Abstract
Background
Citrate has theoretical advantages over heparin for locking hemodialysis central venous catheters (CVCs), but the comparative effectiveness of these agents is not clear.
Objectives
1) To compare the benefits and harms of citrate versus heparin locking solutions among patients undergoing hemodialysis through CVCs; 2) to appraise methodological quality of the supporting evidence.
Data sources
CENTRAL, MEDLINE, EMBASE, CINAHL, ISI Web of Science, and nephrology conference abstracts.
Study eligibility, participants, and interventions
We included randomized, parallel arm clinical trials that enrolled adult patients (>18 years) receiving chronic hemodialysis through CVCs using a citrate locking solution. We excluded studies in which citrate was combined with other agents, such as antibiotics.
Appraisal and synthesis methods
We used the GRADE approach to systematic reviews and quality appraisal. Two reviewers performed data extraction independently and in duplicate. We pooled count data using generic inverse variance with random-effects models, and used fixed-effect models when only two studies were available for pooling. Subgroups included low (≤5%) vs. higher (≥30%) citrate.
Results
We screened 600 citations. Forty-one proceeded to full-text screen; 5 met inclusion criteria. Studies included between 19 and 291 participants (Median N = 61) followed for a total of 174.6 catheter-years; 2 were multi-centred trials. Three studies assessed all-cause mortality; the pooled relative risk for death was 0.71 (95% CI = 0.42-1.24; p = 0.21; I2 = 0%). The rate ratio for bacteremic episodes was 0.54 (95% CI = 0.23-1.29; p = 0.16; I2 = 65%) while the rate ratio for bleeding was 0.48 (95% CI = 0.3-0.75; p = 0.001;I I2 = 5%). Rates of catheter exchange/replacement, all-cause hospitalization and in-situ thrombolysis were not significantly different between groups in any of the pooled analyses. Risk of bias within pooled studies was low.
Limitations
Outcome definitions varied across studies. Imprecision due to small sample sizes and low event rates reduce our overall confidence in the pooled effect estimates.
Implications
Benefits and harms of citrate vs. heparin locking solutions remain unclear; larger studies and standardization of outcome measurement and reporting are warranted.
Trial registration
Protocol Registration Number: CRD42013004781
Electronic supplementary material
The online version of this article (doi:10.1186/s40697-015-0040-2) contains supplementary material, which is available to authorized users.
Abrégé
Contexte
En théorie, il appert que les solutions de citrate présentent certains avantages, comparativement aux solutions héparinées, lorsqu’il s’agit du verrouillage des cathéters veineux centraux (CVC) utilisés en hémodialyse. Cependant, l’efficacité relative de ces deux agents reste incertaine.
Objectifs de l’étude
1) Comparer les avantages et les désavantages des solutions de verrouillage de citrate, d’une part et d’héparine, d’autre part, chez les patients subissant des traitements d’hémodialyse par CVC, et 2) évaluer la qualité méthodologique des données probantes.
Sources d’information
Les bases de données CENTRAL, MEDLINE, EMBASE, CINAHL, ISI Web of Science, et des abrégés de communications à des conférences en néphrologie.
Admissibilité de l’étude, choix des participants et interventions
Nous avons tenu compte des essais cliniques à répartition aléatoire à deux groupes, effectués sur des adultes (18 ans et plus) sous traitements réguliers d’hémodialyse par CVC, et dont le verrouillage utilisait une solution de citrate. Les essais dont la solution de verrouillage combinait le citrate à d’autres agents, comme des antibiotiques, ont été exclus.
Méthodes d’évaluation de la qualité de la preuve et de synthèse
Nous avons utilisé la méthode GRADE pour analyser la qualité des données. Une extraction des données a été réalisée de manière indépendante par chacun des deux évaluateurs. Nous avons regroupé les données de dénombrement en utilisant un modèle générique de type « inverse variance with random-effect » et avons utilisé un modèle de type « fixed-effect » lorsque nous n’avions que deux études à notre disposition. Les sous-groupes ont été divisés selon la concentration de citrate dans la solution de verrouillage : basse (≤5%), ou élevée (≥30%).
Résultats
Nous avons passé 600 titres en revue. 41 d’entre eux menaient au texte plein écran; 5 rencontraient les critères d’admissibilité. Le nombre de participants pour chaque étude se situait en 19 et 291 (médiane N = 61), pour un total de 174,6 années-cathéter; 2 des études étaient des essais cliniques multicentres. Trois études avaient rendu compte de toutes les causes de mortalité; la mesure combinée du risque relatif de mortalité était de 0,71 (95% IC = 0,42-1,24; p = 0,21; I2 = 0%). Le rapport de taux des bactériémies était de 0,54 (95% IC = 0,23-1,29; p = 0,16; I2 = 65%), tandis que le rapport de taux des hémorragies était de 0,48 (95% IC = 0,3-0,75; p = 0,001; I2 = 5%). La variation dans la fréquence de remplacement/changement du cathéter, d’hospitalisation (toutes raisons confondues) ou de thrombolyse in situ n’était pas significative entre les différents groupes et ce, dans toutes les analyses combinées. Le risque de biais à l’intérieur des études regroupées était faible.
Limites de l’étude
La définition des résultats variait selon l’étude. Notre confiance générale dans les estimations regroupées est réduite puisqu’il existe certaines imprécisions résultant de petits échantillons et le taux d’événements est faible.
Implications
Les avantages et désavantages de l’utilisation du citrate, comparativement à l’héparine, dans les solutions de verrouillage restent incertains. Il serait justifié de procéder à des études plus vastes ainsi qu’à une uniformisation de la mesure des résultats et des rapports.
Trial registration
Protocol Registration Number: CRD42013004781
Electronic supplementary material
The online version of this article (doi:10.1186/s40697-015-0040-2) contains supplementary material, which is available to authorized users.
What was known before
Prior reviews have suggested that citrate-containing antimicrobial locking solutions reduce the risk of bacteremia. However, the quality of the supporting evidence has not been extensively reviewed.
What this adds
In this review, we focus on outcomes with citrate alone versus heparin-based locking solutions for hemodialysis catheters, and apply the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to systematic reviews and evidence quality appraisal. We identify a low overall quality of evidence supporting a lower risk of bleeding, but no difference in infection, hospitalization, or patency-related outcomes with citrate versus heparin.
Introduction
Despite guidelines, policies, and initiatives promoting arterio-venous fistulae, prevalent central venous catheter (CVC) rates persistently range between 10-50% internationally, with upward trends in many high-income countries, including Canada [1,2]. Catheter-related bacteremia remains a common and potentially catastrophic hemodialysis complication, resulting in significant morbidity and mortality, as well as hospitalization and resource use. Moreover, thrombotic or patency-related complications reduce dialysis adequacy, are costly, and result in treatment disruptions and inconvenience for patients. While unfractionated heparin has been widely used as a locking solution, it is associated with a number of complications, including inadvertent systemic administration with coagulopathy and bleeding [3], heparin induced thrombocytopenia [4], and allergic reactions [5].
Citrate-based locking solutions are a promising alternative to unfractionated heparin, with a number of small clinical trials recently evaluating its efficacy and safety. Moreover, the unit cost for citrate has recently fallen to below that of heparin in Canada and other jurisdictions, making citrate a potentially cost-effective alternative. Our aims were: 1) to conduct a systematic review and meta-analysis to better understand the benefits and harms of using citrate versus heparin as a CVC locking solution, and 2) to appraise the quality of the supporting body of evidence.
Methods
Overview
Our review adhered to a pre-specified protocol, registered with the PROSPERO register of systematic reviews (registration number: CRD42013004781) [6]. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to systematic reviews and methodological quality appraisal [7-10]. This manuscript was prepared according to the PRISMA Guidelines [11].
Study eligibility
Types of studies
We included randomized clinical trials and systematic reviews published as full-text articles in peer-reviewed journals, as well as abstracts presented at major nephrology conferences. We excluded observational studies, opinion pieces, narrative reviews, and any other articles not containing original data.
Types of participants
We included studies that enrolled adult patients (>18 years) with end-stage renal disease undergoing chronic hemodialysis through CVC’s using a citrate locking solution (intervention) and heparin-based locking solutions (control). Given major potential differences in disease severity and treatment settings, we excluded studies of inpatient or acute kidney injury populations, or those admitted to intensive care units, or receiving continuous renal replacement therapies. We also excluded studies in which patients received hemofiltration or hemodiafiltration. We did not place any restrictions on catheter types (tunneled vs. temporary), incident vs. prevalent patients, or incident vs. prevalent catheters.
Types of interventions
Experimental interventions consisted of citrate-based catheter locking solutions – both low (4%) and higher (15-50%) concentrations. The control intervention included heparin or other non-citrate based locking solutions.
Types of outcomes
Critically important outcomes included patient survival (all-cause mortality), bacteremia rates, and all-cause hospitalization rates. Important but not critical outcomes included access-related hospitalization, catheter replacement/exchange events, bleeding, and local/in situ thrombolysis (tPA, urokinase, or other), as measured by number of thrombolytic administration episodes. We used outcome definitions as they were described in the included studies.
Information sources
We searched The Cochrane Central Register of Controlled Trials (CENTRAL) [12], MEDLINE, EMBASE, CINAHL and ISI Web of Science from inception to June 29, 2013.
Search
All search strategies were developed in collaboration with a Health Information Specialist (NB), and included the following headings and text words: population – dialysis, hemodialysis; intervention – citrate, citric acid, sodium citrate; and comparator – heparin, other locking solutions terms identified during pilot searches. A sample search strategy is in the web Additional file 1. We did not restrict the search strategy based on outcome, language or date of publication. Our primary search was supplemented with hand-searches of narrative and systematic review bibliographies. We also searched conference abstracts from the Canadian Society of Nephrology and the American Society of Nephrology annual meetings spanning 2011–2013.
Study selection
We downloaded all identified reports into a reference manager (Endnote X6 for Macintosh Thomson-Reuters Inc., San Francisco, CA, USA). Two authors (AA and AG) independently assessed all titles and abstracts for relevance and eligibility for full-text screening. Only reports that were obviously irrelevant were discarded. Using the Distiller-SR online software tool [13], we developed a full-text screening form for study selection. Disagreements concerning study inclusion were discussed until a consensus was reached.
Data collection process
Two reviewers (AA and AG) performed the data extraction independently and in duplicate with the use of standard data extraction forms created in Distiller-SR. Predefined reasons for exclusion were documented for each of the excluded studies. Two reviewers assessed methodological quality of the included studies. Discrepancies were resolved by discussion, and if needed, a third reviewer (GN) was consulted. No individual patient data was used.
Data items
For each included study, we extracted data on characteristics of study participants, study design, details of interventions including drug concentration, outcomes (count and rate data as applicable, with measures of dispersion), and methodological factors affecting risk of bias and other quality appraisal criteria.
Methodological quality appraisal
We applied the GRADE quality appraisal criteria [10]. These include risk of bias [14], indirectness (in population, intervention or outcome) [15], imprecision [16], inconsistency (heterogeneity) [17], and publication bias [18]. Risk of bias in individual studies was assessed according to the criteria proposed by Higgins et al., including adequacy of sequence generation, allocation concealment, blinding of participants, outcome assessors, and study personnel, completeness of follow-up, and selective outcome reporting [19].
Summary measures and synthesis of results
All analyses were performed using RevMan 4.3 for Macintosh. We used random effects models to compute rate ratios, but used fixed-effects models when only two studies were available for pooling [20]. We assessed heterogeneity using the I2 statistic.
Additional analyses
Where possible, we performed (pre-specified) subgroup analyses according to citrate concentration, using cut-points defined by included studies (<5% or >30%).
Results
Study selection
We identified a total of 600 studies (Figure 1), including 111 in Medline, 31 in CINAHL, 268 in EMBASE, 31 in CCTR and 159 in Web of Science. We did not identify any relevant conference abstracts. We identified and removed duplicate records (N = 252). Forty-one studies proceeded to full-text screening. Reasons for exclusion were: no original data (including systematic reviews) (N = 33), study population did not consist of outpatients receiving chronic hemodialysis through central venous catheters (N = 2), and crossover design (N = 1). Five studies were therefore included in our review [21-25].
Figure 1.
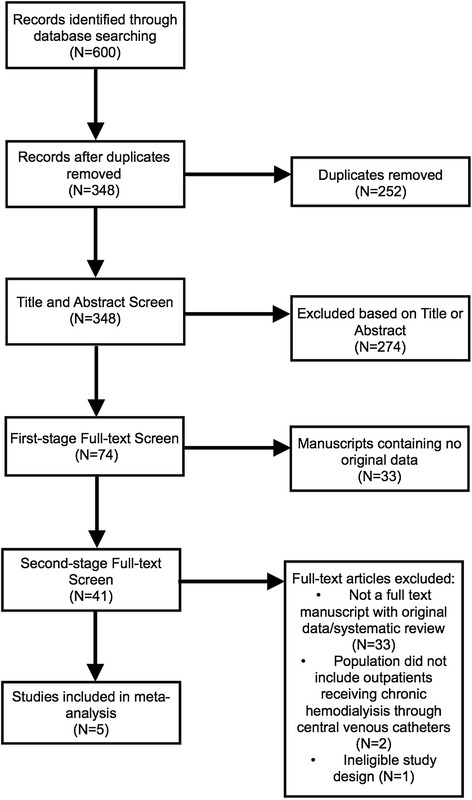
Study inclusion flow diagram.
Study characteristics
All 5 included studies were parallel-arm randomized trials (Table 1). Studies included between 19 and 291 participants (median = 61), followed for a total of 176.4 catheter-years, with study population mean age ranging between 62 to 75 in citrate treatment groups and between 61 to 71 in heparin treatment groups, and percentage of female patients ranging from 34 to 60 in citrate treatment groups and from 41 to 56 in heparin treatment groups. Studies were conducted in Canada, the Unites States, Europe, and Asia; two of the studies were multi-centered [23].
Table 1.
Characteristics of included studies
| Source | Country (#Centres) | Study duration | N | Methods | Participants | Intervention | Control | Outcomes | Funding Source |
|---|---|---|---|---|---|---|---|---|---|
| [22] | Slovenia (1) | Not specifically stated. | 30 | Method of randomization unclear. | ESRD patients with subclavian or jugular single lumen catheters inserted as temporary blood accesses for HD expected to be used for at least 7 days. | 3 ml of 4% trisodium citrate | A mixture of 1 ml (5000 U/ml) of unfractionated heparin and 2 ml of saline. | Catheter removal. | Not specified. |
| [23] | Belgium (1) | April 2000 - October 2000 | 19 | Method of randomization unclear. | Chronic hemodialysis patients with a single lumen central venous catheter as permanent vascular dialysis access. | 5% trisodium citrate | 5000 U/ml heparin solution | Local/in-situ thrombolysis (bolus events for inadequate blood flow). | Not specified. |
| [24] | Canada (1) | December 2004 - June 2005 | 61 | Randomization based on patient surnames. | All patients receiving chronic HD three times a week, 4 h/session, at the in-center Hemodialysis Unit with cuffed catheters as primary vascular access were eligible for the trial. | Prefilled 5 ml syringe containing 4% citrate (MEDXL, Montreal, Quebec, Canada). | 1 ml of 10,000 U/ml heparin diluted to 5000 U/ml with normal saline | Patient survival, bacteremia rates, catheter removal, and local/in-situ thrombolysis. | Not specified. |
| [25] | United Kingdom (4) | 6 mo. period | 232 | Open-label. Randomization was achieved through single-random number allocation. | ESRD patients with IJ CVCs (Bio-Flex TC), on HD > 90 days. | 46.7% trisodium citrate. | 5% heparin | Patient survival, bacteremia rates, catheter-related hospitalization rates, catheter replacement/exchange rates, use of urokinase. | Not specified. |
| [26] | Netherlands (10) | April 2001 - September 2002 | 291 | Randomization by computer-generated random number list. | Patients >18 years, not admitted to the intensive care ward, with chronic or acute renal failure that required hemodialysis through a CVC. Only patients with a newly inserted, well-positioned CVC expected to be in use for 1 wk. | 30% trisodium citrate | Unfractionated sodium heparin 5000 U/ml | Patient survival, bacteremia rates, catheter-related infection hospitalization rates, catheter removal rates, and catheter treatment with urokinase. | Not specified. |
Abbreviations: CVC – central venous catheter; HD – hemodialysis; IJ – internal jugular.
Results of individual studies
Event rates for individual studies are in Table 2. Follow-up ranged between 1,703 and 19,008 catheter days for citrate treatment groups, and between 1,493 and 17,100 catheter days for heparin treatment groups across studies.
Table 2.
Rates of events in individual studies
| Study | Citrate | Heparin | ||||
|---|---|---|---|---|---|---|
| Events | Catheter days | Events/1000 catheter days | Events | Catheter days | Events/1000 catheter days | |
| Mortality | ||||||
| Power | 5 | 19008 | 0.26 | 5 | 17100 | 0.29 |
| Weijmer | 13 | 8181 | 1.59 | 18 | 8049 | 2.24 |
| MacRae | 4 | 2272 | 1.76 | 5 | 1818 | 2.75 |
| Bacteremia | ||||||
| Power | 13 | 19008 | 0.68 | 12 | 17100 | 0.70 |
| Weijmer | 9 | 8181 | 1.10 | 33 | 8049 | 4.10 |
| MacRae | 5 | 2272 | 2.20 | 6 | 1818 | 3.30 |
| Access-related hospitalization | ||||||
| Power | 17 | 19008 | 0.89 | 12 | 17100 | 0.70 |
| Weijmer | 6 | 8181 | 0.70 | 21 | 8049 | 2.70 |
| Catheter replacement | ||||||
| Buturovic | 1 | 2272 | 1.96 | 1 | 1818 | 4.35 |
| MacRae | 8 | 2272 | 3.52 | 5 | 1818 | 2.75 |
| Weijmer | 27 | 8181 | 3.21 | 29 | 8049 | 3.56 |
| Thrombolysis | ||||||
| MacRae | 13 | 2272 | 5.72 | 13 | 1818 | 7.15 |
| Power | 1 | 19008 | 0.06 | 1 | 17100 | 0.04 |
| Weijmer | 69 | 8181 | 8.28 | 63 | 8049 | 7.88 |
| Bleeding | ||||||
| MacRae | 25 | 2272 | 11.00 | 37 | 1818 | 20.35 |
| Weijmer | 5 | 8181 | 0.60 | 16 | 8049 | 2.00 |
Synthesis of results
Forest plots and details of pooled estimates are in Figures 2, 3, 4, 5, 6 and Table 3. Three studies reported all-cause mortality, with a pooled relative risk of 0.71 (95% CI = 0.42-1.24; p = 0.21; I2 = 0%) favouring citrate over heparin. Three studies assessed bacteremic episodes; the rate ratio for this outcome was 0.54 (95% CI = 0.23-1.29; p = 0.16; I2 = 65%) favouring citrate over heparin. Bleeding was reported in two studies, with a rate ratio of 0.48 (95% CI = 0.3-0.75; p = 0.001;I2 = 5%), favouring citrate over heparin. Rates of catheter exchange/replacement and in-situ thrombolysis were not significantly different between groups in any of the pooled analyses. Finally, the pooled rate ratio for all-cause hospitalization was 0.68 (95% CI = 0.38-1.20; p = 0.18; I2 = 86%), favouring citrate over heparin.
Figure 2.
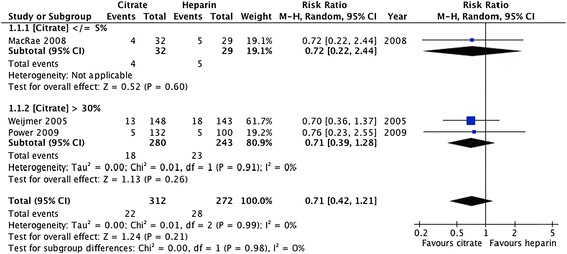
Comparative risk of death (all-cause) with citrate vs. heparin locking solutions.
Figure 3.
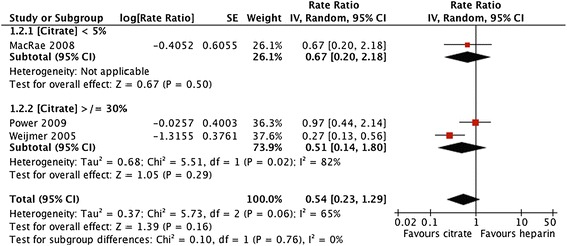
Comparative risk of bacteremia with citrate vs. heparin locking solutions.
Figure 4.
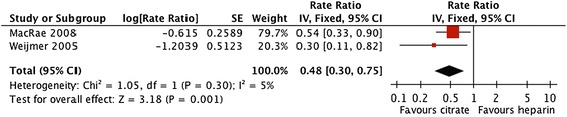
Comparative risk of bleeding with citrate vs. heparin locking solutions.
Figure 5.
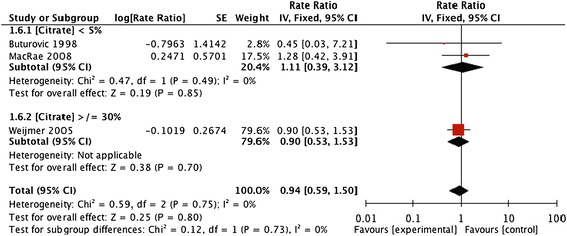
Comparative risk of CVC replacement for impaired patency with citrate vs. heparin locking solutions.
Figure 6.
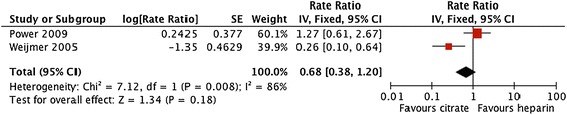
Comparative risk of hospitalization (any cause) with citrate vs. heparin locking solutions.
Table 3.
GRADE evidence profile table: citrate vs. heparin locking solutions for hemodialysis catheters
| Quality assessment | № of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Citrate | heparin | Relative (95% CI) | Absolute (95% CI) | ||
| Survival (follow up: range 56,428 Total Catheter Days) | ||||||||||||
| 3 | randomised trials | not serious | not serious | not serious | very serious 1 | none | 22/312 (7.1%) | 28/272 (10.3%) | RR 0.71 (0.42 to 1.21) | 30 fewer per 1000 (from 22 more to 60 fewer) | ⨁⨁◯◯ LOW | CRITICAL |
| Bacteremia (follow up: range 56,428 Total Catheter Days) | ||||||||||||
| 3 | randomised trials | not serious | serious 2 | not serious | serious 3 | none | 27/312 (8.7%) | 51/272 (18.8%) | Rate Ratio 0.54 (0.23 to 1.29) | 0 fewer per 1000 (from 0 fewer to 0 fewer) 9 | ⨁⨁◯◯ LOW | CRITICAL |
| Thrombolysis (follow up: range 56,428 Total Catheter Days) | ||||||||||||
| 3 | randomised trials | not serious | serious 4 | not serious | serious | none | 235/172 (136.6%) | 150/180 (83.3%) | Rate Ratio 1.25 (0.76 to 2.05) | 0 fewer per 1000 (from 0 fewer to 0 fewer) | ⨁⨁◯◯ LOW | IMPORTANT |
| Bleeding (follow up: range 20,320 Total Catheter Days) | ||||||||||||
| 2 | randomised trials | not serious | not serious | not serious | serious 5 | none | 30/180 (16.7%) | 53/172 (30.8%) | Rate Ratio 0.48 (0.30 to 0.75) | 0 fewer per 1000 (from 0 fewer to 0 fewer) | ⨁⨁⨁◯ MODERATE | IMPORTANT |
| Hospitalization for any reason (follow up: range 52,338 Total Catheter Days) | ||||||||||||
| 2 | randomised trials | not serious | serious 6 | not serious | serious 7 | none | 23/280 (8.2%) | 33/243 (13.6%) | Rate Ratio 0.68 (0.38 to 1.20) | 0 fewer per 1000 (from 0 fewer to 0 fewer) | ⨁⨁◯◯ LOW | IMPORTANT |
| Catheter replacement for patency (follow up: range 24,410 Total Catheter Days) | ||||||||||||
| 3 | randomised trials | not serious | not serious | not serious | serious 8 | none | 36/190 (18.9%) | 35/182 (19.2%) | Rate Ratio 0.94 (0.59 to 1.50 | 0 fewer per 1000 (from 0 fewer to 0 fewer) | ⨁⨁⨁◯ MODERATE | IMPORTANT |
MD – mean difference, RR – relative risk.
1Studies not powered for survival outcome; optimal information siz e criterion not met.
2I2=65% for pooled effect estimate; could not exclude heterogeneity due to study design, duration of follow-up, and citrate concentration.
3Overall event rates were low; studies were likely underpowered to detect statistically significant differences in bacteremia.
4I2=77% for pooled effect estimate, possibly due to unexplained heterogeneity in outcome definitions and study design.
5Vary small event rate and sample siz e; observed effect may be due to random error.
6I2=86% using fixed-effect model; unexplained heterogeneity exists.
7Confidence interval includes no effect.
8Optimal information siz e criterion not met.
9No explanation was provided.
Methodological quality appraisal
Table 3 summarizes the quality appraisal for pooled estimates on an outcome-by-outcome basis. Risk of bias was low in all included studies. One study was open-label, but due to the nature of the reported outcomes, unblinding was unlikely to result in a significant risk of bias [23]. A small overall number of included studies precluded any meaningful analysis of publication bias by funnel plots. Despite a low risk of bias within included studies, the overall quality of evidence was low for both critical and important outcomes, with inconsistency (heterogeneity) and imprecision (due to small sample sizes) affecting the quality of the majority of estimates.
Additional analyses
We created subgroups according to citrate concentration (<5% vs. >30%). However, given the small overall number of studies, no significant subgroup effects were noted.
Discussion
Summary of evidence
Compared with heparin, citrate catheter locking solutions were associated with significantly fewer bleeding episodes. Rates of death and bacteremia tended to be lower with citrate, but pooled effect estimates were not statistically significant. No significant differences in catheter exchange/replacement, thrombolysis, or all-cause hospitalization were evident between groups in any of the pooled analyses.
Our findings are consistent with those of a similar and recently published meta-analysis by Zhao et al., which also found significantly reduced bleeding rates, but no difference in survival, hospitalization, or catheter patency with citrate alone as compared with heparin [26]. The review by Zhao et al. also included studies in which citrate was combined with various antimicrobial agents, including gentamicin, taurolidine, methylene blue, and methylparaben, yielding a pooled rate ratio of 0.39 (95% CI = 0.27 to 0.56; I2 = 27%) for catheter-related bloodstream infection, favouring citrate-containing locking solutions over heparin. Their findings are corroborated by two other similar reviews [27,28]. Given the small overall number of studies comparing the effects of citrate alone versus heparin (N = 3) on bacteremia rates, neither our study, nor that of Zhao et al. could exclude a beneficial effect on this outcome. Whether the protective effect of citrate on blood-stream infection rates noted by Zhao et al. is a result of greater statistical power due to the inclusion of more studies, or attributable to the secondary antimicrobial agents included in these study interventions is unclear. Larger studies would be needed to confirm whether or not citrate alone confers a benefit similar to that of citrate-based locking solutions containing additional antimicrobial agents.
Plausible biological mechanisms exist by which citrate alone might reduce systemic infections when instilled in catheter ports. In vitro studies have demonstrated bactericidal and sporocidal activity with both high (23%) and lower (4%) citrate concentrations, and prevention of biofilm formation, with no evidence of citrate resistance [29,30]. This is in contrast to heparin, which may in fact promote biofilm formation and increase infection risk [31,32]. Citrate’s antibacterial activity appears to be related to the chelation of calcium and magnesium ions, leading to the degradation of bacterial cell membranes, thus reducing bacterial cellular integrity [33]. Given its avidity for divalent cations, inadvertent systemic administration of large volumes of citrate locking solutions can result in calcium complexation and hypocalcemia. One report described a fatal outcome associated with high concentration citrate (46.7%) administration; this ultimately led to FDA and Health Canada bans on highly concentrated citrate locks in the US and Canada [34]. Our review did not identify any evidence of harm due to inadvertent systemic administration with the citrate concentrations used in included studies, and in current routine use [23].
We observed a significantly lower rate of bleeding with citrate as compared with unfractionated heparin (Figure 4). Although the magnitude of effect was similar in the two studies included in this pooled estimate, the event rates were vastly different (~10-fold difference in control group event rate; Table 2), suggesting differences in study populations, co-interventions, and outcome definitions. Assuming that bleeding risk attributable to locking solutions is due to occasional inadvertent systemic administration, it seems reasonable to conclude that the anticoagulant effect of citrate in these two studies (4% and 30%) was lower in vivo than were similar volumes of unfractionated heparin at 5000 IU/ml.
Limitations
We included only randomized trials focusing on patient-important outcomes, and performed a rigorous methodological quality appraisal based on the GRADE approach. Nevertheless, some additional factors limit our overall confidence in the observed pooled estimates. A small overall number of included studies, small sample sizes within studies, and low event rates limit the precision of most of the pooled analyses. We also found some heterogeneity in the pooled estimate for bacteremia, but a small overall number of studies precluded any meaningful subgroup analyses. We also could not exclude publication bias.
Implications for practice
Compared with unfractionated heparin, citrate catheter locking solutions appear to reduce bleeding, and may reduce bacteremia in patients undergoing hemodialysis with a CVC. However, it is unclear from the current published evidence whether citrate alone has the same protective effect against systemic infection as it does when combined with other antimicrobial agents, as observed in the review by Zhao et al. [26]. Although the overall quality of evidence supporting its use is low, both The American Society of Diagnostic and Interventional Nephrology (ASDIN) and European Renal Best Practice (ERBP) consider 4% citrate and heparin acceptable alternatives for locking CVCs [35,36]. Given the availability of 4% citrate in prefilled syringes, and lower unit costs when compared with unfractionated heparin, citrate may be the preferred choice, especially if differences in cost increase over time.
Implications for research
Studies comparing citrate with unfractionated heparin-based catheter locking solutions have generally been small and not adequately powered to provide conclusive evidence of safety and efficacy; hence, larger comparative trials are desirable. Moreover, with the increasingly favourable safety profile and falling costs of low molecular weight heparins, studies comparing these agents with citrate would be useful in informing practice as well. Although not in widespread use, recombinant tissue plasminogen activator (rt-PA) has been used weekly for locking dialysis catheters, with cost effectiveness comparable to that of heparin (costs of rt-PA offset by reduced hospitalization for bacteremia) [37,38]. Future studies comparing the effectiveness of rt-PA with that of citrate might also be of interest. Finally, we, and others have noted significant variability in outcome definitions in hemodialysis vascular access studies [39]. This represents a potential source of heterogeneity and renders the overall body of evidence less amenable to meaningful quantitative synthesis. Standardization of outcome reporting in vascular access research is needed, and published outcome definitions now make this possible [40].
Funding
Amgen Canada provided salary support for AG through an unrestricted research grant. The sponsor had no role in study design, analysis, or reporting.
Additional file
Web Appendix - Search Strategy. Search Strategy Example - MEDLINE.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AG drafted the study protocol, screened studies, abstracted data and drafted the manuscript. NB developed the search strategy and performed the literature search. AA screened studies and abstracted data. GN edited the protocol, performed the meta-analyses, and edited the manuscript. All authors read and approved the final manuscript.
Contributor Information
Alexa Grudzinski, Email: agrudzi2@uwo.ca.
Arnav Agarwal, Email: arnav.mcmaster@gmail.com.
Neera Bhatnagar, Email: bhatnag@mcmaster.ca.
Gihad Nesrallah, Email: gnesrallah@hrh.ca.
References
- 1.Ethier J, Mendelssohn DC, Elder SJ, Hasegawa T, Akizawa T, Akiba T, et al. Vascular access use and outcomes: an international perspective from the dialysis outcomes and practice patterns study. Nephrol Dial Transplant. 2008;23:3219–26. doi: 10.1093/ndt/gfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivan DM, Smith T, Allon M. Does the heparin lock concentration affect hemodialysis catheter patency? Clin J Am Soc Nephrol. 2010;5:1458–62. doi: 10.2215/CJN.01230210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rioux JP, De Bortoli B, Querin S, Deziel C, Troyanov S, Madore F. Measurement of the international normalized ratio (INR) in hemodialysis patients with heparin-locked central venous catheters: evaluation of a novel blood sampling method. J Vasc Access. 2009;10:180–2. doi: 10.1177/112972980901000308. [DOI] [PubMed] [Google Scholar]
- 4.Syed S, Reilly RF. Heparin-induced thrombocytopenia: a renal perspective. Nat Rev Nephrol. 2009;5:501–11. doi: 10.1038/nrneph.2009.125. [DOI] [PubMed] [Google Scholar]
- 5.Berkun Y, Haviv YS, Schwartz LB, Shalit M. Heparin-induced recurrent anaphylaxis. Clin Exp Allergy. 2004;34:1916–8. doi: 10.1111/j.1365-2222.2004.02129.x. [DOI] [PubMed] [Google Scholar]
- 6.Citrate locking solutions for hemodialysis catheters: a systematic review and meta-analysis [Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013004781] [DOI] [PMC free article] [PubMed]
- 7.Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines 11-making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66:151–7. doi: 10.1016/j.jclinepi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Cochrane Library [http://www.thecochranelibrary.com/view/0/index.html]
- 13.DistillerSR [http://systematic-review.net]
- 14.Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–15. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence–indirectness. J Clin Epidemiol. 2011;64:1303–10. doi: 10.1016/j.jclinepi.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol. 2011;64:1283–93. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol. 2011;64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence–publication bias. J Clin Epidemiol. 2011;64:1277–82. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–59. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buturovic J, Ponikvar R, Kandus A, Boh M, Klinkmann J, Ivanovich P. Filling hemodialysis catheters in the interdialytic period: heparin versus citrate versus polygeline: a prospective randomized study. Artif Organs. 1998;22:945–7. doi: 10.1046/j.1525-1594.1998.06268.x. [DOI] [PubMed] [Google Scholar]
- 22.Hendrickx L, Kuypers D, Evenepoel P, Maes B, Messiaen T, Vanrenterghem Y. A comparative prospective study on the use of low concentrate citrate lock versus heparin lock in permanent dialysis catheters. Int J Artif Organs. 2001;24:208–11. [PubMed] [Google Scholar]
- 23.Macrae JM, Dojcinovic I, Djurdjev O, Jung B, Shalansky S, Levin A, et al. Citrate 4% versus heparin and the reduction of thrombosis study (CHARTS) Clin J Am Soc Nephrol. 2008;3:369–74. doi: 10.2215/CJN.01760407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Power A, Duncan N, Singh SK, Brown W, Dalby E, Edwards C, et al. Sodium citrate versus heparin catheter locks for cuffed central venous catheters: a single-center randomized controlled trial. Am J Kidney Dis. 2009;53:1034–41. doi: 10.1053/j.ajkd.2009.01.259. [DOI] [PubMed] [Google Scholar]
- 25.Weijmer MC, van den Dorpel MA, Van de Ven PJG, ter Wee PM, van Geelen JACA, Groeneveld JO, et al. Randomized, clinical trial comparison of trisodium citrate 30% and heparin as catheter-locking solution in hemodialysis patients. J Am Soc Nephrol. 2005;16:2769–77. doi: 10.1681/ASN.2004100870. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Li Z, Zhang L, Yang J, Yang Y, Tang Y, et al. Citrate versus heparin lock for hemodialysis catheters: a systematic review and meta-analysis of randomized controlled trials. Am J Kidney Dis. 2014;63:479–90. doi: 10.1053/j.ajkd.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Labriola L, Crott R, Jadoul M. Preventing haemodialysis catheter-related bacteraemia with an antimicrobial lock solution: a meta-analysis of prospective randomized trials. Nephrol Dial Transplant. 2008;23:1666–72. doi: 10.1093/ndt/gfm847. [DOI] [PubMed] [Google Scholar]
- 28.Yahav D, Rozen-Zvi B, Gafter-Gvili A, Leibovici L, Gafter U, Paul M. Antimicrobial lock solutions for the prevention of infections associated with intravascular catheters in patients undergoing hemodialysis: systematic review and meta-analysis of randomized, controlled trials. Clin Infect Dis. 2008;47:83–93. doi: 10.1086/588667. [DOI] [PubMed] [Google Scholar]
- 29.Ash SR, Mankus RA, Sutton JM, Smeltzer B, Ing T. CONCENTRATED CITRATE (23%) FOR CATHETER LOCK SOLUTION. ASAIO J. 2000;46:222. doi: 10.1097/00002480-200003000-00286. [DOI] [PubMed] [Google Scholar]
- 30.Winnett G, Nolan J, Miller M, Ashman N. Trisodium citrate 46.7% selectively and safely reduces staphylococcal catheter-related bacteraemia. Nephrol Dial Transplant. 2008;23:3592–8. doi: 10.1093/ndt/gfn299. [DOI] [PubMed] [Google Scholar]
- 31.Shanks RM, Sargent JL, Martinez RM, Graber ML, O’Toole GA. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol Dial Transplant. 2006;21:2247–55. doi: 10.1093/ndt/gfl170. [DOI] [PubMed] [Google Scholar]
- 32.Regamey C, Schaberg D, Kirby WM. Inhibitory effect of heparin on gentamicin concentrations in blood. Antimicrob Agents Chemother. 1972;1:329–32. doi: 10.1128/AAC.1.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weijmer MC, Debets-Ossenkopp YJ, Van De Vondervoort FJ, ter Wee PM. Superior antimicrobial activity of trisodium citrate over heparin for catheter locking. Nephrol Dial Transplant. 2002;17:2189–95. doi: 10.1093/ndt/17.12.2189. [DOI] [PubMed] [Google Scholar]
- 34.Grudzinski L, Quinan P, Kwok S, Pierratos A. Sodium citrate 4% locking solution for central venous dialysis catheters–an effective, more cost-efficient alternative to heparin. Nephrol Dial Transplant. 2007;22:471–6. doi: 10.1093/ndt/gfl606. [DOI] [PubMed] [Google Scholar]
- 35.Moran JE, Ash SR, Committee ACP. Locking solutions for hemodialysis catheters; heparin and citrate–a position paper by ASDIN. Semin Dial. 2008;21:490–2. doi: 10.1111/j.1525-139X.2008.00466.x. [DOI] [PubMed] [Google Scholar]
- 36.Vanholder R, Canaud B, Fluck R, Jadoul M, Labriola L, Marti-Monros A, et al. Catheter-related blood stream infections (CRBSI): a European view. Nephrol Dial Transplant. 2010;25:1753–6. doi: 10.1093/ndt/gfq205. [DOI] [PubMed] [Google Scholar]
- 37.Manns BJ, Scott-Douglas N, Tonelli M, Ravani P, LeBlanc M, Dorval M, et al. An Economic Evaluation of rt-PA Locking Solution in Dialysis Catheters. J Am Soc Nephrol. 2014;25:2887–95. doi: 10.1681/ASN.2013050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hemmelgarn BR, Moist LM, Lok CE, Tonelli M, Manns BJ, Holden RM, et al. Prevention of dialysis catheter malfunction with recombinant tissue plasminogen activator. N Engl J Med. 2011;364:303–12. doi: 10.1056/NEJMoa1011376. [DOI] [PubMed] [Google Scholar]
- 39.Mustafa RA, Zimmerman D, Rioux JP, Suri RS, Gangji A, Steele A, et al. Vascular access for intensive maintenance hemodialysis: a systematic review for a canadian society of nephrology clinical practice guideline. Am J Kidney Dis. 2013;62:112–31. doi: 10.1053/j.ajkd.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 40.Lee T, Mokrzycki M, Moist L, Maya I, Vazquez M, Lok CE. Standardized definitions for hemodialysis vascular access. Semin Dial. 2011;24:515–24. doi: 10.1111/j.1525-139X.2011.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


