Abstract
Every animal must learn how to use its limbs within the developmental context of an ever-changing body. Typically, investigations of sensorimotor development focus on waking movements. Here I consider another class of behavior: Twitching movements that occur exclusively during active (REM) sleep. Twitches are particularly abundant in early infancy when critical sensorimotor networks are established. In light of behavioral, electrophysiological, neurophysiological, and computational investigations of this unique behavior, twitches may prove critical for the development and maintenance of the sensorimotor system, as well as its repair after injury or disease.
Keywords: REM sleep, myoclonic twitching, sensorimotor integration, activity-dependent development, spontaneous activity, developmental plasticity, atypical development, recovery of function, REM behavior disorder
Development is wondrous for its capacity to flexibly match form and function. This is readily apparent in people with typically formed bodies, but is even more striking in people with atypical bodies. Children born without legs can learn to walk and run using their arms, and children born without arms can learn to play piano with their toes (Blumberg, 2009). Such anomalies highlight developmental plasticity in ways that typical bodies do not. In fact, typical bodies can mask the wonders of development—in part because we expect species-typical bodies to exhibit species-typical behaviors. So when species-atypical bodies exhibit behaviors that are as elegantly constructed and functional as species-typical ones, only development can resolve the apparent contradiction.
Behavior develops over time through local, moment-to-moment interactions among an array of molecular, cellular, and physiological systems. At each step, these systems process and are modified by a variety of inputs, including genetic activity, sensory stimulation, and physical influences from the environment (Johnston & Edwards, 2002). The quantity of input also matters, including the thousands of steps taken each day by toddlers (Adolph et al., 2012), the hundreds of words heard each day by infants (Hurtado, Marchman, & Fernald, 2008), and the thousands of cumulative hours of practice required for expert performance (Ericsson, Krampe, & Tesch-Römer, 1993). But, there is another behavior—called myoclonic twitching—that is exceptionally abundant in early development. We do it in our sleep.
Sleep Is Not An Absence of Behavior
For as long as people have watched others sleep, we have been aware of twitching: Twitches of the arms and legs and fingers and toes, and the darting of eyes beneath closed lids. When someone is roused in the midst of such activity, we expect to be regaled with stories of dreams filled with action sequences befitting the flurries of jerky limb movements. We imagine that if dogs could tell us about their dreams, we would get an earful about rabbits.
We see twitches and immediately think of dreams. But more than that, the folk theory of twitching—which also happens to be the prevailing scientific theory—is that dreams cause twitches. The scientific theory goes like this: The sleeping brain erects a barrier to movement to prevent animals from acting out the dreams produced by the cerebral cortex. However, this barrier is imperfect and twitches are the bits of dreams that leak through. Twitches are the jetsam of dreams: mere by-products. Or so it is thought.
The philosopher of science Alfred Russell Hanson (Hanson, 1958) famously suggested that “seeing is a ‘theory-laden’ undertaking” (p. 19). Accordingly, perhaps what we see (or don't see) in twitching is distorted by the prevailing theory of twitching. If, as I argue here, that theory is incomplete or even wrong, we are free to consider twitching anew. And when we do, we see that twitching is a complex behavior that is ideally suited to contribute to the development of the sensorimotor system and even, perhaps, to its maintenance and repair across the lifespan.
We Sleep and Twitch Most When We Are Young
Twitching is a defining feature of active (REM) sleep. Other features include suppression of muscle tone (atonia) and, paradoxically, a wake-like cortical electroencephalogram (EEG). In mammals, including humans, active sleep is most prominently expressed early in development, beginning in fetuses and continuing through the early postnatal period (Corner, 1977; Jouvet-Mounier, Astic, & Lacote, 1970; Roffwarg, Muzio, & Dement, 1966). This observation inspired the hypothesis that active sleep contributes to brain development (Roffwarg et al., 1966).
Because rats are altricial (i.e., born in a relatively undeveloped state), they have proved useful for studying the early development of sleep (see Blumberg & Seelke, 2010). A comfortably warm pup cycles between sleep and wake very rapidly, about every 30 seconds or so. An awake pup may lift its head, move around, yawn, stretch, or suckle. With the onset of quiet sleep, muscles relax and the pup is completely motionless except for breathing. Soon thereafter, the initiation of twitching marks the onset of active sleep. Twitching can be so intense that it can be mistaken for a seizure; indeed, early observers of twitching in fetal and neonatal rats described “convulsive-type jerks and twitches” (p. 101) that appeared random and unstructured (Narayanan, Fox, & Hamburger, 1971). However, high-speed video recordings of twitching in rat pups reveals non-random, complex structure at multiple timescales (Blumberg, Coleman, Gerth, & McMurray, 2013). Some combinations of joint movements are more likely to occur than others, reflecting the early expression of motor synergies.
The discrete nature of twitching is readily apparent in recordings of individual muscle activity. Electrographically, twitches appear as brief spikes against a background of low muscle activity, like lightning against the nighttime sky. Simultaneous twitches are rare. Recordings across many different skeletal muscles reveal twitching as a body-wide phenomenon comprising “waves” of discrete jerky movements with brief interludes of quiet. Quantitatively, across all the skeletal muscles of the body, millions of twitches are likely produced each day in newborn rats.
Twitches Are Not Mere By-Products of Dreams
Twitches first appear in rats during the prenatal period when movements rely primarily on neural circuits in the spinal cord (Robinson, Blumberg, Lane, & Kreber, 2000). After birth, as sleep-wake states become easier to discern, circuits within the midbrain are increasingly important for twitching. Surgical separation of the brainstem from the cerebral cortex has no discernible effect on twitching in week-old rats. Twitching survives similar brain transections in cats. Therefore, it is clear that the brainstem can produce twitches without intercession by the cerebral cortex (see Blumberg, 2010).
It is not clear, however, if twitches are produced exclusively by the brainstem, especially in adult humans. For example, people with REM behavior disorder (RBD) exhibit movements during sleep that begin as twitches but evolve into more robust, often violent, activity that is conventionally interpreted as the “acting out of dreams” (Mahowald & Schenck, 2005). RBD is a precursor to other neurodegenerative disorders, including Parkinson's Disease. Interestingly, in people with RBD and Parkinson's, the characteristic tremors of wakefulness are absent from movements during sleep (Oudiette et al., 2011), thus reinforcing the notion that the motor system is controlled very differently during sleep and wake. Delineating the neural mechanisms responsible for this differential control and how they might differ from those in infants are exciting areas for future research.
Sensory Feedback From Twitching Activates the Brain
Sleep is not a period of complete sensory isolation. External stimuli presented across multiple modalities to sleeping people can influence the content of dreams (Schredl et al., 2009). But what about sensory stimuli arising from self-produced movements, as with the twitches produced during sleep? Evidence that the nervous system is sensitive to reafferent signals arising from twitching limbs first emerged a decade ago. Specifically, behavioral experiments and computational modeling in two-week-old rats showed that twitching contributes to the development of the spinally mediated withdrawal reflex (Petersson, Waldenström, Fåhraeus, & Schouenborg, 2003). Reafferent signals from twitching limbs in one-week-old rats also trigger so-called spindle bursts in topographically related areas in primary somatosensory cortex (Khazipov et al., 2004). Human preterm infants exhibit similar activity, suggesting that twitch-related triggering of cortical activity also occurs in human fetuses (Milh et al., 2007). Finally, in newborn rats, neural activity in thalamus and sensorimotor cortex (Tiriac, Del Rio-Bermudez, & Blumberg, 2014; Tiriac, Uitermarkt, Fanning, Sokoloff, & Blumberg, 2012), hippocampus (Mohns & Blumberg, 2010), and cerebellum (Sokoloff, Uitermarkt, & Blumberg, 2014) is much greater during active sleep than during wake.
The nervous system actively participates in its own development. Spontaneous activity of neurons and their associated networks is particularly important for establishing and refining connections in a diverse array of species, at various ages, and in multiple sensory systems (Kirkby, Sack, Firl, & Feller, 2013). For example, spontaneous retinal waves organize patterned activation throughout the visual system (Ackman, Burbridge, & Crair, 2012; Hanganu, Ben-Ari, & Khazipov, 2006) and spontaneous cochlear activity does the same for the auditory system (Tritsch, Yi, Gale, Glowatzki, & Bergles, 2007).
In contrast with spontaneous activity in the retina and cochlea, twitches are spontaneous motor events. They are also very different—in terms of their amplitude, spatiotemporal structure, and occurrence against a background of atonia—from the spontaneous limb movements of awake human infants (Thelen, 1979). As spontaneous movements, twitches are not evoked by an external stimulus, but they are also not random. On the contrary, twitches occur exclusively during periods of active sleep and also exhibit a complex bout structure (Blumberg et al., 2013).
Twitching May Help the Periphery Instruct the Developing Brain
The striking behavioral plasticity evinced by individuals with atypical bodies is mirrored by plasticity within the developing brain. This plasticity has been investigated through experimental manipulation of developing animals as well as assessments of natural variation. For example, in short-tailed opossums, surgical removal of the eyes early in development leads to massive reorganization of inputs to cortical fields (Kahn & Krubitzer, 2002), a finding that helps to explain how speech can activate visual cortex in congenitally blind humans (Roder, Stock, Bien, Neville, & Rosler, 2002). And in star-nosed moles, a mammal that normally has 11 “star” appendages on its snout mapped to 11 distinct areas in somatosensory cortex, an anomalous individual with 12 stars possessed 12 associated areas in cortex (Catania & Kaas, 1997). Thus, inputs from peripheral sensors critically shape sensory system development (Karlen, Hunt, & Krubitzer, 2010).
Developing a sensorimotor system is especially complicated, as it requires the functional integration of sensory and motor maps. For example, a human forelimb comprises an array of bones, muscles, and sensors for touch and proprioception. Bringing all of these components and their neural connections into functional alignment cannot be preprogrammed or hardwired. After all, bones and muscles change and grow throughout our lives. Therefore, the system must be dynamically and continually calibrated and recalibrated. Ultimately, as people with anomalous bodies vividly illustrate, we learn to use the limbs we have, not the limbs we are “supposed” to have. Within this context, twitches can be considered a form of motor exploration by which animals probe the structural features of their limbs and build motor synergies, thereby laying a foundation for goal-directed wake movements (Blumberg et al., 2013).
Figure 1 illustrates the unique challenge faced by a developing sensorimotor system. Consider a bank of switches and a cluster of light bulbs. The person standing at the bank of switches wishes to turn on each bulb in a particular order, but he's unsure how the switches map onto the cluster of bulbs. This problem is analogous to an infant that is first learning to map spinal motoneurons to limb muscles (or is creating higher-order maps linking the spinal cord with the brain). For the person at the switchboard, the obvious solution is to throw one switch at a time and take note of the bulbs that light up. By analogy, for an infant, a twitch might be ideal for mapping the relations among neurons and muscles. Because twitches are discrete events that trigger precise neural activity patterns, they provide an opportunity for the spinal cord and brain to form connections between motor output and sensory input. These ideas have recently put to the test using a computational model of a simulated limb and spinal neural network (Marques, Bharadwaj, & Iida, 2014). Beyond the spinal cord, future work may reveal that the complex structure of twitching at multiple timescales is important for building, refining, and maintaining hierarchically organized sensorimotor loops throughout the brain (Kleinfeld, Berg, & O'Connor, 1999).
Fig. 1.
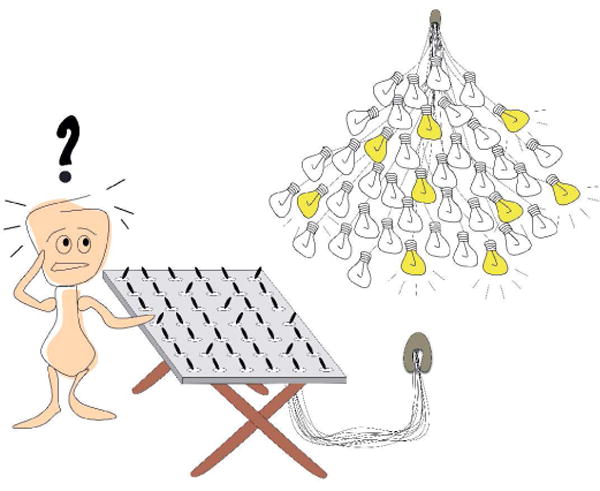
How do animals develop sensorimotor maps linking motoneurons and muscles? Initially, the mapping relations are unknown, metaphorically illustrated here by a bank of switches (neurons) connected in unknown ways to a cluster of light bulbs (muscles). Faced with this confusion, the guy at the switchboard would be wise to throw each switch one at a time and tally the lights that turn on. Analogously, animals may use discrete twitches during sleep to establish, refine, maintain, and repair sensorimotor circuits throughout the nervous system. From Blumberg et al. (2013). Twitching in sensorimotor development from sleeping rats to robots. Current Biology, 23, R532–R537.
Twitching May Contribute to Lifelong Plasticity and Recovery of Function
Twitching continues beyond infancy and into adulthood, albeit at lower rates reflecting the overall lower quantities of active sleep in adults. Sensorimotor plasticity also continues throughout the lifespan as our bodies change, as we learn new skills, and as we respond to traumatic brain or spinal cord injury. In a seminal experiment, Merzenich and colleagues (Clark, Allard, Jenkins, & Merzenich, 1988) fused two adjacent fingers in adult owl monkeys and mapped their somatosensory cortex several months later. Unlike normal monkeys that exhibit distinct cortical representations for each digit, the cortical areas in the monkeys with fused fingers were now also fused—as if the brain were treating the two fingers as one. The authors suggested that fusing the fingers disrupted the uncorrelated input that normally arises from them. If such uncorrelated input is necessary for maintaining individuated cortical representations, twitches seem ideally suited to provide it.
Twitching may similarly benefit people who, due to spinal cord injury, stroke, neurodegenerative disease, or amputation, are learning to use prosthetic limbs (Lebedev & Nicolelis, 2006). The science of brain-machine interfaces is accelerating quickly as scientists are better able to decode neural information and generate neural commands in real time. Ultimately, to attain full functionality, scientists will want the brain to develop the full array of topographic maps of a prosthetic limb just as it would a natural one. Perhaps allowing people to sleep—and thus twitch—while attached to a prosthetic limb will enable the emergence of more precise control in less time. None of these ideas has been tested; indeed, it is not yet known whether twitches trigger brain activity in adults as they do in infants.
Conclusions
Twitches comprise a special class of behavior. They are discrete, jerky movements that occur against a background of muscle atonia. They are ubiquitous, abundant, and highly structured both spatially and temporally (Blumberg et al., 2013). The sensory feedback arising from twitching limbs is processed differently than the feedback arising from wake movements (Tiriac et al., 2014), which enables twitches to trigger synchronized activity throughout the brain. And, as shown in rat pups and using computational models, twitches can contribute to the self-organization of spinal neural circuits (Marques et al., 2014; Petersson et al., 2003). All of these observations belie the folk conception of twitching as random, unstructured, incidental events of little developmental consequence. Our current challenge is to delineate the precise mechanisms by which twitch-triggered neural activity contributes to activity-dependent development and sensorimotor integration.
Researchers still know little about the quantity and patterning of twitching in human infants, how twitching changes across the lifespan, and whether there are individual differences in twitching. If there are individual differences, will they be associated with differences in motor skill or recovery of function after injury or disease? Similarly, comparative studies will be valuable for assessing whether species differences in twitching are related to differences in motor control and behavioral flexibility. We are, in short, only beginning to explore the many dimensions of twitching—a behavior that has been, for so long, tucked quietly away with us in our sleep.
Acknowledgments
I thank Karen Adolph, Greta Sokoloff, Carlos Del Rio Bermudez, Alan Plumeau, and Alex Tiriac for many helpful comments.
Funding: Preparation of this article was supported by National Institutes of Health Grant HD63071.
Footnotes
Declarations of Conflicting Interests: The author declared no conflict of interest with respect to the authorship or the publication of this article.
References
- Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature. 2012;490:219–225. doi: 10.1038/nature11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Cole WG, Komati MM, Garciaguirre JS, Badaly DD, Lingeman JM, et al. How do you learn to walk? Thousands of steps and dozens of falls per day. Psychological Science. 2012;23:1387–1394. doi: 10.1177/0956797612446346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS. Freaks of nature: What anomalies tell us about development and evolution. New York: Oxford University Press; 2009. [Google Scholar]
- Blumberg MS. Beyond dreams: Do sleep-related movements contribute to brain development? Frontiers in Neurology. 2010;1:140. doi: 10.3389/fneur.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Seelke AMH. The form and function of infant sleep: From muscle to neocortex. In: Blumberg MS, Freeman JH, Robinson SR, editors. The Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. pp. 391–423. [Google Scholar]
- Blumberg MS, Coleman CM, Gerth AI, McMurray B. Spatiotemporal structure of REM sleep twitching reveals developmental origins of motor synergies. Current Biology. 2013;23:2100–2109. doi: 10.1016/j.cub.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania K, Kaas J. The mole nose instructs the brain. Somatosensory and Motor Research. 1997;14:56–58. doi: 10.1080/08990229771213. [DOI] [PubMed] [Google Scholar]
- Clark SA, Allard T, Jenkins WM, Merzenich MM. Receptive fields in the body-surface map in adult cortex defined by temporally correlated inputs. Nature. 1988;332:444–445. doi: 10.1038/332444a0. [DOI] [PubMed] [Google Scholar]
- Corner MA. Sleep and the beginnings of behavior in the animal kingdom—Studies of ultradian motility cycles in early life. Progress in Neurobiology. 1977;8:279–295. doi: 10.1016/0301-0082(77)90008-9. [DOI] [PubMed] [Google Scholar]
- Ericsson KA, Krampe RT, Tesch-Römer C. The role of deliberate practice in the acquisition of expert performance. Psychological Review. 1993;100:363–406. [Google Scholar]
- Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. Journal of Neuroscience. 2006;26:6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson N. Patterns of discovery. Cambridge: Cambridge University Press; 1958. [Google Scholar]
- Hurtado N, Marchman VA, Fernald A. Does input influence uptake? Links between maternal talk, processing speed and vocabulary size in Spanish-learning children. Developmental Science. 2008;11:F31–F39. doi: 10.1111/j.1467-7687.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston T, Edwards L. Genes, interactions, and the development of behavior. Psychological Review. 2002;109:26–34. doi: 10.1037/0033-295x.109.1.26. [DOI] [PubMed] [Google Scholar]
- Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Developmental Psychobiology. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Kahn DM, Krubitzer L. Massive cross-modal cortical plasticity and the emergence of a new cortical area in developmentally blind mammals. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11429–11434. doi: 10.1073/pnas.162342799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlen S, Hunt D, Krubitzer L. Cross-modal plasticity in the mammalian cortex. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. pp. 357–374. [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Kirkby LA, Sack GS, Firl A, Feller MB. A role for correlated spontaneous activity in the assembly of neural circuits. Neuron. 2013;80:1129–1144. doi: 10.1016/j.neuron.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld DD, Berg RWR, O'Connor SMS. Anatomical loops and their electrical dynamics in relation to whisking by rat. Somatosensory and Motor Research. 1999;16:69–88. doi: 10.1080/08990229970528. [DOI] [PubMed] [Google Scholar]
- Lebedev M, Nicolelis M. Brain-machine interfaces: past, present and future. TRENDS in Neurosciences. 2006;29:536–546. doi: 10.1016/j.tins.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Mahowald M, Schenck C. Insights from studying human sleep disorders. Nature. 2005;437:1279–1285. doi: 10.1038/nature04287. [DOI] [PubMed] [Google Scholar]
- Marques HG, Bharadwaj A, Iida F. From spontaneous motor activity to coordinated behaviour: A developmental model. PLoS Computational Biology. 2014;10:e1003653. doi: 10.1371/journal.pcbi.1003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milh M, Kaminska A, Huon C, Lapillonne A, Ben-Ari Y, Khazipov R. Rapid cortical oscillations and early motor activity in premature human neonate. Cerebral Cortex. 2007;17:1582–1594. doi: 10.1093/cercor/bhl069. [DOI] [PubMed] [Google Scholar]
- Mohns EJ, Blumberg MS. Neocortical activation of the hippocampus during sleep in newborn rats. Journal of Neuroscience. 2010;30:3438–3449. doi: 10.1523/JNEUROSCI.4832-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan CH, Fox MW, Hamburger V. Prenatal development of spontaneous and evoked activity in the rat (Rattus norvegicus albinus) Behaviour. 1971;40:100–134. doi: 10.1163/156853971x00357. [DOI] [PubMed] [Google Scholar]
- Oudiette D, Leu-Semenescu S, Roze E, Vidailhet M, De Cock VC, Golmard JL, Arnulf I. A motor signature of REM sleep behavior disorder. Movement Disorders. 2011;27:428–431. doi: 10.1002/mds.24044. [DOI] [PubMed] [Google Scholar]
- Petersson P, Waldenström A, Fåhraeus C, Schouenborg J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature. 2003;424:72–75. doi: 10.1038/nature01719. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Blumberg MS, Lane MS, Kreber LS. Spontaneous motor activity in fetal and infant rats is organized into discrete multilimb bouts. Behavioral Neuroscience. 2000;114:328–336. doi: 10.1037//0735-7044.114.2.328. [DOI] [PubMed] [Google Scholar]
- Roder B, Stock O, Bien S, Neville H, Rosler F. Speech processing activates visual cortex in congenitally blind humans. European Journal of Neuroscience. 2002;16:930–936. doi: 10.1046/j.1460-9568.2002.02147.x. [DOI] [PubMed] [Google Scholar]
- Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Schredl M, Atanasova D, Hörmann K, Maurer JT, Hummel T, Stuck BA. Information processing during sleep: the effect of olfactory stimuli on dream content and dream emotions. Journal of Sleep Research. 2009;18:285–290. doi: 10.1111/j.1365-2869.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- Sokoloff G, Uitermarkt BD, Blumberg MS. REM sleep twitches rouse nascent cerebellar circuits: Implications for sensorimotor development. Developmental Neurobiology. 2014 doi: 10.1002/dneu.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E. Rhythmical stereotypies in normal human infants. Animal Behaviour. 1979;27:699–715. doi: 10.1016/0003-3472(79)90006-x. [DOI] [PubMed] [Google Scholar]
- Tiriac A, Del Rio-Bermudez C, Blumberg MS. Self-generated movements with “unexpected” sensory consequences. Current Biology. 2014 doi: 10.1016/j.cub.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A, Uitermarkt BD, Fanning AS, Sokoloff G, Blumberg MS. Rapid whisker movements in sleeping newborn rats. Current Biology. 2012;22:2075–2080. doi: 10.1016/j.cub.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch N, Yi E, Gale J, Glowatzki E, Bergles D. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
Recommended Reading
- Blumberg MS, Coleman CM, Gerth AI, McMurray B. Spatiotemporal structure of REM sleep twitching reveals developmental origins of motor synergies. Current Biology. 2013;23:2100–2109. doi: 10.1016/j.cub.2013.08.055. Using high-speed videography in newborn rats, this paper documents the complex structure of, and synergistic relations among, twitch movements of forelimb joints. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Marques HG, Iida F. Twitching in sensorimotor development from sleeping rats to robots. Current Biology. 2013;23:R532–R537. doi: 10.1016/j.cub.2013.04.075. An accessible overview of recent work on the phenomenology and function of twitching, including recent advances using computational approaches. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. This paper provided the first empirical evidence that sensory feedback from motor activity during sleep triggers topographically organized cortical activity in the form of spindle bursts. [DOI] [PubMed] [Google Scholar]
- Petersson P, Waldenström A, Fåhraeus C, Schouenborg J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature. 2003;424:72–75. doi: 10.1038/nature01719. Using in vivo and computational approaches, this paper was the first to show that twitches have functional consequences for developing spinal circuitry. [DOI] [PubMed] [Google Scholar]
- Tiriac A, Del Rio-Bermudez C, Blumberg MS. Self-generated movements with “unexpected” sensory consequences. Current Biology. 2014 doi: 10.1016/j.cub.2014.07.053. The experiments reported in this paper provide support for the hypothesis that twitches are distinct from wake movements in that they are processed as if they are unexpected—that is, as if they lack corollary discharge. [DOI] [PMC free article] [PubMed] [Google Scholar]


