Summary
Human apolipoprotein (apo) E has three isoforms: apoE2, apoE3 and apoE4. APOE4 is a major genetic risk factor for Alzheimer’s disease (AD), and is associated with dementia in Down’s syndrome and poor neurological outcome after traumatic brain injury, cerebral hemorrhage and other neuropathological disorders. While apoE4 can induce neuropathology by participating in various cellular and molecular pathways, here, I review data supporting the hypothesis that apoE4 has direct toxic effects on the cerebrovascular system which in turn can lead to secondary neuronal dysfunction and degeneration and accumulation of neurotoxins in brain such as Alzheimer’s amyloid-β (Aβ). I review i) Aβ-independent cerebrovascular effects of apoE, particularly activation of a pro-inflammatory cyclophilin A-mediated pathway in brain vascular pericytes by apoE4 that has been recently shown to lead to a loss of cerebrovascular integrity and blood-brain barrier breakdown causing neuronal injury; and ii) Aβ-dependent cerebrovascular effects of apoE such as faulty Aβ clearance from brain to circulation by apoE4. I also discuss isoform-specific interactions of apoE with the low-density lipoprotein receptor related protein-1 on brain vascular cells (i.e., endothelial cells, pericytes) which plays an important role in Aβ-independent and Aβ-dependent effects of apoE on cerebral vasculature.
Apolipoprotein (apo) E was discovered in the early seventies as a protein associated with cholesterol-rich and triglyceride-rich plasma lipoproteins (for review see Mahley et al., 2009).1 ApoE is synthesized by the liver and secreted into the circulation as a protein incorporated into very low density lipoproteins, chylomicron remnants, and a subclass of high density lipoproteins. ApoE regulates transport of cholesterol and lipids throughout the body and mediates clearance of plasma lipoproteins by the low density lipoprotein receptor (LDLR) and other LDLR-related protein family members.1 In the brain, apoE is produced mainly by astrocytes.2 In the cerebrospinal fluid (CSF) and interstitial fluid (ISF) of the central nervous system (CNS), apoE circulates incorporated into small particles and disks resembling high density lipoproteins. Throughout the body and within the CNS, apoE contributes to restorative processes mediating redistribution of lipids to cells that require cholesterol and phospholipids.
Human apoE has three alleles located on a single gene locus on chromosome 19q13 encoding three major apoE isoforms, apoE2, apoE3, and apoE4.3 These apoE isoforms differ by single amino acid substitutions at two residues which have a major effect on the structure and function of apoE protein at the molecular and cellular level, and association with neuropathological conditions. ApoE2 has a cysteine residue at position 158, whereas apoE3 and apoE4 each have arginine. It is believed that this confers greater stability of apoE2 protein and is associated with its protective effect against Alzheimer’s disease (AD).4 ApoE4 is a major genetic risk factor for AD3 and possesses an arginine at residue 112, whereas both apoE2 and apoE3 have cysteines. This single amino acid difference is associated with relative instability of apoE4 protein and its multiple pathogenic effects on cells.1 The most common apoE3 isoform has a cysteine residue at position 112 and an arginine at position 158 which provides greater protein stability compared to apoE4, and is associated with much lower risk for neuropathological disorders compared to APOE4 carriers.
Diverse neuropathological effects of apoE4 on cells within the CNS include, but are not limited to1, 2: i) direct toxic effects on neurons that could be mediated by impaired neurite outgrowth, cytoskeletal disruption, hyperphosphorylation of tau, mitochondrial dysfunction and impaired synaptogenesis; ii) reduced Alzheimer’s amyloid β (Aβ) clearance from brain; and iii) direct toxic effects on the cerebrovascular system causing and/or contributing to neuronal dysfunction and neurodegnerative changes. Several recent reviews provide an excellent overview on apoE isoform-specific toxic effects on neurons and effects on neuropathological disorders.1, 2, 4 Therefore, focus of the present review is on cerebrovascular effects of apoE. Particularly, I discuss apoE isoform-specific effects on cerebrovascular integrity and Aβ vascular clearance, and how apoE4 acting through the cerebrovascular system contributes to development of cognitive and neuropathological changes related to the onset and progression of AD.
ApoE4 and AD
In a recent review on AD genetics, Tanzi summarized multiple genome wide association studies (GWAS) indicating that the APOE4 gene is by far the strongest genetic risk factor for AD increasing by 400% to 1,500% risk of apoE4 carriers for developing disease compared to apoE3/3 individuals.3 For comparison, the 10 other genes most frequently associated with AD by GWAS carry much smaller risk change for AD ranging typically between 10 and 15% (eg, CD33, CLU [clusterin] or PICALM).3 Recent studies have suggested that heterozygous rare variants in TREM2, encoding the triggering receptor expressed on myeloid cells 2 protein, are associated with a significant increase in the risk for AD.5, 6
Approximately 25% of all individuals are carriers of the apoE4 allele which makes the detrimental effects of apoE4 gene expression quite frequent. As illustrated in Table 1, the apoE4 allele is substantially enriched in individuals with AD with 64% and 80% of all patients with sporadic or familial AD carrying at least one copy of the apoE4 allele, respectively.7 A recent study using over 10,000 control subjects and over 7,000 AD cases has shown that the life time risk for developing AD in apoE4 homozygotes is close to that of the BRAC1 gene for breast cancer, which is 57% at 70 years of age.8 Table 2 illustrates that apoE4 homozygotes have the life time risk for developing AD approximately 30% and 60% at 75 and 85 years of age, respectively. In contrast, the life time risk for AD in apoE3 homozygotes is 2% and 10% at 75 and 85 years of age, respectively.
Table 1.
Influence of APOE4 gene on Alzheimer’s disease
| Allelic Frequency | Control | Sporadic AD | Familial AD |
|---|---|---|---|
| ε2 | 8% | 4% | 2% |
| ε3 | 78% | 59% | 48% |
| ε4 | 14% | 37% | 50% |
| APOE4 Carriers | 26% | 64% | 80% |
Modified from Farrer et al., 1997
Table 2.
Effects of APOE Genotype on Lifetime Risk Estimates for Developing Alzheimer’s Disease
| Genotype | 75 years of age | 85 years of age |
|---|---|---|
| ApoE4/E4 | 30% | 60% |
| ApoE4/E3 | 8% | 30% |
| ApoE3/E3 | 2% | 10% |
Modified from Genin et al.8
It has been reported that the apoE4 allele decreases the age of onset of AD by approximately 8 years to 15 years in apoE4 heterozygotes and homozygotes, respectively.3, 7 In addition, APOE4 is associated with dementia in Down’s syndrome and poor neurological outcome after traumatic brain injury and hemorrhage.4 Some studies have suggested that APOE4 is associated with other neuropathologies such as multiple sclerosis, stroke, frontotemporal dementia and Parkinson’s disease. However, the data for these associations are not as strong as for AD and have yet to be reproduced by multiple independent studies.
Neurovascular unit
The neurovascular unit (NVU) comprises vascular cells including endothelium and mural cells such brain capillary pericytes and arterial and/or venous vascular smooth muscle cells, glial cells including astrocytes, microglia and oliogodendroglia, and neurons.9 The endothelial cells of brain capillaries are connected by the tight junctions forming a continuous cellular membrane that underlies the anatomical blood-brain barrier (BBB). The BBB limits the entry of potentially toxic plasma components, red blood cells and leucocytes into the brain. The BBB also regulates the delivery of circulating energy metabolites such as glucose and essential nutrients (e.g., amino acids, vitamins) into the CNS, which are required for proper neuronal and synaptic function. Larger molecules such as peptides and proteins normally do not cross the BBB unless they have specialized transport systems.10
Recent studies have shown that pericytes regulate the BBB permeability and play a major role in maintaining cerebrovascular integrity at the level of brain capillaries which in turn prevents entry of various potentially neurotoxic and vasculotoxic macromolecules in the blood from entering the CNS.11, 12 The BBB also plays a key role in removal of potentially neurotoxic molecules from brain such as glutamate or Alzheimer’s Aβ.9 Neurodegenerative disorders including AD are associated with microvascular dysfunction and/or degeneration in the brain, neurovascular disintegration, defective BBB and vascular risk factors.9 In addition, several genes identified by GWAS studies might affect directly cerebral vascular system and/or vascular clearance of Aβ.3
In AD, a compromised cerebrovascular pathology such as degeneration of brain capillary endothelium, reduced endothelial tight junction protein levels, thickening of the capillary basement membrane and degeneration of small cerebral arteries contribute to reductions in the resting cerebral blood flow (CBF), dysregulation of CBF responses to brain activation and/or impaired BBB function. These microvascular changes typically diminish brain supply of oxygen, energy metabolites and nutrients. Moreover, some studies have suggested that primary vascular dysfunction precedes neuronal dysfunction and neurodegenerative changes and might also contribute to accumulation of Aβ in the brain and the vessel wall resulting in development of cerebral β-amyloidosis and cerebral amyloid angiopathy, both features of AD.9
ApoE Aβ-independent effects on cerebrovascular integrity
As reviewed elsewhere, neurovascular dysfunction may be present in normal APOE4 carriers before cognitive decline and Aβ accumulation occur, and is found in individuals with APOE4-associated neurological disorders including AD, stroke and brain hemorrhage.9, 13 A recent meta-analysis of BBB permeability based on imaging and biochemical CSF studies indicated that AD patients have a greater increase in BBB permeability compared to neurologically healthy human controls which has been also confirmed by post-mortem brain tissue studies (for review see Zlokovic, 2011; Sengillo et al., 2012).9, 14 Importantly, post-mortem analysis indicated that the BBB breakdown is more pronounced in AD individuals carrying the APOE4 allele.15 It has been reported that BBB breakdown in AD patients is associated with significant reductions in the pericyte populations in the cortex and hippocampus.14 However, whether disruption in cerebrovascular integrity precedes cognitive decline in AD patients particularly in the APOE4 carriers remains elusive. In addition, the cellular and molecular mechanisms leading to BBB disruption in AD and particularly in AD cases carrying the APOE4 allele remain largely unknown.
A possible limitation of any study of human brain tissue is the post-mortem sampling, with results reflecting an end stage process. Moreover, longitudinal studies in individuals with mild cognitive impairment or AD patients with different APOE genotypes to compare changes in BBB permeability with a loss of executive functions, cognitive decline, disease onset and/or progression, do not currently exist. Therefore, studies in experimental models such as transgenic mice expressing different human apoE isoforms are needed i) to better characterize age-dependent effects of APOE genotype on brain microcirculation, and determine how changes in cerebrovascular integrity contribute to neuronal dysfunction and neurodegenerative changes; and ii) to elucidate the underlying cellular and molecular mechanisms of BBB disruption.
Recently, we studied transgenic mice with targeted replacement of murine apoE with each human apoE isoform (TR-APOE), mice lacking murine apoE (Apoe−/−), mice expressing each human apoE isoform under control of the astrocyte-specific glial fibrillary acidic protein promoter on an apoE null background, and Apoe−/− and APOE4 transgenic mice with ablation and/or pharmacological inhibition of the proinflammatory cytokine cyclophilin A (CypA).13 In search of molecules that could mediate BBB dysfunction observed in Apoe−/− and APOE4 mice we focused on CypA because CypA has been shown to have deleterious vascular effects in Apoe−/− mice including aortic aneurysms and atherosclerosis.
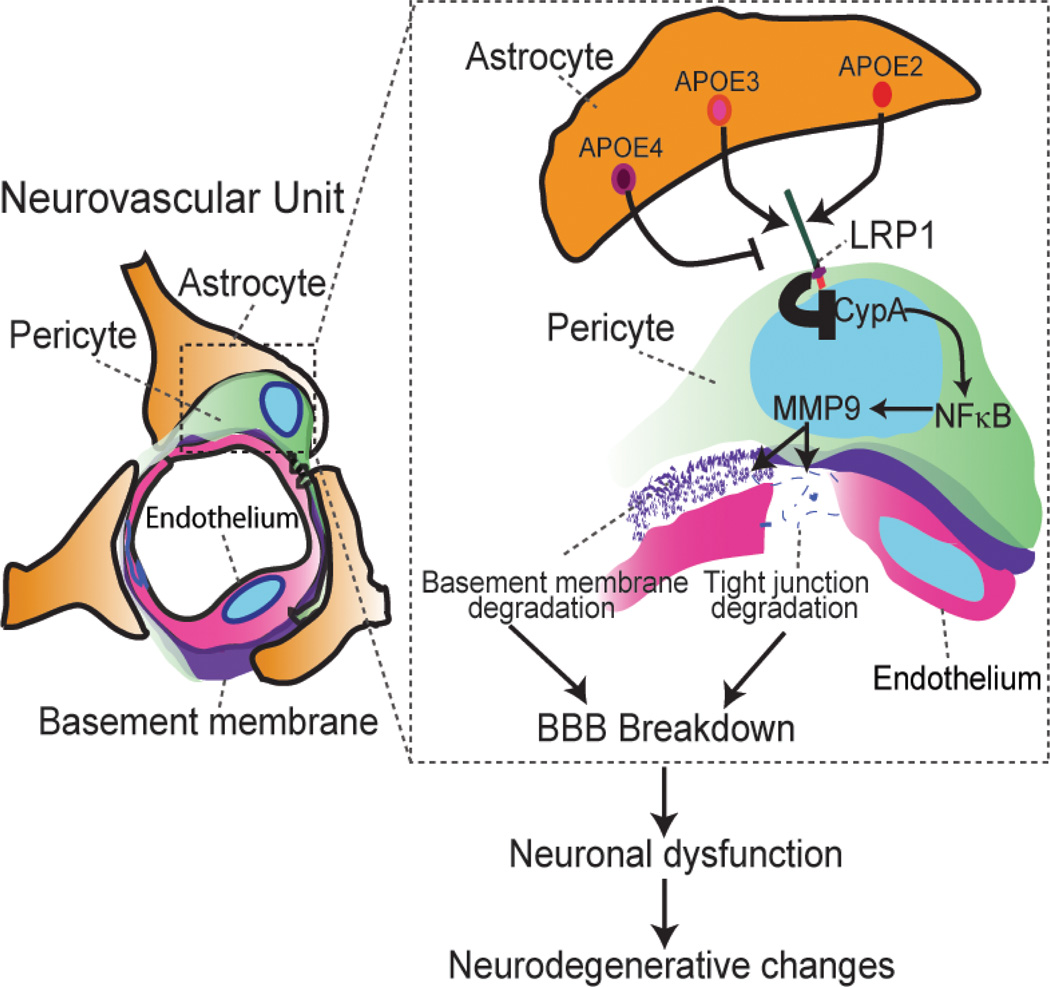
Using different humanized APOE transgenic mouse lines expressing apoE in brain mainly in astrocytes, we showed that astrocyte-derived human apoE4, but not human apoE2 and apoE3, leads to an age-dependent progressive BBB breakdown by activating a proinflammatory CypA-nuclear factor (NF)-κB-matrix-metalloproteinase-9 pathway (MMP-9) in brain capillary pericytes (Figure 1). We next showed that activation of MMP-9 in APOE4 mice leads to enzymatic degradation of the BBB tight junction and basement membrane proteins resulting in BBB breakdown followed by neuronal uptake of multiple blood-derived neurotoxic proteins (e.g., thrombin, fibrin), perivascular deposition of erythrocyte-derived hemosiderin and microvascular and CBF reductions. Importantly, our data show that the vascular defects in APOE4-expressing mice precede neuronal dysfunction and can initiate neurodegenerative changes. In addition, this study showed that astrocyte-secreted apoE3 and apoE2, but not apoE4, suppress the CypA-NF-κB-MMP-9 pathway in pericytes via the low density lipoprotein receptor related protein 1 (LRP1).13 The study confirmed previous findings in cerebral vascular cells showing that apoE3 and apoE2 have a relatively high affinity binding and/or interaction with LRP1, in contrast to apoE4 which shows barely detectable interaction with LRP1 in brain endothelial cells16 and pericytes13. In summary, these findings suggest that CypA is a key target for treating apoE4-mediated neurovascular injury and the resulting neuronal dysfunction and degeneration.
Figure 1.
A schematic showing that astrocyte-secreted APOE2 and APOE3, but not APOE4, signal to pericytes via low density lipoprotein receptor related protein 1 (LRP1), suppressing the CypA–NF-kB–MMP9 pro-inflammatory pathway that causes blood-brain barrier (BBB) breakdown by MMP9-mediated degradation of tight junction and basement membrane proteins. BBB dysfunction is associated with the accumulation of several neurotoxins in the brain which affect neuronal function and contribute to the development of neurodegenerative changes. Modified from ref. 11.
ApoE Aβ-dependent effects on cerebrovascular clearance
According to the two-hit vascular hypothesis of AD, the Aβ peptide and its different forms, particularly the lower molecular weight oligomers are thought to contribute to neuronal and synaptic dysfunction as a second hit in disease process.9 It is well established that there are apoE isoform-specific effects in the Aβ pathway.2, 17 For example, apoE4 expression is associated with a significant increase in amyloid plaques in brain at earlier ages compared with apoE3 or apoE2. Importantly, several experimental studies have shown that apoE4 impairs Aβ clearance from brain and across the BBB in animal models and patients at risk for developing AD16, 17 which in turn promotes Aβ retention in brain and contributes to formation and deposition of amyloid fibrils.
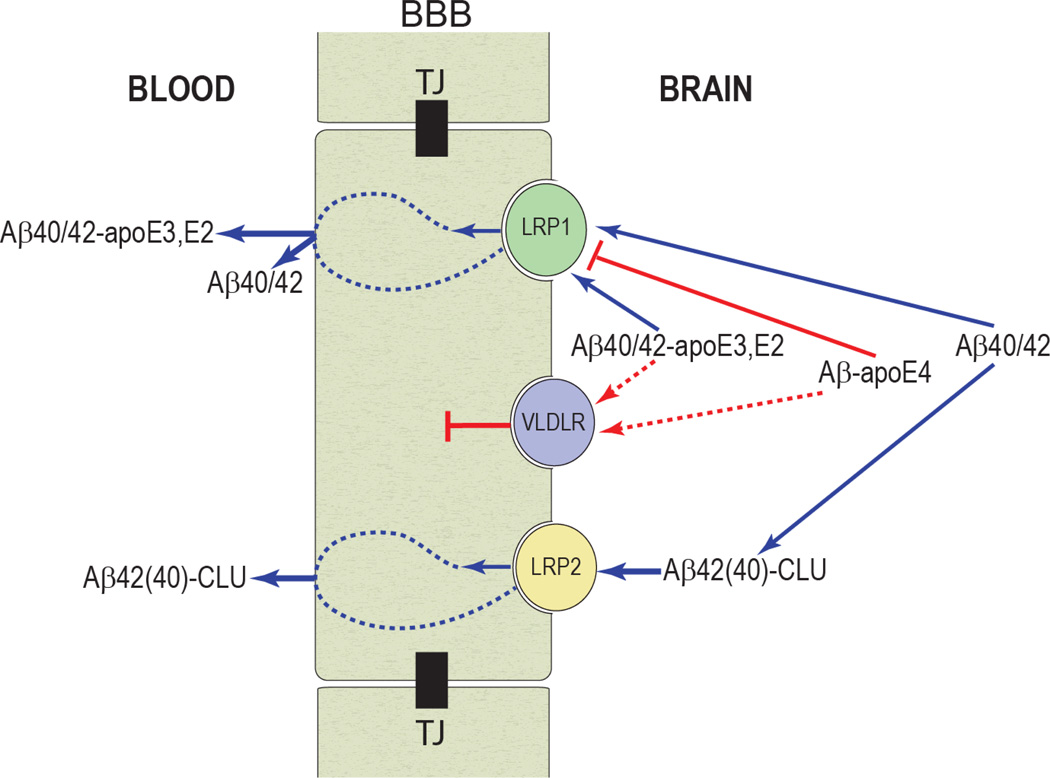
With respect to Aβ clearance across the BBB, studies in rodents have shown that Aβ binding to apoE4 redirects the rapid clearance of free Aβ40/42 from LRP1 to the VLDL receptor (VLDLR), which internalizes apoE4 and Aβ-apoE4 complexes at the BBB more slowly than LRP1 (Figure 2).16 In contrast, apoE2 and apoE3 as well as Aβ-apoE2 and Aβ-apoE3 complexes are cleared at the BBB via both VLDLR and LRP1 at a substantially faster rate than Aβ-apoE4 complexes. Astrocyte-secreted lipidated-apoE2, lipidated-apoE3, and lipidated-apoE4 as well as their complexes with Aβ are cleared at the BBB by mechanisms similar to those of their respective lipid-poor isoforms but at 2- to 3-fold slower rates. Thus, apoE isoforms differentially regulate Aβ clearance from the brain, and this might contribute to the effects of APOE genotype on the disease process in both individuals with AD and animal models of AD.16 Interestingly, it has been also suggested that Aβ binding to clusterin (apoJ) leads to rapid clearance of Aβ across the BBB via LRP2 receptor (Figure 2).18
Figure 2.
The role of blood-brain barrier (BBB) clearance in homeostasis of brain Aβ. Aβ binding to apoE and clusterin (CLU, also referred to as apoJ) in brain interstitial fluid (ISF) influences its aggregation and clearance from brain across the BBB via low-density receptor-related protein 1 (LRP1) and LRP2. In contrast to rapid clearance of free Aβ40/42 and their complexes with apoE2 or apoE3 at the BBB via LRP1, Aβ-apoE4 is redirected to a slow VLDLR-mediated clearance mechanism and is subsequently eliminated from brain at a significantly slower rate. LRP2 contributes to rapid efflux of Aβ-CLU complexes from brain. Modified from refs. 14 and 16.
Conclusions
In summary, several studies have suggested that apoE4 in contrast to apoE2 and apoE3 has direct toxic effects on the cerebrovascular system and may affect neurovascular functions independently of Aβ pathology. In this review, we summarized some recent experimental studies demonstrating differential effects of apoE isoforms on neurovascular functions.
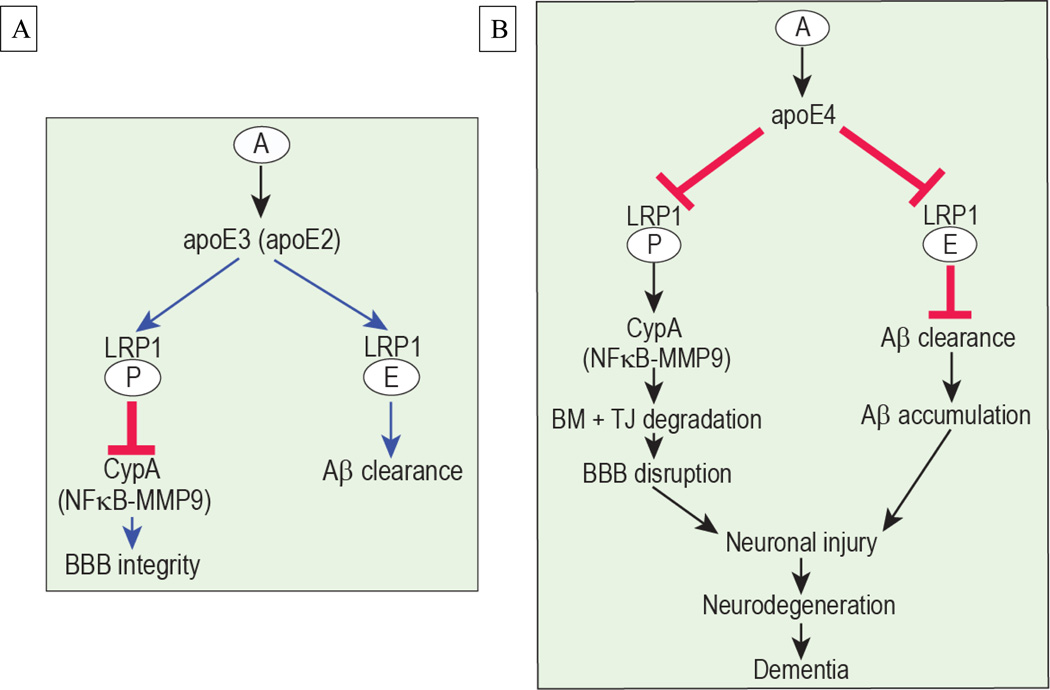
As illustrated in Figure 3A, we propose that astrocyte-secreted apoE3 and apoE2 bind to and interact well with LRP1 on pericytes, which blocks the pro-inflammatory CypA-MMP-9 pathway maintaining the cerebrovascular integrity13, and with LRP1 on brain endothelial cells which results in clearance of Aβ across the BBB16. On the other hand, we propose that poor interaction of apoE4 with LRP1 results in activation of CypA-NFkB-MMP-9 pathway in pericytes causing degradation of the BBB tight junction and basement membrane proteins which results in BBB disruption with accumulation in brain of different blood-derived potentially neurotoxic molecules (e.g., thrombin, fibrin, plasmin) and erythrocyte-derived hemosiderin preceding neuronal injury and degenerative changes (Figure 3B). In Aβ pathway, a weak interaction of apoE4 with LRP1 in brain endothelial cells diminishes Aβ clearance across the BBB resulting in accumulation of Aβ in brain which can injure neurons directly and/or potentiate neurodegenerative changes caused by BBB breakdown.
Figure 3.
Proposed role of apoE isoform-specific effects on the cerebrovascular system via regulation of CypA-NF-κB-MMP9 pathway and Aβ clearance. A. Astrocyte (A) secreted apoE2 and apoE3 interacts with LRP1 on pericytes and suppresses the proinflammatory CypA-NF-κB-MMP9 pathway which maintains blood-brain barrier (BBB) integrity. Additionally, apoE2 and apoE3 interact with LRP1 in the endothelial cells to mediate Aβ clearance from brain to blood. B. Astrocyte secreted apoE4 interacts weakly with LRP1 in pericytes (P) and endothelial cells (E) and its binding to LRP1 on vascular cells is barely detectable. In pericytes, this weak interaction results in increased intracellular CypA which activates the proinflammatory NF-κB-MMP9 pathway leading to BBB disruption and accumulation of neurotoxic blood-derived molecules in brain causing neuronal injury. In endothelial cells, the weak interaction between apoE4 and LRP1 fails to efficiently remove Aβ from brain contributing to Aβ accumulation and Aβ-mediated neuronal injury.
Future studies using transgenic mice with targeted replacement of human APOE gene and reduced or complete deletion of LRP1 from brain endothelium and pericytes and crossed with AD mice should, however, further validate the proposed clearance hypothesis (Fig. 3) exploring whether ApoE/LRP1 interaction both in endothelium and pericytes can regulate Aβ clearance from brain via efflux across the BBB (apoE3 >> apoE4). In addition, studies using transgenic mice with targeted replacement of human APOE gene and reduced or complete deletion of LRP1 from brain endothelium and pericytes should further validate whether apoE/LRP1 deficiency both in endothelium and pericytes can lead to cerebrovascular and BBB disruption causing neuronal dysfunction and degeneration. Moreover, future studies in individuals with apoE4 genotype should explore whether similar early neuroimaging and biochemical markers of BBB disruption are present in humans carrying the APOE4 allele before cognitive decline and/or Aβ accumulation occur.
Acknowledgements
This research was supported by the National Institute of Health grants AG039452, AG23084 and NS34467 to B.V.Z. I am also grateful to Dr. Abhay Sagare for preparing figures.
REFERENCES
- 1.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer's disease to AIDS. J Lipid Res. 2009 Apr;50(Suppl):S183–S188. doi: 10.1194/jlr.R800069-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009 Aug 13;63(3):287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(10) doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011 Mar;10(3):241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerreiro R, Wojtas A, Bras J, et al. TREM2 Variants in Alzheimer's Disease. N Engl J Med. 2012 Nov 14; doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonsson T, Stefansson H, Ph DS, et al. Variant of TREM2 Associated with the Risk of Alzheimer's Disease. N Engl J Med. 2012 Nov 14; doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997 Oct 22–29;278(16):1349–1356. [PubMed] [Google Scholar]
- 8.Genin E, Hannequin D, Wallon D, et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry. 2011 Sep;16(9):903–907. doi: 10.1038/mp.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011 Dec;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zlokovic BV, Lipovac MN, Begley DJ, Davson H, Rakic L. Transport of leucine-enkephalin across the blood-brain barrier in the perfused guinea pig brain. J Neurochem. 1987 Jul;49(1):310–315. doi: 10.1111/j.1471-4159.1987.tb03431.x. [DOI] [PubMed] [Google Scholar]
- 11.Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010 Nov 4;68(3):409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011 Nov;14(11):1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012 May 24;485(7399):512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells Coincides with Blood-Brain Barrier Disruption in Alzheimer's Disease. Brain Pathol. 2012 Nov 5; doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zipser BD, Johanson CE, Gonzalez L, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer's disease. Neurobiol Aging. 2007 Jul;28(7):977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Deane R, Sagare A, Hamm K, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008 Dec;118(12):4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castellano JM, Kim J, Stewart FR, et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011 Jun 29;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell RD, Sagare AP, Friedman AE, et al. Transport pathways for clearance of human Alzheimer's amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007 May;27(5):909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]