Abstract
The development of the nervous system involves cells remaining within the neural tube (CNS) and a group of cells that delaminate from the dorsal neural tube and migrate extensively throughout the developing embryo called neural crest cells (NCC). These cells are a mesenchymal highly migratory group of cells that give rise to a wide variety of cell derivatives: melanocytes, sensory neurons, bone, Schwann cells, etc. But not all NCC can give rise to all derivatives, they have fate restrictions based on their axial level of origin: cranial, vagal, trunk and sacral. Our aim was to provide a thorough presentation on how does trunk neural crest cell migration looks in the chicken embryo, in wholemount and in sections using the unique chicken marker HNK1. The description presented here makes a good guideline for those interested in viewing trunk NCC migration patterns. We show how before HH14 there are few trunk NCC delaminating and migrating, but between HH15 through HH19 trunk NCC delaminate in large numbers. Melanocytes precursors begin to enter the dorsolateral pathway by HH17. We found that by HH20 HNK1 is not a valid good marker for NCC and that HNK1 is a better marker than Sox10 when looking at neural crest cells morphology and migration details.
Keywords: Trunk neural crest, Cell migration, HNK1, Sox10, Neural crest, Peripheral nervous system
Introduction
The development of the nervous system is a fascinating process involving cell induction, determination and movement. Of all embryonic cell types that have key roles during development, the neural crest is unique: it consists of mesenchymal highly migratory cells that are stem cells, which can give rise to a wide variety of cell lineages. In addition to forming neurons and glia of the peripheral nervous system, neural crest cells form craniofacial skeleton, teeth, cornea, melanocytes and adrenal chromaffin cells. This wide variety of cell types arises from multipotent cells that have stem cell properties (Bronner-Fraser and Fraser, 1988; Stemple and Anderson, 1992).
After neural crest cells have been determined at the beginning of neurulation when the neural tube closes and pinches off from the ectoderm, they undergo an epithelial to a mesenchymal cell type change at the dorsal neural tube and transform into a migratory population that moves extensively throughout the developing embryo (Garcia-Castro and Bronner-Fraser, 1999). One key aspect is that neural crest cells migrate along much conserved pathways throughout the embryo (Bronner-Fraser, 1994; Le Douarin, 2004). The molecular nature of the cues guiding these specific movements are only beginning to be understood and appear to differ at different axial levels of the nervous system. Importantly, neural crest cells are classified depending on their point of origin. Thus we have cranial neural crest cells, vagal, trunk and lumbo-sacral (Fig. 1) (Le Douarin et al., 1993). Each population has different potentials regarding their derivatives and target areas. The most versatile is the cranial crest because it is the one that can give rise to peripheral nervous system, but also to cranial and facial structures, corneal keratinocytes and olfactory glia (Baker, 2005; Santagati and Rijli, 2003).
Fig. 1.
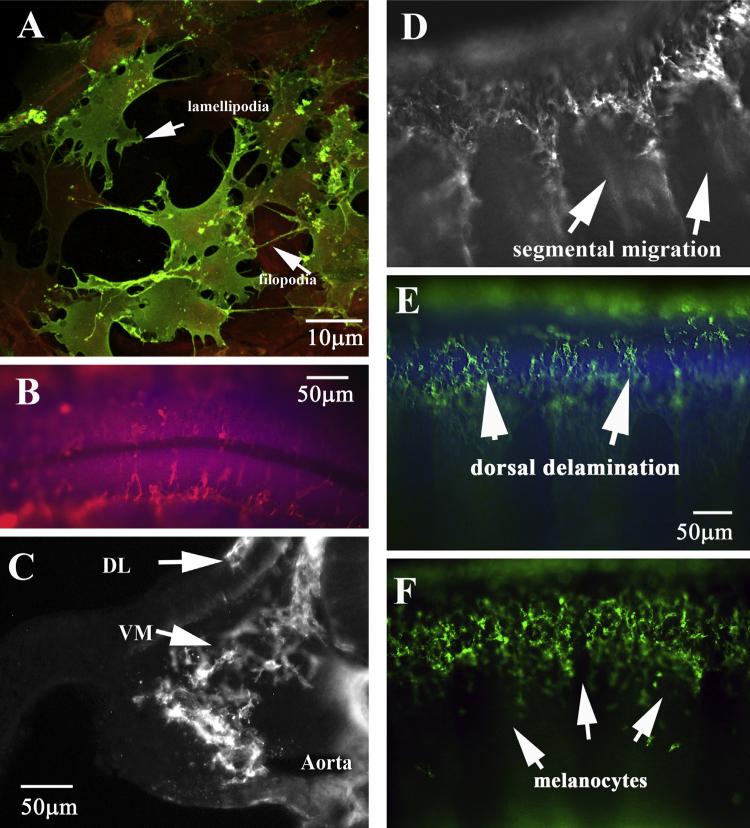
HNK1 labels chicken neural crest cells in great detail. (A) Neural crest cells in culture show classic morphology of mesenchymal cells: many filopodia and lamellipodia. Neural crest cells are stained with HNK1 (green) and other cells with actin (red). (B) Dorsal view of delaminating trunk neural crest cells on top of the two halves of the trunk neural tube. The neural tube is labeled with DAPI. (C) Midtrunk section through HH17 embryo shows migrating trunk crest cells throughout the trunk region. (D–F) Wholemount side views of migrating trunk neural crest cells in caudal (D) or rostral (E) of HH17 embryo and rostral (F) of HH20 embryo. DL: dorso-lateral migrating NCC, VM: ventro-medial migrating NCC. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
With the advent of antibodies and specifically, the discovery that the HNK1 epitope is highly abundant in migrating neural crest cells a new path was opened in the study of neural crest cells and their migratory pathways (Tucker et al., 1984; Vincent et al., 1983). Thus we came to know that trunk and vagal neural crest cells follow a specific stereotypical pathway: they migrate along the rostral 2/3 portions of the somite and they avoid the caudal third (Bronner-Fraser, 1986; Loring and Erickson, 1987; Rickmann et al., 1985). Although this unique migration pattern has been elegantly demonstrated, known and well-studied in large number of manuscripts, it has not been updated with more modern microscopy capabilities since the 1980s to provide a big overview of trunk neural crest migration. Here we want to pay homage and expand on the work done in the labs of Marianne Bronner, Nicole Le Douarin, Carol Erickson and Jean Luc Thiery by presenting a wider range of immunostaining of trunk neural crest cells across several stages ranging from their initial steps onto their peak. The aim here is to provide a thorough presentation on how does trunk neural crest migration look in the chicken embryo, in wholemount and in sections, thus providing what we think will be a valuable representation on trunk neural crest migration for those interested in studying these cells as well as a reference for knowing what to expect for these cells at their different stages of development.
Materials and methods
Animals
Chicken embryos were obtained by incubating fertilized chicken eggs at 38 °C as described by Hamburger and Hamilton (1951) until desired stage of development. Embryos were collected and after gently removing their membranes were fixed overnight at 4 °C in 4% paraformaldehyde.
Wholemount immunofluorescence
After overnight incubation in blocking buffer (PBS with 1% Triton-X100, 10% goat serum), chicken embryos were incubated with 1:50 HNK1 supernatant (DHSB, Iowa) or Tuj1 (Sigma) in PBS overnight at 4 °C, then were extensively washed (minimum of 3 h with at least 6 changes) with PBS and then incubated with and goat-anti-mouse IgM secondary antibodies conjugated Alexa Fluor® Dyes 1:500 (Invitrogen, Carlsbad, CA, USA). Next day embryos were washed extensively (minimum of 3 h with at least 6 changes) in PBS. We also added DAPI to visualize nuclei, 1:5000. Embryos were photographed using a 4× lens objective with a Zeiss A-1 AxioIm-ager and images were aligned and positioned next to each other to create the composite wholemount with Photoshop.
The new developments for high resolution microscopy and improvements over past optics are described here: http://zeiss-campus.magnet.fsu.edu/tutorials/basics/objectivemagnification/indexflash.html.
Immunofluorescence
Chicken embryos were either mounted in 4% agarose and vibratomed (50 μm sections) and photographed with a Zeiss AxioImager microscope or serially dehydrated for paraffin embedding. Paraffin blocks were sectioned (12 μm sections), deparaffinized and serially rehydrated, before rinsing several times in PBS and blocked in 10% normal goat serum for 1 h. HNK1 antibody was diluted in a permeabilizing solution (PBS, 0.2% Triton X-100, 0.1% sodium azide) according to the optimal dilutions (detailed in Table 1) and placed on the slides to incubate overnight at room temperature. Sections were then treated with Alexa Fluor® Dyes 1:500 (Invitrogen, Carlsbad, CA, USA) fluorescently labeled secondary antibodies diluted 1:1000 in PBS.
In situ hybridization
Chicken embryos were fixed in 4% paraformaldehyde (PFA) overnight before being stored in 0.1 M phosphate buffered saline (PBS). Patterns of gene expression were determined by whole-mount in situ hybridization using DIG-labeled RNA antisense probes as described in Grove's lab website (http://www.bcmneuroscience.org/groveslab/protocols.php). Briefly embryos in hybridization buffer are incubated at 65°C overnight with respective probe. Next day after washing with hybridization buffer embryos are further washed in maleic acid buffer before blocking and adding anti-digoxigenin alkaline phosphatase antibody. Next day embryos are extensively washed and color reaction done following Boehringer instructions for NBT/NCBI substrates. The cSox10 probe used is described in (De Bellard et al., 2003). The Seraf chicken plasmid was kindly obtained from Jim Weston (Wakamatsu et al., 2004).
Electroporations of chicken neural tubes
Chicken embryos HH15 were windowed by cutting a small hole on the shell and visualized with India ink (10% in Ringer's solution) to determine their stage of development. After gently removing covering membrane, 1–2 mg/ml solution of DNA (pMES-GFP) was injected into the embryos neural tubes using a mouth pipette and immediately electroporated with two 50 ms pulses of 20 mV each. Embryos were sealed with tape and re-incubated for 48 h until fixation in 4% paraformaldehyde.
Results and discussion
While HNK1 antibody has been known to be a good marker for neurons (Metcalfe et al., 1990) and Schwann cells (Bock et al., 2007), it has been better known/used for its ability to label chicken neural crest cells. The advantage that HNK1 provides when studying neural crest cells is because these cells put large amounts of this carbohydrate epitope on their surface and thus are thoroughly labeled by this antibody (Del Barrio and Nieto, 2004; Loring and Erickson, 1987). Nevertheless, chicken is not the only organisms whose neural crest cells express HNK1 epitope. Other squamata, like turtles, snakes and crocodile neural crest are well labeled with HNK1 (Cebra-Thomas et al., 2007; Kundrat, 2008; Reyes et al., 2010). Although the best universal marker for neural crest cells is the transcription factor Sox10 (Cheng et al., 2000; Sauka-Spengler and Bronner-Fraser, 2006), HNK1 as shown above is a reliable and easy marker to use when studying neural crest cells, thus our present approach following on a well-established method by crest researchers and as a practical way to study in detail the migratory pattern of these cells in the chicken embryo.
A confocal projection of a group of trunk neural crest cells after explant culture with cells labeled with HNK1 highlights the advantage of using HNK1: neural crest cells immunostained with this antibody show their cell morphology to the greatest detail: all the lamellipodia ruffles and filopodia (Fig. 1A). These cultures show in two dimensions, what is the classic mesenchymal shape of trunk neural crest cells in vivo (Fig. 1B–F). The development of more modern and sophisticated optics in microscopes combined with the development of highly sensitive CCD cameras providing higher resolution, allowed views of embryo development not possible in the 1980s and 1990s (Sandell et al., 2012; Yokomizo et al., 2012). Using these new available techniques we are providing here a wider description and showcase of the known trunk neural crest migration patterns across chicken embryo axial levels as well developmental stages.
Neural crest cells delamination and morphology
The neural crest starts as an epithelial population in the dorsal neural tube and becomes migratory after undergoing an epithelial to mesenchymal transition. HNK1 antibody labels the neural crest as soon as they delaminate and start migrating away from the neural tube (Bronner-Fraser, 1986). Thus they can be seen as individual cells with filopodia and lamellipodia on top of the neural tube (Fig. 1B, E). Next step in their migration happens when these cells encounter the medial portion of the developing somite and start what is referred to as their “ventro-medial” pathway (section in Fig. 1C, D, E). At later stages, a “second” wave of migrating trunk neural crest cells that will give rise to melanocytes and skin structures, enter what is referred as the “dorso-lateral” pathway. Here cells migrate under ectoderm and above somitic tissue in a nonsegmental pattern since they are not guided by the caudal somites chemorepellants but by ectoderm signals that attract them toward developing skin like SCF and kept away by somite morphogenesis (Fig. 1C, F) (Belmadani et al., 2009; Grichnik, 2006; Tosney, 2004).
The first aspect that newer microscopy optics allows us to observe is what is commonly referred as wholemount immunofluorescence. Here we can appreciate the sophistication of the neural crest pathways in better resolution and in one single image, not possible or shown when these pathways were demonstrated in mid 80s is the ventromedial and dorsolateral pathways that trunk crest take at later stages (Fig. 1D–F). In this figure not only can we observe cells that are already beginning to migrate toward the dorsal aorta (VM in Fig. 1C), but also those that started to migrate under the ectoderm (not segmentally any longer) as future melanocytes (DL in Fig. 1C, F). Thus with new microscope power we can observe at much greater detail and better resolution what was not feasible in the early 1980s when these seminal studies demonstrated the segmental migration of trunk neural crest (Bronner-Fraser, 1986; Loring and Erickson, 1987; Rickmann et al., 1985). This is the first description of this scope (several stages and higher resolution) that we are aware of for trunk neural crest cell migration after those papers.
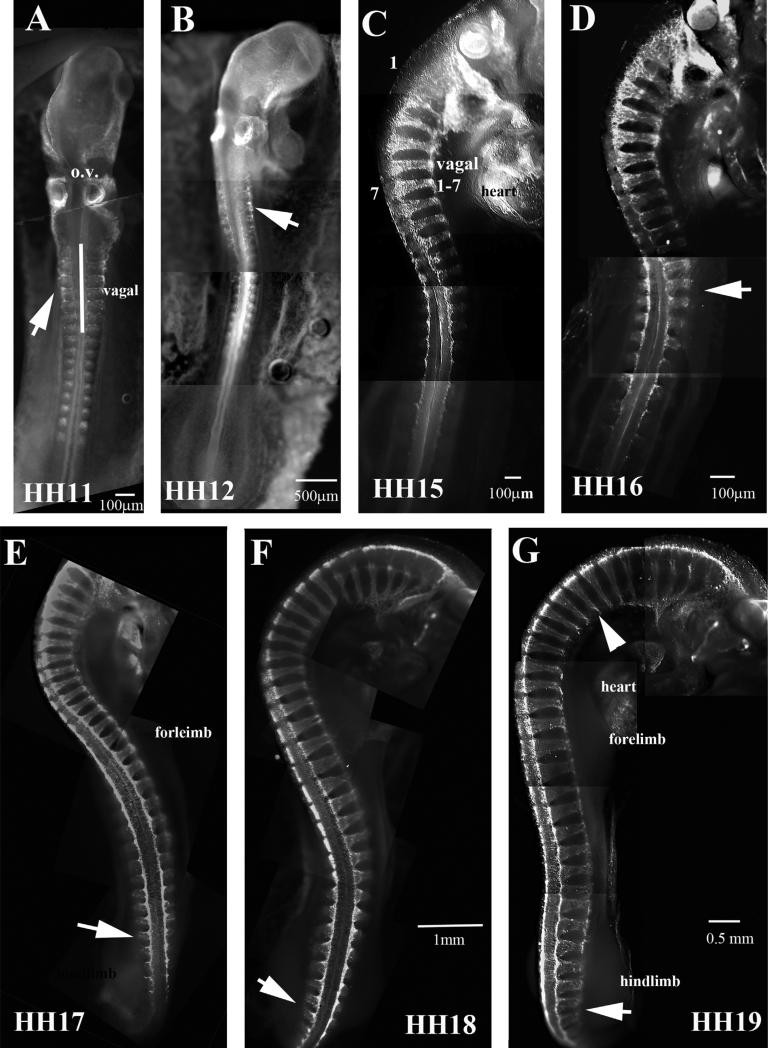
Neural crest cells delaminate in a neat orderly rostral to caudal fashion, starting with cranial crest first and ending with sacral neural crest (Bronner-Fraser and Cohen, 1980; Clay and Halloran, 2010; Kulesa and Gammill, 2010; Le Douarin, 1980). This pattern has been named segmental migration and consists on a wave of delaminating cells as the neural tube develops in the early embryo, thus trunk neural crest does not start migrating until the trunk neural tube has developed at least 7 somites, not counting vagal region (1–7th somite), therefore, trunk crest does not appear before HH12–13 (Bronner-Fraser, 1995a,b; Krull, 2001). Carrying out wholemount immunostaining of chicken embryos ranging from HH11 to HH19 allows us to appreciate the extent of the rapid and abundant migration of the trunk neural crest in early chicken embryo in a rostral to caudal progression (Fig. 2). At HH11–12 we see very few neural crest cells delaminating and migrating at the trunk level, although the cranial crest is migrating extensively and vagal crest is beginning. At this stage is also noticeable that somites are also slightly positive for HNK1 as was demonstrated in the past (Newgreen et al., 1990). But by HH15 and 16 we observe abundant number of cells migrating past the vagal region (somites 1–7). The peak of trunk neural crest migration occurs at HH17–18, when the largest numbers of cells are migrating throughout the embryo, which will continue through HH19 (Fig. 2E–G). Another noticeable observation is that by HH19 the vagal region although it has HNK1 cells, we know from sections that these HNK1 positive cells correspond to Schwann cell precursors and differentiating sensory neurons condensing into spinal ganglia (Fig. 2G).
Fig. 2.
Wholemount views of HH11–19 embryos with HNK1. HNK1 labels migrating trunk neural crest poorly in HH11 and HH12 (A, B), but strongly by HH15 (C). Footnotes indicate the stage of the chicken embryo. Vagal crest is indicated in (C). Arrow in (D) points to 16th somite. Arrow in (E–G) to migrating NCC at tail ends. Arrowhead in (G) point to thinning of NCC streams, indicating that they are beginning to condense. In (A) o.v. stands for otic vesicles.
Trunk neural crest migration pattern at tail end
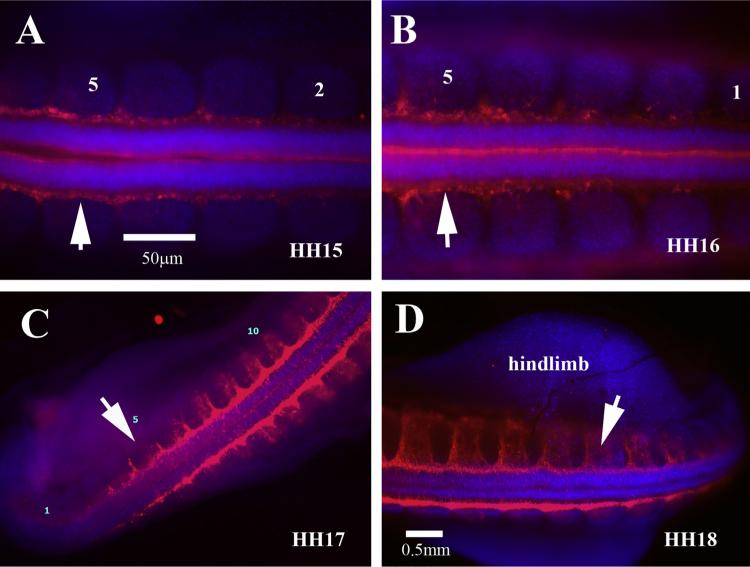
One finding that stands out from doing this side-by-side whole-mounts projections is the difference at the tail end between HH15–16 and HH17–18 in the amount of delaminating neural crest cells. Comparing across figures it is noticeable that at HH15 there are fewer delaminating neural crest cells in the last 5 somites (Fig. 3A, B), while in HH17–18 there is a larger area labeled with HNK1 along the neural tube before entering the somites (Fig. 3C, D). Trunk neural crest cells are seen entering the rostral portion of the somites in the fifth somite formed from tail end in HH15 (Fig. 3A). However, the amount of cells entering the somites is much larger in HH17 embryos, suggesting that there is larger number of cells delaminating at this stage of development than earlier periods (Bronner, 2012; Krispin et al., 2010; Vaglia and Hall, 1999). This is further corroborated in Fig. 8 with Sox10, which shows a much smaller Sox10 labeling in the last somites compared with HH17 (Fig. 8D, E).
Fig. 3.
Wholemount with HNK1 views of chicken tail end. (A) At HH15 there are not many trunk neural crest delaminating from last 5 somites (these are indicated by numbers). (B) By HH16 the amount of cells positive for HNK1 has increased significantly. Tail ends of HH17 (C) and HH18 (D) have twice as many cells delaminating as well as migrating through the last somites.
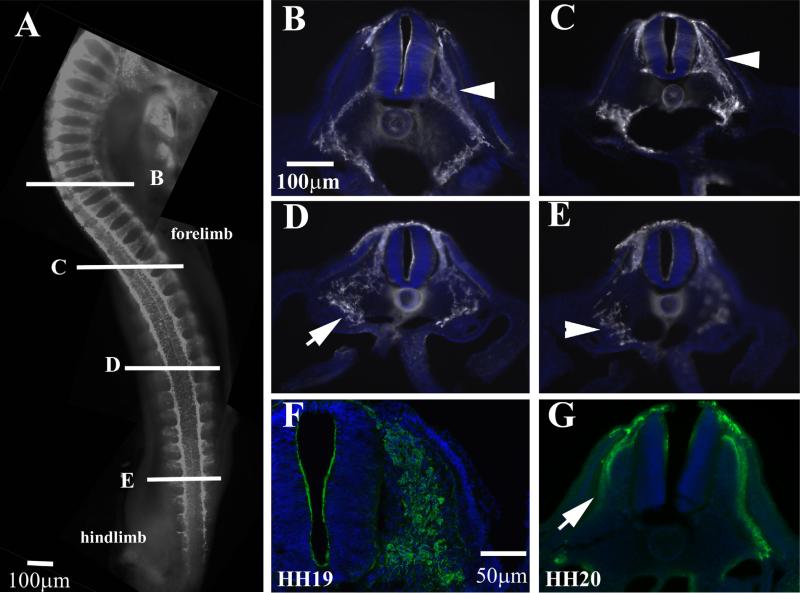
Fig. 8.
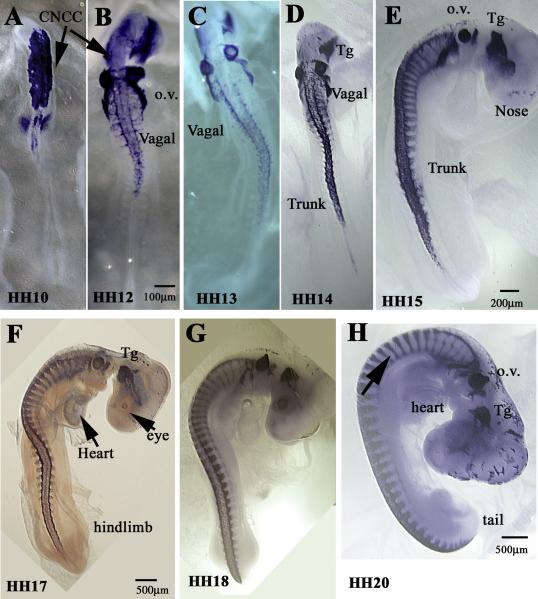
Wholemount views of HH10–20 embryos with Sox10. Sox10 transcription factor labels migrating trunk neural crest very well in chicken embryos. (A–F) Cranial neural crest cells (CNCC) are beginning to migrate at HH10 and continue through HH17 giving rise to cranial ganglia (Tg), cornea (stream around the eye in (F)). Trunk NCC are visible by HH14 as streams on the rostral side of somites posterior to Vagal NCC (D–G). By HH20 there are still migrating NCC along the embryo posterior trunk region, but condensing ganglia and spinal nerves begin to appear in the trunk too (arrow in (H)). Footnotes indicate the stage of the chicken embryo. o.v.: otic vesicles, Tg: trigeminal ganglia.
Another key observation with new camera technologies that was not possible in the past is showing the presence of migrating neural crest cells on top of the neural tube. The first manuscripts that focused on showing neural crest migration did it in sections, not wholemounts. At earlier stages, HH15–16, we found few to none, but by HH17–19 we observed large numbers of trunk neural crest cells visible on the top portion of the neural tube. This implies that at these stages the neural crest is at its peak of delamination (Fig. 3A–D).
Trunk neural crest migration pattern in HH16
A closer look at HH16 chicken embryo immunostained with HNK1 shows vigorous migration along the first 16 somites (Fig. 4A). The vagal neural crest starts showing signs that the first wave of cells had reached the dorsal aorta region and are beginning to condense and fuse into the sympathetic chain (Kuo and Erickson, 2010), while more caudal segments show segmental wave of cells migrating ventrally toward dorsal aorta region where they will form the future sympathetic chain (Fig. 4C, E). A section around the 11th somite shows a cluster of HNK1 positive cells in larger numbers by the future dorsal root ganglion (DRG) region next to neural tube, a scattered group of trunk crest cells migrating toward the aorta and few HNK1 positive cells ventral to aorta corresponding to vagal crest cells that entered the gut mesenchyme to eventually give rise to enteric nervous system (Burns and Le Douarin, 2001) (Fig. 4E).
Fig. 4.
Wholemount views of HH16 embryo with HNK1. (A) Wholemount embryo with HNK1 shows many cells migrating in the first half of the trunk. (B–E) Higher magnification of the rostro to caudal progression of neural crest migration. (F) A trunk section pointed with arrow in (A) wholemount.
Trunk neural crest migration peaks at HH17
The peak of trunk neural crest migration is considered to be at HH17–18 (Bronner-Fraser, 1993; Le Douarin and Dupin, 1993; Le Douarin et al., 1993). It is easy to see why these researchers deemed it so from observing embryos aligned together (Fig. 2) and by observing how much migrating trunk crest cells has increased in numbers and along the growing rostro-caudal axis (Fig. 5A). Serial sections along the trunk at this stage show robust number of cells migrating in “clusters” or better, chains (see also Fig. 1C, D). This pattern has been excellently review by Theveneau and Mayor (2012), and is better known as “collective cell migration” (Theveneau et al., 2010; Theveneau and Mayor, 2010). This type of migration is lost at later stages as can be seen by comparing similar HNK1 midtrunk sections later in development (HH19–20). At HH19 is obvious that neural crest cells are not migrating in chains but are beginning to coalesce and form DRGs (Fig. 5F) so that by HH20 we cannot distinguish neural crest cells by HNK1 which labels the DRG capsule, and we can easily observe a separate group of cells, the DRG (Fig. 5G).
Fig. 5.
Wholemount views of HH17 embryo with HNK1. (A) Wholemount embryo with HNK1 shows many more cells migrating along the trunk. (B–E) Sections pointed in wholemount embryo. (F) A midtrunk section in HH19 and (G) of HH20 embryo to highlight the reduction of cell migrating by these stages. Arrows in (B–E) point to streams of trunk migrating, while in (G) the lack thereof.
Trunk neural crest migration stays strong in HH18–19 embryos
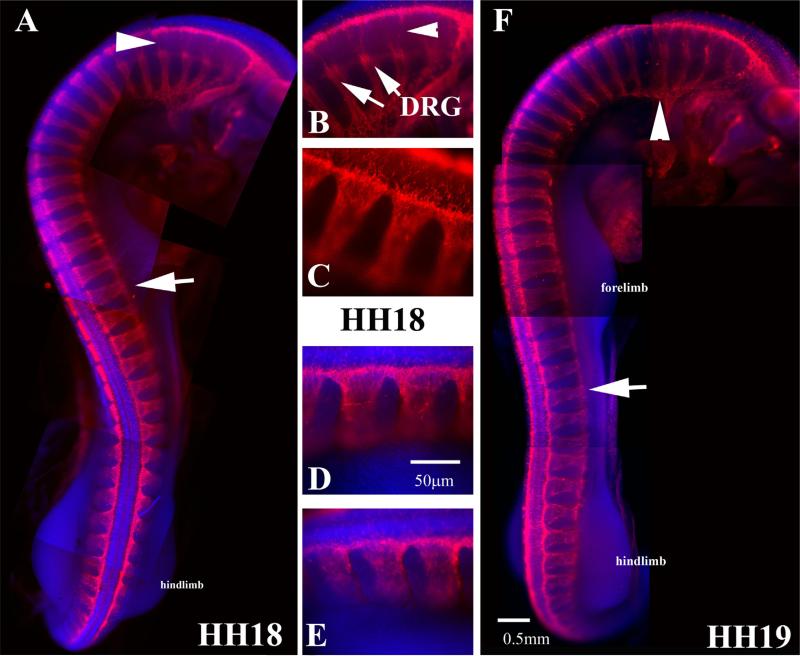
An embryo at HH18 shows many more developments and differences compared with HH16 since organogenesis is well underway (heart, ear, limb buds, etc. are clearly distinguishable) (Hamburger and Hamilton, 1951). The vagal DRGs are already advanced in developments as seen by their condensing into ganglia as well as by lack of HNK1 migrating cells between the dorsal neural tube and DRG (Fig. 6A, B). Secondly, as mentioned for Fig. 2, the thickness of the layer of HNK1 positive cells at practically all trunk levels is quite more robust than HH16 (Fig. 6C–E). Thirdly, although these embryos have noticeable limb buds, trunk neural crest cells in either HH18 or HH19 are not yet seen invading this mesodermal tissue to form the axial plexuses; this will begin by HH21 (Fredette and Ranscht, 1994). Although there are no striking differences between HH18 and HH19 with HNK1 labeling, under careful observation we can notice a reduction in the number of migrating trunk neural crest cells past forelimb region, indicating that neural crest migration is beginning to wean (Fig. 6F).
Fig. 6.
Wholemount views of HH18 and HH19 embryo with HNK1. (A) Wholemount embryo with HNK1 shows the peak of trunk neural crest migration along the whole trunk in HH18 embryo. (B–E) Higher magnification of the rostro to caudal progression of neural crest migration of HH18 embryo in (A). DRGs can be seen in (B) (arrows). (F) HH19 embryo, arrowhead points to vagal region spinal nerves, arrow points midtrunk crest cells still migrating.
Trunk neural crest migration starts slowing down in HH20 embryos
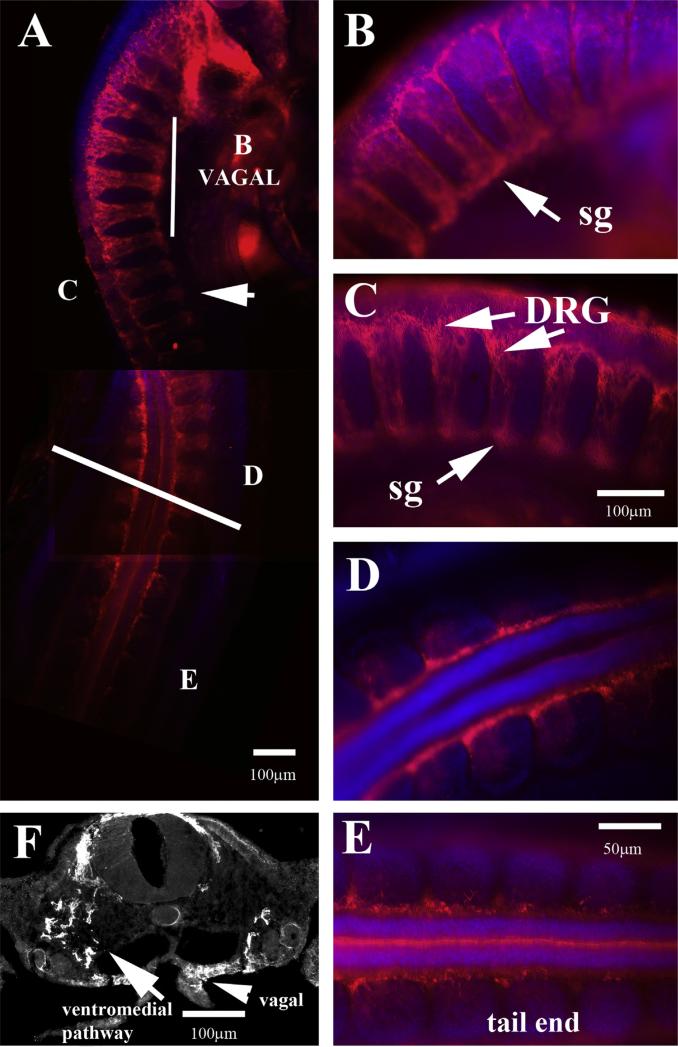
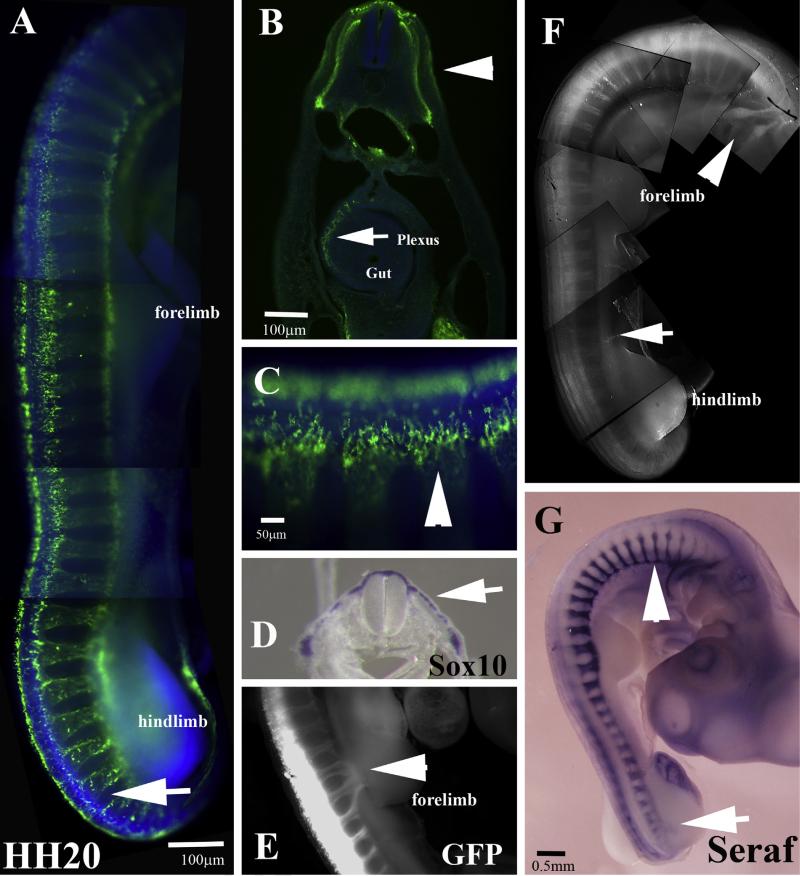
HNK1 labeling in HH20 embryo shows a striking difference with previous stages: the labeling looks punctuated since it corresponds to dorsally migrating neural crest cells (Fig. 7A–C). By HH20 the migrating trunk neural crest entering limb buds are not visible with HNK1, however we can detect them if we carry on GFP electroporation of neural tube (Fig. 7E) or in situ hybridization with a probe for Schwann cell marker Seraf (Wakamatsu et al., 2004) (Fig. 7G). Sections through HH20 embryo before hindlimb show that although HNK1 stains considerably the pathways of the trunk neural crest, it is mostly a remnant of crest derived cells: Schwann cell precursors around DRG and peripheral nerves (Jessen and Mirsky, 1991), enteric neural crest cells and crest cells along the melanocytic pathway (Fig. 7B, C) (Dupin and Le Douarin, 2003). Trunk neural crest cells along the ventromedial pathway do not express much HNK1 any longer, making this marker obsolete at these later stages. However, this does not mean that these cells have stopped being “neural crest cells” since they still express robust levels of Sox10 transcription factor which labels trunk neural crest cells very reliably (Cheng et al., 2000) and see Figs. 8 and 9.
Fig. 7.
Wholemount views of HH20 embryo. (A) Wholemount embryo with HNK1 shows a great reduction in migrating HNK1 labeled trunk neural crest, noticeable in tail end (arrow in (A)). (B) Section through embryo in (A) shows the ventral pathway in HNK1 as well as enteric plexus (arrow) in the gut, arrowhead points to dorsomedial cells along melanocytic pathway. (C) Arrowhead points dorsomedial migrating cells in HNK1. (D) Arrow points Sox10 cells migrating under the ectoderm. (E) Neural tube electroporated with GFP labels peripheral nerves entering forelimb. (F) Neuronal marker Tuj1 labels differentiated neural crest in DRG (arrowhead) and cranial ganglia (arrow). (G) Schwann cell marker Seraf labels their precursors along sympathetic chain (arrowhead) and nerves entering hindlimb (arrow).
Fig. 9.
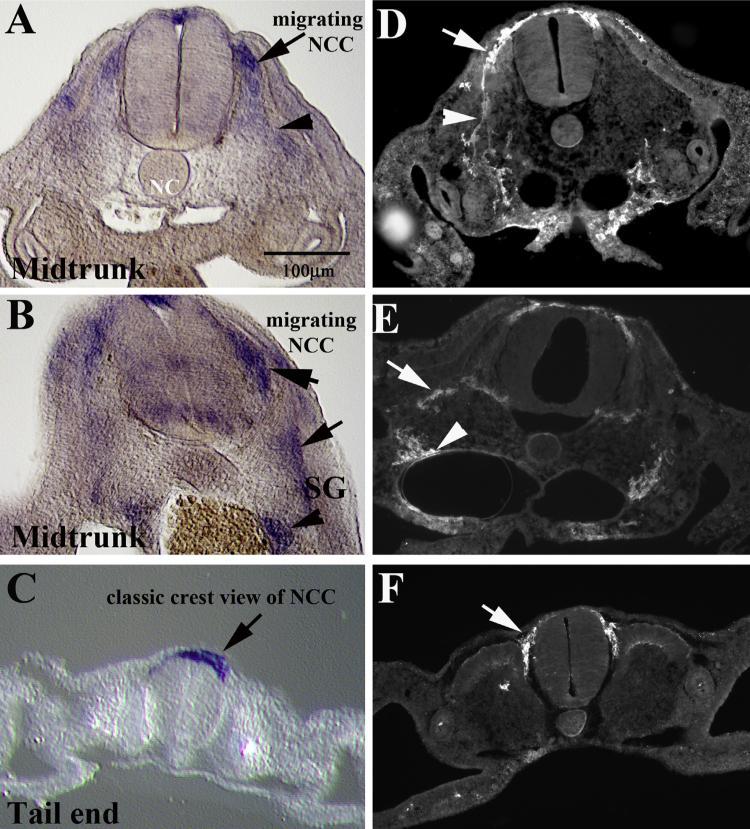
Sox10 and HNK1 comparison. (A–C) Sections along the trunk of a HH17 chicken embryo after wholemount in situ hybridization with Sox10. (D–F) Sections along the trunk of a HH17 chicken embryo immunostained with HNK1. (A, B, D and E) At midtrunk level (posterior to fore limb, anterior to hindlimb) with arrows pointing at NCC along ventromedial pathway and arrowhead to cells reaching toward dorsal aorta (A, D) or sympathetic ganglia (SG) in (B, E). Section at tail end show neural crest as a “crest” above the neural tube (arrows in (C, D)).
The last interesting observation with these HNK1 serial whole-mounts and sections is that the dorsolaterally migrating trunk neural crest cells which will give rise to melanocytes were never seen migrating under the ectoderm past the first half of the dermomyotome. In all our sections we could see a group of HNK1 cells entering the dorsolateral pathway, but we never saw more HNK1 cells migrating further laterally under the ectoderm (see Figs. 1C, 5B–E and 7B). This strongly suggests that these precursors, after they turn off Robo receptors and enter the dorsolateral pathway (Jia et al., 2005), also stop expressing the HNK1 epitope although they still express Sox10 marker (Fig. 7D).
Transcription factor Sox10 labels trunk neural crest migration
In order to underscore the relevance of HNK1 as a marker for neural crest cells, we carried out in situ hybridization with Sox10 across a range of developmental stages in chicken embryos (Fig. 8). Starting as early as HH10 when the first wave of cranial neural crest cells delaminate and start migrating, and continuing until more advanced peripheral nervous system development (HH21), Sox10 labels the same neural crest migration pattern as done by HNK1 in same stages of development. For a better and more extensive material on chicken Sox10, see GEISHA's website: http://geisha.arizona.edu/geisha/search.jsp?entrez gene=449558.
Although this pattern of expression has been already demonstrated for chicken (Cheng et al., 2000), mouse (Kuhlbrodt et al., 1998) and zebrafish (Carney et al., 2006), we re-present it here under the light of our current results of trunk neural crest migration with HNK1. When chicken sections of Sox10 in situ and HNK1 labeling are shown side-by-side it further underscores the validity of HNK1 as a neural crest marker (Fig. 9). Here we show that delaminated migrating trunk neural crest cells express Sox10 visible as streams of cells along the ventromedial pathway (Fig. 9A–C) in the same manner as when using HNK1. However, when compared with the extent and detail of labeling provided by HNK1, this one proves to be a better marker: gives brighter images, better cell morphology since it labels the cell membrane and is easier to work with than an in situ hybridization procedure (Fig. 9D–F).
Conclusions
The development and migration of trunk neural crest cells is an essential embryonic step (Newbern, 2015). Because they are the main providers of all trunk sensory ganglia and Schwann cells, their correct migratory patterns have been heavily studied through functional approaches. The most commonly used is the genetic approach in mouse and zebrafish (Kam et al., 2014; Pryor et al., 2014; Vermillion et al., 2014). However, chicken is the most and best studied model organism given it easiness and hardiness to manipulate. Thus studies in chicken trunk neural crest migration range from gain/loss-of-function experiments to transplants to beads and ex ovo cultures (Ahlgren and Bronner-Fraser, 1999; Giovannone et al., 2012; Klymkowsky et al., 2010; McKeown et al., 2005; Moore et al., 2013; Tucker, 2001). Finally, the best live imaging research on trunk neural crest migration has been done in chicken (Kulesa et al., 2013; McKinney and Kulesa, 2011; Wynn et al., 2013). Altogether, these researches highlight the importance of knowing more thoroughly the pattern and characteristics of chicken trunk neural crest migration.
In conclusion, although there have been many thorough studies using HNK1 as a marker for neural crest cells and their migratory patterns, this is the first comprehensive description of trunk neural crest migration across a wide range of stages of development that also includes Sox10 comparisons. We found that: (A) as previously described trunk neural crest cells delaminate in large numbers starting HH15 and continue through HH20, by which stage it corresponds to melanocytes precursors. (B) That melanocyte precursors begin to enter the dorsolateral pathway by HH17. (C) That by HH20 HNK1 is not a valid marker for neural crest cells on the ventrome-dial or dorsolateral pathway. (D) That HNK1 is a better marker than Sox10 when looking at neural crest cells morphology and migration details.
Acknowledgements
We thank Max Ezin for helpful comments and suggestions during the writing of this manuscript. This work was supported by an NIH/NINDS AREA grant 1R15-NS060099-02 and 5SC3GM096904-03 to MEdB.
References
- Ahlgren SC, Bronner-Fraser M. Inhibition of sonic hedgehog signaling in vivo results in craniofacial neural crest cell death. Curr Biol. 1999;9:1304–14. doi: 10.1016/s0960-9822(00)80052-4. [DOI] [PubMed] [Google Scholar]
- Baker CV. Neural crest and cranial ectodermal placodes. 4th ed. Springer; New York, NY: 2005. [Google Scholar]
- Belmadani A, Jung H, Ren D, Miller RJ. The chemokine SDF-1/CXCL12 regulates the migration of melanocyte progenitors in mouse hair follicles. Differentiation. 2009;77:395–411. doi: 10.1016/j.diff.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock P, Beineke A, Techangamsuwan S, Baumgärtner W, Wewetzer K. Differential expression of HNK-1 and p75NTR in adult canine Schwann cells and olfactory ensheathing cells in situ but not in vitro. J Comp Neurol. 2007;505:572–85. doi: 10.1002/cne.21519. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Dev Biol. 1986;115:44–55. doi: 10.1016/0012-1606(86)90226-5. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Segregation of cell lineage in the neural crest. Curr Opin Genet Dev. 1993;3:641–7. doi: 10.1016/0959-437x(93)90101-t. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Neural crest cell formation and migration in the developing embryo. FASEB J. 1994;8:699–706. doi: 10.1096/fasebj.8.10.8050668. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Origins and developmental potential of the neural crest. Exp Cell Res. 1995a;218:405–17. doi: 10.1006/excr.1995.1173. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Patterning of the vertebrate neural crest. Perspect Dev Neurobiol. 1995b;3:53–62. [PubMed] [Google Scholar]
- Bronner-Fraser M, Cohen AM. Analysis of the neural crest ventral pathway using injected tracer cells. Dev Biol. 1980;77:130–41. doi: 10.1016/0012-1606(80)90461-3. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M, Fraser SE. Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature. 1988;335:161–4. doi: 10.1038/335161a0. [DOI] [PubMed] [Google Scholar]
- Bronner ME. Formation and migration of neural crest cells in the vertebrate embryo. Histochem Cell Biol. 2012;138:179–86. doi: 10.1007/s00418-012-0999-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AJ, Le Douarin NM. Enteric nervous system development: analysis of the selective developmental potentialities of vagal and sacral neural crest cells using quail-chick chimeras. Anat Rec. 2001;262:16–28. doi: 10.1002/1097-0185(20010101)262:1<16::AID-AR1007>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Carney TJ, Dutton KA, Greenhill E, Delfino-Machin M, Dufourcq P, Blader P, et al. A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development. 2006;133:4619–30. doi: 10.1242/dev.02668. [DOI] [PubMed] [Google Scholar]
- Cebra-Thomas JA, Betters E, Yin M, Plafkin C, McDow K, Gilbert SF. Evidence that a late-emerging population of trunk neural crest cells forms the plastron bones in the turtle Trachemys scripta. Evol Dev. 2007;9:267–77. doi: 10.1111/j.1525-142X.2007.00159.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Cheung M, Abu-Elmagd MM, Orme A, Scotting PJ. Chick sox10, a transcription factor expressed in both early neural crest cells and central nervous system. Brain Res Dev Brain Res. 2000;121:233–41. doi: 10.1016/s0165-3806(00)00049-3. [DOI] [PubMed] [Google Scholar]
- Clay MR, Halloran MC. Control of neural crest cell behavior and migration: insights from live imaging. Cell Adhes Migr. 2010;4:586–94. doi: 10.4161/cam.4.4.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellard ME, Rao Y, Bronner-Fraser M. Dual function of Slit2 in repulsion and enhanced migration of trunk, but not vagal, neural crest cells. J Cell Biol. 2003;162:269–79. doi: 10.1083/jcb.200301041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Barrio MG, Nieto MA. Relative expression of Slug, RhoB, and HNK-1 in the cranial neural crest of the early chicken embryo. Dev Dyn. 2004;229:136–9. doi: 10.1002/dvdy.10456. [DOI] [PubMed] [Google Scholar]
- Dupin E, Le Douarin NM. Development of melanocyte precursors from the vertebrate neural crest. Oncogene. 2003;22:3016–23. doi: 10.1038/sj.onc.1206460. [DOI] [PubMed] [Google Scholar]
- Fredette BJ, Ranscht B. T-cadherin expression delineates specific regions of the developing motor axon-hindlimb projection pathway. J Neurosci. 1994;14:7331–46. doi: 10.1523/JNEUROSCI.14-12-07331.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Castro M, Bronner-Fraser M. Induction and differentiation of the neural crest. Curr Opin Cell Biol. 1999;11:695–8. doi: 10.1016/s0955-0674(99)00038-1. [DOI] [PubMed] [Google Scholar]
- Giovannone D, Reyes M, Reyes R, Correa L, Martinez D, Ra H, et al. Slits affect the timely migration of neural crest cells via Robo receptor. Dev Dyn. 2012;241:1274–88. doi: 10.1002/dvdy.23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichnik JM. Kit and melanocyte migration. J Investig Dermatol. 2006;126:945–7. doi: 10.1038/sj.jid.5700164. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chicken embryo. J Morphol. 1951;88:49–52. [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Schwann cell precursors and their development. Glia. 1991;4:185–94. doi: 10.1002/glia.440040210. [DOI] [PubMed] [Google Scholar]
- Jia L, Cheng L, Raper J. Slit/Robo signaling is necessary to confine early neural crest cells to the ventral migratory pathway in the trunk. Dev Biol. 2005;282:411–21. doi: 10.1016/j.ydbio.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Kam MKM, Cheung MCH, Zhu JJ, Cheng WWC, Sat EWY, Tam PKH, et al. Perturbation of Hoxb5 signaling in vagal and trunk neural crest cells causes apoptosis and neurocristopathies in mice. Cell Death Differ. 2014;21:278–89. doi: 10.1038/cdd.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymkowsky MW, Rossi CC, Artinger KB. Mechanisms driving neural crest induction and migration in the zebrafish and Xenopus laevis. Cell Adhes Migr. 2010;4:595–608. doi: 10.4161/cam.4.4.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krispin S, Nitzan E, Kalcheim C. The dorsal neural tube: a dynamic setting for cell fate decisions. Dev Neurobiol. 2010;70:796–812. doi: 10.1002/dneu.20826. [DOI] [PubMed] [Google Scholar]
- Krull CE. Segmental organization of neural crest migration. Mech Dev. 2001;105:37–45. doi: 10.1016/s0925-4773(01)00395-1. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998;18:237–50. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesa PM, Gammill LS. Neural crest migration: patterns, phases and signals. Dev Biol. 2010;344:566–8. doi: 10.1016/j.ydbio.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesa PM, McKinney MC, McLennan R. Developmental imaging: the avian embryo hatches to the challenge. Birth Defects Res C Embryo Today. 2013;99:121–33. doi: 10.1002/bdrc.21036. [DOI] [PubMed] [Google Scholar]
- Kundrat M. HNK-1 immunoreactivity during early morphogenesis of the head region in a nonmodel vertebrate, crocodile embryo. Naturwissenschaften. 2008;95:1063–72. doi: 10.1007/s00114-008-0426-4. [DOI] [PubMed] [Google Scholar]
- Kuo BR, Erickson CA. Regional differences in neural crest morpho-genesis. Cell Adhes Migr. 2010;4:567–85. doi: 10.4161/cam.4.4.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. Migration and differentiation of neural crest cells. Curr Top Dev Biol. 1980;16:31–85. doi: 10.1016/s0070-2153(08)60153-2. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM. The avian embryo as a model to study the development of the neural crest: a long and still ongoing story. Mech Dev. 2004;121:1089–102. doi: 10.1016/j.mod.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Dupin E. Cell lineage analysis in neural crest ontogeny. J Neurobiol. 1993;24:146–61. doi: 10.1002/neu.480240203. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Ziller C, Couly GF. Patterning of neural crest derivatives in the avian embryo: in vivo and in vitro studies. Dev Biol. 1993;159:24–49. doi: 10.1006/dbio.1993.1219. [DOI] [PubMed] [Google Scholar]
- Loring JF, Erickson CA. Neural crest cell migratory pathways in the trunk of the chick embryo. Dev Biol. 1987;121:220–36. doi: 10.1016/0012-1606(87)90154-0. [DOI] [PubMed] [Google Scholar]
- McKeown SJ, Lee VM, Bronner-Fraser M, Newgreen DF, Farlie PG. Sox10 overexpression induces neural crest-like cells from all dorsoventral levels of the neural tube but inhibits differentiation. Dev Dyn. 2005;233:430–44. doi: 10.1002/dvdy.20341. [DOI] [PubMed] [Google Scholar]
- McKinney MC, Kulesa PM. In vivo calcium dynamics during neural crest cell migration and patterning using GCaMP3. Dev Biol. 2011;358:309–17. doi: 10.1016/j.ydbio.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe WK, Myers PZ, Trevarrow B, Bass MB, Kimmel CB. Primary neurons that express the L2/HNK-1 carbohydrate during early development in the zebrafish. Development. 1990;110:491–504. doi: 10.1242/dev.110.2.491. [DOI] [PubMed] [Google Scholar]
- Moore R, Theveneau E, Pozzi S, Alexandre P, Richardson J, Merks A, et al. Par3 controls neural crest migration by promoting microtubule catastrophe during contact inhibition of locomotion. Development. 2013;140:4763–75. doi: 10.1242/dev.098509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbern JM. Molecular control of the neural crest and peripheral nervous system development. In: Paul AT, editor. Current topics in developmental biology. Academic Press; 2015. pp. 201–31. [chapter 7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgreen DF, Powell ME, Moser B. Spatiotemporal changes in HNK-1/L2 glycoconjugates on avian embryo somite and neural crest cells. Dev Biol. 1990;139:100–20. doi: 10.1016/0012-1606(90)90282-n. [DOI] [PubMed] [Google Scholar]
- Pryor SE, Massa V, Savery D, Andre P, Yang Y, Greene NDE, et al. Vangl-dependent planar cell polarity signalling is not required for neural crest migration in mammals. Development. 2014;141:3153–8. doi: 10.1242/dev.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M, Zandberg K, Desmawati I, de Bellard ME. Emergence and migration of trunk neural crest cells in a snake, the California Kingsnake (Lampropeltis getula californiae). BMC Dev Biol. 2010;10:52. doi: 10.1186/1471-213X-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickmann M, Fawcett JW, Keynes RJ. The migration of neural crest cells and the growth of motor axons through the rostral half of the chick somite. J Embryol Exp Morphol. 1985;90:437–55. [PubMed] [Google Scholar]
- Sandell LL, Kurosaka H, Trainor PA. Whole mount nuclear fluorescent imaging: convenient documentation of embryo morphology. Genesis. 2012;50:844–50. doi: 10.1002/dvg.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santagati F, Rijli FM. Cranial neural crest and the building of the vertebrate head. Nat Rev. 2003;4:806–18. doi: 10.1038/nrn1221. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. Development and evolution of the migratory neural crest: a gene regulatory perspective. Curr Opin Genet Dev. 2006;16:360–6. doi: 10.1016/j.gde.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–85. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, et al. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell. 2010;19:39–53. doi: 10.1016/j.devcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theveneau E, Mayor R. Integrating chemotaxis and contact-inhibition during collective cell migration: small GTPases at work. Small GTPases. 2010;1:113–7. doi: 10.4161/sgtp.1.2.13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theveneau E, Mayor R. Neural crest migration: interplay between chemorepellents, chemoattractants, contact inhibition, epithelial–mesenchymal transition, and collective cell migration. Wiley Interdiscip Rev: Dev Biol. 2012;1:435–45. doi: 10.1002/wdev.28. [DOI] [PubMed] [Google Scholar]
- Tosney KW. Long-distance cue from emerging dermis stimulates neural crest melanoblast migration. Dev Dyn. 2004;229:99–108. doi: 10.1002/dvdy.10492. [DOI] [PubMed] [Google Scholar]
- Tucker GC, Aoyama H, Lipinski M, Tursz T, Thiery JP. Identical reactivity of monoclonal antibodies HNK-1 and NC-1: conservation in vertebrates on cells derived from the neural primordium and on some leukocytes. Cell Differ. 1984;14:223–30. doi: 10.1016/0045-6039(84)90049-6. [DOI] [PubMed] [Google Scholar]
- Tucker RP. Abnormal neural crest cell migration after the in vivo knockdown of tenascin-C expression with morpholino anti-sense oligonucleotides. Dev Dyn. 2001;222:115–9. doi: 10.1002/dvdy.1171. [DOI] [PubMed] [Google Scholar]
- Vaglia JL, Hall BK. Regulation of neural crest cell populations: occur-rence, distribution and underlying mechanisms. Int J Dev Biol. 1999;43:95–110. [PubMed] [Google Scholar]
- Vermillion KL, Lidberg KA, Gammill LS. Cytoplasmic protein methylation is essential for neural crest migration. J Cell Biol. 2014;204:95–109. doi: 10.1083/jcb.201306071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M, Duband JL, Thiery JP. A cell surface determinant expressed early on migrating avian neural crest cells. Brain Res. 1983;285:235–8. doi: 10.1016/0165-3806(83)90058-5. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Osumi N, Weston JA. Expression of a novel secreted factor, Seraf indicates an early segregation of Schwann cell precursors from neural crest during avian development. Dev Biol. 2004;268:162–73. doi: 10.1016/j.ydbio.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Wynn ML, Rupp P, Trainor PA, Schnell S, Kulesa PM. Follow-the-leader cell migration requires biased cell–cell contact and local microenvironmental signals. Phys Biol. 2013;10:035003. doi: 10.1088/1478-3975/10/3/035003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomizo T, Yamada-Inagawa T, Yzaguirre AD, Chen MJ, Speck NA, Dzierzak E. Whole-mount three-dimensional imaging of internally localized immunostained cells within mouse embryos. Nat Protoc. 2012;7:421–31. doi: 10.1038/nprot.2011.441. [DOI] [PMC free article] [PubMed] [Google Scholar]