke U, Emrich D, Willms B. Alteration of bile acid metabolism and vitamin-B12-absorption in diabetics on biguanides. Diabetologia. 1977;13:187–193. doi: 10.1007/BF01219698. [DOI] [PubMed] [Google Scholar]
- 5.Scarpello JH, Hodgson E, Howlett HC. Effect of metformin on bile salt circulation and intestinal motility in type 2 diabetes mellitus. Diabet Med. 1998;15:651–656. doi: 10.1002/(SICI)1096-9136(199808)15:8<651::AID-DIA628>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 6.Miles JM, Camilleri M, Vella A. Effects of metformin on bile acid metabolism in type 2 diabetes. Diabetes. 2014;63(Suppl 1):A285–A286. [Google Scholar]
- 7.Camilleri M. Physiological underpinnings of irritable bowel syndrome: neurohormonal mechanisms. J Physiol. 2014;592(Pt 14):2967–2980. doi: 10.1113/jphysiol.2014.270892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundåsen T, Gälman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260:530–536. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 9.Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, le Roux CW. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7:1189–1194. doi: 10.1016/j.cgh.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Wong BS, Camilleri M, Carlson P, et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2012;10:1009–1015. doi: 10.1016/j.cgh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao AS, Wong BS, Camilleri M, et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139:1549–1558. doi: 10.1053/j.gastro.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montagnani M, Love MW, Rössel P, Dawson PA, Qvist P. Absence of dysfunctional ileal sodium-bile acid cotransporter gene mutations in patients with adult-onset idiopathic bile acid malabsorption. Scand J Gastroenterol. 2001;36:1077–1080. doi: 10.1080/003655201750422693. [DOI] [PubMed] [Google Scholar]
- 13.Montagnani M, Abrahamsson A, Gälman C, et al. Analysis of ileal sodium/bile acid cotransporter and related nuclear receptor genes in a family with multiple cases of idiopathic bile acid malabsorption. World J Gastroenterol. 2006;12:7710–7714. doi: 10.3748/wjg.v12.i47.7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajor A, Kilander A, Fae A, et al. Normal or increased bile acid uptake in isolated mucosa from patients with bile acid malabsorption. Eur J Gastroenterol Hepatol. 2006;18:397–403. doi: 10.1097/00042737-200604000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Sadik R, Abrahamsson H, Ung KA, Stotzer PO. Accelerated regional bowel transit and overweight shown in idiopathic bile acid malabsorption. Am J Gastroenterol. 2004;99:711–718. doi: 10.1111/j.1572-0241.2004.04139.x. [DOI] [PubMed] [Google Scholar]
- 16.Valdés Olmos R, den Hartog Jager F, Hoefnagel C, Taal B. Effect of loperamide and delay of bowel motility on bile acid malabsorption caused by late radiation damage and ileal resection. Eur J Nucl Med. 1991;18:346–350. doi: 10.1007/BF02285463. [DOI] [PubMed] [Google Scholar]
- 17.Yeoh EK, Horowitz M, Russo A, Muecke T, Robb T, Chatterton BE. Gastrointestinal function in chronic radiation enteritis: effects of loperamide-N-oxide. Gut. 1993;34:476–482. doi: 10.1136/gut.34.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong BS, Camilleri M, Carlson PJ, et al. A Klothoβ variant mediates protein stability and associates with colon transit in irritable bowel syndrome with diarrhea. Gastroenterology. 2011;140:1934–1942. doi: 10.1053/j.gastro.2011.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camilleri M, Klee EW, Shin A, et al. Irritable bowel syndrome-diarrhea: characterization of genotype by exome sequencing, and phenotypes of bile acid synthesis and colonic transit. Am J Physiol Gastrointest Liver Physiol. 2014;306:G13–G26. doi: 10.1152/ajpgi.00294.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 21.Ward JB, Mroz MS, Keely SJ. The bile acid receptor, TGR5, regulates basal and cholinergic-induced secretory responses in rat colon. Neurogastroenterol Motil. 2013;25:708–711. doi: 10.1111/nmo.12148. [DOI] [PubMed] [Google Scholar]
- 22.Poole DP, Godfrey C, Cattaruzza F, et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22:814–825. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alemi F, Poole DP, Chiu J, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144:145–154. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camilleri M, Shin A, Busciglio I, et al. Genetic variation in GP-BAR1 predisposes to quantitative changes in colonic transit and bile acid excretion. Am J Physiol Gastrointest Liver Physiol. 2014;307:G508–G516. doi: 10.1152/ajpgi.00178.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bunnett NW. Neuro-humoral signalling by bile acids and the TGR5 receptor in the gastrointestinal tract. J Physiol. 2014;592:2943–2950. doi: 10.1113/jphysiol.2014.271155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chadwick VS, Gaginella TS, Carlson GL, Debongnie JC, Phillips SF, Hofmann AF. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med. 1979;94:661–674. [PubMed] [Google Scholar]
- 27.Conley DR, Coyne MJ, Bonorris GG, Chung A, Schoenfield LJ. Bile acid stimulation of colonic adenylate cyclase and secretion in the rabbit. Am J Dig Dis. 1976;21:453–458. doi: 10.1007/BF01072128. [DOI] [PubMed] [Google Scholar]
- 28.Ao M, Sarathy J, Domingue J, Alrefai WA, Rao MC. Chenodeoxycholic acid stimulates Cl(−) secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells. Am J Physiol Cell Physiol. 2013;305:C447–C456. doi: 10.1152/ajpcell.00416.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alrefai WA, Saksena S, Tyagi S, Gill RK, Ramaswamy K, Dudeja PK. Taurodeoxycholate modulates apical Cl−/OH− exchange activity in Caco2 cells. Dig Dis Sci. 2007;52:1270–1278. doi: 10.1007/s10620-006-9090-8. [DOI] [PubMed] [Google Scholar]
- 30.Camilleri M, Murphy R, Chadwick VS. Dose-related effects of chenodeoxycholic acid in the rabbit colon. Dig Dis Sci. 1980;25:433–438. doi: 10.1007/BF01395507. [DOI] [PubMed] [Google Scholar]
- 31.Barcelo A, Claustre J, Toumi F, et al. Effect of bile salts on colonic mucus secretion in isolated vascularly perfused rat colon. Dig Dis Sci. 2001;46:1223–1231. doi: 10.1023/A:1010607127822. [DOI] [PubMed] [Google Scholar]
- 32.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol. 2002;282:G443–F449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann AF, Poley JR. Cholestyramine treatment of diarrhea associated with ileal resection. N Engl J Med. 1969;281:397–402. doi: 10.1056/NEJM196908212810801. [DOI] [PubMed] [Google Scholar]
- 34.Hofmann AF, Poley JR. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology. 1972;62:918–934. [PubMed] [Google Scholar]
- 35.Poley JR, Hofmann AF. Role of fat maldigestion in pathogenesis of steatorrhea in ileal resection. Fat digestion after two sequential test meals with and without cholestyramine. Gastroenterology. 1976;71:38–44. [PubMed] [Google Scholar]
- 36.Wedlake L, A’Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707–717. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 37.Gracie DJ, Kane JS, Mumtaz S, Scarsbrook AF, Chowdhury FU, Ford AC. Prevalence of, and predictors of, bile acid malabsorption in outpatients with chronic diarrhea. Neurogastroenterol Motil. 2012;24:983–e538. doi: 10.1111/j.1365-2982.2012.01953.x. [DOI] [PubMed] [Google Scholar]
- 38.Shin A, Camilleri M, Vijayvargiya P, et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11:1270–1275. doi: 10.1016/j.cgh.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walters JR, Pattni SS. Managing bile acid diarrhoea. Therap Adv Gastroenterol. 2010;3:349–357. doi: 10.1177/1756283X10377126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sayin SI, Wahlström A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Y, Wu J, Li JV, Zhou NY, Tang H, Wang Y. Gut microbiota composition modifies fecal metabolic profiles in mice. J Proteome Res. 2013;12:2987–2999. doi: 10.1021/pr400263n. [DOI] [PubMed] [Google Scholar]
- 42.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes. 2013;4:382–387. doi: 10.4161/gmic.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ou J, Carbonero F, Zoetendal EG, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98:111–120. doi: 10.3945/ajcn.112.056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duboc H, Rajca S, Rainteau D, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62:531–539. doi: 10.1136/gutjnl-2012-302578. [DOI] [PubMed] [Google Scholar]
- 45.Duboc H, Rainteau D, Rajca S, et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:513–520. doi: 10.1111/j.1365-2982.2012.01893.x. [DOI] [PubMed] [Google Scholar]
- 46.Camilleri M, Busciglio I, Acosta A, et al. Effect of increased bile acid synthesis or fecal excretion in irritable bowel syndrome-diarrhea. Am J Gastroenterol. 2014;109:1621–1630. doi: 10.1038/ajg.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vijayvargiya P, Camilleri M, Shin A, Saenger A. Methods for diagnosis of bile acid malabsorption in clinical practice. Clin Gastroenterol Hepatol. 2013;11:1232–1239. doi: 10.1016/j.cgh.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fromm H, Hofmann AF. Breath test for altered bile-acid metabolism. Lancet. 1971;2:621–625. doi: 10.1016/S0140-6736(71)80068-5. [DOI] [PubMed] [Google Scholar]
- 49.Thaysen EH, Orholm M, Arnfred T, Carl J, Rødbro P. Assessment of ileal function by abdominal counting of the retention of a gamma emitting bile acid analogue. Gut. 1982;23:862–865. doi: 10.1136/gut.23.10.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams AJ, Merrick MV, Eastwood MA. Idiopathic bile acid malabsorption: a review of clinical presentation, diagnosis, and response to treatment. Gut. 1991;32:1004–1006. doi: 10.1136/gut.32.9.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gälman C, Arvidsson I, Angelin B, Rudling M. Monitoring hepatic cholesterol 7alpha-hydroxylase activity by assay of the stable bile acid intermediate 7alpha-hydroxy-4-cholesten-3-one in peripheral blood. J Lipid Res. 2003;44:859–866. doi: 10.1194/jlr.D200043-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Camilleri M, Nadeau A, Tremaine WJ, et al. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009;21:734–e43. doi: 10.1111/j.1365-2982.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sauter GH, Münzing W, von Ritter C, Paumgartner G. Bile acid malabsorption as a cause of chronic diarrhea: diagnostic value of 7alpha-hydroxy-4-cholesten-3-one in serum. Dig Dis Sci. 1999;44:14–19. doi: 10.1023/A:1026681512303. [DOI] [PubMed] [Google Scholar]
- 54.Camilleri M, Shin A, Busciglio I, et al. Validating biomarkers of treatable mechanisms in irritable bowel syndrome. Neurogastroenterol Motil. 2014;26:1677–1685. doi: 10.1111/nmo.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pattni SS, Brydon WG, Dew T, et al. Fibroblast growth factor 19 in patients with bile acid diarrhoea: a prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment Pharmacol Ther. 2013;38:967–976. doi: 10.1111/apt.12466. [DOI] [PubMed] [Google Scholar]
- 56.Pattni SS, Brydon WG, Dew T, Walters JR. Fibroblast growth factor 19 and 7alpha-hydroxy-4-cholesten-3-one in the diagnosis of patients with possible bile acid diarrhea. Clin Transl Gastroenterol. 2012;3:e18. doi: 10.1038/ctg.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Covington JA, Westenbrink EW, Ouaret N, et al. Application of a novel tool for diagnosing bile acid diarrhoea. Sensors. 2013;13:11899–11912. doi: 10.3390/s130911899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson AD, Baietto M. Advances in electronic-nose technologies developed for biomedical applications. Sensors. 2011;11:1105–1176. doi: 10.3390/s110101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arasaradnam RP, Ouaret N, Thomas MG, et al. Evaluation of gut bacterial populations using an electronic e-nose and field asymmetric ion mobility spectrometry: further insights into ‘fermentonomics’. J Med Eng Technol. 2012;36:333–337. doi: 10.3109/03091902.2012.690015. [DOI] [PubMed] [Google Scholar]
- 60.Halilbasic E, Claudel T, Trauner M. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J Hepatol. 2013;58:155–168. doi: 10.1016/j.jhep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stotzer PO, Abrahamsson H, Bajor A, Sadik R. Effect of cholestyramine on gastrointestinal transit in patients with idiopathic bile acid diarrhea: a prospective, open-label study. Neuroenterology. 2013;2 doi: 10.4303/ne/235657. Article ID 235657. [DOI] [Google Scholar]
- 62.Bajor A, Törnblom H, Rudling M, Ung KA, Simrén M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut. 2015;64:84–92. doi: 10.1136/gutjnl-2013-305965. [DOI] [PubMed] [Google Scholar]
- 63.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8:159–165. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang JH, Nolan JD, Kennie SL, et al. Potent stimulation of fibroblast growth factor 19 expression in the human ileum by bile acids. Am J Physiol Gastrointest Liver Physiol. 2013;304:G940–G948. doi: 10.1152/ajpgi.00398.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnston IM, Nolan JD, Dew T, Shapiro D, Walters JR. A new therapy for chronic diarrhea? A proof of concept study of the FXR agonist obeticholic acid in patients with primary bile acid diarrhea. Gastroenterology. 2013;144(Suppl 1):S-60. [Google Scholar]
- 66.Mroz MS, Keating N, Ward JB, et al. Farnesoid X receptor agonists attenuate colonic epithelial secretory function and prevent experimental diarrhoea in vivo. Gut. 2014;63:808–817. doi: 10.1136/gutjnl-2013-305088. [DOI] [PubMed] [Google Scholar]