Abstract
Background
Studies on Plasmodium falciparum gametocyte development and dynamics have almost exclusively focused on patients treated with antimalarial drugs, while the majority of parasite carriers in endemic areas are asymptomatic. This study identified factors that influence gametocytaemia in asymptomatic children in the absence and presence of pyrimethamine-sulphadoxine (SP) antimalarial treatment.
Methods
A cohort of 526 children (6 months – 16 years) from western Kenya was screened for asexual parasites and gametocytes and followed weekly up to four weeks. Children with an estimated parasitaemia of ≥1,000 parasites/μl were treated with SP according to national guidelines. Factors associated with gametocyte development and persistence were determined in untreated and SP-treated children with P. falciparum mono-infection.
Results
Gametocyte prevalence at enrolment was 33.8% in children below five years of age and decreased with age. In the absence of treatment 18.6% of the children developed gametocytaemia during follow-up; in SP-treated children this proportion was 29.8%. Age, high asexual parasite density and gametocyte presence at enrolment were predictive factors for gametocytaemia. The estimated mean duration of gametocytaemia for children below five, children from five to nine and children ten years and above was 9.4, 7.8 and 4.1 days, respectively.
Conclusion
This study shows that a large proportion of asymptomatic untreated children develop gametocytaemia. Gametocytaemia was particularly common in children below five years who harbor gametocytes for a longer period of time. The age-dependent duration of gametocytaemia has not been previously shown and could increase the importance of this age group for the infectious reservoir.
Background
The transmission of Plasmodium falciparum parasites from humans to mosquitoes requires the presence of infectious gametocytes in the human peripheral blood. The prevalence of gametocytes is, therefore, commonly used as a parameter of malaria transmission. Any strategy that interferes with gametocyte development or persistence could result in a reduction of malaria transmission. For this purpose, it is important to identify parameters that influence gametocyte development as well as gametocyte dynamics under natural conditions. Although the majority of parasite carriers in endemic countries are asymptomatic [1], studies on gametocyte development and dynamics have almost exclusively focused on patients treated with antimalarial drugs [2-11]. In these studies, gametocytaemia is influenced by the duration of symptoms [3,7], anaemia [4,7], type of antimalarial treatment [2,4,5,9], response to treatment [3,7,10] and age of infected individuals [6,8,11]. The relation between asexual parasites and gametocytes remains equivocal; a positive relation has been described between asexual parasite density at enrolment and gametocyte prevalence after treatment [6], but this was contradicted by several other studies [2-4].
This study describes the dynamics of gametocytaemia in a cohort of asymptomatic children naturally exposed to malaria in western Kenya. Children with microscopically detectable parasitaemia were observed longitudinally. Those developing parasitaemia at levels requiring antimalarial treatment were treated with the first line drug pyrimethamine-sulphadoxine (SP). This study identified factors associated with P. falciparum gametocyte production in the absence or presence of SP-treatment. Within the group of gametocyte carriers, factors associated with gametocyte persistence were determined.
Methods
Study area and design
This five week study was conducted in October – November 2001 in Mbita and Lwanda, small rural villages on the shores of Lake Victoria in Suba district, western Kenya. The transmission of P. falciparum is variable depending upon local environmental conditions that support mosquito populations. The entomological inoculation rate (EIR) was recently estimated at six infectious bites per person per month [12]. Whilst variation in EIR may influence the clinical spectrum of malaria and gametocytaemia it is unlikely to be a factor in this short study. Data were collected as described elsewhere [13]. Briefly, apparently healthy children (aged six months – sixteen years) were recruited from primary schools and the community. Screening for asexual parasites and gametocytes of P. falciparum took place weekly for a period of five weeks. Finger prick blood samples were collected and thick blood smears were dried, stained with 10% Giemsa and examined microscopically. A slide was considered negative if no asexual parasites were seen after examination of 100 fields. Gametocyte and asexual parasite densities were assessed by counting against 500 and 200 leukocytes, respectively, and converted to numbers of parasites per μl by assuming a standard leukocyte count of 8,000/μl. Children with a positive blood smear with an estimated parasite density higher than 1,000 parasites/μl on the initial or any subsequent visits were treated immediately with pyrimethamine-sulphadoxine (SP) according to national guidelines. Only children who were treated once in the first or second week of the study were included in the analyses to achieve a minimum follow up period of three weeks. In case of treatment failure, alternative treatment was administered under supervision of the Clinical Officer. This resulted in the exclusion from the further study and follow-up.
Children or guardians signed an informed consent form. This study was approved by the ethical committee of the Kenya Medical Research Institute (KEMRI) and by the National Institute of Health (NIH) ethical review board of the United States.
Data analyses
Children who presented on at least three consecutive visits were used for analyses. Those who reported the use of antimalarial drugs in the two weeks prior to enrolment or had a Plasmodium malariae mixed infection were excluded. The influence of age on transformed (natural logarithm) parasite densities was analysed in linear regression models. Discrete data were compared using chi-square or Fisher's exact test, trends in binary outcomes using the Cochran-Armitage test for trend. Predictive factors for gametocyte prevalence on days seven or fourteen were tested in non-treated and SP-treated children separately, using multiple regression models with SAS Generalized Estimating Equations (GEE). Presentation parasitaemia was included in the models as a continuous variable and non-parasitaemic children were excluded from the analyses. A random effect was included in the models to allow for correlations within individuals. The Kaplan-Meier estimator was used to determine the proportion of parasitaemic children developing gametocytaemia during the follow-up period. Only for this analysis, gametocyte carriers at enrolment were excluded.
The duration of gametocytaemia in gametocyte carriers was estimated using the method described by Drakeley et al. [14], assuming that a single gametocyte positive slide represented gametocytes circulating in the peripheral blood for at least 2.5 days [15]. When children had gametocyte positive blood films on successive weeks, the estimated duration of gametocytaemia was the interval between observations plus 2.5 days.
Results
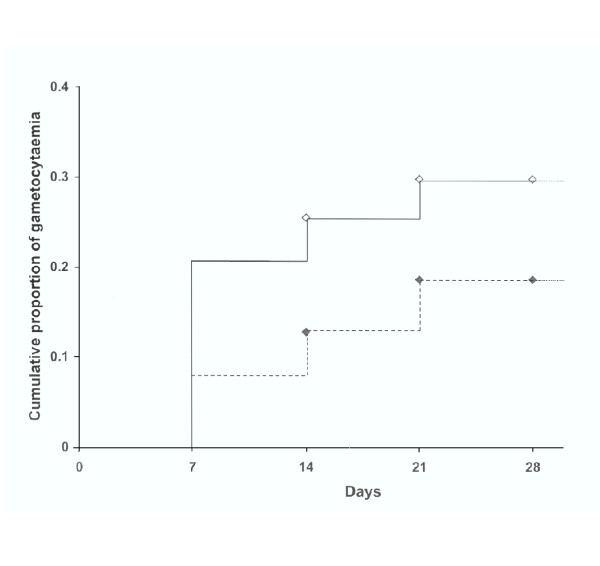
Gametocyte prevalence at enrolment was 33.8% (22/65) in children below five years of age and was negatively associated with age (table 1). Mean gametocyte density at enrolment was 24.5 (IQR 16–32) gametocytes/μl with no difference between age groups, whilst both asexual parasite prevalence and density decreased with age. SP treatment was given to 27.2% (143/526) of the children who presented with asexual parasites on the first or second visit with a geometric mean density of 1,108.0 (IQR 249–4235) parasites/μl. A group of 121 parasitaemic children exhibited relatively low parasitaemia at presentation (geometric mean density of 203.0 (IQR 80–400) parasites/μl) and was not given treatment throughout the study. The Kaplan-Meier estimator of time to gametocytaemia for these two groups of parasitaemic children is presented in figure 1. At the end of the four week follow-up period, the cumulative proportion of gametocytaemia was lower in children not receiving antimalarial treatment than in SP treated children, 18.6% and 29.8%, respectively. In both groups, gametocyte prevalence during the first two weeks of follow-up was independently associated with several factors (table 2). The risk for gametocytaemia during follow-up was positively associated with asexual parasite density at enrolment and negatively with age. Children with detectable gametocytes at enrolment were more likely to show gametocytes during follow-up, after adjustment for age and asexual parasite density at the start of treatment. In children who received SP treatment, the probability of gametocyte prevalence seemed higher on day seven after treatment compared to day fourteen, although this was not statistically significant. R1 resistance did not increase risk of gametocytes during follow-up while R2/R3 resistance did show such an association, OR 3.40 (95% CI 1.61–7.19). The estimated mean duration of gametocytaemia was 9.4 days (range 2.5 – 23.5) for children below five years of age, 7.8 days (range 2.5 – 23.5) for children aged five to nine years and 4.1 days (range 2.5–16.5) for children aged ten years and above. The duration of gametocytaemia was negatively associated with age (β = -1.65, se(β) = 0.73; p = 0.02), after adjustment for asexual parasite density and gametocyte density at enrolment. Treatment with SP did not independently influence the duration of gametocytaemia.
Table 1.
Parasitological data at enrolment
| Age | N | Asexual parasite prevalence, % (n)¥ | Asexual parasite density, mean (IQR)† | Gametocyte prevalence % (n)¶ | Gametocyte density mean (IQR)‡ |
| <5 years | 65 | 73.8 (48) | 2302.4 (530–14149) | 33.8 (22) | 25.3 (16–32) |
| 5–9 years | 211 | 50.2 (106) | 357.8 (120–1040) | 10.4 (22) | 27.9 (16–32) |
| 10–16 years | 250 | 30.4 (76) | 202.7 (80–440) | 5.2 (13) | 18.8 (16–23) |
| Total | 526 | 43.7 (230) | 444.8 (120–1220) | 10.8 (57) | 24.5 (16–32) |
IQR = interquartile range ¥ Cochran-Armitage test for age-dependent trend in prevalence of asexual parasites (Z = -6.75, P < 0.001). † Geometric mean of parasite carriers only (parasites/μl). Linear regression for log-transformed asexual parasite density and age groups, β = -1.155; se(β) = 0.147, P < 0.001. ¶ Cochran-Armitage test for age-dependent trend in prevalence of gametocytes (Z = -5.91, P < 0.001). ‡ Geometric mean of gametocyte carriers only (parasites/μl). Linear regression for log-transformed gametocyte density and age groups, β = -0.123; se(β) = 0.166, P = 0.46.
Figure 1.
The cumulative proportion of gametocytaemia in SP-treated and untreated children. Kaplan-Meier estimator of the time to gametocytaemia for untreated children (dotted line) and SP-treated children (solid line). Groups differed in asexual parasite density at enrolment. Patients with incomplete follow-up are marked on the curve. Gametocyte carriers on day 0 were excluded, as well as children who reported the use of antimalarial drugs prior to enrolment. Log-rank P = 0.05
Table 2.
Adjusted odds ratio (OR) of the probability of gametocyte prevalence, using a multivariate random effect logistic model for untreated and SP-treated children separately.
| Risk factors for gametocytaemia | Adjusted OR (95% CI) | ||
| No treatment | Treatment with SP | ||
| Day of follow-up | Day 7 | 1.48 (0.62 – 3.56)¥ | 1.77 (0.93–3.34)¥ |
| Day 14 | 1 (ref) | 1 (ref) | |
| Age | <5 years | 6.34 (1.15–34.90) | 5.38 (1.91–15.17) |
| 5–9 years | 3.07 (0.98–9.55)¥ | 3.22 (1.19–8.72) | |
| 10–16 years | 1 (ref) | 1 (ref) | |
| Asexual parasite density at enrolment | Per 100 parasites/μl | 1.05 (1.00–1.11) | 1.01 (1.00–1.01) |
| Gametocyte prevalence at enrolment | Present | 3.35 (1.22–9.18) | 4.12 (2.11–8.02) |
| Absent | 1 (ref) | 1 (ref) | |
| SP treatment outcome | R2/3 resistance | - | 3.40 (1.61–7.19) |
| Rl resistance | - | 1.06 (0.45–2.47)¥ | |
| Adequate response | - | 1 (ref) | |
Adjusted = adjusted for all other variables in the model, OR = odds ratio, CI = confidence interval, ref = reference group, ¥ = not statistically significant, - = not applicable. A GEE model was used to allow for correlation between observations from the same individuals. Children reporting the use of antimalarial drugs prior to enrolment were excluded from these analyses.
Discussion
This descriptive study on the largely neglected group of asymptomatic children was part of a larger study on SP and gametocytes. A direct comparison between SP-treated and untreated children was not the aim of the current study and would require a different study design. SP-treated and untreated children differed with regards to treatment and asexual parasite density at enrolment and were therefore analysed separately.
In this study, 18.6% of the asymptomatic non-treated children developed gametocytaemia during a four week follow-up period. Gametocytaemia was particularly common in children <5 years of age, of whom one-third were already carrying gametocytes on enrolment. The high gametocyte prevalence in young children is in line with previous findings [6,11], although the longer duration of gametocytaemia for this age group has not been reported before. The age dependent duration of gametocytaemia could not be explained by asexual parasitaemia, gametocyte density or SP treatment, suggesting a role for age-dependent immune suppression of gametocytaemia. Acquired sexual stage specific immunity has previously been reported to influence gametocyte prevalence [16], but remains poorly defined. Although the exact duration of gametocytaemia could not be determined because of weekly intervals in data-collection, the longer estimated duration of gametocytaemia in the youngest age group combined with their higher gametocyte prevalence could increase the importance of this age group for the infectious reservoir in this area. The importance of children as the infectious reservoir has been stressed elsewhere [17]. Differences in gametocytaemia during follow up should be interpreted carefully since they are at least partially explained by differences in asexual parasite density at enrolment. The observed peak in gametocytaemia on day seven after SP treatment has been previously described [3-6] and may include gametocytes circulating prior to treatment since seven days may be short for gametocytogenesis, as was suggested by in vitro observations [18]. This study determined predictive factors for gametocytaemia for SP-treated and untreated children independently and found that risk factors were similar in both groups. Furthermore, age and gametocyte presence at enrolment were previously described as predictors for gametocytaemia in symptomatic treated children [4,6-8,11]. Asexual parasitaemia at enrolment was positively associated with gametocytaemia during follow-up. Other studies [2-4] may not have found such an association because they only included patients with a high parasitaemia, resulting in little variation in asexual parasite density. Parasitological treatment failure was an important predictor of gametocytaemia in the SP-treated group [13].
Conclusions
This study shows that many asymptomatic children develop gametocytes, even in the absence of treatment. Gametocyte prevalence is especially high in children below five years of age who also appear to harbor gametocytes for a longer period of time. Risk factors for gametocytaemia are similar to those described in symptomatic children and do not appear to differ between treated and untreated children.
Authors' contributions
JTB was responsible for data analyses and manuscript preparation. LCG was responsible for data collection and participated in manuscript preparation. CJD participated in data analyses and manuscript preparation. AMM and NIJA participated in data collection. BAO, JCB and JIG participated in manuscript preparation. RWS participated in data interpretation and manuscript preparation. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
The authors are grateful to C. Akinyi, W. Onyango and A. Ouko at the MOH, Mbita Health Centre, to L. Omukuba, P. Obare at ICIPE-MPRTC and to all the village leaders, for their support, assistance and cooperation.
This study was supported by the National Institutes of Health USA, (Award numbers U19 AI45511, D43 TW01142, D43 TW00920), The Netherlands Organisation for Scientific Research (WOTRO number WR93-369) and an ARPPIS scholarship awarded to BAO by ICIPE. CJD is supported by a research fellowship in tropical medicine (#063516) from the Wellcome Trust.
Contributor Information
J Teun Bousema, Email: t.bousema@ncmls.kun.nl.
Louis C Gouagna, Email: lgouagna@mbita.mimcom.net.
Chris J Drakeley, Email: chris.drakeley@lshtm.ac.uk.
Annemiek M Meutstege, Email: a.meutstege@student.kun.nl.
Bernard A Okech, Email: bokech@nairobi.mimcom.net.
Ikupa NJ Akim, Email: i.akim@mmb.umcn.nl.
John C Beier, Email: jbeier@med.miami.edu.
John I Githure, Email: jgithure@icipe.org.
Robert W Sauerwein, Email: r.sauerwein@mmb.umcn.nl.
References
- Smith T, Schellenberg JA, Hayes R. Attributable fraction estimates and case definitions for malaria in endemic areas. Stat Med. 1994;13:2345–2358. doi: 10.1002/sim.4780132206. [DOI] [PubMed] [Google Scholar]
- Robert V, Awono-Ambene HP, Le Hesran JY, Trape JF. Gametocytemia and infectivity to mosquitoes of patients with uncomplicated Plasmodium falciparum malaria attacks treated with chloroquine or sulfadoxine plus pyrimethamine. Am J Trop Med Hyg. 2000;62:210–216. doi: 10.4269/ajtmh.2000.62.210. [DOI] [PubMed] [Google Scholar]
- Sokhna CS, Trape JF, Robert V. Gametocytaemia in Senegalese children with uncomplicated falciparum malaria treated with chloroquine, amodiaquine or sulfadoxine plus pyrimethamine. Parasite. 2001;8:243–250. doi: 10.1051/parasite/2001083243. [DOI] [PubMed] [Google Scholar]
- von Seidlein L, Drakeley C, Greenwood B, Walraven G, Targett G. Risk factors for gametocyte carriage in Gambian children. Am J Trop Med Hyg. 2001;65:523–527. doi: 10.4269/ajtmh.2001.65.523. [DOI] [PubMed] [Google Scholar]
- von Seidlein L, Jawara M, Coleman R, Doherty T, Walraven G, Targett G. Parasitaemia and gametocytaemia after treatment with chloroquine, pyrimethamine/sulfadoxine, and pyrimethamine/sulfadoxine combined with artesunate in young Gambians with uncomplicated malaria. Trop Med Int Health. 2001;6:92–98. doi: 10.1046/j.1365-3156.2001.00683.x. [DOI] [PubMed] [Google Scholar]
- Akim NI, Drakeley C, Kingo T, Simon B, Senkoro K, Sauerwein RW. Dynamics of P. falciparum gametocytemia in symptomatic patients in an area of intense perennial transmission in Tanzania. Am J Trop Med Hyg. 2000;63:199–203. doi: 10.4269/ajtmh.2000.63.199. [DOI] [PubMed] [Google Scholar]
- Price R, Nosten F, Simpson JA, Luxemburger C, Phaipun L, Ter Kuile F, Van Vugt M, Chongsuphajaisiddhi T, White NJ. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am J Trop Med Hyg. 1999;60:1019–1023. doi: 10.4269/ajtmh.1999.60.1019. [DOI] [PubMed] [Google Scholar]
- Jones TR, McElroy PD, Oster CN, Beier JC, Oloo AJ, Onyango FK, Chumo DK, Sherwood JA, Hoffman SL. Plasmodium falciparum gametocytemia in Kenyan children: associations among age, intensity of exposure to transmission, and prevalence and density of subsequent gametocytemia. Am J Trop Med Hyg. 1997;56:133–136. doi: 10.4269/ajtmh.1997.56.133. [DOI] [PubMed] [Google Scholar]
- Targett G, Drakeley C, Jawara M, von Seidlein L, Coleman R, Deen J, Pinder M, Doherty T, Sutherland C, Walraven G, Milligan P. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J Infect Dis. 2001;183:1254–1259. doi: 10.1086/319689. [DOI] [PubMed] [Google Scholar]
- Tjitra E, Suprianto S, Anstey NM. Higher gametocyte prevalence following failure of treatment of Plasmodium falciparum malaria with sulfadoxine-pyrimethamine and the combination of chloroquine plus sulfadoxine-pyrimethamine: implications for progression of anti-folate resistance. Trans R Soc Trop Med Hyg. 2002;96:434–437. doi: 10.1016/s0035-9203(02)90385-8. [DOI] [PubMed] [Google Scholar]
- Hogh B, Gamage-Mendis A, Butcher GA, Thompson R, Begtrup K, Mendis C, Enosse SM, Dgedge M, Barreto J, Eling W, Sinden RE. The differing impact of chloroquine and pyrimethamine/sulfadoxine upon the infectivity of malaria species to the mosquito vector. Am J Trop Med Hyg. 1998;58:176–182. doi: 10.4269/ajtmh.1998.58.176. [DOI] [PubMed] [Google Scholar]
- Shililu J, Mbogo C, Mutero C, Gunter J, Swalm C, Regens J, Keating J, Yan G, Githure J, Beier J. Spatial distribution of Anopheles gambiae and Anopheles funestus and malaria transmission in Suba District, western Kenya. Proceedings of the 49th Annual Meeting of ASTMH, Houston, TX, USA. 2000.
- Bousema JT, Gouagna LC, Meutstege AM, Okech BE, Akim NIJ, Githure JI, Beier JC, Sauerwein RW. Treatment failure of pyrimethamine-sulphadoxine and induction of Plasmodium. falciparum gametocytaemia in children in western Kenya. Trop Med Int Health. 2003;8:427–430. doi: 10.1046/j.1365-3156.2003.01047.x. [DOI] [PubMed] [Google Scholar]
- Drakeley CJ, Flobbe K, Greenwood BM, Targett GA. Plasmodium falciparum gametocytes in Gambian adults. Ann Trop Med Parasitol. 2000;94:399–401. doi: 10.1080/00034983.2000.11813555. [DOI] [PubMed] [Google Scholar]
- Smalley ME, Sinden RE. Plasmodium falciparum gametocytes: their longevity and infectivity. Parasitology. 1977;74:1–8. doi: 10.1017/s0031182000047478. [DOI] [PubMed] [Google Scholar]
- Baird JK, Jones TR, Purnomo , Masbar S, Ratiwayanto S, Leksana B. Evidence for specific suppression of gametocytemia by Plasmodium falciparum in residents of hyperendemic Irian Jaya. Am J Trop Med Hyg. 1991;44:183–190. doi: 10.4269/ajtmh.1991.44.183. [DOI] [PubMed] [Google Scholar]
- Bonnet S, Gouagna LC, Paul RE, Safeukui I, Meunier JY, Boudin C. Estimation of malaria transmission from humans to mosquitoes in two neighbouring villages in south Cameroon: evaluation and comparison of several indices. Trans R Soc Trop Med Hyg. 2003;97:53–59. doi: 10.1016/s0035-9203(03)90022-8. [DOI] [PubMed] [Google Scholar]
- Sinden RE, Smalley ME. Gametocytogenesis of Plasmodium falciparum in vitro: the cell-cycle. Parasitology. 1979;79:277–296. doi: 10.1017/s003118200005335x. [DOI] [PubMed] [Google Scholar]