Abstract
Background
Arthropod vectors of disease may encounter more than one infected host during the course of their lifetime. The consequences of super-infection to parasite development are rarely investigated, but may have substantial epidemiological and evolutionary consequences.
Methods
Using a rodent malaria model system, behavioural avoidance of super-infection was tested by examining whether already-infected Anopheles stephensi mosquitoes were less responsive to new vertebrate hosts if they were infected. Additionally, a second dose of parasites was given to malaria-infected mosquitoes on a biologically realistic time scale to test whether it impeded the development of a first infection.
Results
No effect of a second infected blood meal on either the prevalence or parasite burden arising from a first was found. Furthermore, it was found that not only were infected mosquitoes more likely to take a second blood meal than their uninfected counterparts, they were disproportionately drawn to infected hosts.
Conclusions
The alterations in mosquito feeding propensity reported here would occur if parasites have been selected to make infected vertebrate hosts more attractive to mosquitoes, and infected mosquitoes are more likely to seek out new blood meals. Although such a strategy might increase the risk of super-infection, this study suggests the cost to parasite development is not high and as such would be unlikely to outweigh the potential benefits of increasing the contact rate between the parasite's two obligate hosts.
Background
Many arthropod disease vectors have multiple opportunities to become infected with the same pathogen species during their lifetime (super-infection). The impact of super-infection within vectors to parasite transmission is largely unknown, and may have substantial impacts on epidemiology. For example, in the laboratory, pathogen transmission can be enhanced when different parasite species co-occur in the same individual vector, a phenomenon that has been observed in some [1-4] but not all mosquito species that have been tested [1,4].
The aim of this study was to investigate the potential epidemiological consequences of super-infection of mosquitoes by malaria parasites. Super-infection of vectors by successive parasite infections has been examined in a variety of infectious diseases [5-7], but to knowledge, the frequency and outcome of malaria super-infection has never been investigated. Malaria parasites are a relevant model for studies of vector super-infection because their biology dictates a substantial risk of super-infection in the wild. First, female Anopheles mosquitoes try to blood feed at least once every two to four days [8], so that mosquitoes can receive two or more separate infections during their life. In the wild, approximately 20 % of An. gambiae mosquitoes live through two feeding cycles, with 6% living four or more [9,10]. Second, a substantial proportion of some Anopheles spp. return to the same house on different feeding cycles [e. g. [11]], so that mosquitoes that encounter infected blood are likely to do so again, especially if infected hosts are more attractive to mosquitoes as indicated in some animal models [12,13].
A model system consisting of the mosquito vector Anopheles stephensi and the rodent malaria parasite Plasmodium chabaudi was used to test whether mosquito feeding behaviour could facilitate or diminish the probability of super-infection. Mosquitoes were fed on infected or uninfected blood and then, four days later, offered a second blood meal of either infected or uninfected blood. The propensity to take a second blood meal was observed, as was whether the development of malaria parasites from the first feed was impeded by the introduction of a second infectious meal on a time-scale mimicking natural blood-feeding behaviour. These experiments provide a first insight into a previously unstudied phenomenon that could affect malaria epidemiology.
Methods
Anopheles stephensi were reared as described elsewhere [14], under standard insectary conditions of 70% RH (± 10%) and 27°C (± 3°C). At this temperature, the sporogonic cycle of P. chabaudi takes approximately 12–16 days [15]. Plasmodium chabaudi was first isolated from its natural host, the thicket rat Thamnomys rutilans, in the Central African Republic in 1969–1970. Since then, this parasite has been stored in liquid nitrogen at the University of Edinburgh. In this experiment one clone of P. chabaudi was used, known as CR, which was isolated from the original samples [16]. Three inbred female mice (C57BL/6J, Harlan England) of similar age and weight were infected with a dose of 105 CR parasites, with three others being left uninfected to act as controls. The control group were given sham injections that contained only the inoculation medium of calf serum and ringers solution. Two days later, a second group of six mice were infected in the same way, with six being sham-injected to act as controls.
Parasitaemia and gametocytaemia were estimated from thin smears taken from tail blood that were examined under a compound microscope (100 ×) as the proportion of red blood cells (RBC) in a random sampling of 300 that were infected with asexual parasites, and the proportion of gametocyte-infected red cells in a random sample of 5000–10,000 RBC respectively. A few hours before mosquitoes were fed on mice, RBC densities were estimated from a 2 μl sample of tail blood by flow cytometry (Coulter Electronics, Luton, England). Asexual and gametocyte densities were estimated as the product of RBC density and parasitemia or gametocytaemia respectively.
Mosquito feeds
Groups of 250 pupae were randomly selected from the rearing trays 10–13 days post-egg hatching and placed in one of six emergence cages (16 × 16 × 16 cm), giving rise to 160–240 adults that were fed ad libitum on a 10% glucose solution supplemented with 0.05% PABA. Mosquito feeds on the first group of infected and control mice (n = 6) took place 16 days after infection, when gametocytes were detectable in all infected mice. Mosquitoes were 4–5 days old at the time of first blood feeding. To increase their appetite, mosquitoes were deprived of glucose for 24 hours before the blood feed. To feed, one anaesthetised mouse was placed on top of each cage and mosquitoes were allowed to bite for 20 minutes.
Immediately after the first blood feeding trial, mosquitoes that had not fed were removed. Ten fully engorged mosquitoes were transferred individually into 30 ml plastic tubes (9 × 2.5 cm) covered with mesh, with the rest being left in the original six cages. A 10% glucose solution (plus 0.05% PABA) was provided ad libitum to mosquitoes held both in cages and in tubes (in cages, glucose was supplied by filter paper wicks, and in tubes by cotton pads soaked in solution that were placed on the top netting).
After 3 days, mosquitoes in tubes were returned to the treatment cages from which they were taken. Hematin within these holding tubes was quantified using a standard photometric assay (as described in [17]) to provide an estimate of blood meal size. On the evening of this same day, a water-filled petri dish was placed in each cage to allow blood-fed females to lay their eggs. These dishes were removed the next morning.
A second blood feed was offered to mosquitoes four days after the first. Prior to the feed, the original six mosquito populations were each split into two new cages, with one receiving an infected host and the other an uninfected host. Twelve mice were prepared for use in this feed (inoculated 18 days before), six infected and six uninfected. However, due to a combination of factors including mouse death prior to and during the feed day, and the failure of gametocytes to develop in some mice, only eight mice (four control and four infected) could be used to feed mosquitoes in each of the 12 cages (with each mouse being used to feed at least two cages). Successive feeding of mosquitoes in different cages from the same mouse was possible because once anaesthetized, mice remain unconscious for over an hour (sufficient for 2 × 20 minute feeding trials), and the relatively small number of mosquitoes per cage (average = 30) ensured that the blood loss per feed was minimal. Any mouse that died during the course of the second blood feed was immediately replaced by a live one from the same infection treatment.
After the second blood feed, all mosquitoes (fed or not) were moved into individual 30 ml tubes for hematin collection, as described above. After 3–4 days (7–8 days after first blood meal), mosquitoes were killed using chloroform, and potentially infected mosquitoes dissected under a microscope (10 ×) in a drop of phosphate-buffered saline (PBS) and inspected for oocysts. From prior experience it is known that at this magnfication, oocysts that have been growing for 7–8 days are easily distinguishable, whereas those that are only 3–4 days old are not. Thus it was certain that all detected oocysts had arisen from the first infectious blood meal.
One wing was removed from all mosquitoes and measured using an ocular micrometer to provide a measure of body size. Haematin that accumulated in the bottom of each tube was quantified as described above. Mosquitoes from tubes where the haematin absorbance was <0.1 nm, the level found in the lithium carbonate control, were classified as a non-feeders. All experiments described above were conducted in accordance with the British Home Office regulations for animal experimentation.
Statistical analysis
The two main questions were (1) does the infection status of the first blood meal (infected or uninfected) influence the propensity of mosquitoes to feed when presented with a second host, and (2) does exposure to parasites in a second blood meal alter the infectivity of parasites from the first? To answer these questions, a series of different statistical models was applied to the data (as described in Table 1[18]). Explanatory variables included the status of the first and second feed (infected or uninfected), with 'mouse'-specific effects (nested within infection treatment) also being fitted to control for additional sources of variation. When the response variable had a binary outcome (presence of parasites, probability of taking a second blood meal), logistic regression was applied to the data using the PROC GENMOD subroutine in SAS, incorporating binomial errors. When the response variable was continuous (number of oocysts, eggs, and size of blood meal), mixed models ANOVA was applied where the main effect of host infection status was treated as a fixed effect, and individual mice as random factors. Differences between mice within treatments were often significant, but as they are of no interest in their own right, they were controlled for by leaving 'mouse-within-treatment' terms in models when they were significant.
Table 1.
Description of statistical models applied to analyse the influence of first and second blood meal type (P. chabaudi infected or uninfected) on the feeding behaviour and infection susceptibility of An. stephensi mosquitoes. In 'Group Analysed', details of the subset of mosquitoes included in a particular analysis are given, with 'first feed' indicating the type of blood meal they were first given (I – only mosquitoes first fed infected blood, I + U – mosquitoes whose first feed was infected or uninfected). In analyses of mosquitoes of whose first feed was infected (models 2,3,5–8), the 'oocyst present' column indicates whether all mosquitoes were included (-), or just those that developed oocysts (Y). The 'took a second feed' column indicates whether analysis was performed on all mosquitoes that had a first blood meal (-), or just those who took a first and second blood meal (Y). N gives the number of mosquitoes included in each analysis. 'Maximal Model' gives the complete set of factors in addition to wing size that were included as explanatory variables for each response variable, with terms in brackets indicating nested variables. Explanatory variables are: FEED1 – status of first blood meal (I or U), FEED2 – status of second blood meal (I or U), with MOUSE1 and MOUSE2 representing the particular mouse within each treatment group that mosquitoes fed on in the first and second feeding trial, respectively. The superscripta denotes variables that were fit as random effects, all others being treated as fixed effects.
| Parameter of Interest | Response Variable | Group Analyzed | N | Model No. | Maximal model (not listing wing size) | SAS subroutine | ||
| First feed | Oocysts present | Took a 2nd feed | ||||||
| Blood Feeding | Had a 2nd blood feed | I + U | - | - | 352 | 1 | F1 + F2(F1) + M1(F1) + M2(F2) | PC |
| I | - | - | 75 | 2 | Oocyst presence + F2 + M2(F2) | PG | ||
| I | Y | - | 45 | 3 | Oocyst number + F2 + M2(F2) | PG | ||
| Log (size of 2nd blood meal) | I+U | . | Y | 167 | 4 | F1+ F2(F1) + Ml(Fl)a + M2(F2)a | PM | |
| I | - | Y | 51 | 5 | Oocyst presence + F2 + Ml(F2)a + M2(F2)a | PM | ||
| I | Y | Y | 34 | 6 | Oocyst number + F2 + Ml(F2)a + M2(F2)a | PM | ||
| Infection Rate | Oocyst presence | I | - | Y | 54 | 7 | F2 + M1 | PG |
| Log(oocyst no.) | I | Y | Y | 34 | 8 | F2 + Ml a | PM | |
Maximal statistical models included the explanatory variables listed in Table 1 and wing size. Mosquito body size (as indexed by wing size) is an important determinant of blood meal size, blood feeding tendency and possibly Plasmodium infection rate [19,20], all of which were response variables in this study. Wing size was included as an explanatory variable in our analyses in order to control for this extra source of variability in order to clarify main treatment effects.
Non-significant terms were sequentially dropped to yield the minimum statistically significant description of the data. Prior to analysis, data on the number of oocysts per mosquito and the size of their second blood meal were log transformed to increase their fit to the normal distribution (as assessed by the Kolmogorov-Smirnov normality test [21]).
Results
Feeding behaviour following infection
A total of 523 mosquitoes were offered an initial blood meal, of which 83% fed during the 20-minute exposure period. Of mosquitoes that took a first blood meal, approximately 47% fed again when offered a second blood meal four days later. Of the 193 mosquitoes whose first blood meal was infected, 55% took a second blood meal when offered 4 days later.
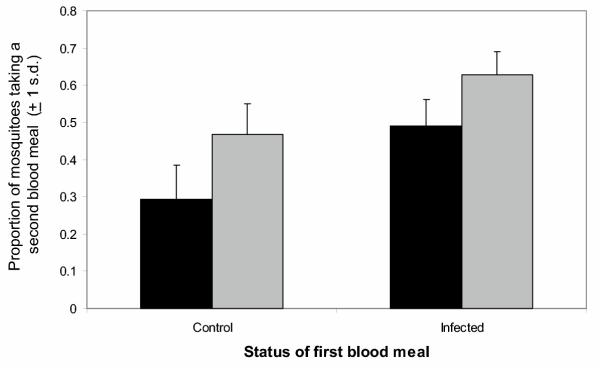
The propensity of mosquitoes to take a second blood meal was strongly associated with the infection status of their first blood meal (Figure 1; χ12 = 22.14, p < 0.01). Mosquitoes whose first meal was infected were almost one and a half times more likely to take a second meal than those whose first blood meal was uninfected (proportion taking a second feed: 0.39 and 0.56 for uninfected and infected mosquitoes respectively). Additionally, mosquitoes were more likely to take a second blood meal if the host they were presented with was infected (Figure 1; Feed2(Feed1): χ12 = 7.95, p < 0.01). Among the 75 mosquitoes first fed infected blood and surviving until dissection on days seven or eight, those that went on to develop oocysts were somewhat more likely to take a second blood feed than those that did not (Model 2: χ12 = 3.06, p = 0.08). There was no evidence that feeding tendency increased with the number of oocysts within a mosquito (Model 3, χ12 = 0.41, p = 0.52, range = 1–249).
Figure 1.
Proportion of mosquitoes that took a second blood meal as a function of the first blood meal they imbibed. Solid black bars indicate that the second blood meal was taken from an uninfected host, and grey bars that the second host was infected with P. chabaudi gametocytes. Error bars represent one standard deviation (calculated for the binomial distribution).
Of mosquitoes that blood-fed twice, the size of the second meal was not influenced by the parasite status of the first (Model 4, Feed2(Feed1): F2,6 = 1.55, p = 0.28). Amongst mosquitoes whose first meal was infected, neither the presence (Model 5, F1,31 = 1.68, p = 0.20) nor number of oocysts (Model 6, F1,26 = 0.36, p = 0.55) influenced the size of the second blood meal. Thus, exposure to parasitized blood in the first or second feed influenced the propensity to take a blood meal, but not the size of the meal.
Re-exposure to parasites and infection
Sixty-three percent of mosquitoes whose first meal was infected went on to develop oocysts, with a mean oocyst intensity of 28.5 (s.e. ± 6.1). Amongst this group, there was no evidence that exposure to parasites in a second feed reduced the development of parasites acquired during the first feed. The presence of gametocytes in the second blood meal had no effect on the oocyst rate arising from the first (Figure 2, χ12 = l.31, p = 0.25). Amongst mosquitoes with oocysts after their first infectious meal, the parasite status of their second blood meal did not influence the number of oocysts they developed (F1,29 = 0.05, p = 0.81). The best predictor of infection rate following the first blood meal was the identity of the first infectious mouse (χ12 = 14.56, p < 0.01), with prevalence being highest in mosquitoes fed on mice with the most gametocytes on the day of blood feeding.
Figure 2.
Proportion of mosquitoes that were initially fed infected blood that developed oocysts as a function of the parasite status of a second blood meal. The second blood meal was given to mosquitoes four days after the first. The data represent the average infection rate across 3 trials (51 mosquitoes), with bars indicating one standard error.
Discussion
This study has demonstrated that the presence of malaria parasites in either of their two obligate hosts increases the probability of contact with the other. Plasmodium-infected mosquitoes were not only more willing to take a second blood meal than their uninfected counterparts, but were almost 1.3 times more likely to initiate feeding if that host was also infected (Figure 1). This phenomenon would increase the risk of super-infection in mosquitoes beyond that expected under the assumption of random contact between hosts and vectors.
Super-infection is probably detrimental to mosquitoes, given that even infections acquired just once reduce mosquito fecundity [22-27] and in some cases, survival [14,28-31]. Why then would vectors, or indeed the parasites that depend upon their survival for transmission, have or induce a feeding strategy that increases the risk of multiple infection? Perhaps the most parsimonious explanation is that natural selection on malaria parasites has acted to make infected vertebrate hosts more attractive to mosquitoes, and infected mosquitoes more willing to blood feed. Both traits would increase contact rate between Plasmodium's two obligate hosts, probably the most important limiting factor to malaria epidemiology. Although such a strategy would engender an increased risk of super-infection, it would none-the-less thrive if the fitness costs of co-existence were minimal.
Quite how Plasmodium infection makes an anaesthetised mouse more attractive is unclear, but there have been numerous reports that other vector-borne parasites increase the attractiveness of their mammalian hosts [32-37]. In malaria, increased feeding on infected hosts has been reported in some [12,38,39], but not all cases [27,40]. Other studies have shown that malaria-infected mosquitoes are more persistent in seeking out blood meals, and bite more often than their uninfected counterparts [41-43]. Some of these studies reported that malaria-infected mosquitoes increased their biting frequency only when infected with the transmissible sporozoite stage of the parasite, and not when oocysts are present [41,44,45]. It has been argued that this apparent stage-specificity is a product of natural selection acting to maximize contact rate with vertebrate hosts only when the parasite is actually capable of being transmitted [44,45]. It is unclear why oocysts apparently enhance feeding propensity in this study (Figure 1) but depress it in others. The energetic demands put on mosquitoes by growing oocysts may, in our system, lead to increased desire for nutrient intake. We have previously observed increased sugar-feeding by oocyst-infected An. stephensi [46], an observation that now appears to extend also to blood-feeding. However, it seems unlikely that the enhanced blood-feeding reported here can be explained solely as a function of parasite energetic demand, because feeding propensity was unrelated to oocyst burden. Further investigation of mosquito blood-feeding behaviour throughout all stages of parasite development, and under resource rich and poor conditions, may help determine the conditions under which oocysts enhance or suppress feeding tendency.
Given that mosquito feeding behaviour appears to enhance the risk of super-infection, the question remains as to what, if any, fitness costs it elicits either on the part of the parasite or the vector. The feeding preferences reported here would not increase the risk of super-infection were infected hosts more likely to exhibit anti-vector behaviour and infected mosquitoes more likely to succumb to it, a possibility we did not test here. Additionally, although no evidence that the early development of malaria parasites was impeded by re-exposure to parasites was found here (Figure 2), the possibility of suppression (or indeed enhancement) during the later part of the sporogonic cycle cannot be ruled out. Indeed, we only tested for a negative development effect of super-infection over a limited period of the sporogonic cycle (oocyst development between day 4 and 7). Had we tracked infection success through to the sporozoite stage, we may have detected a developmental effect of superinfection. Malaria parasite growth in mosquitoes can be hindered by subsequent infection with filarial worms [47], and this may have happened here at a later stage of infection. Additionally, this study was restricted to examining the development of a first Plasmodium infection in the presence of a second (not the development of a second infection in the presence of the first). Thus we have only described the fitness consequences to one party in the super-infection. Although the successful development of a first infection may not be hindered by a second, it is possible that the second cannot develop in the presence of the first. Quantitative PCR protocols to track the relative frequencies of primary and superinfecting clones in sporozoite populations are currently being developed. Such tools will allow testing of whether any of the additional potential costs of super-infection proposed here exist, and whether they could impose sufficient selection to counteract the feeding-behaviour enhancement we report in this study.
Conclusions
This study demonstrated that malaria-infected mosquitoes are not only more likely to seek out a second blood meal than their uninfected counterparts, but also that on second feeding, infected mosquitoes are disproportionately drawn to infected vertebrate hosts. This phenomenon may be the product of natural selection acting on parasites to enhance their transmission risk by increasing the contact rate between their two obligate hosts. One potential drawback of this strategy is that it would engender an increased risk of super-infection in mosquitoes, which could hamper parasite and/or vector fitness. However, we found no evidence that parasite development in mosquitoes was impeded by re-exposure to Plasmodium on a second meal. Future studies that examine the impact of Plasmodium super-infection on mosquito longevity will be of great use to resolving whether the potential transmission benefits of the feeding behaviour shifts described here could be counteracted by increases in vector mortality.
Authors' contributions
HF designed, conducted and analysed the experiments described here, and wrote the manuscript. AR assisted in drafting the final manuscript, planning the experiments, and obtained the Wellcome Trust grant with which this work was funded.
Acknowledgments
Acknowledgements
We thank Brian Chan for experimental assistance, and the staff of the Edinburgh University Animal House. This study was funded by the Wellcome Trust.
Contributor Information
Heather M Ferguson, Email: Heather.Ferguson@wur.nl.
Andrew F Read, Email: a.read@ed.ac.uk.
References
- Vaughan JA, Trpis M, Turell MJ. Brugia malayi microfilariae (Nematoda: Filaridae) enhance the infectivity of Venezuelan equine encephalitis virus to Aedes mosquitoes (Diptera: Culicidae) J Med Entomol. 1999;36:758–763. doi: 10.1093/jmedent/36.6.758. [DOI] [PubMed] [Google Scholar]
- Vaughan JA, Turell MJ. Facilitation of Rift Valley fever virus transmission by Plasmodium berghei sporozoites in Anopheles stephensi mosquitoes. Am J Trop Med Hyg. 1996;55:407–409. doi: 10.4269/ajtmh.1996.55.407. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Rossignol PA, Spielman A, Rossi CA. Enhanced arboviral transmission by mosqitoes that concurrently ingested microfilariae. Science. 1984;225:1039–1041. doi: 10.1126/science.6474165. [DOI] [PubMed] [Google Scholar]
- Paulson SL, Poirier SJ, Grimstad PR, Craig GB. Vector competence of Aedes hendersoni (Diptera, Culicidae) for LaCrosse virus – lack of impaired function in virus-infected salivary glands and enhanced virus transmission by sporozoite-infected mosquitoes. J Med Entomol. 1992;29:483–488. doi: 10.1093/jmedent/29.3.483. [DOI] [PubMed] [Google Scholar]
- Bosworth W, Ewert A. Superinfection of Aedes togoi with Brugia malayi. J Med Entomol. 1973;10:217–219. doi: 10.1093/jmedent/10.2.217. [DOI] [PubMed] [Google Scholar]
- File SK. Superinfection of Biomphalaria glabrata with Schistosoma mansoni. J Parasitol. 1975;61:75–78. [PubMed] [Google Scholar]
- Sundin DR, Beaty BJ. Interference to oral superinfection of Aedes triseratus infected with La Crosse virus. Am J Trop Med Hyg. 1988;38:428–432. doi: 10.4269/ajtmh.1988.38.428. [DOI] [PubMed] [Google Scholar]
- Gillies M. The duration of the gonotrophic cycle in Anopheles gambiae and Anopheles funestus, with a note on the efficiency of hand catching. East Afr Med J. 1953;30:129–135. [PubMed] [Google Scholar]
- Charlwood JD, Vij R, Billingsley PF. Dry season refugia of malaria-transmitting mosquitoes in a dry savannah zone of east Africa. Am J Trop Med Hyg. 2000;62:726–732. doi: 10.4269/ajtmh.2000.62.726. [DOI] [PubMed] [Google Scholar]
- Charlwood JD, Pinto J, Sousa CA, Ferreira C, Gil V, Do Rosario VE. Mating does not affect the biting behaviour of Anopheles gambiae from the islands of Sao Tome and Principe, West Africa. Ann Trop Med Parasitol. 2003;97:751–756. doi: 10.1179/000349803225002345. [DOI] [PubMed] [Google Scholar]
- McCall PJ, Mosha FW, Njunwa KJ, Sherlock K. Evidence for memorized site-fidelity in Anopheles arabiensis. Trans R Soc Trop Med Hyg. 2001;95:587–590. doi: 10.1016/S0035-9203(01)90087-2. [DOI] [PubMed] [Google Scholar]
- Ferguson HM, Rivero A, Read A. The influence of malaria parasite genetic diversity and anaemia on mosquito feeding and fecundity. Parasitology. 2003;127:9–19. doi: 10.1017/S0031182003003287. [DOI] [PubMed] [Google Scholar]
- Day JF, Edman JD. Malaria renders mice susceptible to mosquito feeding when gametocytes are most infective. J Parasitol. 1983;69:163–170. [PubMed] [Google Scholar]
- Ferguson HM, Read AF. Genetic and environmental determinants of malaria parasite virulence in mosquitoes. Proc R Soc Lond B. 2002;269:1217–1224. doi: 10.1098/rspb.2002.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killick-Kendrick R, Peters W. Rodent Malaria. Academic Press Inc; 1978. [Google Scholar]
- Beale GH, Carter R, Walliker D. Genetics. In: Killick-Kendrick R, Peters W, editor. In Rodent Malaria. London: Academic Press; 1978. [Google Scholar]
- Briegel H. Determination of uric acid and hematin in a single sample of excreta from blood-fed insects. Experientia. 1980;36:1428. [Google Scholar]
- SAS II SAS/STAT Software: Changes and enhancements through Release 612, SAS Institute Inc. 1997.
- Takken W, Klowden MJ, Chambers GM. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): The disadvantage of being small. J Med Entomol. 1998;35:639–645. doi: 10.1093/jmedent/35.5.639. [DOI] [PubMed] [Google Scholar]
- Hurd H, Hogg JC, Renshaw M. Interactions between bloodfeeding, fecundity and infection in mosquitoes. Parasitol Today. 1995;11:411–416. doi: 10.1016/0169-4758(95)80021-2. [DOI] [Google Scholar]
- SPSS, I in SPSS 61: Guide to Data Analysis. 1995.
- Hogg JC, Hurd H. Malaria-induced reduction of fecundity during the first gonotrophic cycle of Anopheles stephensi mosquitoes. Med Vet Entomol. 1995;9:176–180. doi: 10.1111/j.1365-2915.1995.tb00175.x. [DOI] [PubMed] [Google Scholar]
- Hogg JC, Hurd H. Plasmodium yoelii nigeriensis: the effect of high and low intensity of infection upon the egg production and bloodmeal size of Anopheles stephensi during three gonotrophic cycles. Parasitology. 1995;111:555–562. doi: 10.1017/s0031182000077027. [DOI] [PubMed] [Google Scholar]
- Hogg JC, Hurd H. The effects of natural Plasmodium falciparum infection on the fecundity and mortality of Anopheles gambiae s.l. in north east Tanzania. Parasitology. 1997;114:325–331. doi: 10.1017/S0031182096008542. [DOI] [PubMed] [Google Scholar]
- Hacker CS. The differential effect of Plasmodium gallinaceum on the fecundity of several strains of Aedes aegypti. J Invertebr Pathol. 1971;18:373–377. doi: 10.1016/0022-2011(71)90040-1. [DOI] [PubMed] [Google Scholar]
- Hacker CS, Kilama WL. The relationship between Plasmodium gallinaceum density and the fecundity of Aedes aegypti. J Invertebr Pathol. 1974;23:101–105. doi: 10.1016/0022-2011(74)90079-2. [DOI] [PubMed] [Google Scholar]
- Freier JE, Friedman S. Effect of host infection with Plasmodium gallinaceum on the reproductive capacity of Aedes aegypti. J Invertebr Pathol. 1976;28:161–166. doi: 10.1016/0022-2011(76)90117-8. [DOI] [PubMed] [Google Scholar]
- Klein TA, Harrison BA, Andre RG, Whitmire RE, Inlao I. Detrimental effects of Plasmodium cynomolgi infections on the longevity of Anopheles dirus. Mosquito News. 1982;42:265–271. [Google Scholar]
- Anderson RA, Knols BG, Koella JC. Plasmodium falciparum sporozoites increase feeding-associated mortality of their mosquito hosts Anopheles gambiae s.l. Parasitology. 2000;129:329–333. doi: 10.1017/S0031182099005570. [DOI] [PubMed] [Google Scholar]
- Buxton PA. The effect of Proteosoma upon the survival of Culex. Parasitology. 1935;27:547–550. [Google Scholar]
- Ferguson HM, Read AF. Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol. 2002;18:256–261. doi: 10.1016/S1471-4922(02)02281-X. [DOI] [PubMed] [Google Scholar]
- Baylis M, Mbwabi A. Feeding-behavior of Tsetse-flies (Glossina pallidipes austen) on trypanosoma-infected oxen in Kenya. Parasitology. 1995;110:297–305. doi: 10.1017/s0031182000080884. [DOI] [PubMed] [Google Scholar]
- Coleman RE, Edman JD, Semprevivo LH. Interactions between malaria (Plasmodium yoelii) and leishmaniasis (Leishmania mexicana amazonensis) – effect of concomitant infection on host activity, host body temperature, and vector engorgement success. J Med Entomol. 1988;25:467–471. doi: 10.1093/jmedent/25.6.467. [DOI] [PubMed] [Google Scholar]
- Coleman RE, Edman JD. Feeding site selection of Lutzomyia longipalpis (Diptera, Psychodidae) on mice infected with Leishmania mexicana amazonensis. J Med Entomol. 1988;25:229–233. doi: 10.1093/jmedent/25.4.229. [DOI] [PubMed] [Google Scholar]
- Mahon R, Gibbs A. Arbovirus-infected hens attract more mosquitoes. In: Mackenzie JD, editor. In Viral Diseases in Southeast Asia and the western Pacific. Sydney: Academic Press; 1982. pp. 502–504. [Google Scholar]
- Turell MJ, Bailey CL, Rossi C. Increased mosquito feeding on Rift Valley fever virus-infected lambs. Am J Trop Med Hyg. 1984;33:1232–1238. doi: 10.4269/ajtmh.1984.33.1232. [DOI] [PubMed] [Google Scholar]
- O'Shea B, Rebollar-Tellez E, Ward RD, Hamilton JGC, El Naiem D, Polwart A. Enhanced sandfly attraction to Leishmania-infected hosts. Trans R Soc Trop Med Hyg. 2002;96:117–118. doi: 10.1016/S0035-9203(02)90273-7. [DOI] [PubMed] [Google Scholar]
- Day JF, Ebert KM, Edman JD. Feeding patterns of mosquitos (Diptera, Culicidae) simultaneously exposed to malarious and healthy mice, including a method for separating blood meals from conspecific hosts. J Med Entomol. 1983;20:120–127. doi: 10.1093/jmedent/20.2.120. [DOI] [PubMed] [Google Scholar]
- Rossignol PA, Ribeiro JMC, Jungery M, Turell MJ, Spielman A, Bailey CL. Enhanced mosquito blood-finding success on parasitemic hosts – evidence for vector parasite mutualism. Proc Natl Acad Sci U S A. 1985;82:7725–7727. doi: 10.1073/pnas.82.22.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkot T, Narara A, Paru R, Graves P, Garner P. Human host selection by anophelines: no evidence for preferential selection of malaria or microfilariae-infected individuals in a hyperendemic area. Parasitology. 1989;98:337–342. doi: 10.1017/s0031182000061400. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Koella JC, Hurd H. The effect of Plasmodium yoelii nigeriensis infection on the feeding persistence of Anopheles stephensi Liston throughout the sporogonic cycle. Proc R Soc Lond B. 1999;266:1729–1733. doi: 10.1098/rspb.1999.0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koella JC, Sorensen FL, Anderson RA. The malaria parasite, Plasmodium falciparum, increases the frequency of multiple feeding of its mosquito vector, Anopheles gambiae. Proc R Soc Lond B. 1998;265:763–768. doi: 10.1098/rspb.1998.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koella JC, Packer MJ. Malaria parasites enhance blood-feeding of their naturally infected vector Anopheles punctulatus. Parasitology. 1996;113:105–109. doi: 10.1017/s0031182000066348. [DOI] [PubMed] [Google Scholar]
- Koella J., Rieu L, Paul R. Stage-specific manipulation of a mosquito's host-seeking behavior by the malaria parasite Plasmodium gallinaceum. Behav Ecol. 2002;13:816–820. doi: 10.1093/beheco/13.6.816. [DOI] [Google Scholar]
- Schwartz A, Koella JC. Trade-offs, conflicts of interest and manipulation in Plasmodium-mosquito interactions. Trends Parasitol. 2001;17:189–194. doi: 10.1016/S1471-4922(00)01945-0. [DOI] [PubMed] [Google Scholar]
- Rivero A, Ferguson HM. The energetic budget of Anopheles stephensi infected with Plasmodium chabaudi: is energy depletion a mechanism for virulence? Proc R Soc Lond B. 2003;270:1365–1371. doi: 10.1098/rspb.2003.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque CMR, Ham PJ. Concomitant malaria (Plasmodium gallinaceum) and filaria (Brugia pahangi) infections in Aedes aegypti – effect on parasite development. Parasitology. 1995;110:1–6. doi: 10.1017/s0031182000080987. [DOI] [PubMed] [Google Scholar]