Abstract
Thymocyte development requires the coordinated input of signals that originate from numerous cell surface molecules. Although the majority of thymocyte signal initiating receptors are lineage-specific, most trigger ‘ubiquitous’ downstream signaling pathways. T-lineage specific receptors are coupled to these signaling pathways by lymphocyte-restricted adapter molecules. We and others recently identified a new putative adapter protein, Themis1, whose expression is largely restricted to the T lineage. Mice lacking Themis1 exhibit a severe block in thymocyte development and a striking paucity of mature T cells revealing a critical role for Themis1 in T cell maturation. Themis1 orthologs contain three conserved domains: a proline rich region (PRR) that binds to the ubiquitous cytosolic adapter Grb2, a nuclear localization sequence (NLS), and two copies of a novel cysteine-containing globular (CABIT) domain. In the present study, we evaluated the functional importance of each of these motifs by retroviral reconstitution of Themis1−/− progenitor cells. The results demonstrate an essential requirement for the PRR and NLS motifs but not the conserved CABIT cysteines for Themis1 function.
Introduction
Themis1 is the founding member of a new gene family with orthologs in almost all animal species. In mammals, three related family members, Themis1, Themis2 and Themis3 have been detected that exhibit highly tissue-specific expression in the T-cell (Themis1), B-cell, myeloid and dendritic cell (Themis2) and intestinal epithelial cell (Themis3) lineages 1. Analysis of induced loss of function Themis1 mutants by several independent groups has revealed a critical function for Themis1 in later stages of T cell development including positive selection and the generation of mature T cells 1–5. Specifically, Themis1−/− mice show a severe block at the CD4+CD8+ (Double positive, DP) to CD4+CD8− (CD4-Single Positive, CD4-SP) or CD4−CD8+ (CD8-Single Positive, CD8-SP) stages that results in a marked reduction in numbers of CD4-SP and CD8-SP thymocytes and T cells 1–5. To date, only Themis1 has been inactivated in the mouse germline and it therefore remains unknown if Themis2 and Themis3 have similarly important roles in the cell lineages in which they are expressed.
Themis family members do not contain any known catalytic domains. However, sequence alignment of Themis1 and Themis2 identifies three highly conserved regions of potential functional relevance: 1) a carboxy-terminal proline-rich region (PRR) (PPPRPPKxP) that matches a Class II SH3 recognition motif (PxxPx+), 2) a bipartite nuclear localization sequence (NLS) (PKR-X12-KRRPR), and 3) two copies of a newly described Cysteine-containing All-Beta in Themis (CABIT) domain (φXCX7-26φXLPφX3GXF with X = any amino acid and φ = any hydrophobic residue) 1, 2. Outside of these three domains Themis1 and Themis2 are poorly conserved 1. Compelling evidence that one or all of the conserved regions are important for function is the recent finding that a Themis2 transgene is capable of restoring normal T cell development in mice lacking Themis1 6.
In this study, we investigated the importance of each of the three conserved domains for Themis1 function by generating retroviral constructs that encode Themis1 proteins in which one domain has been mutated and testing the ability of these constructs to rescue T cell development in Themis1−/− thymocytes. Our results demonstrate that whereas retention of key cysteine residues within the CABIT domain are not required for function, the PRR and NLS motifs are each essential for Themis1 activity. Our data also suggest that interaction of Themis1 with the adapter Grb2, and possibly Themis1-mediated nuclear localization of Grb2, may be critical for Themis1 function and for normal T cell maturation.
Results
Generation and in vitro characterization of Themis1 mutants
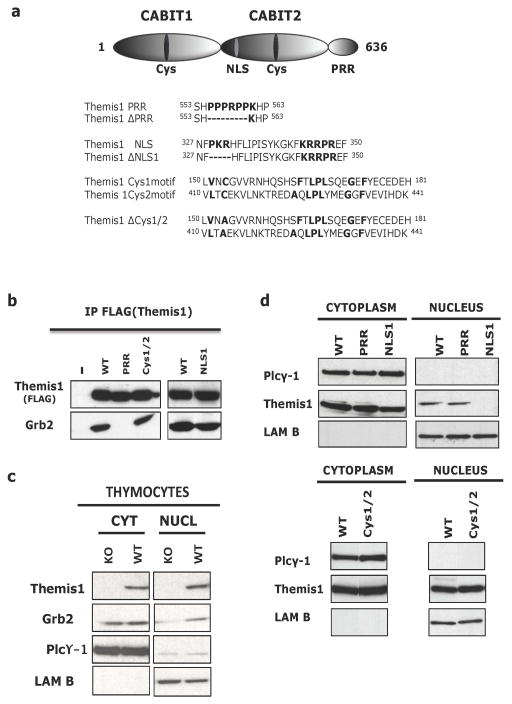
Vertebrate Themis1 orthologs contain three highly conserved motifs that represent potential functional domains: 1) a carboxy-terminal proline-rich region (PRR), 2) a bipartite nuclear localization sequence (NLS), and 3) two CABIT domains 1, 2. To investigate the importance of each of these motifs for Themis1 function, we generated FLAG-epitope tagged Themis1 cDNAs encoding either wild-type Themis1 or proteins in which one of the conserved domains of Themis1 has been mutated. Mutant versions of Themis1 included Themis-ΔPRR in which the PPPRPP amino acids of the PRR were deleted, Themis-ΔNLS1 in which the PKR amino acids of the N-terminal domain of the bipartite NLS were deleted, and Themis-ΔCys153/413 (Themis-ΔCys) in which both of the conserved CABIT domain cysteine residues was changed to alanine (Fig. 1a).
Figure 1. Generation and characterization of Themis1 mutants lacking individual conserved domains.
a. Top, Schematic of Themis1 protein structure showing the location of the two novel globular CABIT domains (CABIT-1 and CABIT-2) each containing a conserved cysteine (Cys) residue, the bipartite nuclear localization signal (NLS), and the proline-rich region (PRR). Bottom, amino-acid sequences of relevant regions that are mutated or deleted in the three Themis1 variants used in this study (ΔPRR, ΔNLS and ΔCys). b. Association of Themis1 and Themis1 mutants with the adaptor Grb2. Flag epitope tagged wild-type (WT) Themis1 or Themis1 mutant proteins were overexpressed in HEK-293T cells. Themis1 proteins were immunoprecipitated with anti-FLAG-Sepharose beads, resolved by SDS-PAGE and blotted for Grb2. c. Subcellular localization of Grb2 in Themis1−/− and Themis1+/+ thymocytes. Cytoplasmic and nuclear fractions of thymocytes were immunobloted for Themis1, PLCγ-1(cytoplasmic control) and Lamin B (nuclear control). d. Subcellular localization of Themis1. Cytoplasmic and nuclear fractions of HEK-293T cells overexpressing WT or mutant Themis1 proteins were immunobloted for Themis1, PLCγ-1(cytoplasmic control) and Lamin B (nuclear control).
Transfection of the HEK 293T cell line with plasmids encoding wild-type or mutant Themis1 proteins confirmed expression of FLAG epitope tagged proteins of the predicted size (Fig. 1b). We and others have shown that, in thymocytes, Themis1 binds constitutively to the ubiquitous adapter protein Grb2 1–3, 7. This association is mediated by the Themis1 PRR which can bind to either the N-terminal SH3 domain or the C-terminal SH3 domain of Grb2. As expected, deletion of the Themis1 PRR motif resulted in complete loss of Grb2 binding potential (Fig. 1b). In contrast, mutation of the conserved cysteines within the two CABIT domains did not affect Themis1:Grb2 association. Grb2 association with Themis-ΔNLS1 was reduced in comparison to wild-type Themis1 but mutation of the NLS did not abrogate Grb2 binding (Fig. 1b and data not shown).
We also previously demonstrated that Themis1 protein is detectable in both the nucleus and cytoplasm in thymocytes 1, 6. In addition, we found that nuclear Grb2 is markedly reduced in Themis1−/− thymocytes suggesting that Themis1 may be important for nuclear translocation and/or retention of Grb2 in thymocytes (Fig. 1c). Consequently, we evaluated the importance of each of the three conserved domains for nuclear localization of Themis1. Themis-ΔNLS1 was undetectable in the nuclear subcellular fraction indicating that an intact bipartite NLS sequence is necessary for Themis1 nuclear translocation and/or retention (Fig. 1d). In contrast, the relative cytoplasmic/nuclear distribution of Themis1 protein was similar in wild-type Themis1, Themis-ΔPRR and Themis-ΔCys transfected cells indicating that the conserved CABIT cysteines and PRR sequence are not essential for Themis1 nuclear localization/retention (Fig. 1d). Moreover, the observation that nuclear localization of Themis-ΔPRR is unimpaired demonstrates that Themis1 nuclear transport and/or retention is not dependent upon Grb2 association.
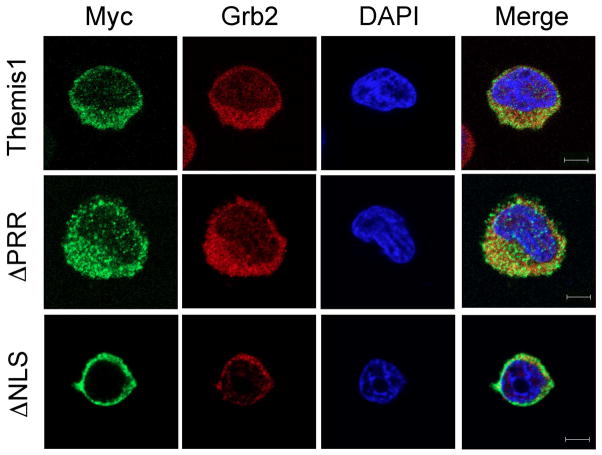
To directly visualize Themis1 localization in T cells, we next transfected the human Jurkat T cell line with Myc epitope tagged wild-type, ΔPRR, or ΔNLS1 versions of Themis1. Examination of Themis1 localization in transfected Jurkat cells confirmed a requirement for the NLS domain, but not the PRR domain, for nuclear localization of Themis1 (Fig. 2). We also examined cellular localization of endogenous Grb2 in transfected cells using anti-Grb2 antibody. Overall, there was no significant effect on the cellular distribution of Grb2 even in cells expressing Themis-ΔNLS1 (Fig. 2 and data not shown). However, it is important to note that these cells also contain endogenous wild-type Themis1 which is capable of binding to Grb2.
Figure 2.
Immunostaining of Themis mutants. Jurkat E6.1 cells transiently expressing myc-tagged Themis constructs were plated onto stimulatory coverslips, fixed after incubation at 37°C for 3 min and immunostained with anti-myc (green) and anti-Grb2 (red) antibodies followed by staining with DAPI (blue). A representative confocal slice is shown. Upper panels: Wild-type Themis1; Middle panels: Themis1-ΔPRR mutant; Lower panels: Themis1-ΔNLS mutant. Bar= 10 μm.
In vivo testing of Themis1 mutants by retroviral genetic reconstitution
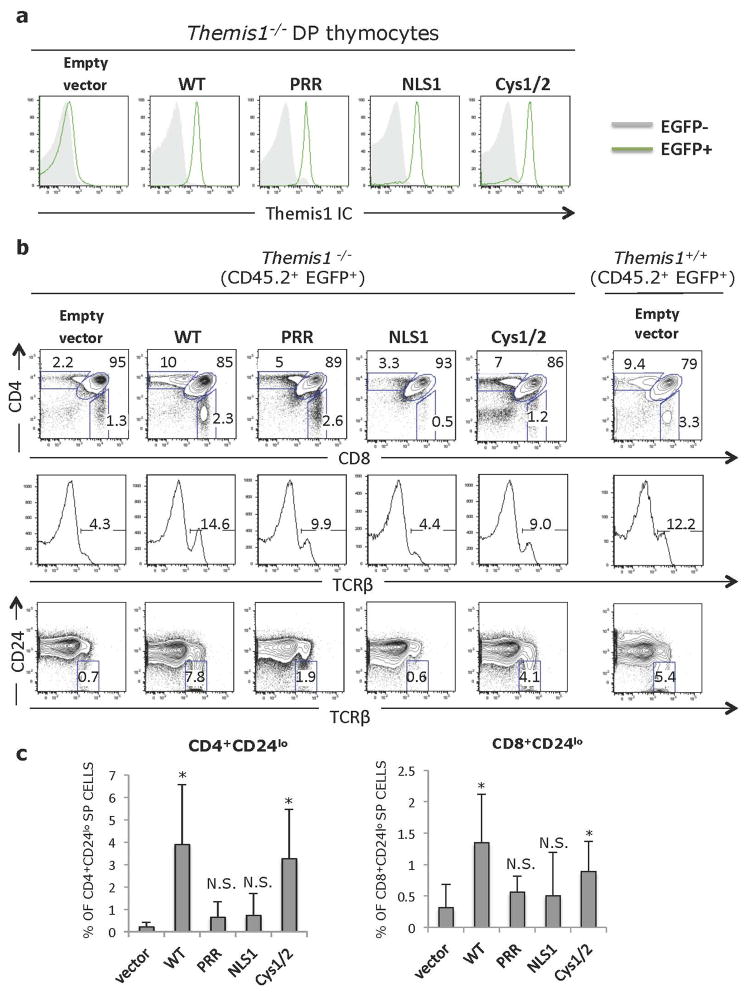
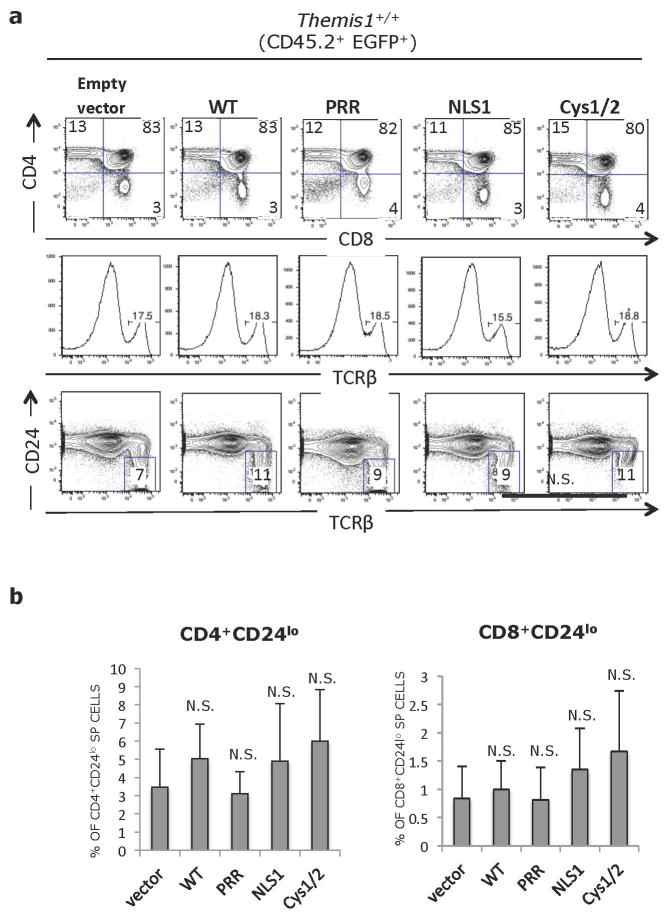
To evaluate the importance of each of the Themis1 conserved domains for in vivo function, we generated retroviral constructs that encoded wild-type Themis1, Themis-ΔPRR, Themis-ΔNLS1 or Themis-ΔCys. Lineage-negative bone marrow cells from C57BL/6 (CD45.2+) Themis−/− mice were infected with retrovirus then injected intravenously into lethally irradiated congenic CD45.1+ C57BL/6 mice. Eight weeks later, recipient mice were sacrificed and thymocytes and T cells from peripheral lymphoid organs were examined by flow cytometry. Since the retroviral vectors contained tandem Themis1-IRES-EGFP cassettes, expression of retroviral-encoded genes could be identified by gating on CD45.2+ EGFP+ cells. Intracellular staining of CD45.2+ EGFP+ thymocytes from bone marrow chimeric mice with antibody against Themis1 demonstrated similar expression of wild-type Themis1, Themis-ΔPRR, Themis-ΔNLS1 or Themis-ΔCys retroviral infected cells (Fig. 3a). Having established equivalent expression of the retroviral-encoded Themis1 proteins, we next validated the experimental system by comparing mice reconstituted with Themis1−/− bone marrow cells that had been infected with either empty retrovirus (Empty) or retrovirus encoding wild-type (WT) Themis1. As expected, the phenotype of thymocytes derived from Empty retrovirus infected Themis1−/− bone marrow cells closely resembled that of Themis1−/− mice exhibiting a profound block at the DP to CD4-SP or CD8-SP stages (Fig. 3b). Moreover, the percentage of TCRbhi thymocytes, which include both late-stage positively selected DP thymocytes and SP thymocytes, as well as CD24lo thymocytes which are composed primarily of mature CD4-SP and CD8-SP thymocytes, was strongly reduced in thymocytes derived from Empty retrovirus infected Themis1−/− progenitors (Fig. 3b). Also similar to Themis1−/− mice, the percentage of peripheral lymph node CD45.2+ EGFP+ CD4-SP and CD8-SP T cells was dramatically reduced in these mice (Fig. 4a, b). In contrast, based on the same criteria, the phenotype of thymocytes and peripheral T cells derived from wild-type Themis1 retrovirus infected Themis1−/− bone marrow cells exhibited an essentially normal phenotype (as compared to Themis1+/+ mice) indicating that genetic reconstitution of WT Themis1 can completely ‘rescue’ the developmental defects in Themis1−/− mice (Figs. 3&4).
Figure 3. Importance of conserved Themis1 protein domains in thymocyte development.
Lethally irradiated C57BL6/J (CD45.1+) mice were reconstituted with lineage depleted Themis1−/− bone marrow cells (CD45.2+) that had been infected with a bicistronic EGFP expressing retroviral vector (Empty vector) or the same vector encoding either wild type Themis1 cDNA (WT) or mutant Themis1 proteins: Themis-ΔPRR (PRR), Themis-ΔNLS (NLS) or Themis-ΔCys (Cys1/2). Eight weeks post-reconstitution, bone marrow chimeras sacrificed and thymocytes and splenocytes were analysed by flow cytometry. a. Intracellular staining for Themis1 in CD45.2+ EGFP+ CD4+CD8+ (DP) thymocytes from bone marrow chimeric mice. Grey histograms represent Themis1 expression in EGFP− DP thymocytes. Green histograms represent expression of Themis1 in EGFP+ DP thymocytes, expressing the indicated Themis1 proteins. b. Representative flow cytometry analysis of CD45.2+ EGFP+ thymocytes from bone marrow chimeras. Upper plots show CD4 versus CD8 profiles, middle histograms show percent of TCRβ+ cells. Bottom plots show percent of mature CD45.2+ EGFP+ TCRβhiCD24lo thymocytes. Plots in right column show Themis1+/+ thymocytes infected with empty retroviral vector for reference. c. Percentage of mature (TCRβhiCD24lo) CD45.2+ EGFP+ CD4-SP and CD8-SP thymocytes in the indicated bone marrow chimeras (n=6 each). *p ≤0.05, N.S., not significant (Two-tailed T-test, unequal variance) All comparisons are to vector only.
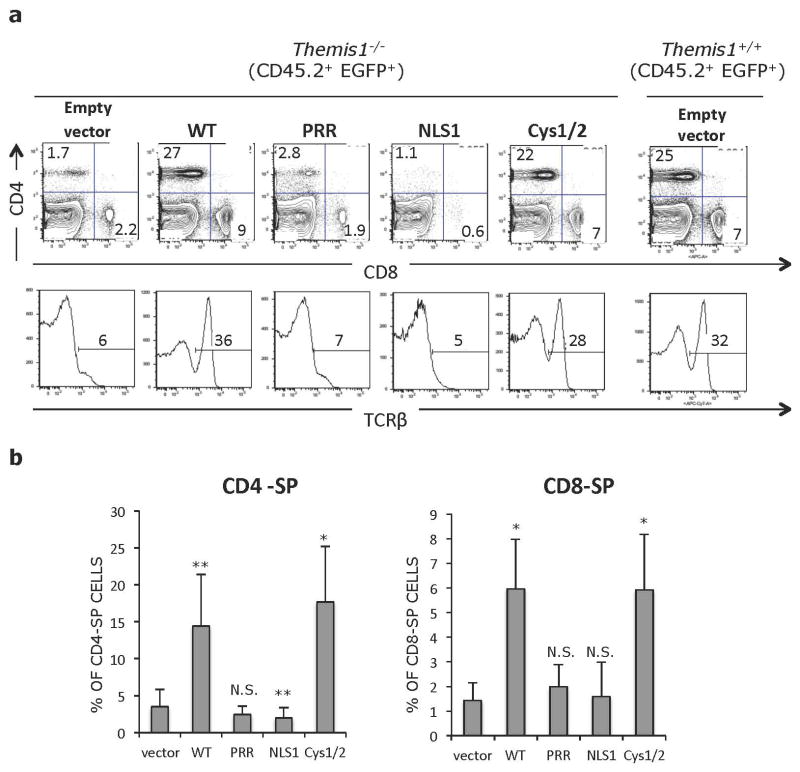
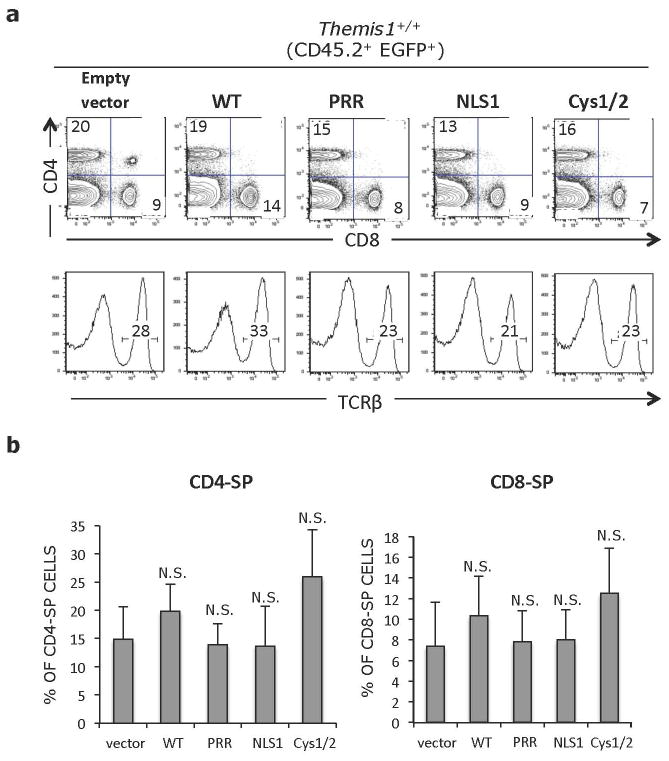
Figure 4.
a. Representative flow cytometry analysis of CD45.2+ EGFP+ splenocytes from bone marrow chimeras shown in Figure 2. Upper plots show CD4 versus CD8 profiles, lower histograms show percent of TCRβ+ T cells within the CD45.2+ EGFP+ population. Plots in right column show Themis1+/+ splenocytes infected with empty retroviral vector for reference. b. Percentage of CD45.2+ EGFP+ CD4-SP and CD8-SP splenocytes in the indicated bone marrow chimeras (n=6 each). *p ≤0.05, **p ≤0.01, N.S., not significant (Two-tailed T-test, unequal variance). All comparisons are to vector only.
Themis-ΔCys was capable of restoring thymocyte development when expressed in Themis1−/− progenitor cells. The percentage of EGFP+ CD4-SP and CD8-SP thymocytes, and more importantly, mature CD24lo CD4-SP and CD8-SP thymocytes was significantly increased relative to mice reconstituted with Empty vector infected bone marrow cells and relative to non-retroviral infected host Themis1−/− thymocytes, though the extent of rescue appeared to be slightly less than observed with WT Themis1 (Fig. 3b, c). However, the percentage of CD45.2+ EGFP+ CD4-SP and CD8-SP peripheral T cells was similar to that observed in mice where Themis1−/− progenitors were infected with WT Themis1 (Fig. 4a, b). In contrast to Themis-ΔCys, Themis-ΔPRR and Themis-ΔNLS1 failed to rescue thymocyte development when introduced into Themis1−/− progenitors. Neither protein was capable of promoting the maturation of CD4-SP or CD8-SP thymocytes (Fig. 3b, c) and the percentage of peripheral EGFP+ T cells was not significantly different than in mice that had been reconstituted with Empty retrovirus (Fig. 4a, b).
To determine if Themis-ΔPRR, Themis-ΔNLS1 or Themis-ΔCys effect thymocyte development in the presence of wild-type Themis1, we infected bone marrow progenitors from wild-type (B6-CD45.1) mice with retrovirus encoding wild-type Themis1 or the three Themis1 mutants. Interestingly, expression of either WT Themis1 or Themis-ΔCys resulted in a consistent though not statistically significant increase in CD4-SP and CD8-SP T thymocytes and peripheral CD4-SP and CD8-SP T cells (Figs. 5&6). These results are consistent with data obtained with Themis1 transgenic mice (data not shown) and suggest that T cell maturation can be enhanced by augmentation of Themis1 expression by either WT Themis1 or Themis-ΔCys. Expression of Themis-ΔPRR or Themis-ΔNLS1 in wild-type bone marrow progenitors did not appreciably enhance or diminish T cell maturation indicating that neither protein exerted dominant-negative effect on thymocyte development in the presence of WT Themis1 (Figs. 5&6).
Figure 5.
Effect of Themis1 or Themis1 mutant protein expression on Themis+/+ thymocyte development. Lethally irradiated C57BL6/J (CD45.1+) mice were reconstituted with lineage depleted Themis1+/+ bone marrow cells (CD45.2+) that had been infected with a bicistronic EGFP expressing retroviral vector (Empty vector) or the same vector encoding either wild type Themis1 cDNA (WT) or mutant Themis1 proteins: Themis-ΔPRR (PRR), Themis-ΔNLS (NLS) or Themis-ΔCys (Cys1/2). Eight weeks post-reconstitution, bone marrow chimeras sacrificed and thymocytes and splenocytes were analysed by flow cytometry. a. Intracellular staining for Themis1 in CD45.2+ EGFP+ CD4+CD8+ (DP) thymocytes from bone marrow chimeric mice. Grey histograms represent Themis1 expression in EGFP− DP thymocytes. Green histograms represent expression of Themis1 in EGFP+ DP thymocytes, expressing the indicated Themis1 proteins. b. Representative flow cytometry analysis of CD45.2+ EGFP+ thymocytes from bone marrow chimeras. Upper plots show CD4 versus CD8 profiles, middle histograms show percent of TCRβ+ cells. Bottom plots show percent of mature CD45.2+ EGFP+ TCRβhiCD24lo thymocytes. Plots in right column show Themis1+/+ thymocytes infected with empty retroviral vector for reference. c. Percentage of mature (TCRβhiCD24lo) CD45.2+ EGFP+ CD4-SP and CD8-SP thymocytes in the indicated bone marrow chimeras (n=6 each). N.S., not significant (Two-tailed T-test, unequal variance). All comparisons are to vector only.
Figure 6.
a. Representative flow cytometry analysis of CD45.2+ EGFP+ splenocytes from bone marrow chimeras shown in Supplemental Figure 1. Upper plots show CD4 versus CD8 profiles, lower histograms show percent of TCRβ+ T cells within the CD45.2+ EGFP+ population. Plots in right column show Themis1+/+ splenocytes infected with empty retroviral vector for reference. b. Percentage of CD45.2+ EGFP+ CD4-SP and CD8-SP splenocytes in the indicated bone marrow chimeras (n=6 each). N.S., not significant (Two-tailed T-test, unequal variance). All comparisons are to vector only.
Discussion
The current results identify a critical role for the PRR and NLS domains of Themis1 for its function in regulating T cell maturation. The observation that the PRR domain, which mediates Grb2 binding, is required for Themis1 activity is consistent with recently published data demonstrating that Grb2 association is important for recruitment of Themis1 to the scaffolding adapter LAT in thymocytes 7. The PRR domain may also be required for the interaction of Themis1 with other cytoplasmic effector proteins such as the tyrosine phosphatase SHP-1 that are potential targets of Themis1 regulatory activity 8.
Notably, our results demonstrate a role for the NLS, and by extension, nuclear localization, for Themis1 activity. Previous studies have shown that Grb2 can be detected in the nucleus as well as in the cytoplasm 9–11. In conjunction with our finding that nuclear Grb2 is reduced in Themis1−/− thymocytes, this raises the possibility that an important function of Themis1 may be to regulate transport of Grb2, and perhaps other Grb2 associated proteins such as the guanine nucleotide exchange factor Vav112 to the nucleus. We did not observe a defect in T cell development in mice overexpressing Themis1-ΔNLS1. The absence of a discernable dominant-negative effect of Themis1-ΔNLS1 suggests that either Grb2 nuclear localization may not be essential for thymocyte maturation or that overexpression of Themis1-ΔNLS1 may not affect Grb2 nuclear translocation in thymocytes that also express wild-type Themis1.
Based on the high degree of conservation of the CABIT domain, which extends from mammals to cnidarians, it was proposed that the CABIT cysteine residues may be important for catalytic activity, possibly involving ubiquitination or thiol redox reactions 2. Our finding that the conserved cysteine residues are not essential for Themis1 activity argues against such a role, or alternatively, that the function performed by the CABIT domains is not essential for T cell development. It is also possible that the conserved cysteine residues are not absolutely required for CABIT domain activity. Further in depth analysis of the PRR, NLS and CABIT domains, including complementation studies, should provide valuable additional insight into Themis1 function and its precise role in T cell signaling and thymocyte maturation.
Methods
Mice
Themis1 −/− mice have been described previously 1. CD45.1 (B6.SJL-Ptprca Pepcb/BoyJ) mice were obtained from Jackson laboratory. Animal experiments were approved by the Animal Care and Use Committee of the National Institute of Child Health and Human Development, NIH.
Cells and Plamids
293T cell lines were maintained in DMEM medium supplemented with 10% FCS, penicillin, streptomycin and L-glutamine. Wild type (E6.1) Jurkat T cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum and antibiotics. Transient transfections were performed using the Amaxa electroporation system kit T (Amaxa Biosystems, Gaithersburg MD). For Myc-tagged Themis1 constructs, cDNA of Themis1 was amplified from thymocytes and cloned into pcDNA3.1 (Life Technologies). This plasmid was used as a template to delete the PRR region of Themis1 (RxPXXP), the N-terminal (PKR) part of the nuclear localization sequence (NLS), or to generate Cys to Ala amino acid mutations in the CABIT1 and CABIT2 domains of Themis1. Mutagenesis reactions were performed using the GeneTailer mutagenesis kit from Invitrogen. Mutations were verified by sequencing. pMYs-IresEGFP retroviral plasmids (Cell Biolabs) were used for expression of wild type or mutant Themis1 proteins in vivo.
Retroviral transduction of Bone Marrow
Themis1−/− or Themis1+/+ mice were treated with 5-fluorouracil (3mg/mice) for 5 days. Bone marrow cells from treated mice were incubated with SCF (100 ng/ml), and TPO (50ng/ml) in IMDM medium supplemented with 15% FBS, Pen/Strep/Glut, 2-ME, and nonessential amino acids in 24-well plates at a concentration of 2×106 cells/well. 24 hours later cells were placed on retronectin (Takara Bio) pre-coated 24 well plates with viral supernatant containing Polybrene (8ug/ml; Sigma) and centrifuged at RT for 1 hour at 1000g. Cells were washed once with IMDM media and placed in the culture with cytokines. A second round of infection was performed one day after. 3–5×105 cells were intravenously injected into lethally (950rad) irradiated CD45.1 recipients. For virus generation, retroviral vectors (i.e., empty vector, WT-Themis1 vector, or mutated Themis1 vector) were transfected into Plat-E packaging cells using polyjet reagent (Signagen). Viral supernatants were collected 48 hours after transfection and stored at +4C. The infection efficiency of bone marrow cells was determined by analyzing the percentage of GFP+ cells by flow cytometry.
Antibodies and reagents
Sources for antibodies and reagents used in this study include: anti-laminB (M-20), anti-PLCγ (SC-81) obtained from Santa Cruz Biotechnology, and anti-Grb2 obtained from BD Pharmingen; Anti-Themis1 rabbit antibodies were previously described 1.
Subcellular fractionation
2×106 293T cells were incubated in 120 μl of hypotonic buffer [Hepes 100 mM, KCl 10 mM, EDTA 1 mM, Na3VO4 2 mM, protease inhibitor tablet (Roche)] for 20 min on ice. After incubation, 1.2 μl of 10% NP40 was added. Lysates were mixed vigorously and centrifuged at 3000 r.p.m. for 5 min. Supernatants, which contained plasma membrane and cyotosol, were collected. Pellets were washed with hypotonic buffer and incubated with 50 μl of nuclear lysis buffer (Tris-HCL 100mM, NaCl 300 mM, EDTA 1 mM, Na3VO4 2 mM, NP-40 1%, SDS 0.1%, protease inhibitor tablet) for 10 min on ice. Lysates were centrifuged at 14000 r.p.m., for 10 min. Supernatants, which contain nuclear extract, were collected.
Intracellular staining and Flow cytometry
Thymocytes and spleen cells (1.6×108/ml) were surface stained with the following antibodies: CD4(RM4.5), CD8 (53-6.7), CD69 (H1.2F3), CD24 (30-F1), CD45.2 (104), CD5 (53-7.3), TCRβ (H57) obtained from eBioscience. For intracellular staining, cells were fixed in 2% PFA, permeabilized in 0.75% Triton-PBS buffer and stained using rabbit anti-Themis1 followed by goat anti-rabbit Alexa647 antibody (Invitrogen). Acquisition was performed on a BD Biosciences LSR II and analysis was performed with FACS Diva software.
In vitro binding experiments and Immunoprecipitations
2×106 293T cells were lysed in Lysing buffer (Tris-HCL 100mM, NaCl 150 mM, EDTA 1 mM, Na3VO4 2 mM, NP-40 1%, protease inhibitors tablet). Lysates were pre-cleared by centrifugation (10 min 14000 rpm at +4C) and incubated for 2 hours at 4 °C with anti-Flag (M2 clone) beads from Sigma. Beads were washed and proteins were resuspended in NuPage LDS sample buffer (Invitrogen). Proteins were resolved by SDS-PAGE and transferred to Immobilon-P membranes (Millipore).
Fixation and immunostaining
Cells were allowed to spread on coverslips as described 13. Briefly, poly-lysine covered four-chambered glass coverslips (LabTek II, Nunc/Nalgene) were coated with10 μg/ml of antibody (anti-CD3 HIT3a, or anti-CD45). The chambers were loaded with 300 μl of normal media without phenol red supplemented with 25 mM HEPES, pH 7.0, and warmed. Cells were resuspended in the same buffer, plated into the bottom of the chamber and incubated at 37°C. After 3 min, cells were fixed in 2.4% paraformaldehyde for 30 min. The cells were permeabilized with TritonX-100, incubated with blocking buffer for 30 min and then incubated with primary antibodies for 60 min, followed by washes and 60 min incubation with Alexa conjugated secondary antibodies.
Imaging
Images from fixed cells were collected with a Zeiss 510 LSCM, using a 63X, 1.4 NA objective (Carl Zeiss Inc, Thornton NY). Z stacks of complete cells were taken.
Image processing
Zeiss AIM software was used to produce images. Adobe PhotoShop and Illustrator (Adobe Systems Inc, San Jose CA) were used to prepare composite figures. Scale bars were cut from the original images and then were pasted in a more visible position on the final composite image.
Acknowledgments
We thank Jan Lee and Dalal El-Khoury for technical assistance. This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver, NICHD (PEL: Project number: 1ZIAHD001803-19).
References
- 1.Lesourne R, Uehara S, Lee J, Song KD, Li L, Pinkhasov J, et al. Themis, a T cell-specific protein important for late thymocyte development. Nature immunology. 2009;10(8):840–7. doi: 10.1038/ni.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson AL, Aravind L, Shulzhenko N, Morgun A, Choi SY, Crockford TL, et al. Themis is a member of a new metazoan gene family and is required for the completion of thymocyte positive selection. Nature immunology. 2009;10(8):831–9. doi: 10.1038/ni.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patrick MS, Oda H, Hayakawa K, Sato Y, Eshima K, Kirikae T, et al. Gasp, a Grb2-associating protein, is critical for positive selection of thymocytes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(38):16345–50. doi: 10.1073/pnas.0908593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu G, Vallee S, Rybakin V, McGuire MV, Ampudia J, Brockmeyer C, et al. Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nature immunology. 2009;10(8):848–56. doi: 10.1038/ni.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kakugawa K, Yasuda T, Miura I, Kobayashi A, Fukiage H, Satoh R, et al. A novel gene essential for the development of single positive thymocytes. Molecular and cellular biology. 2009;29(18):5128–35. doi: 10.1128/MCB.00793-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesourne R, Zvezdova E, Song KD, El-Khoury D, Uehara S, Barr VA, et al. Interchangeability of Themis1 and Themis2 in thymocyte development reveals two related proteins with conserved molecular function. J Immunol. 2012;189(3):1154–61. doi: 10.4049/jimmunol.1200123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paster W, Brockmeyer C, Fu G, Simister PC, de Wet B, Martinez-Riano A, et al. GRB2-mediated recruitment of THEMIS to LAT is essential for thymocyte development. J Immunol. 2013;190(7):3749–56. doi: 10.4049/jimmunol.1203389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu G, Casas J, Rigaud S, Rybakin V, Lambolez F, Brzostek J, et al. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature. 2013;504(7480):441–5. doi: 10.1038/nature12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verbeek BS, Adriaansen-Slot SS, Rijksen G, Vroom TM. Grb2 overexpression in nuclei and cytoplasm of human breast cells: a histochemical and biochemical study of normal and neoplastic mammary tissue specimens. The Journal of pathology. 1997;183(2):195–203. doi: 10.1002/(SICI)1096-9896(199710)183:2<195::AID-PATH901>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 10.Sorkin A, McClure M, Huang F, Carter R. Interaction of EGF receptor and grb2 in living cells visualized by fluorescence resonance energy transfer (FRET) microscopy. Current biology: CB. 2000;10(21):1395–8. doi: 10.1016/s0960-9822(00)00785-5. [DOI] [PubMed] [Google Scholar]
- 11.Romero F, Ramos-Morales F, Dominguez A, Rios RM, Schweighoffer F, Tocque B, et al. Grb2 and its apoptotic isoform Grb3-3 associate with heterogeneous nuclear ribonucleoprotein C, and these interactions are modulated by poly(U) RNA. The Journal of biological chemistry. 1998;273(13):7776–81. doi: 10.1074/jbc.273.13.7776. [DOI] [PubMed] [Google Scholar]
- 12.Blanchet F, Cardona A, Letimier FA, Hershfield MS, Acuto O. CD28 costimulatory signal induces protein arginine methylation in T cells. The Journal of experimental medicine. 2005;202(3):371–7. doi: 10.1084/jem.20050176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunnell SC, Barr VA, Fuller CL, Samelson LE. High-resolution multicolor imaging of dynamic signaling complexes in T cells stimulated by planar substrates. Science STKE. 2003;177:PL8. doi: 10.1126/stke.2003.177.pl8. [DOI] [PubMed] [Google Scholar]