Anti-tumor necrosis factor (TNF) therapies have provided an invaluable therapeutic alternative to immunomodulators, corticosteroids, and mesalamine compounds for the induction and maintenance of remission in inflammatory bowel disease (IBD). However, sustained remission drops significantly at 1 year, and the efficacy of manipulating other cytokines (eg, interleukin [IL]-6, IL-10, and IL-11) has been limited.1–3 Therefore, there is an unmet need for alternative biological therapies. Restoring the balance of pro- and anti-inflammatory pathways in IBD is a potential strategy that deserves further examination. A prospective novel approach is to target molecular pathways upstream of the proinflammatory cytokines that mediate IBD, such as nuclear factor (NF)-κB and the mitogen activated protein kinases (MAPKs). MAPKs comprise a large family of highly conserved serine/threonine protein kinases implicated in the regulation of a vast array of key cellular processes, such as production of cytokines, migration and accumulation of leukocytes, and angiogenesis, all of which contribute to the pathogenesis of IBD. Because of their key regulatory role in the production of inflammatory cytokines and in mediating their downstream effects, their inhibition has received considerable attention as a novel therapeutic strategy for inflammatory diseases.
In mammals, there are 3 major classes of MAPKs, the extracellular signal-regulated kinases and the 2 stress-activated protein kinase families, c-jun N-terminal kinase (JNK) and p38. The p38 family is composed of 4 isoforms (α, β, γ, and δ). The α, β, and δ isoforms are expressed widely in leukocyte subsets including CD4+ T cells, neutrophils, monocytes, macrophages, and endothelial cells.4 The p38α isoform has received the most attention as a therapeutic target owing to its critical role in the post-transcriptional regulation of inflammatory genes.4,5 Inhibitors of p38α have been shown to block proinflammatory cytokines such as interferon-γ, TNF, IL-1β, IL-8, and cyclooxygenase-2 production from myeloid cells, in addition to reducing mortality from endotoxin-induced shock and inhibiting the development of collagen-induced arthritis in animal models.5–7 Furthermore, activation of p38α stabilizes the 3′-untranslated region of mRNA from rapidly turned over inflammatory cytokines such as TNF and IL-1β, via adenosine-uridine–rich elements. As such, p38α seems to be critical to the regulation of proinflammatory cytokine synthesis, both at the transcriptional and translational levels.8
In human IBD, numerous studies have described increased p38 phosphorylation, corresponding with increased cytokine expression and leukocytic infiltration. Furthermore, in patients with ulcerative colitis and Crohn’s disease, p38α expression was localized within invading macrophages and neutrophils near the intestinal epithelium.9,10 Although a major emphasis has been placed on the role of p38α in the regulation of inflammatory cytokines, a critically important observation pertaining to IBD is that the TNF-dependent expression of mucosal address in cell adhesion molecule-1 (MAdCAM-1) is linked to p38α. MAdCAM-1 orchestrates lymphocyte homing to both Peyer’s patches and gastrointestinal-associated lymphoid tissues and plays a role in the pathogenesis of IBD.11,12
The first synthetic p38 inhibitors, the pyridinyl imidazoles (eg, SB203580 and SB202190), showed promising anti-inflammatory results in vitro and in vivo, in models of arthritis,13 chronic obstructive pulmonary disease, and some models of IBD.14 Moreover, there is now a large arsenal of new p38 inhibitors (including triazanapthalenones, diaryl ureas, benzophenones, pyrazole ketones, indole amides, diamides, quinazolinones, pyrimido-pyri-midinones),13,15 displaying efficacy in several preclinical IBD models, whereas others are currently undergoing clinical trials.16 However, many of these studies have encountered less than satisfactory efficacy or undesired side effects. One of the limitations of current p38 inhibitors stems from their competitive inhibition of the adenosine triphosphate binding pocket, resulting in drugs with excellent potency but insufficient specificity. This lack of specificity resulted in the withdrawal of candidate molecules from clinical trials, owing to unexpected side effects such as unexplained transaminase elevation.17 It may also explain the results of the most successful clinical trial of a p38 inhibitor in Crohn’s disease (ie, CNI-1493), in which 50% of patients were in remission after 4 months, yet further analyses revealed no decreased phosphorylation of p38 in colonic biopsies. Thus, its clinical effect was attributed to JNK inhibition rather than to altered p38 activity.10 Although attempts to improve the selectivity and pharmacodynamics of p38 inhibitors continue, this strategy remains mostly as a disappointing footnote in the search for novel IBD therapies.
Contrary to the conflicting results of broad spectrum p38 inhibitors, the cell-specific deletion of p38α has previously shown marked anti-inflammatory effects. Targeted deletion of p38α in macrophages impaired the response to the Toll-like receptor-4 ligand lipopolysaccharide (LPS) with reduced TNF, IL-12, and IL-18, in addition to reduced activation of transcription factors C/EBP-β and CREB in LPS-treated p38α-deficient macrophages. Furthermore, after in vivo challenge with LPS, p38α conditional knockout mice showed significantly lower TNF levels in sera and prolonged survival times.18 The selective transgenic inhibition of p38α in macrophages was also protective in mice, in both acute and chronic models of cutaneous inflammation.19 Further recent findings have elaborated on the use of cell-specific therapies in preclinical models of IBD. Delivery of anti-sense oligonucleotides targeting TNF into infiltrating colonic macrophages markedly protected mice from CD45RBhigh and trinitrobenzene sulfonic acid–induced colitis.20 These selective inhibition studies highlight the possibility of targeted cellular therapies for chronic inflammatory diseases, perhaps with lessened interference with physiologic immune responses, which is a significant concern with current “biologic” therapies.
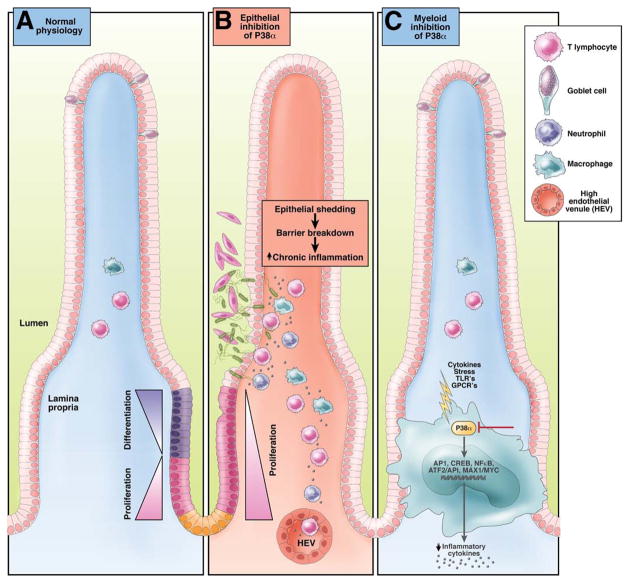
In this issue of Gastroenterology, Otsuka et al21 have elegantly identified a dichotomy between the actions of p38 in mucosal epithelial cells and those of the infiltrating myeloid lineage, which may shed new light on the current failings of broad-spectrum p38 inhibitors in IBD (Figure 1). The authors demonstrate that, although the use of p38α/β inhibition with the antagonist SB203580 had no significant beneficial effects on clinical indices, selective deletion of p38α in myeloid cells had a protective effect in colitis induced by acute dextran sulphate sodium treatment. This protection was mediated in part by the reduced activation of the MAPK-responsive transcription factors AP-1, GAS, and NF-κB. As has been reported previously through p38 inhibition of macrophages, the expression of the colonic proinflammatory cytokines IL-1β, IL-6, IL-12p40, and TNF was reduced.18 The authors also emphasized the critical role for p38α during physiologic epithelial cell homeostasis. Treatment of mice with the p38α/β antagonist, SB203580, and epithelial-specific p38α-deletion inhibited the homeostatic control of epithelial cell differentiation and proliferation and resulted in a decreased production of goblet cells. Because the levels of mucus secreted by goblet cells have a protective role in the intestine,22 broad-spectrum p38α/β inhibition may predispose the intestine to epithelial damage, bacterial invasion, and dysregulated immune responses (Figure 1).
Figure 1.
Effect of selective epithelial or myeloid p38α inhibition on intestinal homeostasis. (A) Intestinal homeostasis requires the maintenance of a balance between proliferation and differentiation of epithelial precursors and the regulation of physiologic leukocyte recruitment. Mucus-secreting goblets cells have a protective role in maintaining barrier function. (B) Inhibition of P38α in epithelial cells leads to hyper proliferation and lack of goblet cell differentiation culminating in barrier breakdown and tissue injury. (C) Conversely, specific P38α inhibition in the myeloid lineage results in reduced inflammatory signaling, production of proinflammatory cytokines and marked protection from colitis.
Clearly, MAPKs play important roles in transducing inflammatory signals and therefore are key molecular targets for therapeutic intervention in chronic inflammatory diseases such as IBD. However, because multiple isoforms of these kinases are implicated in the regulation of essential cellular responses, broad-spectrum MAPK inhibition is likely to result in serious side effects. By contrast, cell-specific p38α inhibition or small molecular inhibitors of the downstream, inflammation-restricted MAPK signaling of infiltrating pathogenic leukocytes during chronic inflammation is a potential novel therapeutic strategy that may further the physician’s armamentarium for the treatment of IBD in the near future.
Acknowledgments
Funding
Supported by US PHS/NIH grants: DK080212 to JRN.
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002;122:512–530. doi: 10.1053/gast.2002.31072. [DOI] [PubMed] [Google Scholar]
- 2.Sands BE, Bank S, Sninsky CA, et al. Preliminary evaluation of safety and activity of recombinant human interleukin 11 in patients with active Crohn’s disease. Gastroenterology. 1999;117:58–64. doi: 10.1016/s0016-5085(99)70550-0. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber S, Fedorak RN, Nielsen OH, et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Crohn’s Disease IL-10 Cooperative Study Group. Gastroenterology. 2000;119:1461–1472. doi: 10.1053/gast.2000.20196. [DOI] [PubMed] [Google Scholar]
- 4.Ashwell JD. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat Rev Immunol. 2006;6:532–540. doi: 10.1038/nri1865. [DOI] [PubMed] [Google Scholar]
- 5.Lee JC, Laydon JT, McDonnell PC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 6.Westra J, Doornbos-van der Meer B, de Boer P, et al. Strong inhibition of TNF-alpha production and inhibition of IL-8 and COX-2 mRNA expression in monocyte-derived macrophages by RWJ 67657, a p38 mitogen-activated protein kinase (MAPK) inhibitor. Arthritis Res Ther. 2004;6:R384–392. doi: 10.1186/ar1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Winzen R, Kracht M, Ritter B, et al. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waetzig GH, Seegert D, Rosenstiel P, et al. p38 mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J Immunol. 2002;168:5342–5351. doi: 10.4049/jimmunol.168.10.5342. [DOI] [PubMed] [Google Scholar]
- 10.Hommes D, van den Blink B, Plasse T, et al. Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn’s disease. Gastroenterology. 2002;122:7–14. doi: 10.1053/gast.2002.30770. [DOI] [PubMed] [Google Scholar]
- 11.Waetzig GH, Schreiber S. Review article: mitogen-activated protein kinases in chronic intestinal inflammation—targeting ancient pathways to treat modern diseases. Aliment Pharmacol Ther. 2003;18:17–32. doi: 10.1046/j.1365-2036.2003.01642.x. [DOI] [PubMed] [Google Scholar]
- 12.Gorfu G, Rivera-Nieves J, Ley K. Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med. 2009;9:836–850. doi: 10.2174/156652409789105525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signaling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 14.Hollenbach E, Neumann M, Vieth M, et al. Inhibition of p38 MAP kinase- and RICK/NF-kappaB-signaling suppresses inflammatory bowel disease. FASEB J. 2004;18:1550–1552. doi: 10.1096/fj.04-1642fje. [DOI] [PubMed] [Google Scholar]
- 15.Cirillo PF, Pargellis C, Regan J. The non-diaryl heterocycle classes of p38 MAP kinase inhibitors. Curr Top Med Chem. 2002;2:1021–1035. doi: 10.2174/1568026023393390. [DOI] [PubMed] [Google Scholar]
- 16.Schindler JF, Monahan JB, Smith WG. p38 pathway kinases as anti-inflammatory drug targets. J Dent Res. 2007;86:800–811. doi: 10.1177/154405910708600902. [DOI] [PubMed] [Google Scholar]
- 17.Dominguez C, Powers DA, Tamayo N. p38 MAP kinase inhibitors: many are made, but few are chosen. Curr Opin Drug Discov Dev. 2005;8:421–430. [PubMed] [Google Scholar]
- 18.Kang YJ, Chen J, Otsuka M, et al. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. J Immunol. 2008;180:5075–5082. doi: 10.4049/jimmunol.180.7.5075. [DOI] [PubMed] [Google Scholar]
- 19.Kim C, Sano Y, Todorova K, et al. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9:1019–1027. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuo L, Huang Z, Dong L, et al. Targeting delivery of anti-TNF-{alpha} oligonucleotide into activated colonic macrophages protects against experimental colitis. Gut. 2009 Dec 1; doi: 10.1136/gut.2009.184556. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Otsuka M, Kang YJ, Ren J, et al. Distinct effects of p38alpha deletion in myeloid lineage and gut epithelia in mouse models of inflammatory bowel disease. Gastroenterology. 2010;138:1255–1265. doi: 10.1053/j.gastro.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell B, Deschner E, Almy TP, et al. Patterns of cell proliferation in gastrointestinal disease. Dis Colon Rectum. 1967;10:107–111. doi: 10.1007/BF02617356. [DOI] [PubMed] [Google Scholar]