Abstract
Background
People of South Asian origin are at high risk of chronic kidney disease (CKD). Some have suggested that the usual level of glomerular filtration rate (GFR) in South Asians may be lower than in populations of European origin. However, measured GFR in a general adult population of South Asian origin has not been studied.
Design
Cross-sectional observational study
Setting and Participants
530 subjects aged >40 years randomly selected from communities in Karachi, Pakistan, using multi-stage cluster sampling. Subjects with both diabetes and hypertension were excluded.
Predictor
Age, sex, diabetes, hypertension.
Outcome
Measured GFR using urinary clearance of inulin.
Results
The mean age of participants was 49.7 +/− 9.5 (SD) years, 51% were men, 34.9% had hypertension, 30.5% had diabetes. The mean measured GFR was 94.1 +/− 28.6 (SD) ml/min/1.73 m2. GFR was lower by 0.79 +/− 0.11 ml/min/1.73 m2 for each 1 year higher age. The 5-year age- and sex- specific mean GFR of the study population was generally within 1 standard deviation of the mean of previously reported values for US adults. The factors independently associated with GFR were younger age (beta coefficient, −3.84 [95% CI, −5.46 to −2.21] ml/min/1.73 m2 per 5 year older), higher serum albumin (4.58 [95% CI, 0.74 to 8.42] ml/min/1.73 m2 per 0.5 g/dl increase), higher fasting plasma glucose (0.81 [95% CI, 0.44 to 1.18] ml/min/1.73 m2 per 10 mg/dl increase ), high versus low meat intake (7.81 [95% CI, 1.14 to 14.48] ml/min/1.73 m2 for >11 versus <5 servings per week), and higher estimated protein intake (1.46 [95% CI, 0.41 to 2.51] ml/min/1.73 m2 per 1.0 g/d increase) from urine urea nitrogen.
Limitations
Moderate sample size, lack of validation of some items in the dietary assessment for this study population.
Conclusions
The mean measured GFR in South Asian adults from the general population in Karachi, Pakistan, is only modestly lower than in European-origin counterparts, with similar age association. This may reflect lower dietary protein intake in South Asians.
Keywords: kidney function, glomerular filtration rate, ethnicity, South Asian
Chronic kidney disease (CKD) has become a global public health problem. (1) Adverse outcomes of CKD include kidney failure, accelerated cardiovascular disease, and premature mortality, regardless of geographic location.(2) (3) (4)Some of these outcomes can be prevented or delayed by early detection and treatment of CKD. Unfortunately, CKD is under-diagnosed and under-treated. (5)
This problem is compounded in the South Asian populations of India, Pakistan, Bangladesh, and Sri Lanka, in whom social determinants and conventional risk factors for CKD, including high blood pressure and diabetes, are widely prevalent and poorly controlled.(6, 7) (8) One in three adults aged 40 years or older has hypertension and one in five has diabetes. (9) In addition, there are concerns that low birth weight, and prematurity, both common in this population, may be associated with reduced nephron mass and kidney function, thus further enhancing the risk of CKD. (10)
Moreover, studies on migrant South Asians living in the United States and elsewhere suggest a faster rate of progression to end stage kidney failure than white counterparts. (11) (12)
Glomerular filtration rate (GFR) is accepted generally as the best overall indicator of kidney function in health and disease. The gold standard for measuring GFR is urinary clearance of inulin, an inert substance that is freely filtered by the glomeruli and neither reabsorbed nor secreted by the renal tubules. GFR in healthy individuals of European-origin at age 20 years is approximately 125 ml/min/1.73 m2 and is lower by about 1 ml/min/1.73 m2 per year thereafter (ie around 105 ml/min/1.73m2 at age 40 years). (13) (14)However there is lack of consensus on the “normal” level of kidney function in South Asians. (15) This stems primarily from earlier reports of small kidneys in South Asian children as well as low levels of measured GFR (mean of about 81 ml/min/1.73 m2) in young potential kidney donors in India. (16) (17) Moreover, dietary protein consumption, which is known to be associated with lower GFR, may be lower in South Asians than in Western counterparts. (18) (19) Hence, some have suggested that low GFR levels in this population are usual and do not suggest the presence of CKD(17) (20). However, there are no studies of measured GFR in unselected South Asians from the general population, so it is not possible to evaluate this suggestion. Clearly, this information would have significant implications for the 1.5 billion and rapidly growing native and migrant South Asian population worldwide.
We performed a cross sectional, population-based study on adults aged 40 years and above residing in the low to middle income communities in Karachi, Pakistan, to determine the level of measured GFR using the gold standard of inulin clearance, and to examine the demographic, dietary, and co-morbid determinants of measured GFR in this population. We were particularly interested in assessing the dietary profile in this population with regards to protein intake and its relationship with GFR levels, and compare with the reported levels for the US population. The results have significant implications for defining normal kidney function and assessing the burden of kidney disease in South Asian population globally.
Methods
Study Design and setting
This was a cross sectional study in 10 randomly selected communities in Karachi (Clinicaltrials.gov registration number NCT00800878). The inclusion criteria were subjects aged 40 years or above, and able to consent. Poorly mobile patients unable to attend clinic, those mentally incompetent, with known advanced liver disease, with both hypertension and diabetes, or pregnant were excluded. Ethical approval was obtained by the Ethics Review Committee at the Aga Khan University. The participants were provided with results of blood and urine tests (at no cost to them), and appropriate referral.
Sampling details
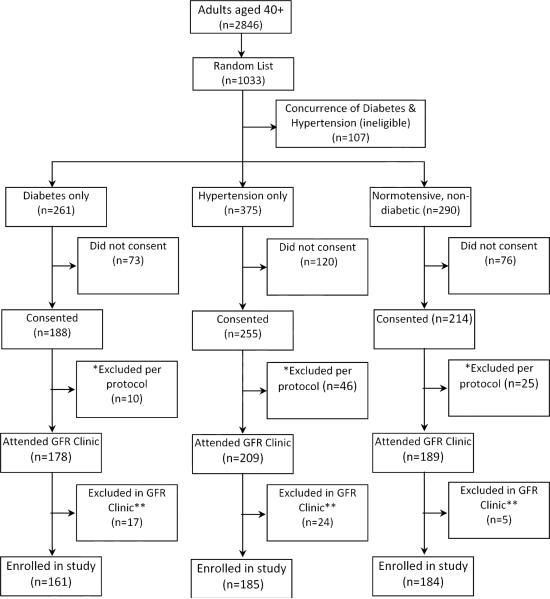
In brief, the Federal Bureau of Statistics has divided the city of Karachi into 5000 clusters each of about 250 households. We used multistage random cluster sampling to identify 10 clusters in which 1033 subjects were randomly selected from all 2468 subjects aged 40 years residing in these communities (study flow diagram in Figure 1). The selected subjects were then screened for presence of known hypertension, known diabetes, and neither of these conditions, and invited from the three stratified groups (n=926) to consent for GFR measurement. A total of 657 (71%) consented.
Figure 1.
Study flow. * Excluded per protocol due to presence of symptoms suggestive of medical illness such as shortness of breath, resting tachycardia (heart rate ≥ 120 beats per minutes), joint pains, jaundice, fever. ** Excluded due to febrile illness, or detection of concurrence of hypertension and diabetes, or symptoms suggestive of minor allergic reaction following inulin infusion (n=5).
Screening visits
Trained research field staff paid home visits to invite all subjects who consented and were eligible to participate. Those who have severe co-morbid conditions (active systemic disease defined as any acute febrile illness, or any other debilitating disease reported by the subject) were excluded. In addition, since the prevalence of co-morbid conditions potentially affecting GFR are higher with the concomitant presence of hypertension and diabetes, subjects with both conditions were also excluded. Blood pressure was measured with a calibrated automated device “Omron HEM-537 ™ Blood Pressure Monitor” in the sitting position after 5 minutes of rest using an appropriate sized cuff. Three consecutive readings were taken and the mean of the final two used in the analysis. Diabetes status was ascertained via history. Those eligible (n=576) were revisited by the field staff, and a standardized questionnaire was administered to collect information on demography, medical history, food frequency questionnaire, smoking, exercise, education, socioeconomic status, ethnicity. Physical examination was performed including anthropometry, (ie height, weight, waist to hip ratio, upper arm circumference, wrist circumference, triceps, biceps and sub-scapular and supra-iliac skin fold thickness). Body fat and lean body mass were estimated with the bio-impedance device QuadScan 4000 (Bodyscan Ltd, Isle of Mann,UK). (21, 22) Blood pressure was re-measured. Instructions on performing 24- hour urine collection were given with appropriate bottles, and picked up by field staff on the date agreed upon by the subjects. On average, eight to ten subjects were enrolled weekly.
Visit to Research Laboratory
Subjects were given an appointment at their convenience to visit the research laboratory in the morning after an over-night fast along with the 24 hour urine collections. At the laboratory, all subjects underwent a standardized physical examination by a medical doctor for confirmation of absence of significant co-morbid conditions (as defined above). Those found to have evidence of clinical heart failure (defined as two major or one major and two minor criteria as per Framingham Heart Study protocol) were excluded from the study. (23) Blood samples were collected on eligible subjects for measurement of fasting blood glucose (glucose oxidase method), serum albumin (bromocresol purple, Beckman) and serum creatinine (Jaffe rate reaction method with alkaline picrate solution) with Synchron / Delta-CX7 analyzer. Fasting serum lipid levels were measured (CHOD-PAP enzymatic End Point method) with a Colorimetric / Selectra Hitachi 912 analyzer. Urine urea nitrogen (enzymatic method), urine creatinine and urine albumin (nephrometry method on an Array machine) were measured in the 24 hour collections.
Diabetes status was confirmed (fasting plasma glucose > 126 mg/dl or receiving anti-diabetes medications). All assessments were performed to a standard protocol that conformed to international standards for definitions and measurements, and external quality control were performed by the Bio-Rad Laboratories Inc, Irvine, CA.
GFR measurements
GFR was measured using inulin clearance as the reference standard. The procedure started at 9.00 AM. A blank urine sample was voided and fasting blood sample was collected. An oral water load of 8 to 10 ml/kg body weight was given to initiate an appropriate urine flow.(24) The level of hydration was maintained by oral administration of 100 ml liquid / hour. An indwelling catheter was inserted intravenously for inulin infusion. A loading dose of 30 mg/kg of Inutest® 25% (Fresenius Kabi, Austria) was given followed by a continuous infusion of 40 mg/kg of inulin diluted in a 10% mannitol solution. After an equilibration period of 45 minutes, two clearance periods of 30 minutes each were analyzed. Urine sample was collected by spontaneous voiding. Blood samples were drawn at the midpoint of each clearance period.(24) In case urine flow rate differed by more than 0.1 ml/min between each period, a third 30-minute clearance was performed. Subjects rested in a semi-reclining position throughout the procedure. Blood samples were drawn from the arm opposite the infusion site, and were centrifuged and stored in EDTA tubes at −20°C in the research laboratory. Urine was collected and also stored at −20°C until shipment of frozen samples. Blood and urine samples were sent to Renal Laboratories, Saint-Etienne Hospital, University of Jean Monnet, Saint-Etienne, France for measurement. Inulin concentration was assessed according to enzymatic method by ELx 808 Absorbance Microplate Reader Biotek Instruments. GFR was measured as the mean of at least two urinary clearance periods of inulin with the formula U*V/P, where U and P are inulin concentrations in urine and plasma, respectively, and V is the urine flow rate in ml/min. GFR was expressed per 1.73 m2 of body surface area (BSA) by multiplying measured values by 1.73/ BSA (BSA= Height 0.725 (cm) x body weight0.425 (kg) x 0.007184). (25)
Statistical Analysis
The mean (SD) level of measured GFR (adjusted for BSA) was computed for all participants. GFR was compared between men and women, and among the subgroups with hypertension, diabetes, and those without these conditions using analysis of variance and difference in proportion were assessed by Pearson's chi-square test.
GFR in Pakistani subjects was compared with a previous report of a compilation of inulin clearance measurements in healthy adult men and women in the United States, presumably of European origin. (13) (Individual patient data were not available from this study.)
Multivariable models were built to assess the factors associated with GFR and linear regression analysis was performed. The candidate determinants list included: A) Demographic characteristics: age, sex, level of education, tobacco use, and physical activity B) Body composition, diet and nutritional biomarkers: Height, weight, body mass index, overweight (BMI cut- off for Asians >23.5 kg/m2) (26) (27), central obesity (waist circumference), serum albumin, urine urea nitrogen excretion) (28) and food frequency questionnaire assessing weekly intake of items in the following food groups: 1) meat including poultry, fish, eggs 2) vegetables, 3) fresh fruits, 4) low fat or non-fat dairy, 5) nuts, seeds and legumes, 6) fats and oil rich food, 7) sweets and calorie dense food, 8) food with high salt content, 9) grain and grain products (29), and C) Co-morbid conditions and biomarkers: Presence of hypertension (defined as systolic blood pressure ≥140 or diastolic blood pressure ≥90 mm Hg, or on antihypertensive medications (30), diabetes (fasting blood glucose ≥126 mg/dl or on anti-diabetic medications) (31), level of fasting plasma glucose, and lipids. Urine urea nitrogen excretion was converted into estimated protein intake using the Maroni formula. (32, 33)
All factors associated with GFR with p<0.05 in the univariate analysis, and sex were retained in the final model. We also searched for interactions between age with sex.
Results
Study Design
The subject enrolment diagram is illustrated in Figure 1. The clearance measurements were completed as per protocol on 530 subjects: 336 subjects had two and 194 had three urinary clearance periods.
Characteristics of the Population and measured GFR Levels
The mean age of subjects was 49.7 +/− 9.5 (SD) years; 52.1 +/− 9.7 in men and 47.3 +/− 8.5 in women (p<0.001). About 50% of all participants were men, and mean serum albumin level was 3.4 g/dl. The characteristics of the participants are listed in Table 1. A total of 184 subjects were normotensive and non-diabetic, 185 were hypertensive and non-diabetic, and 161 were diabetic and normotensive.
Table 1.
Characteristics of South Asian Subjects with Measured GFR
| Characteristics | All (n= 530) | ‡Normotensive, non-diabetic (n= 184) | §Hypertensive, Non-diabetic (n=185) | ∥Diabetic, normotensive (n=161) | P value |
|---|---|---|---|---|---|
| Age years | 49.7 +/− 9.5 | 48.1 +/− 9.0 | 50.0 +/− 9.2 | 51.3 +/− 10.0 | 0.01 |
| Men | 268 (50.6) | 99 (53.8) | 78 (42.2) | 91 (56.5) | 0.02 |
| Weight kg | 66.0 +/− 13.4 | 63.8 +/− 14.0 | 67.6 +/− 14.0 | 66.6 +/− 11.5 | 0.02 |
| Height cm | 159.8 +/− 9.2 | 161.3 +/− 9.6 | 158.7 +/− 9.3 | 159.5 +/− 8.2 | 0.02 |
| *BSA m2 | 1.68 +/− 0.18 | 1.67 +/− 0.19 | 1.69 +/− 0.18 | 1.69 +/− 0.16 | 0.4 |
| †BMI kg/m2 | 25.9 +/− 5.1 | 24.5 +/− 5.1 | 26.9 +/− 5.6 | 26.2 +/− 4.3 | <0.001 |
| ¶Overweight | 370 (69.8) | 108 (58.7) | 143 (77.0) | 119 (73.9) | <0.001 |
| Waist circumference in all (cm) | 92.8 +/− 11.5 | 89.8 +/− 12.2 | 94.2 +/− 11.4 | 94.6 +/− 10.1 | <0.001 |
| Waist circumference in women (cm) | 92.5 +/− 12.2 | 88.3 +/− 11.5 | 94.3 +/− 12.9 | 94.8 +/− 10.5 | 0.004 |
| Waist circumference in men (cm) | 94.2 +/− 11.0 | 91.4 +/− 12.4 | 95.6 +/− 9.8 | 96.1 +/− 9.9 | 0.005 |
| Total body fat percent | 34.6 +/− 9.0 | 33.4 +/− 8.9 | 36.6 +/− 9.2 | 33.8 +/− 8.2 | 0.001 |
| Lean body mass percent | 43.1 +/− 10.1 | 42.7 +/− 11.0 | 42.8 +/− 9.6 | 44.1 +/− 9.6 | 0.40 |
| Systolic BP mm Hg | 129.2 +/− 20.5 | 120.0 +/− 13.6 | 140.4 +/− 23.5 | 126.8 +/− 17.4 | <0.001 |
| Diastolic BP mm Hg | 80.9 +/− 12.0 | 76.2 +/− 9.1 | 87.0 +/− 13.5 | 79.1 +/− 10.0 | <0.001 |
| Serum albumin g/dl | 3.7 +/− 0.3 | 3.7 +/− 0.3 | 3.7 +/− 0.3 | 3.6 +/− 0.4 | 0.03 |
| **Dietary meat intake | 0.01 | ||||
| <5 servings/wk | 111 (20.9) | 27 (14.7) | 43 (23.2) | 41 (25.5) | |
| 5-10 servings/wk | 276 (52.1) | 97 (52.7) | 100 (54.1) | 79 (49.1) | |
| ≥11 servings/wk | 143 (27.0) | 60 (32.6) | 42 (22.7) | 41 (25.5) | |
| Hemoglobin g/dl | 12.6 +/− 1.8 | 12.6 +/− 1.7 | 12.5 +/− 1.8 | 12.8 +/− 1.8 | 0.4 |
| Fasting plasma glucose mg/dl | 126.7 +/− 65.3 | 98.2 +/− 31.8 | 98.3 +/− 14.7 | 192.0 +/− 80.7 | <0.001 |
| Total cholesterol mg/dl | 184.6 +/− 39.4 | 181.5 +/− 36.5 | 188.6 +/− 36.3 | 183.6 +/− 45.4 | 0.2 |
| LDL cholesterol mg/dl | 111.5 +/− 28.8 | 109.0 +/− 26.1 | 114.5 +/− 27.5 | 110.9 +/− 32.8 | 0.2 |
| VLDL cholesterol mg/dl | 32.5 +/− 17.8 | 27.6 +/− 13.9 | 32.4 +/− 15.6 | 38.3 +/− 22.1 | <0.001 |
| HDL cholesterol mg/dl | 40.0 +/− 10.3 | 42.5 +/− 10.3 | 41.1 +/− 9.7 | 36.0 +/− 9.8 | <0.001 |
| Triglycerides mg/dl | 162.6 +/− 89.0 | 138.0 +/− 69.4 | 161.8 +/− 77.9 | 191.5 +/− 110.4 | <0.001 |
| Urine creatinine g/d | 1.05 +/− 2.9 | 0.95 +/− 0.5 | 1.25 +/− 0.5 | 0.93 +/− 0.4 | 0.6 |
| Urine creatinine in all (mg/kg/d) | 14.0 +/− 4.9 | 14.9 +/− 5.0 | 13.1 +/− 4.6 | 13.9 +/− 5.0 | 0.5 |
| Urine creatinine in women (mg/kg/d) | 16.1 +/− 4.7 | 17.0 +/− 4.9 | 15.5 +/− 4.4 | 15.7 +/− 4.7 | 0.07 |
| Urine creatinine in men (mg/kg/d) | 11.8 +/− 4.1 | 12.5 +/− 4.0 | 11.4 +/− 4.0 | 11.5 +/− 4.4 | 0.5 |
| Urine urea nitrogen g/d | 5.0 +/− 2.4 | 5.2 +/− 2.7 | 4.5 +/− 1.9 | 5.5 +/− 2.4 | <0.001 |
| 24 h urine volume (L/d) | 1.4 +/− 5.7 | 1.4 +/− 6.3 | 1.4 +/− 5.1 | 1.6 +/− 5.7 | 0.004 |
| Estimated protein intake g/d | 43.9 +/− 15.8 | 44.3 +/− 17.8 | 40.9 +/− 13.1 | 47.1 +/− 15.8 | 0.001 |
| Estimated protein intake g/kg/d | 0.67 +/− 0.21 | 0.69 +/− 0.23 | 0.62 +/− 0.17 | 0.71 +/− 0.21 | <0.001 |
| Serum creatinine in all mg/dl | 0.8 +/− 0.6 | 0.8 +/− 0.4 | 0.8 +/− 0.5 | 0.9 +/− 0.7 | 0.3 |
| Serum creatinine in women mg/dl | 0.7 +/− 0.6 | 0.7 +/− 0.6 | 0.7 +/− 0.5 | 0.7 +/− 0.8 | 0.8 |
| Serum creatinine in men mg/dl | 0.9 +/− 0.1 | 0.84 +/− 0.2 | 1.0 +/− 0.4 | 1.0 +/− 0.7 | 0.1 |
| Serum urea nitrogen mg/dl | 11.8 +/− 7.3 | 11.3 +/− 4.6 | 11.1 +/− 7.0 | 13.3 +/− 9.6 | 0.007 |
| Urine albumin excretion mg/d | 5.2 (3.4, 11.9) | 3.9 (2.9, 6.0) | 5.2 (3.5, 11.7) | 8.9 (4.4, 22.0) | 0.003 |
| Urine albumin-creatinine ratio (mg/g) | 6.0 (4.0, 13.0) | 4.7 (3.4, 7.6) | 6.7 (4.2, 13.8) | 10.2 (5.0, 25.1) | 0.006 |
| Measured GFR ml/min | 91.7 +/− 30.5 | 91.0 +/− 29.9 | 89.3 +/− 27.1 | 95.3 +/− 34.5 | 0.2 |
| Measured GFR ml/min/1.73 m2 | 94.1 +/− 28.6 | 94.1 +/− 27.8 | 91.2 +/− 25.3 | 97.4 +/− 32.7 | 0.1 |
Abbreviations:
BSA=body surface area
BMI=body mass index; GFR, glomerular filtration rate; BP, blood pressure; HDL, high density lipoprotein; LDL, low-density lipoprotein; VLDL, very low density lipoprotein. P values refer to differences among the three subgroups and are based on chi-squared test or ANOVA, as indicated.
Subjects with both hypertension and diabetes were excluded.
Hypertension defined as systolic blood pressure ≥140/90 mm Hg, or on antihypertensive medications.
Diabetes defined as fasting blood glucose ≥126 mg/dl or on anti-diabetic medications
Overweight defined on the basis of Asian specific cut-off of BMI ≥ 23 kg/ m2.
Overall, the characteristics on subjects on whom GFR measurements were obtained (n=530) did not differ from those who did not consent to participate (n=269) in terms of age, diastolic blood pressure, family history of diabetes, or heart disease. However, the former had lower systolic blood pressure (133 +/− 20 vs 138 +/− 23 mm Hg, p=0.002), lower prevalence of hypertension (34% vs 44%) and higher prevalence of diabetes (30% vs 27%) (p=0.03), and were more likely to be men (51% vs 42%, p=0.01).
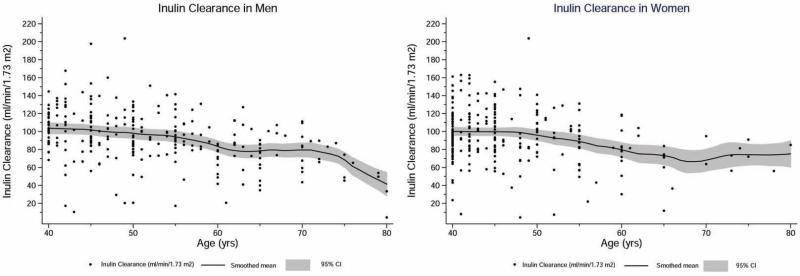
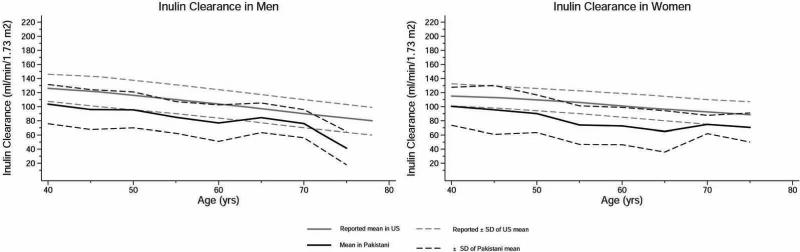
Amongst the three groups, diabetics had the worse risk factor profile (Table 1). The GFR during the three clearance periods are shown in Table 2. There was no significant differences among the three periods (p=0.1), indicating no systematic effect of hydration or mannitol on GFR. (34) (35) The overall mean measured GFR adjusted for BSA was 94.1 +/− 28.6 ml/min/1.73 m2: 93.4 +/− 28.0 in men vs 94.8 +/− 29.2 in women (p=0.6). The levels were lower by 0.79 +/− 0.11 ml/min/1.73 m2 for each 1 year higher age. The age- and sex- specific GFR levels of the study population are illustrated in Figure 2, and along with previously reported mean estimates at 5 year age intervals in adults of European origin (or Caucasians) in figure 3. (13) Interestingly, the mean GFR levels of study subjects generally fell within 1 standard deviation of the reported values for US adults, especially in men, and also in women, except at older age (>60 years) when data were relatively sparse for Pakistani adults. The mean GFR level for the Pakistani subjects after accounting for the clustering by census in the study design was 94.0 (95% CI, 90.3-97.8) ml/min/1.73 m2.
Table 2.
mGFR by Urinary Clearance Periods
| All | Men | Women | ||||
|---|---|---|---|---|---|---|
| No. | mGFR | No. | mGFR | No. | mGFR | |
| Clearance period 1 | 526 | 94.7 +/− 33.0 | 266 | 94.9 +/− 32.5 | 260 | 94.5 +/− 33.7 |
| Clearance period 2 | 528 | 93.7 +/− 30.7 | 267 | 92.0 +/− 29.6 | 261 | 95.5 +/− 31.7 |
| Clearance period 3 | 194 | 97.4 +/− 28.5 | 93 | 98.4 +/− 26.4 | 101 | 96.4 +/− 30.4 |
| Mean of clearance periods | 530 | 94.1 +/− 28.6 | 268 | 93.4 +/− 28.0 | 262 | 94.8 +/− 29.2 |
Measured glomerular filtration rate (mGFR) given as mean +/− SD in ml/min/1.73 m2 (conversion factor for mGFR in ml/s/1.73 m2, ×0.01667) in South Asians Aged 40 years and Older from the General Population in Karachi, Pakistan.
Figure 2.
Measured values for GFR are shown for men and women of various ages, with the GFR measured as the urinary clearance of inulin in Pakistani adults Solid lines represent mean GFR per 5 year age group and shaded areas represent corresponding 95% CI of the mean.
Figure 3.
Measured values for GFR are shown for men and women of various ages, with the GFR measured as the urinary clearance of inulin in Pakistani adults and compared to GFR in US adults also measured as inulin clearance reported by Wesson et al (13). Black lines are for Pakistani adults and gray lines for US. Solid lines represent the mean value of GFR per 5 year age group, and dotted black lines represent corresponding 1 SD from the mean value of GFR.
Factors Associated with Measured GFR
The factors associated with measured GFR in the Pakistani subjects are shown in Table 3. The beta coefficients (95% CIs) in model 1 are based on univariate analysis, while those in model 2 to 3 are adjusted incrementally for sociodemographic and dietary factors, and co-morbid biomarkers. In the final multivariable model (Table 3, model 3), the factors independently associated with r GFR (beta coefficient) were: younger age (−3.84 [95% CI, −5.46 to - 2.21] ml/min/1.73 m2 per 5 year older), higher serum albumin (4.58 [95% CI, 0.74 to 8.42] ml/min/1.73 m2 per 0.5 g/dl increase) higher fasting plasma glucose (0.81 [95% CI, 0.44 to 1.18] ml/min/1.73 m2 per 10 mg/dl increase), high (>11 servings or more per week) versus low (<5 servings per week) meat intake (7.81 [95% CI, 1.14 to 14.48] ml/min/1.73 m2) and higher estimated protein intake (0.22 [95% CI, 0.06, 0.38] ml/min/1.73 m2 per 1.0 g/d increase). The interaction between age and sex was not significant (p=0.5).
Table 3.
Factors Associated with Measured GFR in South Asians
| Factor | Unadjusted | †Model 1 | ‡Model 2 | §Model 3 (Final) |
|---|---|---|---|---|
| Age (/5 y increase) | −4.94(−6.16 to−3.72)* | −5.18 (−6.44,−3.92)* | −4.20 (−5.76 to −2.63)* | −3.84 (−5.46 to −2.21)* |
| Men vs women | 1.37(−3.52 to 6.26) | −3.61 (−8.38,1.16) | 5.04 (−5.36 to 15.43) | 7.84 (−2.98 to 18.65) |
| BMI (/1 kg/m2 increase | 0.50 (0.02 to 0.97)* | 0.63 (−0.44 to 1.71) | 0.57 (−0.48 to 1.62) | |
| Dietary Meat | ||||
| >11 vs ≤ 5_servings/wk | 11.59(4.55 to 18.63)* | 11.19 (4.38 to 17.99)* | 7.81 (1.14 to 14.48)* | |
| 6-10 vs ≤ 5 servings/wk | 3.16 (−3.09to 9.42) | 4.24 (−1.74 to 10.21) | 3.84 (−1.96 to 9.64) | |
| Serum albumin (/0.5 g/dl increase) | 6.84(3.00 to 10.70)* | 4.58 (0.74 to 8.42)* | ||
| Hemoglobin (/1g/dl increase) | 1.38 (0.02 to 2.73)* | 0.87 (−0.79 to 2.52) | ||
| Estimated protein intake (/1 g/d increase) | 0.30(0.17 to0.43)* | 0.22 (0.06 to 0.38)* | ||
| Fasting plasma glucose (/10 mg/dl increase) | 0.96 (0.60 to 1.33)* | 0.81 (0.44 to 1.18)* | ||
| 24 h urine volume (/100 ml increase) | 0.27 (−0.20 to 0.68) | |||
| Current smokers vs non- smokers | 2.30(−4.60 to 9.19) | |||
| SBP (/10 mm Hg increase) | −1.69(−2.88 to−0.51)* | −0.12 (−2.24 to 2.00) | ||
| DBP (/10 mm Hg increase) | 0.24 (−1.80 to2.27) | |||
| Pulse pressure (/10 mm Hg increase) | −4.06(−5.83 to −2.29)* | −0.71 (−1.45 to 2.65 | ||
| Total body fat percent (/1% increase) | −0.29(−0.56 to −0.02)* | −0.61 (−1.43 to 0.23) | −0.60 (−1.41 to 0.21) | |
| Lean body mass percent (/1% increase) | 0.29 (0.02 to 0.56)* | −0.05 (−0.66 to 0.55) | −0.26 (−0.84 to 0.34) | |
| HDL cholesterol (/1 mg/dl increase) | −0.29(−0.53 to −0.06)* | −0.10 (−0.35 to 0.15) | ||
| LDL cholesterol (/1 mg/dl increase) | 0.03(−0.05 to 0.12) | 7.72 (−0.19 to 15.63) | ||
| VLDL cholesterol (/1 mg/dl increase) | 0.24(0.10 to 0.38)* | |||
| Triglycerides (/1 mg/dl increase) | 0.05(0.02 to 0.07)* | −1.54 (−3.12 to 0.05) |
Values shown are β coefficient in ml/min/1.73 m2 (95%CI). Based on 530 participants. GFR was measured via inulin clearance.
Abbreviations: BMI, body mass index; GFR, glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low-density lipoprotein; VLDL, very low density lipoprotein.
Model I was adjusted for demographic variables
Model 2 adjusted for demographic, anthropometric and dietary factors
Model 3 adjusted for demographic, anthropometric, dietary and co-morbid biomarkers (final model)
[originally bolded]
In a subgroup analysis restricted to 161 subjects with diabetes, younger age (beta coefficient, −5.44 [95% CI, −3.12 to −7.76] ml/min/1.73 m2 per 5 year older), fasting plasma glucose (0.90 [95% CI, 0.36 to 1.44] ml/min/1.73 m2 per 10 mg/dl increase) and serum albumin (12.2 [95% CI, 6.15 to 18.25] ml/min/1.73 m2 per 0.5 g/dl increase), were each significantly associated with higher GFR.
Discussion
Using the reference standard measurement of inulin clearance, we found that the mean level of measured GFR in unselected South Asian adults aged 40 years and older in communities in Karachi, Pakistan, is about 94 +/− 28.6 (SD) ml/min/1.73 m2, which is only modestly lower than levels reported in European-origin counterparts (figures 2 and 3). 23 Nutritional parameters including dietary protein intake and serum albumin were related to GFR levels in the study population. Our findings have significant implications for clinical practice and public health policy, globally, and do not support the concept of reduced kidney function in people of South Asian descent. (5)
There is a dearth of studies of GFR measurement in unselected general population. Data collected in the 1960s using the reference standard of inulin clearance on healthy European-origin women and men indicate GFR levels of about 105-120 ml/min/1.73 m2(Figure 3). (13, 14, 36) (37) Recent studies in kidney donors of European-descent with an average age approximately 39 years showed a mean GFR levels of 107 +/− 16 ml/min/1.73 m2. (38) (39) The decline in GFR with older age (−3.84 [95% CI, −5.46 to −2.21] ml/min/1.73 m2 for every 5 year increase) in men and women in our study was also consistent with Western data, which would suggest a mean GFR of about 99 ml/min/1.73 m2 at 49 years---the average age in our study. As described below, this minor difference from about 94 ml/min/1.73 m2 may be due to lower habitual dietary protein intake than the kidney donors.
We found that serum albumin, dietary protein intake estimated from urinary urea excretion and food frequency questionnaires (40) were associated with measured GFR. (Table 3, model 3) As documented in other studies, the nature of the relationship between dietary protein intake, and serum albumin, a nutritional parameter, and GFR is complex. Higher habitual dietary protein intake and short-term increases in protein intake and amino acid infusions are associated with higher GFR in healthy subjects, perhaps related to physiological effects of protein intake on renal hemodynamic determinants of GFR. (20) Previous studies have also demonstrated that in patients with chronic kidney disease, lower GFR is associated with lower protein intake, likely reflecting malnutrition in chronic disease. (18, 41)
The estimated protein intake of 43.9 +/− 15.8 g/d (or 0.7 mg/kg body weight/day) our study population is lower than reported estimates for the US population in NHANES (91 +/− 22 g/d in adults aged 19-30 years, and about 66 +/−17 g/d in the elderly; or approximately 0.9- 1.0 mg/kg/d in adults aged 50 and older). (42) Based on the relationship observed in the multivariable model (Table 3, column 5) a 30 g/d lower mean estimated protein intake is associated with GFR that is lower by approximately 6.6 ml/min/1.73m2.
The levels of GFR in our study were considerably higher than those reported in vegetarian potential kidney donors in India (81 ml/min/1.73 m2), especially accounting for the younger age of subjects recruited in those studies (about 8-15 years younger than in our study); the estimated age-corrected difference in GFR is about 25-30 ml/min/1.73 m2. (16) (20) This was true even for the normotensive, non-diabetic adults in our study whom the mean GFR level was 94.1 +/− 27.8 ml/min/1.73 m2. It is possible that variation in dietary protein intake and nutritional status may explain some of the marked differences in GFR levels among studies. (20) However, methodological differences in GFR measurement or the concomitant presence of underlying CKD in potential kidney donors is equally possible, as many donors are family members of patients with kidney disease, and are therefore at higher risk for pathological reduction in GFR. (20) (41)
Our findings have significant implications for clinical practice and public health policy globally. Accurate distinction between normal kidney function and kidney disease is important for early detection and treatment of kidney disease, as well as for appropriate selection of kidney donors. We found only modest differences in GFR levels in South Asians compared to previously reported direct GFR values in Caucasians, and do not suggest reduced kidney function in the vast majority of unselected South Asians in Karachi.
The main limitation of our study is that the sample size was limited for ascertainment of factors across the entire range of GFR (especially levels less than 60 ml/min/1.73 m2). While the food frequency questionnaire was modified version of Willet FFQ, it was not validated for dietary ascertainment in this population. (43) (44) Likewise, the Quadscan measurements of lean body mass and fat mass have not been validated in this population. However, the main aim of the study was to determine the normal level of GFR in South Asians from the general population in Karachi, which we were able to achieve. (13)
The major strengths include the use of inulin clearance which is the reference standard for GFR assessment, and the inclusion of unselected subjects from the general population, which enhances the validity and generalizability of our findings. Finally, it is also important to underscore that meat consumption was not very low in our study, which is consistent with the current dietary practices not just in Pakistan but in the majority of native and migrant South Asians worldwide. (27, 45) (19) Thus, our findings are applicable to the majority of South Asians globally.
In conclusion, our population based study of middle aged South Asian adults from the general population in Karachi, Pakistan, provides strong evidence that mean measured GFR level (94 ml/min/1.73 m2) is only modestly lower than levels reported in European-origin counterparts, possibly related to lower dietary protein intake. Our findings do not support the notion that the usual level of kidney function is low in people of South Asian descent.
Acknowledgements
Abstracts based on this work were presented at the 43rd Annual Renal Week during November 16-21, 2010 at Denver, Colorado.
We would like to thank all research staff for their assistance, and would like to acknowledge the cooperation of Mr Ibrahim Mustafa at the Aga Khan University Hospital for logistical assistance with the GFR clinic for research subjects, and Ms Lisa Thiabuddin for inulin assays at Renal Laboratories, Saint-Etienne Hospital, University of Jean Monnet, Saint-Etienne, France.
Support: The study was financially supported by a research award (1R03TW007588-01A1) from the NIH-Fogarty International Center (Principal Investigator, Dr Jafar; Co-Investigators, Drs Inker and Levey). The design, conduct, analysis, interpretation and presentation of the data were the responsibility of the authors, with no involvement from the funder.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
References
- 1.Eknoyan G, Lameire N, Barsoum R, et al. The burden of kidney disease: improving global outcomes. Kidney Int. 2004;66:1310–1314. doi: 10.1111/j.1523-1755.2004.00894.x. [DOI] [PubMed] [Google Scholar]
- 2.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 3.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prasad GV, Vangala SK, Silver SA, et al. South Asian Ethnicity as a Risk Factor for Major Adverse Cardiovascular Events after Renal Transplantation. Clin J Am Soc Nephrol. 2011 doi: 10.2215/CJN.03100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 6.Jafar TH. The growing burden of chronic kidney disease in Pakistan. N Engl J Med. 2006;354:995–997. doi: 10.1056/NEJMp058319. [DOI] [PubMed] [Google Scholar]
- 7.Hossain MP, Goyder EC, Rigby JE, El Nahas M. CKD and poverty: a growing global challenge. Am J Kidney Dis. 2009;53:166–174. doi: 10.1053/j.ajkd.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal SK, Srivastava RK. Chronic kidney disease in India: challenges and solutions. Nephron Clin Pract. 2009;111:c197–203. doi: 10.1159/000199460. [DOI] [PubMed] [Google Scholar]
- 9.Jafar TH, Qadri Z, Chaturvedi N. Coronary artery disease epidemic in Pakistan: more electrocardiographic evidence of ischaemia in women than in men. Heart. 2008;94:408–413. doi: 10.1136/hrt.2007.120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingelfinger JR. Weight for gestational age as a baseline predictor of kidney function in adulthood. Am J Kidney Dis. 2008;51:1–4. doi: 10.1053/j.ajkd.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Hall YN, Hsu CY, Iribarren C, Darbinian J, McCulloch CE, Go AS. The conundrum of increased burden of end-stage renal disease in Asians. Kidney Int. 2005;68:2310–2316. doi: 10.1111/j.1523-1755.2005.00691.x. [DOI] [PubMed] [Google Scholar]
- 12.Barbour SJ, Er L, Djurdjev O, Karim M, Levin A. Differences in progression of CKD and mortality amongst Caucasian, Oriental Asian and South Asian CKD patients. Nephrol Dial Transplant. 2010;25:3663–3672. doi: 10.1093/ndt/gfq189. [DOI] [PubMed] [Google Scholar]
- 13.Wesson L. Physiology of the human kidney. Grune & Stratton; New York: 1969. pp. 96–108. [Google Scholar]
- 14.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 15.Kaligotla VD, Chirravoori S, Tangirala M, Taduri G, Vishnubhatla S, R R Is There a Chronic Kidney Disease Epidemic? Profile of Chronic Kidney Disease in an Urban Renal Camp in Southern India. Hong Kong Journal of Nephrology. 2008;10:27–33. [Google Scholar]
- 16.Dharnidharka VR, Sortur AS, Kandoth P, Atiyel P, Dabbagh S. Effect of body size and malnutrition on renal size in childhood. Nephrology. 1998;4:361–365. [Google Scholar]
- 17.Barai S, Bandopadhayaya GP, Patel CD, et al. Do healthy potential kidney donors in india have an average glomerular filtration rate of 81.4 ml/min? Nephron Physiol. 2005;101:p21–26. doi: 10.1159/000086038. [DOI] [PubMed] [Google Scholar]
- 18.King AJ, Levey AS. Dietary protein and renal function. J Am Soc Nephrol. 1993;3:1723–1737. doi: 10.1681/ASN.V3111723. [DOI] [PubMed] [Google Scholar]
- 19.Ganguli D, Das N, Saha I, et al. Major dietary patterns and their associations with cardiovascular risk factors among women in West Bengal, India. Br J Nutr. 2011:1–11. doi: 10.1017/S0007114510005131. [DOI] [PubMed] [Google Scholar]
- 20.Barai S, Gambhir S, Prasad N, et al. Levels of GFR and protein-induced hyperfiltration in kidney donors: a single-center experience in India. Am J Kidney Dis. 2008;51:407–414. doi: 10.1053/j.ajkd.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Houtkooper LB, Lohman TG, Going SB, WH H. Why bioelectrical impedance analysis should be used for estimating adiposity. American Journal of Clinical Nutrition. 1996;64:436S–448S. doi: 10.1093/ajcn/64.3.436S. [DOI] [PubMed] [Google Scholar]
- 22.Segal KR, Van Loan M, Fitzgerald PI, Hodgdon JA, Van Itallie TB. Lean body mass estimation by bioelectrical impedance analysis: a four-site cross-validation study. Am J Clin Nutr. 1988;47:7–14. doi: 10.1093/ajcn/47.1.7. [DOI] [PubMed] [Google Scholar]
- 23.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 24.Mariat C, Alamartine E, Barthelemy JC, et al. Assessing renal graft function in clinical trials: can tests predicting glomerular filtration rate substitute for a reference method? Kidney Int. 2004;65:289–297. doi: 10.1111/j.1523-1755.2004.00350.x. [DOI] [PubMed] [Google Scholar]
- 25.Rolin HA, 3rd, Hall PM, Wei R. Inaccuracy of estimated creatinine clearance for prediction of iothalamate glomerular filtration rate. Am J Kidney Dis. 1984;4:48–54. doi: 10.1016/s0272-6386(84)80026-8. [DOI] [PubMed] [Google Scholar]
- 26.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 27.Jafar TH, Chaturvedi N, Pappas G. Prevalence of overweight and obesity and their association with hypertension and diabetes mellitus in an Indo-Asian population. Cmaj. 2006;175:1071–1077. doi: 10.1503/cmaj.060464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bingham SA. Urine nitrogen as a biomarker for the validation of dietary protein intake. J Nutr. 2003;133(Suppl 3):921S–924S. doi: 10.1093/jn/133.3.921S. [DOI] [PubMed] [Google Scholar]
- 29.Lin PH, Aickin M, Champagne C, et al. Food group sources of nutrients in the dietary patterns of the DASH-Sodium trial. J Am Diet Assoc. 2003;103:488–496. doi: 10.1053/jada.2003.50065. [DOI] [PubMed] [Google Scholar]
- 30.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 31.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 32.Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985;27:58–65. doi: 10.1038/ki.1985.10. [DOI] [PubMed] [Google Scholar]
- 33.Masud T, Manatunga A, Cotsonis G, Mitch WE. The precision of estimating protein intake of patients with chronic renal failure. Kidney Int. 2002;62:1750–1756. doi: 10.1046/j.1523-1755.2002.00606.x. [DOI] [PubMed] [Google Scholar]
- 34.Atherton JC, Hai MA, Thomas S. The time course of changes in renal tissue composition during mannitol diuresis in the rat. J Physiol. 1968;197:411–428. doi: 10.1113/jphysiol.1968.sp008567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warren SE, Blantz RC. Mannitol. Arch Intern Med. 1981;141:493–497. [PubMed] [Google Scholar]
- 36.Hallan S, Astor B, Lydersen S. Estimating glomerular filtration rate in the general population: the second Health Survey of Nord-Trondelag (HUNT II). Nephrol Dial Transplant. 2006;21:1525–1533. doi: 10.1093/ndt/gfl035. [DOI] [PubMed] [Google Scholar]
- 37.Berg UB. Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrol Dial Transplant. 2006;21:2577–2582. doi: 10.1093/ndt/gfl227. [DOI] [PubMed] [Google Scholar]
- 38.Poggio ED, Rule AD, Tanchanco R, et al. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int. 2009;75:1079–1087. doi: 10.1038/ki.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rule AD, Gussak HM, Pond GR, et al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43:112–119. doi: 10.1053/j.ajkd.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 40.Rafoth RJ, Onstad GR. Urea synthesis after oral protein ingestion in man. J Clin Invest. 1975;56:1170–1174. doi: 10.1172/JCI108193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kopple JD, Greene T, Chumlea WC, et al. Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney Int. 2000;57:1688–1703. doi: 10.1046/j.1523-1755.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 42.Fulgoni VL., 3rd Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003-2004. Am J Clin Nutr. 2008;87:1554S–1557S. doi: 10.1093/ajcn/87.5.1554S. [DOI] [PubMed] [Google Scholar]
- 43.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 44.Willett WC. Future directions in the development of food-frequency questionnaires. Am J Clin Nutr. 1994;59:171S–174S. doi: 10.1093/ajcn/59.1.171S. [DOI] [PubMed] [Google Scholar]
- 45.Radhika G, Sathya RM, Ganesan A, et al. Dietary profile of urban adult population in South India in the context of chronic disease epidemiology (CURES - 68). Public Health Nutr. 2010:1–8. doi: 10.1017/S136898001000203X. [DOI] [PubMed] [Google Scholar]