Abstract
Objective
We sought to determine whether muscle density, an index of skeletal muscle fat content, was predictive of 2-year changes in weight-bearing bone parameters in young girls.
Methods
Two-year prospective data from 248 girls, aged 8–13 years at baseline. Peripheral quantitative computed tomography was used to measure changes in bone strength indices (bone strength index [BSI, mg2/mm4] and strength-strain index [SSIp, mm3]) and volumetric bone mineral density [vBMD, mg/cm3] at distal metaphyseal and diaphyseal regions of the femur and tibia, as well as calf and thigh muscle density (mg/cm3), and muscle cross-sectional area (MCSA, mm2), indices of skeletal muscle fat content and muscle force production, respectively.
Results
After controlling for potential confounders, greater gains in femur BSI (44%, P<0.002), total femur vBMD (114%, P<0.04) and femur trabecular vBMD (306%, P<0.002) occurred in girls in the lowest versus the highest groups of baseline thigh muscle density. Greater gains in tibial BSI (25%, P<0.03) and trabecular vBMD (190%, P<0.002) were also observed in the lowest versus the highest baseline calf muscle density groups.
Conclusion
Baseline muscle density is a significant predictor of changes in bone density and bone strength in young girls during a period of rapid skeletal development.
Keywords: Skeletal Muscle Fat Content, Bone Development, Girls, volumetric Bone Mineral Density (vBMD), peripheral Quantitative Computed Tomography (pQCT)
Introduction
Peak bone strength achieved in early adulthood is a primary predictor of fracture risk later in life1,2. Notably, >90% of bone mineral is accrued by the end of adolescence3 making this period the most opportune time to achieve maximal volumetric bone mineral density (vBMD) and promote adaptations in bone structure, the primary determinants of bone strength4. Disruption of normal bone development, resulting in suboptimal bone strength, would likely increase risk for developing future osteoporotic fractures1,5.
The relationship between adiposity and bone in children is complex and uncertain. While some studies in children have found a link between adiposity and impaired bone development5–7, others have found that adiposity is protective of the skeleton8. The conflicting results are perhaps due to a failure to distinguish between fat depots, which are likely to have different consequences for bone9,10. Indeed, recent studies have shown that abdominal visceral fat and skeletal muscle fat depots are inversely associated with vBMD in adults11 and may impair skeletal growth and mineralization in young children7,12. Adults with high muscle fat content also have higher marrow fat content13, which has been linked to a weaker skeleton14. These findings suggest the same metabolic processes may be regulating fat infiltration of skeletal muscle and bone remodeling15. Type 2 diabetes mellitus (T2DM) is also independently associated with both fatty infiltration of skeletal muscle and reductions in vBMD in adults16,17 and youth18,19. Given that nearly 20% of the pediatric population is obese20, and that overweight and obese children are over-represented in childhood fracture cases21,22, excess skeletal muscle fat may serve as an important risk factor for suboptimal bone development as well as metabolic dysfunction16,23,24, in youth.
Previous cross-sectional studies in children and adults have reported significant positive associations between muscle density, which is inversely associated with skeletal muscle fat content25, and bone parameters assessed by peripheral quantitative computed tomography (pQCT)26–28. For example, in a cross-sectional analysis, we found that muscle density was independently associated with bone strength of the femur and tibia after adjusting for potential confounders in girls26. To our knowledge, only one other study has assessed prospectively, the relationship between muscle, particularly muscle size, and bone growth during puberty29. However, because longitudinal data on the relationships between skeletal muscle fat, and bone strength and development in children and adolescents are scarce, we conducted a 2-year longitudinal study in 248 girls to show that changes in muscle density of the calf an thigh was positively associated with changes in weight-bearing vBMD and bone strength30. These findings suggest that skeletal muscle fat may serve as a predictor of poor skeletal development in children and adolescents. However, whether baseline levels of skeletal muscle fat content are a useful predictor of longitudinal changes in bone parameters in pediatric patients is not known.
Therefore, in the present analysis, we sought to determine whether baseline calf and thigh muscle density, an index of skeletal muscle fat content, predicted 2-year changes in vBMD and indices of bone strength at weight-bearing skeletal sites (i.e., femur and tibia) in girls. On the basis of our previous findings26,30, as well as the contention that osteoporosis-prone individuals may be identified even before puberty by virtue of low bone parameters for their age31, we hypothesized that muscle density would be associated with 2-year changes in vBMD and bone strength at weight-bearing skeletal sites in girls.
Methods
Participants
The study was approved by the University of Arizona Human Subjects Protection Committee and was conducted in accordance with the Helsinki Declaration. All girls and guardians provided written informed consent. Details regarding subject recruitment as well as the study protocol have been published previously26,30. The data presented in this paper came from healthy fourth and sixth grade girls (n=248; aged 8 to 13 years at baseline), who were recruited as participants in the Jump-In: Building Better Bones study24. The primary aim of Jump-In was to evaluate the effects of a school-based exercise intervention on bone development in girls. The exercise intervention, lasting 5–10 minutes/session, was delivered 3 times per week in physical education class and/or recess, depending on school schedules. Additional details regarding the exercise intervention have been previously reported30. Exclusion criteria included medications known to affect bone metabolism and medical disorders associated with altered skeletal structure or function. Baseline bone and soft tissue composition data were available on 444 girls26 after exclusion of 65 pQCT scans due to the presence of motion artifact. Of those girls, 248 completed 2-year laboratory assessments and acceptable pQCT scans for soft tissue analysis; thus, these subjects were included in the present analysis. Motion artifact was determined by trained staff who rated the level of movement as described previously32. Each scan was visually inspected and rated using a linear, ordinal scale of 1 to 5 to assess the level of motion artifact present. A score of 1 represented a scan with no movement and 5 represented extreme movement such that significant image streaking and disruption of the cortical shell was present. Images graded 4 or 5 were deemed to have unacceptable motion artifact for bone and soft tissue analysis.
Anthropometry
Measures of body mass, standing height, sitting height and bone lengths were obtained following standardized protocols33. Body mass was measured (nearest 0.1 kg) using a calibrated scale (Seca, Model 881, Hamburg, Germany). Standing and sitting height were measured at full inhalation (nearest millimeter, mm) using a calibrated stadiometer (Shorr Height Measuring Board, Olney, MD). Femur and tibia lengths (nearest mm) were measured on the non-dominant leg. Leg dominance was determined by asking participants which foot they would use to kick a ball when playing soccer/kickball. If the subject was uncertain, she was asked to identify the hand used for writing, and that was determined to be her side of dominance. Femur length was measured from the proximal aspect of the patella to the inguinal crease. Tibia length was measured from the proximal end of the medial border of the tibial plateau to the distal edge of the medial malleolus. Baseline coefficients of variation (CVs; intra-operator variability) for femur and tibia lengths (n=444 girls), calculated as described by Glüer et al34, are 0.34% and 0.51%, respectively35. For each anthropometric variable, two measurements were taken and averaged. Both measurements were repeated if the first two trials differed by more than 4 mm for height, sitting height and bone lengths and 0.3 kg for body mass, and the average of the second set of measures was used.
Physical maturation
Maturation was assessed using maturity offset over the more conventional method of Tanner staging due to its reliance on objective anthropometric measurements of linear growth. Maturity offset is based on estimated years from peak height velocity (PHV) using Mirwald’s equation36. These algorithms include interactions among anthropometric measures (i.e., height, weight, sitting height, leg length) and chronologic age to derive a maturity-offset value. The following equation from Mirwald et al.36 was used to derive maturity offset in our sample of young females: Maturity offset (y)= −9.376+0.0001882*Leg Length (cm) and Sitting Height (cm) interaction+0.0022*Age (y) and Leg Length (cm) interaction+0.005841*Age (y) and Sitting Height (cm) interaction – 0.002658*Age (y) and Weight (kg) interaction+0.07693*Weight (kg) by Height (cm) ratio. Positive maturity offset values represent years after PHV while a negative maturity offset value represents years before PHV. In Mirwald’s sample, the maturity offset equation for girls explained 89% of the variance in years from PHV.36
Physical activity
Physical activity (PA) was assessed by the modified Past Year Physical Activity Questionnaire (PYPAQ), a survey of all sport and leisure-time physical activity in which the respondent engaged at least 10 times in the past year outside of physical education class37,38. The modified PYPAQ (mPYPAQ) solicits information regarding the average duration, weekly frequency and the number of months of participation for each activity. Previous cross-sectional analyses from our laboratory have shown that the modified version of the PYPAQ is positively associated with geometric adaptations of the femur and tibia and is a stronger predictor of bone strength compared to other assessments of physical activity (i.e., 3-day physical activity recall questionnaire (3DPAR) pedometer, bone-specific physical activity questionnaire, BPAQ)38. The mPYPAQ was administered in an interview with the participant and guardian. Total mPYPAQ score was computed using a modified equation from Shedd and colleagues39, which incorporated weight-bearing load, frequency and duration of each activity reported: PYPAQ score=Σ1−n [duration (minutes/session) × frequency (days/week) × load (peak strain score)], where n was the number of activities a subject reported during the past year35.
Bone and body composi\tion assessment
pQCT – Bone measures
Changes in vBMD and bone strength were assessed using pQCT (XCT 3000, Stratec Medizintechnik GmbH, Pforzheim, Germany, Division of Orthometrix; White Plains, NY, USA) at the distal 4% and 20% femur and distal 4% and 66% tibia sites relative to the respective distal growth plates on the non-dominant limb. Scout scans were performed to locate the distal growth plates, with the scanner programmed to find the sites of interest based on skeletal lengths. Slice thickness was set to 2.3 mm and voxel size was set to 0.4 mm. Scanner speed was set at 25 mm/s. Additional details regarding pQCT bone measurements, image processing, calculations, and analysis, are published elsewhere40. pQCT data acquisition and analyses followed guidelines provided by Bone Diagnostics, Inc. (Fort Atkinson, WI, USA). All pQCT scans were performed by a single operator, and one investigator (J.N.F) analyzed all scans using the Stratec software (version 6.0). The pQCT instrument was calibrated and quality assurance procedures were completed daily in order to ensure precision of measurements.
Trabecular vBMD (mg/cm3) and bone strength index (BSI, mg2/mm4) were assessed at the distal metaphyseal (4%) sites of the femur and tibia, and cortical vBMD (mg/cm3) and polar strength-strain index (SSIp, mm3) at the femur (20%) and tibia (66%) diaphyseal sites. BSI estimates the bone’s ability to withstand compression at metaphyseal regions, and is calculated as the total area×total vBMD.2 41 SSIp is used to estimate the bone’s ability to resist torsion and bending forces at diaphyseal regions. Diaphyseal SSIp was calculated using Stratec software as the integrated product of the geometric properties (i.e., section modulus) with the material properties of bone: Strength-strain index (SSIp, mm3)=Σi=1, 39 Σi=1; section modulus is calculated as , where a is the area of a voxel (mm2), r is the distance of a voxel from the center of gravity (mm), and rmax is the maximum distance of a voxel from the center of gravity (mm). The material properties of bone are calculated as the quotient of measured cortical density (cortical vBMD, mg/cm3) and normal physiologic cortical density (ND,1200 mg/cm3). To establish precision errors, 29 subjects were scanned twice with repositioning between scans; CVs calculated as described by Glüer and colleagues34 were less than 1.1% for vBMD and indices of bone strength (i.e., BSI and SSIp)26.
pQCT – Soft tissue measures
Muscle density (mg/cm3) and muscle cross sectional area (MCSA, mm2) were assessed at the 20% femur (thigh) and 66% tibia (calf) sites of the non-dominant limb using pQCT as described previously26. Tissue characteristics (i.e., adipose, muscle, and bone) were separated using edge detection and threshold techniques based on attenuation characteristics, which are directly related to tissue composition and density. Details describing edge detection and image filtration for tissue analysis in our laboratory have been previously reported26. All images were filtered subsequently with a 7×7 image filter that clearly defined the edge of the muscle and eliminated all bone above 120 mg/cm3, thereby ensuring that muscle density was the only soft-tissue component being measured within the edge of the muscle. A limitation of pQCT is the inability to distinguish between intra- and extramyocellular fat compartments; however, several controlled studies have established a clear relationship between lower muscle density and higher skeletal muscle fat content25,42. Thus, muscle density was used as a composite index of skeletal muscle fat content in the intraand extramyocellular stores. Coefficients of variation (CVs; intra-investigator) for MCSA and muscle density obtained at the calf and thigh regions were 1.4% and 0.9%, respectively, whereas CVs for these parameters at the thigh region were 1.2% and 0.4%, respectively (n=29)26.
Statistical analysis
Data were checked for outliers and normality using histograms, and skewness and kurtosis were calculated for all variables. All bone variables were normally distributed; thus, no transformations were applied. Descriptive statistics (means, SDs, and ranges) were calculated for the entire sample. Quintiles were subsequently used to divide the sample into 5 muscle density groups (fifths). Bone strength and density outcomes did not differ significantly among the three middle fifths of muscle density; thus, we collapsed these groups into a single group and compared bone parameters among the lowest, middle (average of the middle three fifths), and highest groups. Analysis of covariance (ANCOVA) was used to determine whether there were significant differences in bone development parameters among the 3 groups of baseline calf and thigh muscle density, respectively, after adjusting for ethnicity, randomization, baseline measures of maturity offset, bone lengths (femur or tibia), MCSA, 2-year change in maturity offset, and average physical activity level. Bonferroni post hoc tests were used to adjust for multiple comparisons among groups of baseline thigh and calf muscle density. Prior to ANCOVA analyses, linear regression analyses were used to assess the influence of the model covariates (e.g., ethnicity, randomization, baseline measures of maturity offset, bone lengths (femur or tibia), MCSA, 2-year change in maturity offset and average physical activity level) on bone outcomes. All regression models were assessed for linearity, normality and homoscedasticity using residual plots. The average value for physical activity (average PYPAQ) was used as a covariate over baseline or 2-year PYPAQ scores, to control for the overall effect of physical activity on bone development over the 2-year study period. The 2-year change in maturity offset was included as a covariate to capture changes in maturation, which is known to significantly influence linear growth and body composition2. Femur length or tibia length (without height) was included in regression models for thigh and calf muscle density, respectively. It should be noted that 52% of the girls included in this analysis participated in a school-based exercise intervention. However, additional analyses showed that the relationships were essentially identical in models with and without control for the exercise intervention with a categorical variable Previous results from our laboratory30 confirm that, after adjusting for similar covariates, no interactions or effect modifications were present for any of the independent variables, supporting the use of a collapsed versus stratified group. Nevertheless, to control for any potential confounding introduced by the intervention, all analyses were adjusted for inclusion in the intervention versus control group. All analyses were performed using the Statistical Package for the Social Sciences for Windows, Version 20.0 (SPSS, Chicago, IL,USA). The level of statistical significance was set at P<0.05 (two-tailed).
Results
Descriptive characteristics
Descriptive statistics for the study subjects are provided in Table 1. Sample ethnicity was 23% Hispanic and 77% non-Hispanic. Sample race was 90% white, 6.4% Asian, 2.4% black or African American, 0.8% Native American or Pacific Islander, and 0.4% other. Based on body mass index (BMI, kg/m2), at baseline, 3.2% of the sample was underweight (BMI<5th percentile), 75.4% of the sample was healthy weight (BMI 5th to 85th percentile), 13.7% of the sample was overweight (BMI 85th to 95th percentile), and 7.7% of the sample was obese (BMI>95th percentile)43. At the 2-year follow up, 2.3% of the sample was underweight, 72.7% of the sample was healthy weight, 17.7% of the sample was overweight, and 7.3% of the sample was obese. At baseline, 67% of the girls were early pubertal (Tanner stages II) and 7% of the girls had reached menarche. By the 2-year follow-up, 48% (n=118) of the girls were postmenarcheal. On average, participants were 1.2 years away from achieving PHV at baseline, ranging from 3.2 years prior to PHV to 1.0 years post PHV. At the two-year follow-up, maturity offset averaged 0.70 years post PHV (range: −1.73 to 3.21).
Table 1.
Sample descriptive characteristics (n=248).
| Baseline | 24-months | % changea | |
|---|---|---|---|
| Age (years) | 10.6±1.1 | 12.7±1.1 | - |
| Maturity offset (years) | −1.2±1.0 | 0.70±1.0 | - |
| Tanner (%; 1/2/3/4/5) | 35/33/28/4/0 | 6/13/37/37/8 | - |
| Menarche (%; Post) | 6 | 48 | - |
| Height (cm) | 144.2±9.9 | 156.9±9.1 | 8.8a |
| Weight (kg) | 38.6±9.9 | 50.0±12.2 | 29.5a |
| BMI (kg/cm2) | 18.3±3.2 | 20.1±3.8 | 9.8a |
| Femur length (cm) | 34.0±3.1 | 36.8±2.6 | 8.0a |
| Tibia length (cm) | 33.1±2.9 | 36.4±2.5 | 9.9a |
| Physical activity score | 5322.8±4670.0 | 5182.8±4204.6 | −2.6 |
| Total body fat mass (kg) | 11.0±6.0 | 15.4±7.9 | 39.5a |
| Whole body lean mass (kg) | 25.5±5.0 | 32.2±5.6 | 26.3a |
| Thigh muscle density (mg/cm3) | 76.3±1.5 | 77.5±1.4 | 1.6a |
| Calf muscle density (mg/cm3) | 79.0±1.2 | 80.0±1.2 | 1.2a |
| Thigh muscle cross-sectional area (mm2) | 3557.3±713.7 | 4407.5±916.0 | 23.9a |
| Calf muscle cross-sectional area (mm2) | 3193.6±576.7 | 3876.0±659.1 | 21.3a |
| Femur BSI (mg2/mm4) | 94.3±27.0 | 123.4±36.1 | 30.8a |
| Femur SSI (mm3) | 1320.0±399.3 | 1881.1±521.6 | 42.5a |
| 4% Femur total density (mg/cm3) | 274.5±33.5 | 289.4±40.1 | 5.4a |
| 20% Femur cortical density (mg/cm3) | 1046.5±22.9 | 1068.5±31.7 | 2.1a |
| 4% Femur trabecular density (mg/cm3) | 236.2±31.9 | 246.1±37.0 | 4.2a |
| 4% Tibia BSI (mg2/mm4) | 50.6±12.8 | 68.0±19.7 | 34.4a |
| 66%Tibia SSI (mm3) | 1153.3±328.4 | 1593.3±417.4 | 38.1a |
| 4% Tibia total density (mg/cm3) | 293.7±34.1 | 321.2±46.1 | 9.4a |
| 66% Tibia cortical density (mg/cm3) | 1027.9±31.9 | 1057.1±36.7 | 2.8a |
| 4% Tibia trabecular density (mg/cm3) | 221.9±25.3 | 229.2±30.7 | 3.3a |
Values are presented as mean ± SD. P values represent paired samples t-Test for difference between the baseline and 2-year study visit.
BSI=bone strength index (mg2/mm4); SSI=strength–strain index (mm3);
Significant at P<0.0001.
As expected, significant increases in age, maturity, height, body weight, body mass index (BMI), femur length, tibia length, total body fat mass and lean mass, calf and thigh MCSA, calf and thigh muscle density, and femur and tibia bone strength and bone density indices were observed (all p values <0.001) from baseline to the 2-year follow-up.
Associations between covariates and changes in bone outcomes
Multiple linear regression analyses were used to assess the independent relationships between model covariates and bone parameters. Baseline maturity offset was positively associated with change in total vBMD at the femur (all r=0.31) and tibia (r=0.37) and with change in cortical vBMD at the diaphyseal femur (r=0.31) (all P<0.0001). Similar positive relationships were observed between the 2-year change in maturity offset and changes in total and trabecular vBMD at the femur (r=0.32; r=0.41) and tibia (r=0.23; r=0.21) sites, as well as with all indices of bone strength (femur: r=0.48–0.64; tibia: r=0.40–0.61) (all P<0.0001). Lastly, significant, positive associations were found between baseline thigh MCSA and change in BSI (r=0.24) and SSIp (r=0.29) at the femur and baseline calf MCSA and change in bone strength indices at the tibia (r=0.30–0.34) (all P<0.0001). In contrast, in all analyses, covariates including randomization, ethnicity, physical activity, and bone length did not significantly influence the relationships between thigh and calf muscle density and bone outcomes.
Comparison of bone parameters across muscle density groups
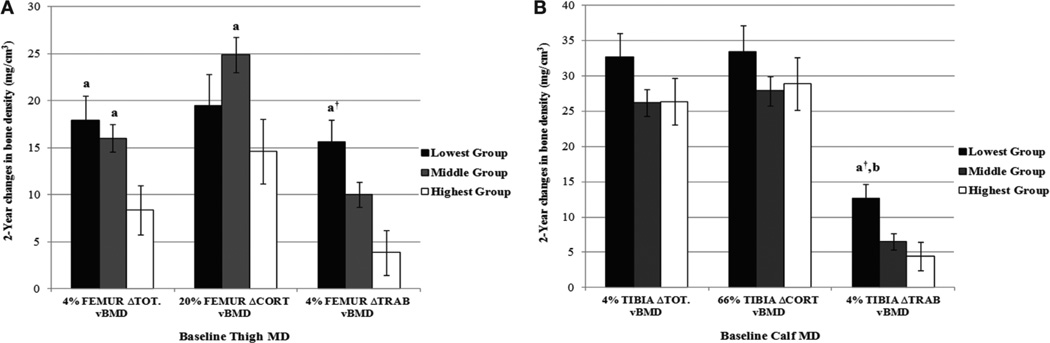
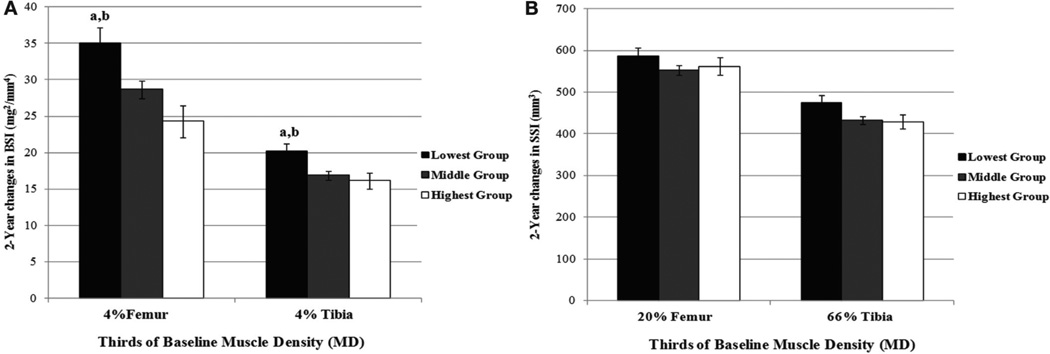
Figures 1 and 2 show the adjusted means (± standard errors) for change in vBMD (Figure 1) and bone strength (Figure 2) across groups of baseline thigh (Panel A) and calf muscle density (panel B). Participants in the lowest compared to the highest group of baseline thigh muscle density gained significantly more total vBMD (1.14 fold) at the femur whereas the middle versus highest group of baseline thigh muscle density increased more in cortical vBMD at the diaphyseal femur (all P<0.04). The largest increase in trabecular vBMD occurred at femur and tibia metaphyseal (4%) sites. Participants in the lowest group of baseline thigh muscle density had a 3.06 fold greater increase at the distal femur in trabecular vBMD (P<0.002) compared to participants in the highest group of baseline thigh muscle density. Participants in the lowest group of baseline calf muscle density similarly experienced a 1.9 fold greater gain in trabecular vBMD at the distal tibia versus the highest group of calf muscle density (P<0.013). Importantly, these changes reflected similar gains in bone strength. Participants in the lowest group had 44% (P<0.002) greater increase in femur BSI compared to the highest group of thigh muscle density and a 22% (P<0.032) gain in BSI compared to the middle group of thigh muscle density. Similarly, participants in the lowest group of baseline calf muscle density gained 19% and 25% more BSI (all P<0.03) at the tibia versus girls in the middle and highest group of calf muscle density, respectively.
Figure 1.
Adjusted (±SE) change in femur and tibia vBMD across groups of baseline thigh (A) and calf (B) muscle density. Differences among groups of baseline muscle density were evaluated by ANCOVA using randomization, ethnicity, baseline measures of maturity offset, bone lengths, MCSA, 2-year change in maturity offset and average physical activity. MD= muscle density (mg/cm3); Tot BMD= total (average) volumetric bone mineral density (mg/cm3); Cort BMD=cortical volumetric bone mineral density (mg/cm3); Trab BMD=trabecular volumetric bone mineral density (mg/cm3). aSignificantly different (P<0.05) from highest group; ANCOVA; †Significantly different (P<0.01) from highest group; ANCOVA; bSignificantly (P<0.05) different from middle group; ANCOVA.
Figure 2.
Adjusted (±SE) changes in femur and tibia BSI (A) and SSIp (B) across groups of baseline thigh and calf muscle density. Differences among groups of baseline muscle density. were evaluated by ANCOVA using randomization, ethnicity, baseline measures of maturity offset, bone lengths, MCSA, 2-year change in maturity offset and average physical activity. MD=muscle density (mg/cm3); BSI=bone strength index (mg2/mm4); SSI=strength–strain index (mm3). aSignificantly different (P<0.03) from highest group; ANCOVA; bSignificantly (P<0.05) different from middle group; ANCOVA.
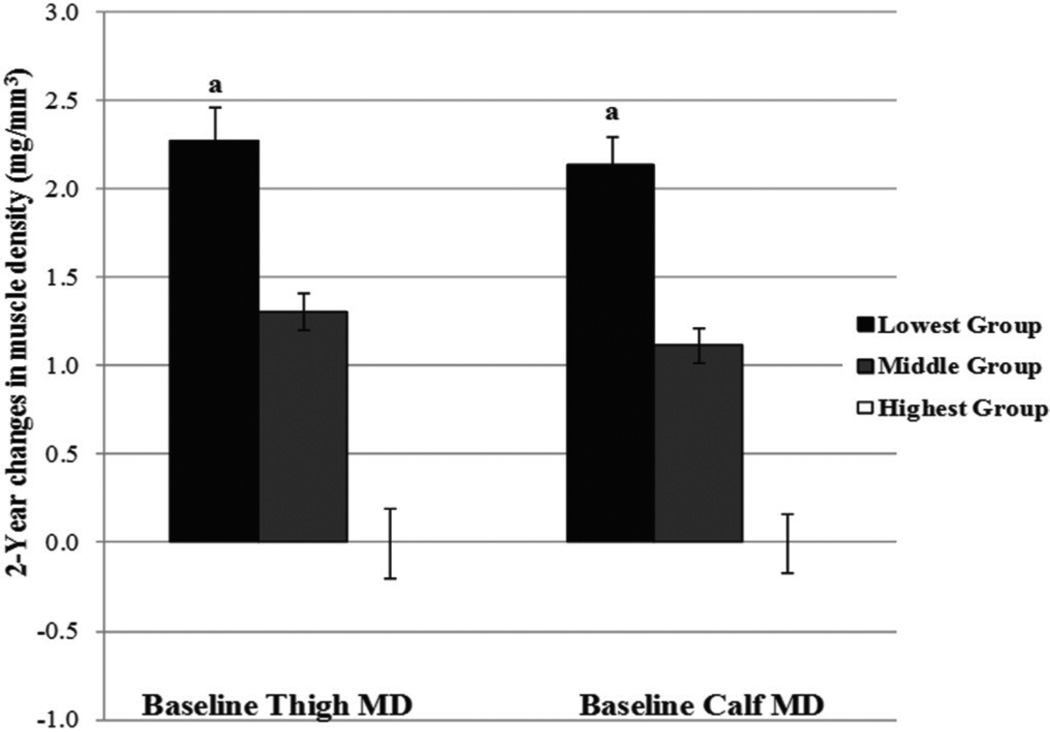
Comparisons of the change in muscle density across the lowest, middle (average of the middle three fifths), and highest groups of baseline muscle density of the thigh and calf, after adjusting for the same covariates are shown in Figure 3. Girls in the lowest group of muscle density at baseline experienced the greatest increase in muscle density as well as the greatest increase in vBMD and bone strength compared to girls in the middle and highest groups (all P<0.0001).
Figure 3.
Adjusted (±SE) changes in thigh and calf across groups of baseline thigh and calf muscle density. Differences among groups for respective groups of baseline muscle density were evaluated by ANCOVA using randomization, ethnicity, baseline measures of maturity offset, bone lengths, MCSA, 2-year change in maturity offset and average physical activity. MD=muscle density (mg/cm3). aSignificantly different (P<0.0001) from highest group; ANCOVA.
Discussion
In the present longitudinal analysis, we investigated the association of baseline muscle density, an index of skeletal muscle fat, with changes in vBMD and indices of bone strength in girls. Based on our previous work showing positive associations between muscle density and bone strength23,26, we hypothesized that girls with higher levels of calf and thigh muscle density would gain more bone over a critical 2-year period of skeletal development. Our results show that across differences of baseline muscle density, changes in bone parameters were evident, with the greatest gains in muscle density and bone parameters occurring in girls with the lowest muscle density (Figures 1–3). These results were independent of an exercise intervention effect on baseline and 2-year physical activity levels. Further, because no differences in age or maturation were observed among the muscle density groups at baseline, these findings suggest that increases in bone development parameters occur independent of changes in maturation and related biological factors that are known to affect linear growth, and therefore rules out these potential confounders as explanations. Indeed, these longitudinal data support our previous cross-sectional26 and longitudinal findings30, and suggest that muscle density is a significant predictor of changes in weight-bearing bone density and strength during rapid growth. Together, these findings support the idea that muscle and bone are related and suggest that changes in muscle density help to predict changes in bone parameters during growth. These results have important implications given that muscle development plateaus at an earlier age in females, resulting in a greater accrual of fat relative to muscle mass during adolescence44. Because 25% of bone mass is accrued in the two years surrounding peak height velocity1, understanding the contributory effects of skeletal muscle quality on bone during this “window of opportunity” could inform preventive strategies aimed to optimize gains in vBMD and bone strength during growth and possibly reduce fracture risk later in life.
Our previous cross-sectional work26 showed that higher skeletal muscle fat was associated with lower bone strength in pre- and early-pubertal girls. In the present analysis, we show that greater gains in vBMD and bone strength at metaphyseal and diaphyseal sites of the femur and tibia as well as greater increases in muscle density, occurred in girls with the lowest baseline muscle density, which supports our previous analysis showing changes in muscle density predict changes in bone outcomes30. Thus, given that lower muscle density is an index of greater skeletal muscle fat content25, our findings suggest that fatty infiltration of the skeletal muscle may impair bone development during growth and act as an important risk factor for the development of osteoporosis later in life. These results are consistent with previous studies in adults reported by Yerges-Armstrong et al.15 and Lang et al.28 who both found that indices of skeletal muscle fat content, measured by pQCT, were inversely associated with vBMD at weight-bearing skeletal sites, as well as the results of recently published longitudinal analyses from our laboratory30 showing that reductions in skeletal muscle density (increased skeletal muscle fat content) were related to lesser gains in vBMD and bone strength at the femur and tibia during a 2-year follow up. Further studies will be necessary to determine how muscle density predicts fracture risk.
The underlying mechanisms that explain the relations between skeletal muscle fat and bone are not fully understood, although evidence linking metabolic dysfunction to musculoskeletal abnormalities is mounting. For example, studies in children and adults have suggested a strong link between low muscle mass and muscular strength45 and fat deposition in skeletal muscle (i.e., intramyocellular fat stores), insulin resistance, and Type 2 diabetes mellitus23. Likewise, because the insulin signaling is involved in maintaining skeletal muscle mass as well as bone remodeling, skeletal muscle atrophy and subsequent compromises in bone strength could occur as a consequence of increased skeletal muscle triglyceride deposition46. In adults, increased storage of triglycerides and smaller lipid droplets that form along the muscle membrane has been shown to contribute to the loss of muscle strength and a decline in lower extremity performance and an increased risk of falls and fractures24. Epidemiologic studies in older adults have reported relationships between muscle mass measurements and fractures that were related to lower BMD, functional decline, and metabolic dysfunction47. In support of these data, a recent study24 reported that in elderly men and women, a 1 SD decrease in thigh muscle Hounsfield units (HU) resulted in a 50% increase in hip fracture risk. After appropriate adjustments for chronic disorders (e.g., diabetes, hypertension), the association of thigh muscle HU value with hip fracture risk remained significant (40% risk), although the decline in risk supports the additional effect of metabolic dysregulation on bone maintenance and skeletal integrity24. The relationship between skeletal muscle fat content and muscle function in young girls is unclear. Nevertheless, from our prior and current analyses, it is clear that skeletal muscle density, an established surrogate of skeletal muscle fat content25, is an important factor to consider in understanding the developing bone.
Increasing evidence demonstrates a molecular link between adipocytes, osteocytes and myocytes. Adipocytes, skeletal myocytes and osteoblasts originate from a common progenitor, multipotential mesenchymal stem cells, located within the bone marrow microenvironment. Upon activation by various growth factors47, these cells have an equal propensity to differentiate into the adipogenic, myogenic and osteogenic cells48,49. Proliferation of existing muscle satellite cells into myogenic precursor cells is specifically activated by transcription factors (MyoD, Myf5, and Pax7) released in response to increased weight bearing and mechanical stimulation from muscle contractions. Recent in vitro studies show that muscle satellite cells are capable of differentiating into osteocytes and adipocytes as well as skeletal myocytes46. Thus enhanced use and growth of muscle fibers during development contributing to gains in muscle density and muscle strength also helps drive the bone modeling and remodeling processes. Consequently, lack of mechanical stimulation from muscle contraction contributes to the differentiation of muscle satellite cells into adipocytes rather than myocytes or osteocytes. Further evidence is needed to examine whether it is possible for adipogenic cell differentiation into myocytes to occur in response to reductions in skeletal muscle fat (gains muscle density).
This study has several important strengths. The large sample size and the longitudinal design improves upon the limitations of past cross-sectional studies7,50, by providing an opportunity to assess the effects of soft tissue composition, such as fat within skeletal muscle, independent of bone loading physical activity, on bone development in girls. A novel aspect was the use of pQCT-derived measures of thigh and calf-specific muscle cross-sectional areas, surrogates for muscle size and strength, to control for mechanical stimulation from muscle forces that undoubtedly influence bone development during the pubertal transition51. Evaluating vBMD and bone strength in the context of muscle strength and bone length components is important when evaluating pediatric bone health52–54. Given the large range of physical maturation among individuals of the same chronological age our use of Mirawald’s36 equation to objectively assess bone age assessment was an additional study strength.
It should be noted that our study had a number of limitations. For example, as noted earlier, while muscle density is directly related to skeletal muscle fat content, it does not distinguish between intramyocellular (IMCL) and extramyocellular (EMCL) fat compartments, although previous studies using proton magnetic resonance spectroscopy (MRS) to measure intra- and extramyocellular fat stores in adults55 and in children23 have demonstrated that composite measures of IMCL and EMCL such as skeletal muscle density are acceptable indices of skeletal muscle fat content. Thus, muscle density from pQCT is a cost-effective and low-radiation (<0.001 mSV) surrogate for skeletal muscle fat content that is feasible for relatively large-scale studies. An additional concern was that we did not measure functional skeletal muscle parameters, which may also serve as useful surrogates for skeletal muscle quality and force production. However, in addition to the assessment of muscle density, as noted above, a significant strength was the measurement of muscle-cross sectional area, which is strongly related to skeletal muscle force production56. Another potential concern is the limited range in growth of muscle density in this population, which may have resulted in the underestimation of the impact of skeletal muscle fat content on changes in bone parameters. Indeed, in a longer follow-up study, we would expect that the differences in changes in bone parameters between the highest and lowest baseline muscle density groups would be even larger than those observed in our 2-year study. Finally, it is important to note that during the 2-year period of the present study, 52% of the girls included in this analysis participated in a school-based exercise intervention. In order to account for any bias the intervention may have introduced, we included randomization as a covariate in all statistical analyses. Nevertheless, we acknowledge that this approach may not have completely removed all potential bias.
Conclusion
In conclusion, we show that increases in bone density and bone strength vary significantly across levels of initial muscle density. Girls with lower muscle density experienced the greatest increases in bone density and strength compared to girls with higher muscle density. Girls with lower initial muscle density also experienced the greatest gains in muscle density supporting previous findings that gains in muscle density are related to gains in bone density and bone strength. Given that muscle density is a surrogate for skeletal muscle fat, these findings support the premise that skeletal muscle fat may contribute to impaired bone development in peri-pubertal girls. Increased skeletal muscle fat may serve as an important risk factor for suboptimal bone development in children and adolescents as well as for metabolic disorder16,23,24.
Acknowledgements
The project was supported by Award Number HD-050775 (SG) from the National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health. DRL is supported by the United States department of Agriculture (USDA) National Needs Fellowship: Graduate Training in Nutritional Sciences (Grant Support: NIH/NICHD #HD-050775).
All phases of this study were supported by an NIH/NICHD grant #HD-050775.
Footnotes
The authors have no conflict of interest.
References
- 1.Faulkner RA, Bailey DA. Osteoporosis: a pediatric concern? Medicine and sport science. 2007;51:1–12. doi: 10.1159/000102993. [DOI] [PubMed] [Google Scholar]
- 2.Foley S, Quinn S, Jones G. Tracking of bone mass from childhood to adolescence and factors that predict deviation from tracking. Bone. 2009;44(5):752–757. doi: 10.1016/j.bone.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14(10):1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 4.Seeman E, Delmas PD. Bone quality - the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354(21):2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 5.Dimitri P, Wales JK, Bishop N. Fat and bone in children: differential effects of obesity on bone size and mass according to fracture history. J. Bone Miner Res. 2010;25(3):527–536. doi: 10.1359/jbmr.090823. [DOI] [PubMed] [Google Scholar]
- 6.Manias K, McCabe D, Bishop N. Fractures and recurrent fractures in children; varying effects of environmental factors as well as bone size and mass. Bone. 2006;39(3):652–657. doi: 10.1016/j.bone.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Goulding A, Taylor RW, Jones IE, McAuley KA, Manning PJ, Williams SM. Overweight and obese children have low bone mass and area for their weight. Int J Obes Relat Metab Disord. 2000;24(5):627–632. doi: 10.1038/sj.ijo.0801207. [DOI] [PubMed] [Google Scholar]
- 8.Sayers A, Tobias JH. Fat mass exerts a greater effect on cortical bone mass in girls than boys. J Clin Endocrinol Metab. 2010;95(2):699–706. doi: 10.1210/jc.2009-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94(9):3387–3393. doi: 10.1210/jc.2008-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell M, Mendes N, Miller KK, et al. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab. 2010;95(3):1247–1255. doi: 10.1210/jc.2009-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi T, Kanazawa I, Yamamoto M, et al. Associations between components of the metabolic syndrome versus bone mineral density and vertebral fractures in patients with type 2 diabetes. Bone. 2009;45:174–179. doi: 10.1016/j.bone.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Russell M, Mendes N, Miller KK, et al. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab. 2010;95(3):1247–1255. doi: 10.1210/jc.2009-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pluijm SM, Visser M, Smit JH, Popp-Snijders C, Roos JC, Lips P. Determinants of bone mineral density in older men and women: body composition as mediator. J Bone Miner Res. 2001;16(11):2142–2151. doi: 10.1359/jbmr.2001.16.11.2142. [DOI] [PubMed] [Google Scholar]
- 14.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing research reviews. 2009;8(4):339–348. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Yerges-Armstrong LM, Miljkovic I, Cauley JA, et al. Adipose tissue and volumetric bone mineral density of older Afro-Caribbean men. J Bone Miner Res. 2010;25(10):2221–2228. doi: 10.1002/jbmr.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodpaster B, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 17.Miljkovic-Gacic I, Gordon CL, Goodpaster BH, et al. Adipose tissue infiltration in skeletal muscle: age patterns and association with diabetes among men of African ancestry. Am J Clin Nutr. 2008;87(6):1590–1595. doi: 10.1093/ajcn/87.6.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afghani A, Cruz ML, Goran MI. Impaired glucose tolerance and bone mineral content in overweight latino children with a family history of type 2 diabetes. Diabetes Care. 2005;28(2):372–378. doi: 10.2337/diacare.28.2.372. [DOI] [PubMed] [Google Scholar]
- 19.Pollock NK, Bernard PJ, Wenger K, et al. Lower bone mass in prepubertal overweight children with prediabetes. J Bone Miner Res. 2010;25(12):2760–2769. doi: 10.1002/jbmr.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Statistics NCfH. Health, United States, 2011: With Special Features on Socioeconomic Status and Health. Vol. 2011. Hyattsvillem MD: US: Department of Health and Human Services; 2012. [PubMed] [Google Scholar]
- 21.Goulding A, Grant AM, Williams SM. Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res. 2005;20(12):2090–2096. doi: 10.1359/JBMR.050820. [DOI] [PubMed] [Google Scholar]
- 22.Goulding A, Cannan R, Williams SM, Gold EJ, Taylor RW, Lewis-Barned NJ. Bone mineral density in girls with forearm fractures. J Bone Miner Res. 1998;13(1):143–148. doi: 10.1359/jbmr.1998.13.1.143. [DOI] [PubMed] [Google Scholar]
- 23.Sinha R, Dufour S, Petersen KF, et al. Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, central adiposity. Diabetes. 2002;51(4):1022–1027. doi: 10.2337/diabetes.51.4.1022. [DOI] [PubMed] [Google Scholar]
- 24.Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25(3):513–519. doi: 10.1359/jbmr.090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89(1):104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 26.Farr JN, Funk JL, Chen Z, et al. Skeletal muscle fat content is inversely associated with bone strength in young girls. J Bone Miner Res. 2011;26(9):2217–2225. doi: 10.1002/jbmr.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yerges-Armstrong LM, Miljkovic I, Cauley JA, et al. Adipose tissue and volumetric bone mineral density of older Afro-Caribbean men. J Bone Miner Res. 2010;,25(10):2221–2228. doi: 10.1002/jbmr.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25(3):513–519. doi: 10.1359/jbmr.090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L, Nicholson P, Wang Q, Alen M, Cheng S. Bone and muscle development during puberty in girls: a seven-year longitudinal study. J Bone Miner Res. 2009;24(10):1693–1698. doi: 10.1359/jbmr.090405. [DOI] [PubMed] [Google Scholar]
- 30.Farr JN, Laddu DR, Blew RM, Lee VR, Going SB. Effects of physical activity and muscle quality on bone development in girls. Med Sci Sports Exerc. 2013;45(12):2332–2340. doi: 10.1249/MSS.0b013e31829c32fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrari SL, Chevalley T, Bonjour JP, Rizzoli R. Childhood fractures are associated with decreased bone mass gain during puberty: an early marker of persistent bone fragility? J Bone Miner Res. 2006;21(4):501–507. doi: 10.1359/jbmr.051215. [DOI] [PubMed] [Google Scholar]
- 32.Blew RM, Lee VR, Farr JN, Schiferl DJ, Going SB. Standardizing evaluation of pQCT image quality in the presence of subject movement: qualitative versus quantitative assessment. Calcif Tissue Int. 2014;94(2):202–211. doi: 10.1007/s00223-013-9803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohman TGRA, Martorell RA. Anthropometric Standardization Reference Manual. Champaign, IL: 1988. [Google Scholar]
- 34.Gluer C, Blake G al YLe. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int. 1995;5:262–270. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- 35.Farr JN, Funk JL, Chen Z, et al. Skeletal muscle fat content is inversely associated with bone strength in young girls. J Bone Miner Res. 2011;26(9):2217–2225. doi: 10.1002/jbmr.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34(4):689–694. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 37.Aaron DJ, Kriska AM, Dearwater SR, Cauley JA, Metz KF, LaPorte RE. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am J Epidemiol. 1995;142(2):191–201. doi: 10.1093/oxfordjournals.aje.a117618. [DOI] [PubMed] [Google Scholar]
- 38.Farr JN, Lee VR, Blew RM, Lohman TG, Going SB. Quantifying bone-relevant activity and its relation to bone strength in girls. Med Sci Sports Exerc. 2011;43(3):476–483. doi: 10.1249/MSS.0b013e3181eeb2f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shedd KM, Hanson KB, Alekel DL, Schiferl DJ, Hanson LN, Van Loan MD. Quantifying leisure physical activity and its relation to bone density and strength. Med Sci Sports Exerc. 2007;39(12):2189–2198. doi: 10.1249/mss.0b013e318155a7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farr JN, Tomas R, Chen Z, Lisse JR, Lohman TG, Going SB. Lower trabecular volumetric BMD at metaphyseal regions of weight-bearing bones is associated with prior fracture in young girls. J Bone Miner Res. 2011;26(2):380–387. doi: 10.1002/jbmr.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kontulainen SA, Johnston JD, Liu D, Leung C, Oxland TR, McKay HA. Strength indices from pQCT imaging predict up to 85% of variance in bone failure properties at tibial epiphysis and diaphysis. Journal of musculoskeletal & neuronal interactions. 2008;8(4):401–409. [PubMed] [Google Scholar]
- 42.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54(3):509–515. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 43.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;(246):1–190. [PubMed] [Google Scholar]
- 44.Ashby RL, Adams JE, Roberts SA, Mughal MZ, Ward KA. The muscle-bone unit of peripheral and central skeletal sites in children and young adults. Osteoporos Int. 2011;22(1):121–132. doi: 10.1007/s00198-010-1216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCarthy HD, Samani-Radia D, Jebb SA, Prentice AM. Skeletal muscle mass reference curves for children and adolescents. Pediatric obesity. 2013 doi: 10.1111/j.2047-6310.2013.00168.x. [DOI] [PubMed] [Google Scholar]
- 46.Sishi B, Loos B, Ellis B, Smith W, du Toit EF, Engelbrecht AM. Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp Physiol. 2011;96(2):179–193. doi: 10.1113/expphysiol.2010.054189. [DOI] [PubMed] [Google Scholar]
- 47.Di Monaco M, Vallero F, Di Monaco R, Tappero R, Cavanna A. Fat mass and skeletal muscle mass in hip-fracture women: a cross-sectional study. Maturitas. 2007;56(4):404–410. doi: 10.1016/j.maturitas.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Zhao LJ, Jiang H, Papasian CJ, et al. Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res. 2008;23(1):17–29. doi: 10.1359/JBMR.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68(4–5):245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 50.Roemmich JN, Clark PA, Lusk M, et al. Pubertal alterations in growth and body composition.. VI. Pubertal insulin resistance: relation to adiposity, body fat distribution and hormone release. Int J Obes Relat Metab Disord. 2002;26(5):701–709. doi: 10.1038/sj.ijo.0801975. [DOI] [PubMed] [Google Scholar]
- 51.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219(1):1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 52.Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am J Clin Nutr. 2007;86(5):1530–1538. doi: 10.1093/ajcn/86.5.1530. [DOI] [PubMed] [Google Scholar]
- 53.Petit MA, Beck TJ, Kontulainen SA. Examining the developing bone: What do we measure and how do we do it? Journal of musculoskeletal & neuronal interactions. 2005;5(3):213–224. [PubMed] [Google Scholar]
- 54.Klein GL, Fitzpatrick LA, Langman CB, et al. The state of pediatric bone: summary of the ASBMR pediatric bone initiative. J Bone Miner Res. 2005;20(12):2075–2081. doi: 10.1359/JBMR.050901. [DOI] [PubMed] [Google Scholar]
- 55.Jacob S, Machann J, Rett K, et al. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes. 1999;48(5):1113–1119. doi: 10.2337/diabetes.48.5.1113. [DOI] [PubMed] [Google Scholar]
- 56.Maughan RJ, Watson JS, Weir J. Relationships between muscle strength and muscle cross-sectional area in male sprinters and endurance runners. Eur J Appl Physiol Occup Physiol. 1983;50(3):309–318. doi: 10.1007/BF00423237. [DOI] [PubMed] [Google Scholar]