Abstract
Accumulating evidence suggests that mental simulation of the future and past relies on common processes supported by the hippocampus. However, it is currently unknown whether the hippocampus also supports the ability to share these mental simulations with others. Recently, it has been proposed that language and language-related structures in the brain are particularly important for communicating information not tied to the immediate environment, and indeed specifically evolved so that humans could share their mental time travels into the future and the past with others. The current study investigated whether processes supported by the hippocampus are necessary for effectively communicating the contents of one's mental simulations by examining the discourse of amnesic patients with medial temporal lobe damage. In Experiment 1 we tested whether patients can produce integrated discourse about future and past events by measuring lower-level discourse cohesion and higher-level discourse coherence. Striking reductions in both measures were observed in amnesic patients’ narratives about novel future events and experienced past events. To investigate whether these deficits simply reflected concurrent reductions in narrative content, in Experiment 2 we examined the status of discourse integration in patients’ verbal narratives about pictures, which contained an equivalent amount of narrative content as controls’. Discourse cohesion and coherence deficits were also present when patients generated narratives based on pictures, and these deficits did not depend on the presence of neural damage outside the hippocampus. Together, these results reveal a pervasive linguistic integration deficit in amnesia that is not limited to discourse about the past or the future and is not simply secondary to reductions in narrative content. More broadly, this study demonstrates that the hippocampus supports the integration of individual narrative elements into coherent and cohesive discourse when constructing complex verbal accounts, and plays a critical role in the effective communication of information to others.
Keywords: amnesia, hippocampus, binding, language, memory
1. Introduction
The ability to mentally project into the future and past supports a range of adaptive behaviors and allows us to build predictions and plans for the future based on prior experience. Recent evidence suggests that mental simulation of the future is compromised in medial temporal lobe amnesia. Specifically, amnesic patients with adult-onset hippocampal damage have difficulty not only projecting back in time to mentally simulate the past (retrospection), but also projecting forward in time to mentally simulate novel and specific future scenarios (prospection) (Andelman, Hoofien, Goldberg, Aizenstein, & Neufeld, 2010; Hassabis, Kumaran, Vann, & Maguire, 2007; Klein, Loftus, & Kihlstrom, 2002; Race, Keane, & Verfaellie, 2011, 2013; Tulving, 1985). Interestingly, patients’ impairments in retrospection and prospection are strongly positively correlated (Race et al., 2011), suggesting that common hippocampal mechanisms support both functions. Candidate hippocampal mechanisms include the retrieval and recombination of mnemonic details and the integration of these details into coherent mental representations (Addis & Schacter, 2011; Hassabis & Maguire, 2007; Schacter & Addis, 2009).
While mental simulation of the future and past has been closely linked to hippocampal function, it is currently unknown whether the hippocampus also supports the communication of these mental simulations. The ability to effectively communicate one's mental simulations of the future and past confers important adaptive advantages, enabling experiences, plans, and ideas to be shared so that others may benefit (Corballis, 2009, 2013). Recently, it has been proposed that language and language-related structures in the brain are particularly important for communicating information not tied to the immediate environment, and indeed evolved so that humans could share their mental time travels into the future and the past with others (Corballis, 2009, 2013; Gardenfors, 2004; Suddendorf, Addis, & Corballis, 2009). Specifically, Corballis (2009) has argued that events in the present are shared by mutual experience and can be communicated through simple signals that direct attention or convey the importance of visible referents. In contrast, conveying information about the past and future requires symbolic linguistic elements and the combination of these elements into integrated discourse units that can be easily understood (Corballis, 2009). The link between language and mental simulation, and their co-evolution in humans, has been related to the development of brain regions such as the hippocampus that allow events to be situated in different points in time (Suddendorf et al., 2009). However, many aspects of language production are intact following hippocampal damage (Kensinger, Ullman, & Corkin, 2001; Milner, Corkin, & Teuber, 1968; Race et al., 2011; Skotko, Andrews, & Einstein, 2005) and it is currently unknown whether functions supported by the hippocampus are particularly important for creating integrated discourse about the past and future.
Preliminary evidence supporting the role of the hippocampus in discourse integration comes from a handful of prior studies that have investigated whether amnesic patients with medial temporal lobe damage can construct integrated verbal narratives about the past. Discourse cohesion and coherence are two linguistic measures that have been investigated, and serve to index lower-level and higher-level aspects of narrative integration, respectively. Discourse cohesion is a measure of the connection of individual narrative elements using linguistic devices (e.g., grammatical and lexical links), whereas discourse coherence is a measure of the overall continuity and organization of the narrative into a unified, integrated whole (Caspari & Parkinson, 2000; Louwerse & Graesser, 2005). MacKay and colleagues (1998) were the first to suggest that the hippocampus may play an important role in creating coherent discourse about the past (MacKay, Burke, & Stewart, 1998). They found that the amnesic patient H.M. produced verbal narratives about childhood events (as well as verbal narratives about ambiguous sentences) that were less coherent and less focused compared to the narratives produced by controls. Based on these results, MacKay and colleagues proposed that the hippocampus supports discourse-level integration through its role in linguistic binding (MacKay, James, Hadley, & Fogler, 2011; MacKay, James, Taylor, & Marian, 2007; MacKay et al., 1998). Specifically, they proposed that the same hippocampal binding processes that support episodic memory also enable the rapid formation of new connections between disparate lexical, semantic, or phonological representations during verbal discourse. Congruent with this hypothesis, recent neuroimaging evidence suggests that the hippocampus plays a role in syntactic integration during language comprehension (Meyer et al., 2005) and discourse-level semantic integration of pictures (West & Holcomb, 2002). It has also been suggested that the hippocampus plays a role in linking sentence information across event boundaries in the service of memory (DuBrow & Davachi, 2013; Ezzyat & Davachi, 2011; Swallow et al., 2011).
While these results support the hypothesis that the hippocampus enables the integration of individual narrative elements into cohesive and coherent discourse when describing the past, prior results have not always been consistent across studies. In particular, Caspari and Parkinson (2000) found evidence for cohesion reductions in the autobiographical discourse of the amnesic patient M.R., but did not find evidence for reductions in M.R.'s discourse coherence. More recently, Kurczek and Duff (2011) found suggestive evidence for impairments in both discourse cohesion and discourse coherence in amnesic patients’ narratives about the past, but these impairments did not reach significance. Thus, important questions remain about the presence and nature of discourse-level integration impairments in amnesia and whether processes supported by the hippocampus are particularly critical for creating cohesive and coherent discourse about the past.
In addition, it is currently unknown whether hippocampal damage impacts amnesic patients’ ability to create cohesive and coherent discourse about the future. Describing novel future events that have yet to occur places high demands on combinatorial processes to form new linguistic connections and to integrate elements from past experience in new and creative ways (Schacter & Addis, 2009). Hippocampal binding processes have been proposed to be particularly critical when creating new linguistic connections that do not have pre-existing internal representations that can be automatically retrieved and verbalized (MacKay et al., 2011; MacKay et al., 1998). In a similar vein, it has been proposed that hippocampal damage is particularly disruptive to verbal communication when generating novel utterances that require the creative and flexible use of language (Duff & Brown-Schmidt, 2012). Thus, we hypothesized that creating discourse about novel future events may place increased demands on hippocampal processes that enable the flexible binding of linguistic information, such that damage to the hippocampus is particularly disruptive.
In Experiment 1, we investigated whether the hippocampus is necessary for creating unified and meaningful narrations of mental simulations by measuring discourse cohesion and coherence when amnesic patients construct complex verbal narratives about possible future events and experienced past events. If hippocampal binding processes play a critical role in both lower and higher levels of discourse integration, we would expect patients’ narratives to be characterized by deficits in both cohesion and coherence. Furthermore, if hippocampal binding processes are particularly critical for creating new linguistic associations that do not have preexisting internal representations, deficits in discourse cohesion and coherence should be more prevalent in patients’ descriptions of novel future events than of past events.
2. Experiment 1
2.1. Materials and Methods
2.1.1. Participants
Participants included nine amnesic patients with MTL lesions. Eight of these patients had participated in our prior study (Race et al., 2011) that analyzed the informational content of the same past and future narratives used in the present study. Patient P09 was new to this study. The neuropsychological profiles of all patients indicate impairments isolated to the domain of memory with profound impairments in new learning (see Table 1). Twelve healthy controls also participated, all of whom had participated in Race et al. (2011). The control subjects were matched to the patient group in terms of mean age (mean = 60 ± 12.2 years), education (14 ± 2.0 years), and verbal IQ (105 ± 15.7). As reported by Race and colleagues (2011), quantitative assessment revealed that patients’ descriptions of the future and past contained fewer episodic details than those of controls. This pattern of impairment was also present in the additional amnesic patient included in the present study (P09), who provided fewer episodic details than controls in his future and past narratives (z scores < -2). All participants were paid for their participation and provided informed consent in accordance with the procedures of the Institutional Review Boards at Boston University and the VA Boston Healthcare System.
Table 1.
Patient Demographic, Neuropsychological and Neurological Characteristics
| Patient | Etiology | Age | Edu | WAIS. Ill VIQ | WMS. Ill |
Hipp Vol Loss | Subhipp Vol Loss | |||
|---|---|---|---|---|---|---|---|---|---|---|
| GM | VD | AD | WM | |||||||
| P01 | Encephalitis | 55 | 14 | 92 | 45 | 56 | 55 | 85 | 73% | 78%* |
| P02 | Encephalitis | 66 | 12 | 106 | 69 | 68 | 77 | 111 | 66% | 72%+ |
| P03 | Anoxia | 60 | 12 | 83 | 52 | 56 | 55 | 91 | N/A | N/A |
| P04 | Anoxia + left temporal lobectomy | 46 | 16 | 86 | 49 | 53 | 52 | 93 | 63% | 60%^ |
| P05 | Anoxia | 54 | 14 | 111 | 59 | 72 | 52 | 96 | 22% | - |
| P06 | Encephalitis | 82 | 18 | 135 | 45 | 53 | 58 | 141 | N/A | N/A |
| P07 | Anoxia | 58 | 17 | 134 | 70 | 75 | 67 | 126 | N/A | N/A |
| P08 | Anoxia | 60 | 16 | 110 | 62 | 68 | 61 | 92 | N/A | N/A |
| P09 | Anoxia | 55 | 18 | 119 | 67 | 75 | 55 | 93 | 58% | - |
Note. Age = Age (years); Edu = Education (years); WAIS, III = Wechsler Adult Intelligence Scale, III; VIQ = Verbal IQ; WMS, III = Wechsler Memory Scale, III; GM = General Memory; VD = Visual Delayed; AD = Auditory Delayed; WM = Working Memory; Hipp Vol Loss = Bilateral Hippocampal Volume Loss; Subhipp Vol Loss = Parahippocampal Gyrus Volume Loss.
a = volume loss in bilateral anterior parahippocampal gyrus and left posterior parahippocampal gyrus.
b = volume loss in bilateral anterior parahippocampal gyrus and right posterior parahippocampal gyrus.
c = volume loss in left anterior parahippocampal gyrus.
To assess the extent of patients’ neural damage, structural magnetic resonance imaging (MRI) scans were collected for five of the patients. Information about the acquisition and analysis of MRI scans and lesion volumetrics has been previously reported for patients P01, P02, P04, P05, and P09 (Kan, Giovanello, Schnyer, Makris, & Verfaellie, 2007; Race, LaRocque, Keane, & Verfaellie, 2013). Quantitative analysis compared each patient's regional brain volumes (corrected for intracranial volume) to volumes from eight age- and gender-matched control subjects. Two of the anoxic patients (P05 and P09) had damage limited to the hippocampus, and two of the encephalitic patients (P01 and P02) and one of the anoxic patients (P04) had damage to the hippocampus and surrounding parahippocampal gyrus (volume reductions > 2 SDs from the control mean; see Table 1). Measurements of frontal, parietal, occipital, and lateral temporal cortex were also made to assess the possibility of additional damage outside the MTL). No common volume reductions were found outside the MTL. MRI could not be obtained for the remaining patients because of medical contraindications. For the encephalitic patient P06, a computerized tomography (CT) scan was available and visual inspection indicated extensive hippocampal and parahippocampal gyrus damage. For the remaining patients, MTL pathology can be inferred on the basis of etiology and neuropsychological profile.
2.1.2. Stimuli
Two narratives about the future and two narratives about the past were randomly selected from a larger sample obtained by Race and colleagues (2011). These narratives were generated by having participants imagine specific personal events about the future (e.g., winning the lottery), and recall specific personal events about the past (e.g., graduation ceremony). Participants were given three minutes to describe each event in as much detail as possible. Within the allotted three minutes, participants continued with their descriptions without interference from the examiner until they came to a natural ending point. Narratives were audiotaped and transcribed into word processing documents for analysis.
2.1.3. Scoring
Narrative Cohesion
Narrative cohesion was scored using a coding scheme that measures the microanalytic dimensions of narrative connectedness and the degree to which information in a sentence or phrase is linked to prior narrative elements (Halliday & Hasan, 1976). Narratives were first segmented into distinct phrases, and then cohesive ties across phrases were identified. Cohesive ties included references, substitutions, ellipses, conjunctions, or lexical cohesions. References are defined as two linguistic elements that are related in what they refer to (e.g., pronouns: “The man drove the car. The car belongs to him.”). Substitutions refer to alternate words that are used in place of repetition of an item (“My pencil is broken. I need a new one.”). Ellipses are instances in which one of the identical linguistic elements is omitted (“The whole family had dessert. Charles chose cookies.”). Conjunctions represent semantic relations that presuppose the presence of other discourse components (“The dinner ended at seven. After dinner, the family went for a walk). Lexical cohesions reflect ties based on vocabulary (“James ran into the street. The approaching car didn't seem to scare the man.”). For each participant, the number of phrases and the number of ties were counted in each narrative and then averaged across the narratives about the past and the narratives about the future. A ratio of the number of ties per phrase was then calculated for each participant for each type of narrative.
Narrative Coherence
Narrative coherence was measured using a multidimensional method of coding narrative coherence (Narrative Coherence Coding Scheme; NaCCS) (Reese et al., 2011) that consists of three macroanalytic dimensions of narrative integration (Context, Chronology, and Theme), each of which are scored using a four-point rating scale. Narrative context refers to the degree to which a narrative is oriented in time and space. Narratives that do not contain any information about time or location are scored as 0. Narratives that provide partial information about time or location are scored as 1 (e.g., if time or location is mentioned at any level of specificity). Narratives receive a score of 2 if both time and place are mentioned, but one of these aspects is vague (e.g., time is referred to as “a while ago”). A narrative receives a score of 3 if both time and place are mentioned and both are specific (e.g., “At 8 o'clock this morning I drove to the my brother's house”). Chronology refers to the degree to which the actions included in the narrative can be ordered on a timeline. A narrative that contains little or no information about the order of events is scored as a 0. A narrative in which less than half of the actions can be ordered on a timeline receives a 1. A narrative in which 50-75% of the actions can be ordered on a timeline receives a 2. A narrative in which greater than 75% of the relevant actions can be ordered on a timeline receives a 3. Theme refers to the extent the narrator stays on topic, develops a theme using causal linkages or elaborations, and provides a resolution. Narratives that are substantially off-topic receive a 0. A narrative that has an identifiable topic but which is not developed through elaborations, evaluations, or causal linkages receives a 1. A narrative that substantially develops the topic via elaborations, evaluations, interpretations or causal linkages receives a 2. A narrative that includes all of the above and in addition incorporates a resolution to the story receives a 3. For each participant, the three dimensions of narrative coherence were scored for each narrative and these scores were averaged across the two narratives about the past and the two narratives about the future.
Interrater reliability of narrative cohesion and coherence scoring was established on the basis of 17 event narratives (20% of the total narratives) scored by two raters. The primary scorer was not blind to subject status, but the second trained scorer was blind to subject status. Intraclass correlation analysis indicated acceptable agreement across scorers for discourse cohesion (Cronbach's alpha = .97 for ties, .98 for phrases) as well as discourse coherence (Cronbach's alpha = .89 for temporal order, Cronbach's alpha = .76 for context; Cronbach's alpha = .80 for theme).
2.2. Results
2.2.1. Narrative Cohesion
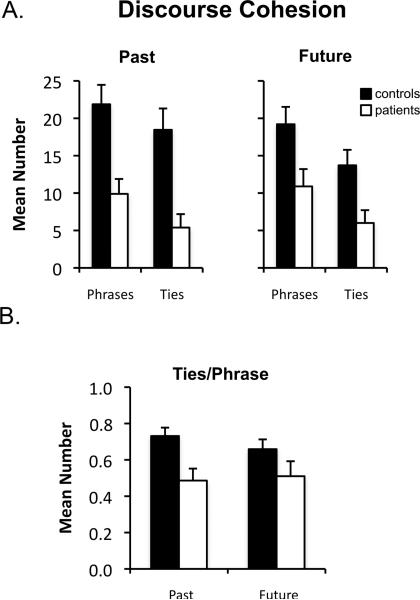
To investigate the level of narrative cohesion in patients’ descriptions of the past and future, the average number of phrases and cohesive ties in participants’ narratives was first calculated (Figure 1A). A two-way mixed factorial ANOVA with factors of group (controls, patients) and time period (past, future) showed that patients produced fewer cohesive ties than did controls (main effect of group; F(1,19) = 13.88, p < .001), and that the magnitude of this reduction did not differ between future and past narratives (group x time period interaction; F(1,19) = 2.88, p = .11). However, patients also produced fewer phrases than did controls regardless of whether they were describing the past or future: A two-way mixed factorial ANOVA yielded a main effect of group (F(1,19) = 12.05, p < .005) and no group x time period interaction (F(1,19) = 1.10, p = .31). To take into account this difference in the number of phrases produced across groups, the average number of cohesive ties per phrase was calculated and entered into a mixed factorial ANOVA with factors of group (patients, controls) and time period (future, past). As can be seen in Figure 1B, patients produced fewer cohesive ties per phrase compared to controls (main effect of group; F(1,19) = 8.01, p < .01), and the magnitude of the deficit in narrative cohesion did not differ between future and past narratives (group x time period; F(1,19) = .89, p = .36).
Figure 1.
Discourse cohesion scores for descriptions of the past and future in controls (black bars) and patients (white bars). (A) Mean number of phrases and mean number of ties in participants’ past and future narratives. (B) Mean number of ties per phrase in participants’ past and future narratives. Error bars indicate SEM.
2.2.2. Narrative Coherence
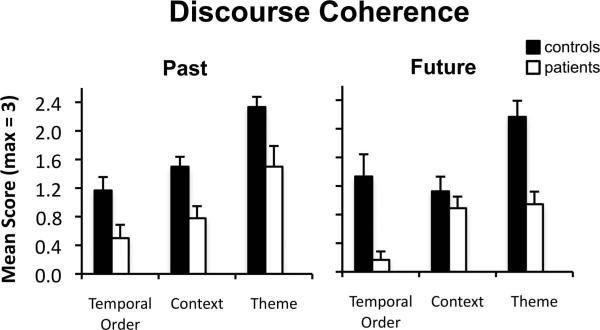
To investigate the level of narrative coherence in patients’ descriptions of the past and future, mean narrative coherence scores were entered into a three-way mixed factorial ANOVA with factors of group (patients, controls), time period (future, past), and coherence dimension (temporal order, context, theme). Patients’ descriptions of the past and future were less coherent than controls’ (main effect of group; F(1,19) = 13.26, p < .002; Figure 2). A significant group x dimension interaction (F(2,38) = 3.97, p < .03) was modified by a group x dimension x time period interaction (F(2,38) = 4.12, p < .03), indicating that patients’ pattern of impairment across dimensions differed for past and future narratives. Follow-up group x dimension analyses revealed that the magnitude of patients’ coherence impairment did not differ across coherence dimensions for past narratives (F(2,38) = .24, p = .79) but did differ across coherence dimensions for future narratives (F(2,38) = 6.44, p < .01). Follow-up pairwise comparisons revealed that patients’ coherence scores were reduced compared to controls’ across all coherence dimensions for past narratives (t values > 2.46, p values < .05) and in the dimensions of theme (t(19) = 4.05, p < .001) and temporal order (t(19) = 3.52, p < .005) for future narratives.
Figure 2.
Discourse coherence scores for descriptions of the past and future in controls (black bars) and patients (white bars) separated by coherence dimension (temporal order, context, theme). Error bars indicate SEM.
2.3. Discussion: Experiment 1
The results from Experiment 1 demonstrate that amnesic patients’ descriptions of the past and future are reduced in measures of discourse-level integration. Specifically, patients’ descriptions of past and future personal events were characterized by reductions in both lower-level discourse integration (narrative cohesion) and higher-level discourse integration (narrative coherence). These results suggest that the hippocampus plays a critical role in organizing ongoing narratives about the past and future into linguistically cohesive and coherent discourse. Interestingly, deficits in discourse cohesion and coherence were not greater in patients’ descriptions of the future than of the past, suggesting that the contribution of hippocampal binding processes to discourse-level integration does not vary with the novelty of the scenario being described.
Although these findings suggest a pervasive impairment in discourse integration in amnesia, it is important to note that the observed impairments in discourse cohesion and coherence occurred in the context of deficits in narrative content. Specifically, we found that amnesic patients’ descriptions of the past and future contained fewer phrases than controls’, which aligns with our prior observation that patients’ descriptions of the past and future also contain fewer narrative details (Race et al., 2011). Although impairments in discourse cohesion remained significant when controlling for patients’ reduction in narrative content, the Narrative Coherence Coding Scheme used for analysis of discourse coherence does not provide a way to control for differences in narrative content. Thus, it is possible that deficits in narrative content may contribute to some of the observed deficits in discourse integration in amnesia. Before drawing conclusions about the presence and scope of discourse integration deficits in amnesia, it is important to confirm that discourse integration deficits are not simply secondary to reductions in narrative content. We address this question in Experiment 2 by measuring discourse cohesion and coherence in a condition in which narrative content is equivalent in patients and controls (picture narratives) (Race et al., 2011). In doing so, we were also able to evaluate whether discourse integration impairments in amnesia are limited to past and future narratives.
3. Experiment 2
3.1. Material and Methods
3.1.1. Participants
The participants in Experiment 2 were the same nine amnesic patients and twelve healthy controls who participated in Experiment 1.
3.1.2. Stimuli
Stimuli consisted of five picture narratives that were elicited by presenting participants with detailed drawings of scenes that depicted characters engaged in various activities (Race et al., 2011). For each picture, participants were instructed to imagine that the picture was a scene taken from a movie and to tell a story about what was going on in the scene. Participants were given three minutes for each narrative and continued with their narratives without interference from the examiner until they came to a natural ending point. As reported by Race and colleagues (2011), quantitative assessment of these narratives for eight patients revealed that the number of episodic details in the picture narratives was not reduced compared to controls. In addition, the number of episodic details in the picture narratives from patient P09 was not reduced compared to controls (z score > 0). In the present experiment, we investigated whether patients’ picture narratives were less coherent and cohesive compared to those of controls despite having a normal amount of narrative content.
3.1.3. Scoring
Narrative coherence and cohesion were scored using the same procedure as Experiment 1 and then averaged across the five picture narratives for each participant. Interrater reliability of scoring was established on the basis of 21 event narratives (20% of the total narratives) scored by two raters. The primary scorer was not blind to subject status, but the second trained scorer was blind to subject status. Intraclass correlation analysis indicated acceptable agreement across scorers for discourse cohesion (Cronbach's alpha = .97 for total phrases, Cronbach's alpha = .99 for total ties) as well as discourse coherence (Cronbach's alpha = .81 for temporal order, Cronbach's alpha = .77 for context; Cronbach's alpha = .75 for theme).
3.2. Results
3.2.1. Narrative Cohesion
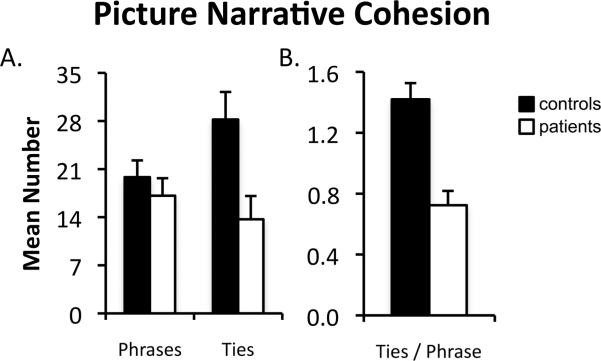
Narrative cohesion was investigated by calculating the average number of cohesive ties, phrases, and cohesive ties per phrase in participants’ picture narratives. As can be seen in Figure 3A, patients’ picture narratives contained fewer cohesive ties compared to controls’ (t(1,19) = 2.65, p < .05) but did not contain fewer phrases (t(1,19) = .76, p = .46). Critically, patients produced fewer cohesive ties per phrase compared to controls (t(1,19) = 4.90, p < .001; Figure 3B).
Figure 3.
Discourse cohesion scores for picture narratives produced by controls (black bars) and patients (white bars). (A) Mean number of phrases and mean number of ties in participants’ picture narratives. (B) Mean number of ties per phrase in participants’ picture narratives. Error bars indicate SEM.
3.2.2. Narrative Coherence
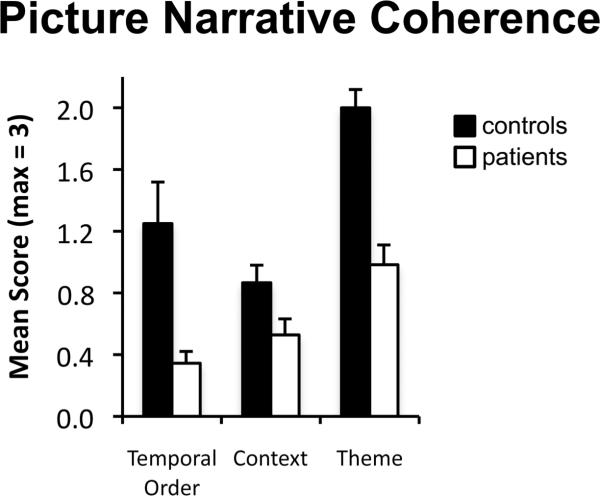
To investigate the coherence of participants’ picture narratives, mean scores for the three dimensions of discourse coherence were entered into a two-way mixed factorial ANOVA with factors of group (patients, controls) and coherence dimension (temporal order, context, theme). As can be seen in Figure 4, patients’ picture narratives were less coherent than controls’ (main effect of group; F(1,19) = 17.31, p < .001). A significant interaction between group and coherence dimension (F(2,38) = 4.43, p < .03) indicates that the magnitude of the impairment in amnesia differed across coherence dimensions. However, follow-up pairwise comparisons revealed that patients’ coherence scores were reduced compared to controls’ across all coherence dimensions (temporal order, context, theme; t values > 2.20, p values < .05).
Figure 4.
Discourse coherence scores for picture narratives produced by controls (black bars) and patients (white bars) separated by coherence dimension (temporal order, context, theme). Error bars indicate SEM,
3.2.3. Anatomical basis of deficits
In order to investigate more precisely the anatomical basis of the discourse cohesion and coherence impairments in amnesia, picture narrative performance was separately analyzed for the patients with volumetrically confirmed damage limited to the hippocampus (P05 and P09; H-only group) and for the patients with volumetrically or visually confirmed MTL damage that included the hippocampus and MTL cortex (P01, P02, P04, and P06; H+ group).
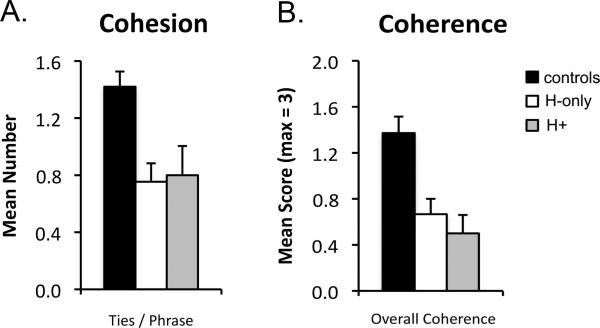
To investigate the anatomical basis of the impairment in discourse cohesion, participants’ mean number of ties per phrase was entered into a one-way ANOVA with factor of group (controls, H-only patients, H+ patients). Results from the ANOVA indicated that cohesion differed across groups (main effect of group; F(2,17) = 6.44, p = .01). Follow-up analysis revealed that impairments in discourse cohesion did not depend on the presence of MTL damage outside the hippocampus. Specifically, discourse cohesion was reduced compared to controls both in H-only patients (mean ties/phrase = .75 vs. 1.42; t(12) = 2.50, p < .05, 1-tailed) and in H+ patients (mean ties/phrase = .80 vs. 1.42; t(14) = 3.00, p < .005, 1-tailed; Figure 5A) and did not differ between the two patient groups (t(4) = .08, p = .94).
Figure 5.
Results for picture narrative (A) cohesion and (B) coherence separated by patient subgroup: Controls (black bars), patients with selective hippocampal damage (H-only; white bars), and patients whose neural damage included the hippocampus and MTL cortex (H+; grey bars). Error bars indicate SEM.
To investigate the anatomical basis of the impairment in discourse coherence, participants’ mean coherence scores were entered into a two-way mixed factorial ANOVA with factors of group (controls, H-only patients, H+ patients) and coherence dimension (temporal order, context, theme). Coherence differed across groups (main effect of group; F(2,15) = 6.69, p < .01) regardless of coherence dimension (group x dimension; F(4,30) = 1.79, p =.17). Follow-up analysis revealed that impairments in coherence, like impairments in cohesion, were present in both patient groups. Specifically, overall coherence (average of the three coherence dimensions) was reduced both in H-only patients (mean coherence = .67 vs. 1.37; t(12) = 2.02, p < .05, 1-tailed ) and in H+ patients (mean coherence = .50 vs. 1.37; t(14) = 3.43, p < .005, 1-tailed; Figure 5B). In addition, overall coherence did not differ between the two patient groups (t(4) = .70, p = .52).
Together, these results suggest that patients’ impairments in discourse coherence and cohesion do not depend on the presence of MTL damage outside the hippocampus and that isolated hippocampal damage is sufficient to impair both levels of discourse integration.
3.3. Discussion: Experiment 2
The results from Experiment 2 demonstrate that discourse-level integration deficits in amnesia do not simply reflect reductions in narrative content and are not limited to narratives about the past and future. Specifically, patients’ narratives about pictures available in the present were less linguistically cohesive and coherent than those of controls, despite containing an equivalent number of phrases (this study) and narrative details (Race et al., 2011). These results suggest that even when relevant narrative details are readily available and can be verbalized, the hippocampus plays a critical role in the integration of individual narrative elements into coherent and cohesive discourse. Furthermore, the deficits in discourse integration observed in amnesia can be attributed specifically to damage in the hippocampus given their presence in patients with restricted hippocampal lesions.
4. Discussion
Our capacity to mentally relive the past and imagine possible futures (mental time travel) is essential to adaptive behavior and enables us to build predictions and plans for the future based on prior experience (Suddendorf & Corballis, 1997, 2007). Communicating our mental time travels into the past and future enables us to share our memories, plans and ideas and to thereby benefit from the experience of others (Corballis, 2009, 2013). Indeed, it has been proposed that language itself evolved to allow us to share our mental simulations of the past and future with others and to communicate information not tied to the immediate environment (Corballis, 2009, 2013). However, in order to effectively communicate our thoughts, linguistic elements describing these thoughts not only must be verbally produced but also must be integrated into cohesive and coherent discourse units that are easily understood. The current study provides novel evidence that the hippocampus plays a critical role in these integrative functions. Specifically, we found that the hippocampus supports discourse coherence and cohesion when constructing complex verbal accounts about the past and future. In addition, we found that the hippocampus also supports discourse coherence and cohesion when constructing complex verbal accounts about events in pictures, when demands on retrieving narrative elements from long-term memory are low and narrative content is intact. Together, these results suggest that the hippocampus makes a critical contribution to the coherence and cohesion of multiple types of complex verbal accounts whenever narrative elements must be linked into coherent and cohesive units.
Experiment 1 demonstrated that amnesic patients’ descriptions of the past and future are characterized by reductions in both lower-level measures of linguistic integration (discourse cohesion) and higher-level measures of linguistic integration (discourse coherence). A handful of prior studies have investigated discourse cohesion and coherence when amnesic patients have constructed narratives about the past, but results have been mixed. While discourse cohesion impairments in patients’ descriptions of the past have been previously reported in some studies (Caspari & Parkinson, 2000), they have not reached significance in others (Kurczek & Duff, 2011). Similarly, coherence impairments have previously been reported in some amnesic patients’ descriptions of the past (Kurczek & Duff, 2011; MacKay et al., 1998), but other studies have not observed such deficits (Caspari & Parkinson, 2000). Procedural differences across prior studies, such as the discourse elicitation procedure and the metric used to measure cohesion and coherence, may have contributed to these mixed results. For example, coherence impairments have been observed when using detailed rating metrics (Kurczek & Duff, 2011; MacKay et al., 1998), but not when using more general subjective assessments (Caspari & Parkinson, 2000). In addition, discourse cohesion impairments have been reported when patients produced narratives in response to an autobiographical prompt (e.g. senior prom) (Caspari & Parkinson, 2000), but were less robust when patients’ autobiographical narratives were produced in a conversational setting using the Mediated Discourse Elicitation Protocol (Kurczek & Duff, 2011). The current study replicates and extends these prior results by demonstrating that patients’ non-mediated descriptions of specific past experiences are significantly reduced both in terms of discourse cohesion and discourse coherence when assessed by detailed rating metrics.
To our knowledge, no prior study has investigated whether processes supported by the hippocampus are critical for creating integrated discourse about future events. The current results provide novel evidence that the hippocampus is critical for creating integrated discourse about the future, in addition to its role in creating integrated discourse about the past. While we hypothesized that creating narratives about novel future events might place higher demands on integrative processes supported by the hippocampus, and thereby result in more severe cohesion and coherence deficits in amnesia for narratives about the future than about the past, this prediction was not borne out as patients’ verbal narratives about the past and future were equally fragmented linguistically. One potential reason for this result is that while discourse about the future may contain more novel narrative content than discourse about the past, the way in which descriptions of the future and past are put into language may be equally novel and thereby place similar demands on linguistic integration.
The discourse integration deficits observed in Experiment 1 cannot be explained simply as a consequence of broader deficits in narrative content or long-term memory, as deficits in discourse cohesion and coherence were also observed in patients’ verbal narratives about pictures (Experiment 2) – a condition in which long-term memory demands are low and the amount of narrative content was equivalent in patients and controls (Race et al., 2011). Importantly, the fact that discourse integration deficits in amnesia were present in the context of intact narrative content reveals that the hippocampal processes supporting the combination of narrative elements are dissociable from the hippocampal processes that support the retrieval or perception of relevant narrative elements. These results are congruent with prior suggestions of reduced discourse integration in picture narratives in amnesia (Caspari & Parkinson, 2000; Kurczek & Duff, 2011; MacKay et al., 1998). However, these prior studies did not take into account potential differences in verbal output, and discourse integration impairments in amnesia may have been secondary to reductions in narrative content. The current study goes beyond prior work in demonstrating unequivocally that discourse integration is impaired in amnesia, even in the context of intact narrative content. Together with the results from Experiment 1, the finding of reduced discourse integration in patients’ picture narratives is congruent with the notion that the combinatorial properties of language that enable expression of “who did what to whom, what is true of what, where, when, and why” are important not only for recounting episodic memories and imagining future events, but also for telling fictional stories (Pinker, 2003; Corballis, 2009). More broadly, the results across Experiments 1 and 2 indicate that the hippocampus plays a critical role in the construction of verbal narratives whenever linguistic elements must be combined to effectively communicate mental simulations and stories.
4.1 Mechanisms underlying impairments in discourse cohesion
Our findings raise important questions about the mechanisms underlying the observed discourse impairments across conditions in amnesia. With regard to narrative cohesion, one possibility is that the observed deficits are secondary to deficits in long-term memory in amnesia that may operate over the course of narrative production (Kurczek & Duff, 2011). Specifically, discourse cohesion requires forming linguistic ties between verbal information in distinct portions of the narrative and may be impaired if referents previously mentioned in the narrative cannot be remembered. Arguments against this possibility come from the prior finding by Race and colleagues (2011) that patients produce very few repetitions in their narratives and do not produce a greater number of repetitions than controls (see also Skotko et al., 2005). In addition, further investigation of the current data revealed that the discourse cohesion impairment in amnesia occurred even over very short time scales. Specifically, amnesic patients produced fewer cohesive ties than controls even between immediately adjacent phrases (p < .001) in their picture narratives, and this deficit was also present when analysis was restricted to ties between immediately adjacent phrases with only one or zero words between cohesive markers (p < .05). These results argue against long-term forgetting as the sole root of the discourse cohesion deficit in amnesia.
An alternative possibility is that discourse cohesion deficits in amnesia reflect impaired relational binding mechanisms supported by the hippocampus that operate both in short-term memory and in long-term memory (Eichenbaum & Cohen, 2001; Olsen, Moses, Riggs, & Ryan, 2012). Binding mechanisms supported by the hippocampus may be critical not only for integrating features of an event in support of episodic memory, but also for integrating linguistic features during ongoing discourse. This possibility was first suggested by MacKay and colleagues to explain the language impairments observed in patient H.M. (MacKay et al., 2011; MacKay et al., 2007; MacKay et al., 1998). More recent studies provide additional support for this notion by suggesting that hippocampal processes support the integration of distinct verbal elements during language comprehension and memory (DuBrow & Davachi, 2013; Ezzyat & Davachi, 2011; Meyer et al., 2005; Swallow et al., 2011).
Discourse cohesion may also be supported by hippocampal binding processes that integrate information across working memory and long-term memory. Building a cohesive narrative requires holding previously reported narrative information in mind while continuously updating this information with new linguistic elements drawn from semantic memory. Construction-integration models of discourse (Kintsch, 1988; Mar, 2004) emphasize the importance of such interactions between working memory and long-term memory to support the mapping of new semantic information onto information already encountered in a narrative. According to these models, working memory processes are thought to select, sequence, and integrate information from long-term memory in order to create holistic verbal productions (Mar, 2004). The integration of information across working memory and long-term memory has also been associated with the function of an episodic buffer, which provides a modeling space for developing hypothetical situations and provides a foundation for narrative processes (Baddeley, 2000; Baddeley & Wilson, 2002; Mar, 2004). Recent neuroimaging evidence has linked hippocampal activity with the integrative functions of an episodic buffer (Berlingeri et al., 2008; Rudner, Fransson, Ingvar, Nyberg, & Ronnberg, 2007; Rudner & Ronnberg, 2008). Of particular relevance is the observation of hippocampal activity during the creation and maintenance of multimodal representations in the linguistic domain (Rudner et al., 2007; Rudner & Ronnberg, 2008).
4.2 Mechanisms underlying impairments in discourse coherence
Discourse coherence reflects the development of a unified theme, spatiotemporally-specific context, and chronologically ordered narrative. Development of a unified theme may depend on similar hippocampal processes that support discourse cohesion. Specifically, thematic measures of discourse coherence reflect the degree to which the narrator stays on topic and develops a global theme using causal linkages and elaborations. This combinatorial process may depend on hippocampal functions that support integration across working memory and long-term memory so that new linguistic details drawn from semantic memory can be added to online representations of an unfolding discourse topic.
In contrast, contextual and chronological aspects of discourse coherence may be supported by hippocampal functions that are distinct from those supporting discourse cohesion. Contextual measures of discourse coherence reflect the degree to which a narrative is oriented in space and time. The hippocampus is known to play an important role in the representation of space and time (Burgess, Maguire, & O'Keefe, 2002; Kraus, Robinson, White, Eichenbaum, & Hasselmo, 2013; O'Keefe, Burgess, Donnett, Jeffery, & Maguire, 1998; O'Keefe & Nadel, 1978) and the association of event details with their spatial and temporal contexts (Eichenbaum, 2004; Eichenbaum & Cohen, 2001; Hannula, Tranel, & Cohen, 2006; Hartley et al., 2007; Konkel, Warren, Duff, Tranel, & Cohen, 2008; Olson, Page, Moore, Chatterjee, & Verfaellie, 2006). One possibility is that the hippocampus supports the creation of narrative context by structuring linguistic elements around spatiotemporally-specific details.
Chronological measures of discourse coherence reflect the ability to create cross-temporal links that chain together narrative elements into a continuous, sequentially ordered narrative that unfolds over time (Mar, 2004). It is known that the hippocampus supports the representation of temporal order and sequence information (Eichenbaum, 2004; Fortin, Agster, & Eichenbaum, 2002; Heuer & Bachevalier, 2013; Hsieh, Ekstrom, & Ranganath, 2011; Hsieh, Gruber, Jenkins, & Ranganath, 2014; Kesner, Gilbert, & Barua, 2002; Kumaran & Maguire, 2006), and these hippocampal functions could underlie the constructive causal-temporal ordering of linguistic information. Further support for this hypothesis comes from prior studies reporting impairments in amnesia when patients are required to make temporal order judgments or to acquire temporal information about new stimuli (Bowers, Verfaellie, Valenstein, & Heilman, 1988; Hurst & Volpe, 1982; Konkel et al., 2008; Mayes et al., 2001; Rosenbaum, Gilboa, Levine, Winocur, & Moscovitch, 2009).
4.3. Conclusions
The current study provides novel evidence that the hippocampus plays a critical role in the integration of linguistic elements into cohesive and coherent discourse units when constructing several different types of complex verbal narratives, and suggests several hippocampal mechanisms that may support these integrative functions. It is important to note, however, that it is unlikely that the hippocampus acts alone in its support of discourse integration. Rather, interactions between the hippocampus and multiple cortical regions supporting language are likely crucial to this function. Interactions with the prefrontal cortex may be particularly critical, given that the prefrontal cortex has been implicated in many of the same integrative and associative functions that support the structuring of language (e.g., selecting and sequencing, temporal ordering, on-line maintenance, and integration across working memory and long-term memory) (Baddeley, 2000; Fuster, Bodner, & Kroger, 2000; Koechlin & Summerfield, 2007; Mar, 2004; Schlesewsky & Bornkessel-Schlesewsky, 2012; Yasuno et al., 1999). Indeed, frontal lobe lesions have been closely associated with impaired narrative production and deficits in the sequential organization of linguistic information (Kazmarek, 1984). Understanding how medial temporal and frontal mechanisms contribute both independently and interactively to the organization of discourse represents an important area for future research.
Acknowledgements
The authors thank Margaret Cadden and Keely Burke for help with data collection and analysis.
Research Support: This research was supported by NIH grants RO1MH093431 and F32NS073212 and by the Department of Veterans Affairs Clinical Science Research and Development Service.
Appendix
Cue: Imagine an encounter with an animal one year from now.
Patient: I happen to see a rabbit | that's outside on the side of our house. | I can't really describe it that much, | except that it sees me, | and it goes hopping back to the woods. | Aside from that, | I can't say anything that particular, | of me really seeing it or anything of the sorts. | Just that it's in our side portion of our house | and we have woods there, | it just sees me, | maybe 30 feet away or so, | it sees me, | and squirts or scoots back into the woods. | I can't really say anything else. |
Coherence Score
Context: 1 (only location is mentioned)
Chronology: 1 (less than half of the actions can be ordered on timeline)
Theme: 1 (narrative has identifiable topic but is not substantially developed)
Cohesion Score (ties underlined; phrases indicated by vertical bar)
Ties: 10
Phrases: 15
Ties/Phrase: .67
Control: We'll say a raccoon in the back yard. | There's nobody around. | It's early in the morning five o'clock. | Go in the yard to put the sprinkler on. | Don't know if he's going to attack. | He looks, | he stares. | What should I do? | Should I get a pitchfork? | Should I get a baseball bat? | I'm anticipating and wondering what should I do. | Throw a rock at it. | He jumps for the stairs. | I throw something else at him | and he goes up the fence up a tree, | but he's not gone. | So I throw from another angle a rock. | He just goes to the next tree. | Then I go around the block and come back | and he's in another part of the yard. | So I throw another rock at him, | or a piece of trash, | and he goes over the fence | and I don't see him any more. |
Coherence Score
Context: 3 (both time and location are mentioned and are specific)
Chronology: 3 (greater than 75% of relevant actions can be ordered on a timeline)
Theme: 3 (narrative stays on-topic, topic is developed, and narrative has a resolution)
Cohesion Score
Ties: 18
Phrases: 24
Ties/Phrase: .75
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Schacter DL. The hippocampus and imagining the future: where do we stand? Front Hum Neurosci. 2011;5:173. doi: 10.3389/fnhum.2011.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andelman F, Hoofien D, Goldberg I, Aizenstein O, Neufeld MY. Bilateral hippocampal lesion and a selective impairment of the ability for mental time travel. Neurocase. 2010;16(5):426–435. doi: 10.1080/13554791003623318. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4(11):417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Wilson BA. Prose recall and amnesia: implications for the structure of working memory. Neuropsychologia. 2002;40(10):1737–1743. doi: 10.1016/s0028-3932(01)00146-4. [DOI] [PubMed] [Google Scholar]
- Berlingeri M, Bottini G, Basilico S, Silani G, Zanardi G, Sberna M, Paulesu E. Anatomy of the episodic buffer: a voxel-based morphometry study in patients with dementia. Behav Neurol. 2008;19(1-2):29–34. doi: 10.1155/2008/828937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers D, Verfaellie M, Valenstein E, Heilman KM. Impaired acquisition of temporal information in retrosplenial amnesia. Brain Cogn. 1988;8(1):47–66. doi: 10.1016/0278-2626(88)90038-3. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Caspari I, Parkinson SR. Effects of memory impairment on discourse. Journal of Neurolinguistics. 2000;13:15–36. [Google Scholar]
- Corballis MC. Mental time travel and the shaping of language. Exp Brain Res. 2009;192(3):553–560. doi: 10.1007/s00221-008-1491-9. [DOI] [PubMed] [Google Scholar]
- Corballis MC. Wandering tales: evolutionary origins of mental time travel and language. Front Psychol. 2013;4:485. doi: 10.3389/fpsyg.2013.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBrow S, Davachi L. The influence of context boundaries on memory for the sequential order of events. J Exp Psychol Gen. 2013;142(4):1277–1286. doi: 10.1037/a0034024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Brown-Schmidt S. The hippocampus and the flexible use and processing of language. Front Hum Neurosci. 2012;6:69. doi: 10.3389/fnhum.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44(1):109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. Oxford University Press; New York: 2001. [Google Scholar]
- Ezzyat Y, Davachi L. What constitutes an episode in episodic memory? Psychol Sci. 2011;22(2):243–252. doi: 10.1177/0956797610393742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5(5):458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM, Bodner M, Kroger JK. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature. 2000;405(6784):347–351. doi: 10.1038/35012613. [DOI] [PubMed] [Google Scholar]
- Gardenfors P. Cooperation and the evolution of symbolic communication. In: Oller D, Griebel U, editors. Cooperation and the evolution of symbolic communication. MIT Press; Cambridge: 2004. pp. 237–256. [Google Scholar]
- Halliday MAK, Hasan R. Cohesion in English. London: Longman. 1976 [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: relational memory impairments in amnesia, even at short lags. J Neurosci. 2006;26(32):8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha-Khadem F, Burgess N. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17(1):34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci U S A. 2007;104(5):1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cogn Sci. 2007;11(7):299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Heuer E, Bachevalier J. Working memory for temporal order is impaired after selective neonatal hippocampal lesions in adult rhesus macaques. Behav Brain Res. 2013;239:55–62. doi: 10.1016/j.bbr.2012.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh LT, Ekstrom AD, Ranganath C. Neural oscillations associated with item and temporal order maintenance in working memory. J Neurosci. 2011;31(30):10803–10810. doi: 10.1523/JNEUROSCI.0828-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh LT, Gruber MJ, Jenkins LJ, Ranganath C. Hippocampal Activity Patterns Carry Information about Objects in Temporal Context. Neuron. 2014;81(5):1165–1178. doi: 10.1016/j.neuron.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst W, Volpe BT. Temporal order judgments with amnesia. Brain Cogn. 1982;1(3):294–306. doi: 10.1016/0278-2626(82)90030-6. [DOI] [PubMed] [Google Scholar]
- Kan IP, Giovanello KS, Schnyer DM, Makris N, Verfaellie M. Role of the medial temporal lobes in relational memory: neuropsychological evidence from a cued recognition paradigm. Neuropsychologia. 2007;45(11):2589–2597. doi: 10.1016/j.neuropsychologia.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmarek BLJ. Neurolinguistic analysis of verbal utterances in patients with focal lesions of the frontal lobes. Brain and Language. 1984;21:52–58. doi: 10.1016/0093-934x(84)90035-x. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Ullman MT, Corkin S. Bilateral medial temporal lobe damage does not affect lexical or grammatical processing: evidence from amnesic patient H.M. Hippocampus. 2001;11(4):347–360. doi: 10.1002/hipo.1049. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Barua LA. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav Neurosci. 2002;116(2):286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- Kintsch W. The role of knowledge in discourse comprehension: a construction- integration model. Psychol Rev. 1988;95(2):163–182. doi: 10.1037/0033-295x.95.2.163. [DOI] [PubMed] [Google Scholar]
- Klein SB, Loftus J, Kihlstrom J. Memory and temporal experience: The effects of episodic memory loss on an amnesic patient's ability to remember the past and imagine the future. Social Cognition. 2002;20:353–379. [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11(6):229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. Front Hum Neurosci. 2008;2:15. doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus BJ, Robinson RJ, 2nd, White JA, Eichenbaum H, Hasselmo ME. Hippocampal “time cells”: time versus path integration. Neuron. 2013;78(6):1090–1101. doi: 10.1016/j.neuron.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. The dynamics of hippocampal activation during encoding of overlapping sequences. Neuron. 2006;49(4):617–629. doi: 10.1016/j.neuron.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Kurczek J, Duff MC. Cohesion, coherence, and declarative memory: Discourse patterns in individuals with hippocampal amnesia. Aphasiology. 2011;25(6-7):700–712. doi: 10.1080/02687038.2010.537345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwerse MM, Graesser AC. Coherence in discourse. In: Strazny P, editor. Encyclopedia of linguistics. Fitzroy Dearborn; Chicago: 2005. pp. 216–218. [Google Scholar]
- MacKay DG, James LE, Hadley CB, Fogler KA. Speech errors of amnesic H.M.: unlike everyday slips-of-the-tongue. Cortex. 2011;47(3):377–408. doi: 10.1016/j.cortex.2010.05.009. [DOI] [PubMed] [Google Scholar]
- MacKay DG, James LE, Taylor JK, Marian DE. Amnesic H.M. exhibits parallel deficits and sparing in language and memory: Systems versus binding theory accounts. Language and Cognitive Processes. 2007;22(3):377–452. [Google Scholar]
- MacKay DG, Burke DM, Stewart R. H.M.'s language production deficits: Implications for relations between memory, semantic binding, and the hippocampal system. Journal of Memory and Language. 1998;38:28–69. [Google Scholar]
- Mar RA. The neuropsychology of narrative: story comprehension, story production and their interrelation. Neuropsychologia. 2004;42(10):1414–1434. doi: 10.1016/j.neuropsychologia.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Isaac CL, Holdstock JS, Hunkin NM, Montaldi D, Downes JJ, Roberts JN. Memory for single items, word pairs, and temporal order of different kinds in a patient with selective hippocampal lesions. Cogn Neuropsychol. 2001;18(2):97–123. doi: 10.1080/02643290125897. [DOI] [PubMed] [Google Scholar]
- Meyer P, Mecklinger A, Grunwald T, Fell J, Elger CE, Friederici AD. Language processing within the human medial temporal lobe. Hippocampus. 2005;15(4):451–459. doi: 10.1002/hipo.20070. [DOI] [PubMed] [Google Scholar]
- Milner B, Corkin S, Teuber H-L. Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of H.M. Neuropsychologia. 1968;6:215–234. [Google Scholar]
- O'Keefe J, Burgess N, Donnett JG, Jeffery KJ, Maguire EA. Place cells, navigational accuracy, and the human hippocampus. Philos Trans R Soc Lond B Biol Sci. 1998;353(1373):1333–1340. doi: 10.1098/rstb.1998.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon Press; Oxford: 1978. [Google Scholar]
- Olsen RK, Moses SN, Riggs L, Ryan JD. The hippocampus supports multiple cognitive processes through relational binding and comparison. Front Hum Neurosci. 2012;6:146. doi: 10.3389/fnhum.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. Working memory for conjunctions relies on the medial temporal lobe. J Neurosci. 2006;26(17):4596–4601. doi: 10.1523/JNEUROSCI.1923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race E, Keane MM, Verfaellie M. Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J Neurosci. 2011;31(28):10262–10269. doi: 10.1523/JNEUROSCI.1145-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race E, Keane MM, Verfaellie M. Losing sight of the future: Impaired semantic prospection following medial temporal lobe lesions. Hippocampus. 2013;23(4):268–277. doi: 10.1002/hipo.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race E, LaRocque KF, Keane MM, Verfaellie M. Medial temporal lobe contributions to short-term memory for faces. J Exp Psychol Gen. 2013;142(4):1309–1322. doi: 10.1037/a0033612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese E, Haden CA, Baker-Ward L, Bauer P, Fivush R, Ornstein PA. Coherence of Personal Narratives across the Lifespan: A Multidimensional Model and Coding Method. J Cogn Dev. 2011;12(4):424–462. doi: 10.1080/15248372.2011.587854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum RS, Gilboa A, Levine B, Winocur G, Moscovitch M. Amnesia as an impairment of detail generation and binding: evidence from personal, fictional, and semantic narratives in K.C. Neuropsychologia. 2009;47(11):2181–2187. doi: 10.1016/j.neuropsychologia.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Rudner M, Fransson P, Ingvar M, Nyberg L, Ronnberg J. Neural representation of binding lexical signs and words in the episodic buffer of working memory. Neuropsychologia. 2007;45(10):2258–2276. doi: 10.1016/j.neuropsychologia.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Rudner M, Ronnberg J. The role of the episodic buffer in working memory for language processing. Cogn Process. 2008;9(1):19–28. doi: 10.1007/s10339-007-0183-x. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philos Trans R Soc Lond B Biol Sci. 2009;364(1521):1245–1253. doi: 10.1098/rstb.2008.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesewsky M, Bornkessel-Schlesewsky I. Preface: The neurobiology of syntax. Brain Lang. 2012;120(2):79–82. doi: 10.1016/j.bandl.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Skotko BG, Andrews A, Einstein G. Language and the medial temporal lobe: Evidence from H.M.'s spontaneous discourse. Journal of Memory and Language. 2005;53:397–415. [Google Scholar]
- Suddendorf T, Addis DR, Corballis MC. Mental time travel and the shaping of the human mind. Philos Trans R Soc Lond B Biol Sci. 2009;364(1521):1317–1324. doi: 10.1098/rstb.2008.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. Mental time travel and the evolution of the human mind. Genet Soc Gen Psychol Monogr. 1997;123(2):133–167. [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behav Brain Sci. 2007;30(3):299–313. doi: 10.1017/S0140525X07001975. discussion 313-251. [DOI] [PubMed] [Google Scholar]
- Swallow KM, Barch DM, Head D, Maley CJ, Holder D, Zacks JM. Changes in events alter how people remember recent information. J Cogn Neurosci. 2011;23(5):1052–1064. doi: 10.1162/jocn.2010.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness Canadian Psychologist. 1985;25:1–12. [Google Scholar]
- West WC, Holcomb PJ. Event-related potentials during discourse-level semantic integration of complex pictures. Brain Res Cogn Brain Res. 2002;13(3):363–375. doi: 10.1016/s0926-6410(01)00129-x. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Hirata M, Takimoto H, Taniguchi M, Nakagawa Y, Ikejiri Y, Takeda M. Retrograde temporal order amnesia resulting from damage to the fornix. J Neurol Neurosurg Psychiatry. 1999;67(1):102–105. doi: 10.1136/jnnp.67.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]