Abstract
Scleroderma is a progressive autoimmune disease affecting multiple organs. Fibrosis, the hallmark of scleroderma, represents transformation of self-limited wound healing into a deregulated self-sustaining process. The factors responsible for maintaining persistent fibroblast activation in scleroderma and other conditions with chronic fibrosis are not well understood. Toll-like receptor 4 (TLR4) and its damage-associated endogenous ligands are implicated in immune and fibrotic responses. We now show that fibronectin extra domain A (FnEDA) is an endogenous TLR4 ligand markedly elevated in the circulation and lesional skin biopsies from patients with scleroderma, as well as in mice with experimentally induced cutaneous fibrosis. Synthesis of FnEDA was preferentially stimulated by transforming growth factor–β in normal fibroblasts and was constitutively up-regulated in scleroderma fibroblasts. Exogenous FnEDA was a potent stimulus for collagen production, myofibroblast differentiation, and wound healing in vitro and increased the mechanical stiffness of human organotypic skin equivalents. Each of these profibrotic FnEDA responses was abrogated by genetic, RNA interference, or pharmacological disruption of TLR4 signaling. Moreover, either genetic loss of FnEDA or TLR4 blockade using a small molecule mitigated experimentally induced cutaneous fibrosis in mice. These observations implicate the FnEDA-TLR4 axis in cutaneous fibrosis and suggest a paradigm in which aberrant FnEDA accumulation in the fibrotic milieu drives sustained fibroblast activation via TLR4. This model explains how a damage-associated endogenous TLR4 ligand might contribute to converting self-limited tissue repair responses into intractable fibrogenesis in chronic conditions such as scleroderma. Disrupting sustained TLR4 signaling therefore represents a potential strategy for the treatment of fibrosis in scleroderma.
INTRODUCTION
Scleroderma is a chronic disease of unknown etiology and substantial mortality characterized by autoimmunity, inflammation, and intractable tissue fibrosis. Because it has no validated biomarkers or effective disease-modifying therapies, scleroderma represents a major unmet medical need (1). The early inflammatory stage of scleroderma is often followed by tissue deposition of collagen-rich scar that disrupts the normal architecture and leads to dysfunction and eventual failure of the skin, lungs, and other organs (2). Although transforming growth factor–β (TGF-β) is recognized as an important trigger for fibroblast activation (3), the factors responsible for maintaining chronic fibrosis remain incompletely understood (4). As the primary extra-cellular matrix (ECM)–producing stromal cells, myofibroblasts serve as the key effectors of fibrogenesis (5). Multiple extracellular cues including soluble cytokines and chemokines, reactive oxygen species, and biomechanical signals induce stimulation of collagen and ECM molecule synthesis, and acquisition of a contractile myofibroblast phenotype. Ultimately, the establishment of self-amplifying feed-forward loops in lesional tissues may account for the failure to restrain fibro-blast activation, and a fundamental unanswered question in scleroderma is the nature of the autocrine and paracrine signaling pathways that underlie these loops (6).
Toll-like receptors (TLR) recognize both microbial pathogen-associated molecular patterns and nonmicrobial endogenous ligands (7). Endogenous TLR4 ligands display molecular patterns that are normally inaccessible to the immune system but are released passively into the extracellular space upon cell injury or necrosis, or activation after chronic injury. Matrix molecules such as biglycan, tenascin C, and hyaluronic acid are up-regulated or undergo oxidation or fragmentation upon tissue injury and serve as potential endogenous TLR4 ligands (8). Because they are normally inert and are recognized by TLRs only upon injury, these “damage-associated molecular patterns” (DAMPs) serve as danger signals that enable the innate immune system to sense and respond to sterile tissue damage (9, 10). Accumulating evidence implicates DAMP-triggered aberrant TLR signaling in chronic inflammatory and fibrotic disorders, as well as in mouse models of disease (11–14). Skin and lung biopsies from patients with scleroderma show elevated levels of endogenous TLR4 ligands and constitutive TLR4 signaling, but the signals responsible for TLR4 activation and their role in pathogenesis remain unknown (15, 16).
Fibronectins are high–molecular weight modular glycoproteins that circulate in soluble form in plasma or accumulate in tissue as insoluble ECM components (17). Because of alternate splicing of the fibronectin gene, cellular fibronectin contains extra domains A (EDA) and B (EDB), which are excluded from plasma fibronectin (18). The EDA-containing fibronectin variant (FnEDA) fulfills dual function as both structural ECM scaffold and signaling molecule regulating adhesive, proliferative, and migratory cellular responses, and plays an important role in myofibroblast differentiation and wound healing (19, 20). Although there is little FnEDA expression in adult tissues, marked transient up-regulation is seen during normal wound healing and tissue repair (21–24). Cellular responses elicited by FnEDA are mediated via both surface integrins and TLR4 (24–28).
The present studies were undertaken to investigate the expression and regulation of FnEDA in scleroderma, and its role and mechanism of action in fibrosis. The results reveal significant elevation of FnEDA in the serum and skin from patients with scleroderma, as well as in lesional tissues from mice with cutaneous fibrosis. Exogenous FnEDA had potent effects on collagen gene expression, myofibroblast differentiation and in vitro wound healing, and increased matrix stiffness and collagen cross-linking in human skin equivalents. These ex vivo fibrotic responses were abolished by either genetic or pharmacological disruption of fibroblast TLR4 signaling. Moreover, mice lacking FnEDA or treated with a TLR4 inhibitor showed attenuation of inducible cutaneous fibrosis. Together, our results indicate that FnEDA is aberrantly expressed in patients with scleroderma, induces fibrotic response via TLR4, and is required for maximal cutaneous fibrogenesis in the mouse. On the basis of these findings, we propose the following paradigm for pathological fibrogenesis: Injury triggers increased production and persistent extracellular accumulation of FnEDA, which in turn induces TLR4-dependent fibrotic responses including FnEDA synthesis. The ensuing positive feedback loop is likely to contribute to the persistence and progression of fibrosis in scleroderma. The results from these studies might inform the development of therapeutic approaches to control fibrosis in scleroderma.
RESULTS
Elevated FnEDA levels in scleroderma
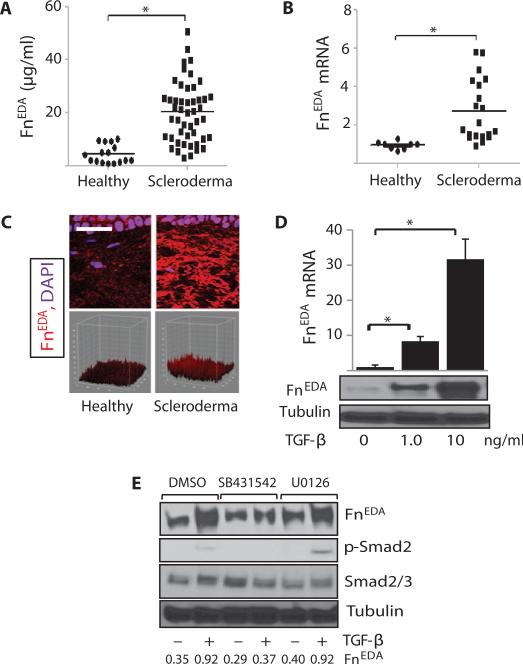
To assess FnEDA expression in scleroderma, we pursued two approaches. First, serum levels were determined in 48 patients with diffuse cutaneous scleroderma and 16 healthy adults recruited from a single institution. The clinical characteristics of the subjects are shown in Table 1. Ninety-five percent of the scleroderma patients were women, and 18 had early-stage (<2 years) disease. A fivefold increase in serum FnEDA levels was seen in scleroderma patients compared to control subjects (mean, 20.2 ± 1.6 μg/ml versus 4.5 ± 0.8 μg/ml; P < 0.0001) (Fig. 1A). The 95% confidence interval in control subjects was <6.3 μg/ml; 94% of scleroderma patients had elevated FnEDA levels. No significant correlation was found between FnEDA levels and disease duration, modified Rodnan skin score (mRSS), forced vital capacity (FVC) or diffusing capacity for carbon monoxide (DLco), or change in mRSS during a 6-month follow-up period (tables S1 and S2).
Table 1.
Subjects for serum determination of FnEDA. The serum creatinine level was 0.74 ± 0.2mg/dl (mean±SD), and only 3 of 48 patients had serum creatinine level >1.0 mg/dl; none had levels >1.35 mg/dl. Controls were healthy women (median age, 49 years; range, 34 to 62 years). dcSSc diffuse cutaneous SSc DLco, diffusing capacity for carbon monoxide; F, female; FVC, forced vital capacity; lcSSc limited cutaneous SSc; M, male; mRSS, modified Rodnan skin score (1 to 51).
| Study code | Age (years) | Sex | Disease subtype | Disease stage (early/late)* | mRSS | FVC (% predicted) | DLco (% predicted) |
|---|---|---|---|---|---|---|---|
| SScReg_1010_2 | 57 | F | dcSSc | Late | 15 | 99 | 74 |
| SScReg_1024_2 | 54 | F | dcSSc | Late | 22 | 67 | 86 |
| SScReg_1073_2 | 61 | F | dcSSc | Late | 9 | 73 | 60 |
| SScReg_1080_2 | 55 | F | dcSSc | Early | 19 | 81 | 82 |
| SScReg_1084_2 | 57 | M | dcSSc | Late | 10 | 80 | 72 |
| SScReg_1100_2 | 39 | F | dcSSc | Early | 14 | 61 | 43 |
| SScReg_1107_2 | 48 | M | dcSSc | Early | 25 | 96 | 94 |
| SScReg_1121_2 | 47 | F | dcSSc | Late | 26 | 71 | 75 |
| SScReg_1046_2 | 37 | F | dcSSc | Late | 21 | 73 | 62 |
| SScReg_1154_2 | 56 | F | dcSSc | Late | 13 | 87 | 85 |
| SScReg_1156_2 | 48 | F | dcSSc | Early | 23 | 77 | |
| SScReg_1141_2 | 52 | F | dcSSc | Early | 16 | 75 | 53 |
| SScReg_1182_2 | 50 | F | dcSSc | Late | 9 | 77 | 61 |
| SScReg_1213_3 | 30 | F | dcSSc | Late | 10 | 81 | 82 |
| SScReg_1266_3 | 66 | F | dcSSc | Early | 14 | 64 | 72 |
| SScReg_1068_2 | 49 | F | dcSSc | Late | 27 | 83 | 88 |
| SScReg_1097_2 | 64 | F | dcSSc | Early | 9 | 66 | 65 |
| SScReg_1103_2 | 54 | F | dcSSc | Late | 13 | 70 | 70 |
| SScReg_1043_2 | 61 | F | dcSSc | Late | 7 | 78 | 72 |
| SScReg_1095_2 | 42 | F | dcSSc | Late | 11 | 83 | 60 |
| SScReg_1102_3 | 29 | F | dcSSc | Late | 20 | 72 | 56 |
| SScReg_1048_3 | 39 | M | dcSSc | Late | 12 | 89 | 81 |
| SScReg_1117_3 | 45 | F | dcSSc | Late | 23 | 94 | 65 |
| SScReg_1221_3 | 57 | F | dcSSc | Early | 12 | 95 | 81 |
| SScReg_1248_2 | 63 | F | dcSSc | Late | 36 | 97 | 64 |
| SScReg_1258_1 | 57 | F | dcSSc | Late | 2 | 90 | 55 |
| SScReg_1271_2 | 50 | F | dcSSc | Late | 25 | 56 | 43 |
| SScReg_1279_3 | 59 | F | dcSSc | Late | 12 | 75 | 60 |
| SScReg_1313_3 | 62 | F | dcSSc | Late | 49 | 86 | 44 |
| SScReg_1332_2 | 47 | F | dcSSc | Late | 26 | 50 | 21 |
| SScReg_1354_3 | 42 | F | dcSSc | Early | 29 | 100 | 76 |
| SScReg_1370_2 | 45 | F | dcSSc | Early | 23 | 100 | 71 |
| SScReg_1380_2 | 56 | F | dcSSc | Early | 7 | 91 | 42 |
| SScReg_1386_2 | 63 | F | dcSSc | Early | 22 | 102 | 53 |
| SScReg_1366_2 | 55 | F | dcSSc | Early | 23 | 76 | 75 |
| SScReg_1392_3 | 47 | F | dcSSc | Early | 19 | 67 | 76 |
| SScReg_1397_3 | 52 | F | dcSSc | Late | 22 | 105 | 64 |
| SScReg_1402_3 | 48 | F | dcSSc | Early | 7 | 72 | 21 |
| SScReg_1405_3 | 52 | F | dcSSc | Early | 13 | 77 | 58 |
| SScReg_1410_3 | 56 | F | dcSSc | Early | 17 | 81 | 77 |
| SScReg_1006_3 | 47 | F | dcSSc | Late | 3 | 101 | 99 |
| SScReg_1412_3 | 65 | F | dcSSc | Late | 6 | 100 | 96 |
| SScReg_1119_3 | 56 | M | dcSSc | Late | 38 | 41 | 26 |
| SScReg_1198_3 | 59 | F | dcSSc | Late | 5 | 76 | 53 |
| SScReg_1393_3 | 41 | F | dcSSc | Late | 14 | 40 | 42 |
| SScReg_1320_3 | 31 | F | dcSSc | Late | 10 | 44 | 60 |
| SScReg_1070_3 | 62 | F | dcSSc | Late | 13 | 25 | 15 |
| SScReg_1426_2 | 28 | F | dcSSc | Late | 18 | 89 | 82 |
Early, <2 years from first non-Raynaud disease manifestation; late, >2 years from first non-Raynaud manifestation.
Fig. 1. FnEDA is elevated in scleroderma, and its expression is stimulated by TGF-β.
(A) FnEDA serum levels in scleroderma patients (n = 48) and healthy adults (n = 16) were determined by enzyme-linked immunosorbent assay (ELISA). Each data point is the mean ± SD of trip-licate determinations from a single subject. *P < 0.0001, Mann-Whitney U test. (B) RNA isolated from skin biopsies from scleroderma patients (n = 20) and healthy adults (n = 8) was analyzed by real-time qPCR. Results, expressed relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), are the means ± SD of duplicate determinations. *P = 0.0002, Mann-Whitney U test. (C) Immunofluorescence analysis. Skin biopsies from scleroderma patients (n = 13) and healthy controls (n = 6) in parallel were immunostained with antibodies to FnEDA and examined by immunofluorescence confocal microscopy. Upper panels: Representative images. Scale bar, 25 μm. Lower panels: 3D plots of fluorescence intensity (see Table 3). P = 0.0159, Mann-Whitney U test. (D and E) Confluent human skin fibroblasts were incubated for 24 hours with TGF-β (1 and 10 ng/ml) alone (D) or in the presence or absence of SB431542 or U0126 (E). (D) Upper panel: Levels of mRNA were determined by real-time quantitative PCR. Results, normalized with GAPDH, are means ± SD of triplicate determinations. *P < 0.0001, one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test. Lower panel: Whole-cell lysates were subjected to Western analysis. Representative immunoblots.
Next, tissue levels of FnEDA mRNA and protein were examined. RNA was isolated from biopsies of lesional skin from 20 scleroderma patients or forearms of 8 healthy volunteers (Table 2). Real-time quantitative polymerase chain reaction (qPCR) results showed a nearly threefold increase (P = 0.0002) in FnEDA mRNA in scleroderma biopsies compared to matched controls (Fig. 1B). Immunofluorescence showed that healthy control skin (n = 6) had only barely detectable FnEDA in the dermis, whereas scleroderma biopsies (n = 13) displayed a more than twofold increase (P = 0.016) (Fig. 1C and Table 3). Together, these results indicate that the FnEDA splice variant is selectively elevated in both serum and lesional skin biopsies from patients with scleroderma.
Table 2.
Subjects providing skin biopsies for mRNA analysis. None of the subjects had renal failure. Controls were healthy subjects (median age, 37 years; range, 30 to 63 years); 70% of the patients are female. PM, polymyositis overlap; EMS, eosinophilia-myaglia syndrome.
| Study code | Age (years) | Sex | Disease subtype | Disease stage* | mRSS |
|---|---|---|---|---|---|
| S1001 | 52 | F | dcSSc | Late | 24 |
| SSc01LA | 48 | F | dcSSc | Early | 21 |
| SSc01BLA | 46 | F | dcSSc | Early | 3 |
| SSc02BLA | 41 | F | dcSSc | Early | 21 |
| SSc03LA | 55 | F | lcSSc | Early | 11 |
| SSc04LA | 60 | F | lcSSc | Late | 4 |
| SSc05RA | 66 | F | dcSSc | Early | 9 |
| SSc06BRA | 53 | F | dcSSc | Early | 13 |
| SSc07BLA | 52 | F | dcSSc | Early | 17 |
| SSc08BLA | 34 | F | dcSSc | Late | 32 |
| SSc10BLA | 27 | F | dcSSc | Late | 26 |
| SSc12 BLA | 21 | M | dcSSc | Early | 15 |
| SSc1002BLA | 56 | F | dcSSc | Late | 34 |
| SSc1004BLA | 55 | F | dcSSc | Early | 19 |
| SSc1066LA | 26 | F | lcSSc | Late | 5 |
| SSc1067LA | 54 | F | dcSSc | Late | 13 |
| SSc1080LA | 48 | F | SSc/PM | Early | 20 |
| SSc1096LA | 45 | F | EMS | Early | 23 |
| SSc1103BLA | 30 | F | dcSSc | Late | 4 |
| SSc1156BLA | 50 | F | dcSSc | Early | 14 |
Early, <2 years from first non-Raynaud disease manifestation;late, >2 years from first non-Raynaud manifestation.
Table 3.
Subjects providing skin biopsies for immunofluorescence analysis. None of the subjects had renal failure. Controls were healthy subjects (median age, 54 years; range, 45 to 63 years); 95% of the patients are female.
| Study code | Age (years) | Sex | SSc type | Early/late (<2 years for early)* | mRSS | FnEDA† |
|---|---|---|---|---|---|---|
| SScMH_03_Base_LA | 48 | F | dcSSc | Early | 21 | 10.59 |
| SScMH_04_Base_LA | 45 | F | dcSSc | Early | 9 | 24.20 |
| SScMH_05_Base_RA | 40 | F | dcSSc | Early | 32 | 5.64 |
| SScMH_06_Base_RA | 54 | F | lcSSc | Early | 16 | 15.00 |
| SScMH_08_Base_LA | 65 | F | dcSSc | Early | 12 | 17.55 |
| SScMH_12_Base_LA | 51 | F | dcSSc | Early | 14 | 7.52 |
| SScMH_13_Base_LA | 57 | F | dcSSc | Early | 17 | 17.03 |
| SScMH_15_Base_LA | 63 | F | dcSSc | Late | 36 | 39.26 |
| SScMH_17_Base_LA | 53 | M | dcSSc | Early | 35 | 16.26 |
| SScMH_18_Base_LA | 57 | F | dcSSc | Late | 11 | 16.92 |
| SScMH_20_Base_LA | 59 | F | dcSSc | Late | 12 | 12.84 |
| SScMH_27_Base_LA | 47 | F | dcSSc | Early | 29 | 29.07 |
| SScMH_31_Base_LA | 56 | F | dcSSc | Early | 15 | 18.82 |
Early, <2 years from first non-Raynaud disease manifestation; late, >2 years from first non-Raynaud manifestation.
Immunofluorescence intensity. Each data point represents the mean intensity from four randomly selected high-power fields (hpfs) per sample. Mean immunofluorescence intensity in healthy control biopsies (n = 6) was 7.86 ± 4.62. P < 0.016 (healthy versus SSc) (Mann-Whitney U test).
Preferential stimulation of FnEDA in TGF-β– treated normal fibroblasts
The elevated expression of FnEDA in scleroderma patients prompted us to investigate the mechanisms underlying its regulation. For this purpose, quiescent foreskin fibroblasts were incubated with TGF-β for up to 48 hours, and RNA and protein were analyzed by real-time qPCR and immunoblotting. The results showed that TGF-β induced a dose-dependent increase in levels of both FnEDA mRNA (maximal >30-fold) and protein (>9-fold) in these fibroblasts (Fig. 1D). In contrast to the marked up-regulation of the EDA splice variant, fibronectin showed only a modest (about threefold) increase in response to TGF-β. To identify signaling pathways underlying TGF-β stimulation of FnEDA, fibroblasts were pretreated with the selective activin receptor-like kinase 5 (ALK5) inhibitor SB431542 or the mitogen-activated protein kinase kinase (MEK1/2) inhibitor U0126 for 30 min before the addition of TGF-β. The stimulatory effects of TGF-β on FnEDA were completely abolished in the presence of SB431542, whereas U0126 had no effect, indicating a primary role for the canonical Smad pathway (Fig. 1E).
Attenuated dermal fibrosis in FnEDA-null mice
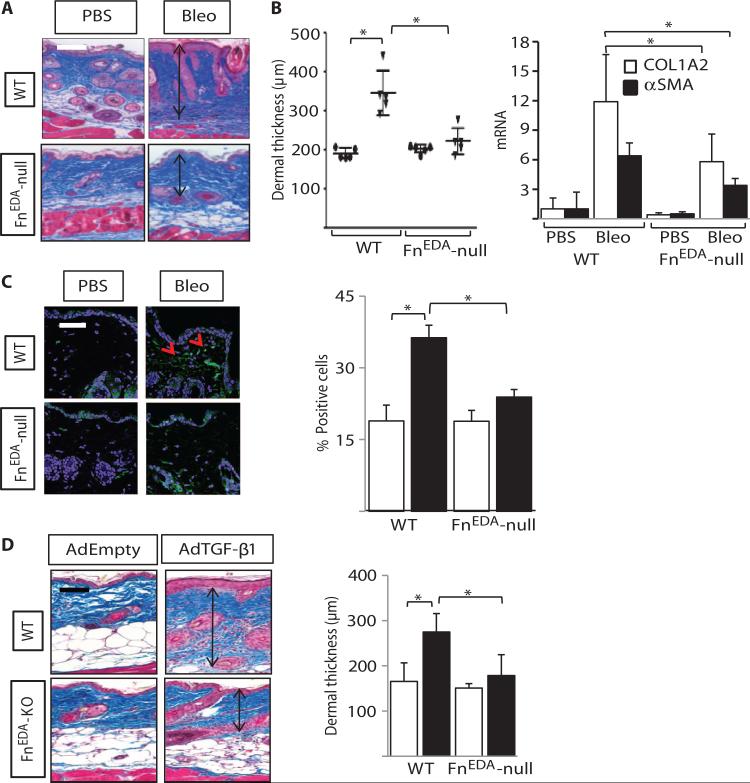
To explore the potential role of FnEDA in tissue remodeling in vivo, mice with homozygous exclusion of the EDA were studied (21). FnEDA-null mice were viable, developed normally, and showed no overt pheno-type. Cutaneous fibrosis was induced by daily subcutaneous injections of bleomycin (15). Mice were sacrificed at day 24, and lesional skin was harvested for analysis. A twofold increase in dermal thickness (P < 0.0001) and collagen accumulation (P = 0.02) was evident in wild-type C57BL/6 mice treated with bleomycin (Fig. 2A). Similarly treated FnEDA-null mice showed significantly reduced dermal thickness (P < 0.001) and collagen (P = 0.015). Immunofluorescence demonstrated a marked increase in FnEDA accumulation in the dermis in wild-type mice injected with bleomycin, whereas no FnEDA could be detected in FnEDA-null mice, as expected (fig. S1, A and B). Real-time qPCR results showed that bleomycin-induced up-regulation of collagen and α smooth muscle actin (αSMA) mRNA in the fibrotic skin was significantly attenuated, and the proportion of αSMA-positive fibroblasts markedly decreased, in FnEDA-null mice (Fig. 2, B and C). Moreover, the increase in the number of phospho-Smad2–positive cells in the fibrotic dermis was significantly attenuated in FnEDA-null mice (fig. S2, A and B).
Fig. 2. Skin fibrosis is attenuated in mice lacking FnEDA.
FnEDA-null mice and wild-type (WT) mice in parallel received daily subcutaneous injections of phosphate-buffered saline (PBS) or bleomycin (Bleo) for 14 days and sacrificed at day 24 (A to C), or two subcutaneous injections of AdTGF-β1 or empty vector 14 days apart and sacrificed at 28 days (D). Lesional skin was harvested for analysis. (A) Left panel: Masson's trichrome stain. Representative images. Arrows indicate dermis. Scale bar, 100 μm. Right panel: Dermal thickness (distance from dermal-epidermal junction to adipose layer), shown as the means ± SD of triplicate determinations per hpf from five mice per group. Ovals, PBS; inverted triangles, bleomycin. *P < 0.0001, PBS versus bleomycin; P < 0.001, WT versus FnEDA-null bleomycin, one-way ANOVA followed by Bonferroni's multiple comparison test. (B) mRNA levels were determined by real-time quantitative PCR. The results, normalized with GAPDH, represent the means ± SD of triplicate determinations from four mice per group. *P = 0.014, Mann-Whitney U test. (C) Immunofluorescence using antibodies against αSMA (green) and 4′,6′-diamidino-2-phenylindole (DAPI) (blue). Left panel: Representative images. Scale bar, 50 μm. Red arrows indicate αSMA-positive cells in the dermis. Right panel: The proportion of αSMA-immunopositive cells in the lesional dermis was determined in five randomly selected hpf per slide from four mice per group. Open bars, PBS; closed bars, bleomycin. PBS versus bleomycin. *P < 0.0001, WT bleomycin versus null bleomycin; P < 0.001, one-way ANOVA followed by Bonferroni's multiple comparison test. (D) Masson's trichrome stain. Left panel: Representative images. Scale bar, 100 μm. Right panel: Dermal thickness, shown as the means ± SD of triplicate determinations per hpf from four mice per group. Open bars, PBS; closed bars, AdTGF-β1. *P = 0.02, PBS versus bleomycin; P = 0.03, WT versus FnEDA-null bleomycin, Sidak's multiple comparison test.
Multiple ECM components are implicated in the progression of organ fibrosis (29). Elastin, the main component of elastic fibers, provides structural integrity and is responsible for the elastic properties of the skin and other organs (30, 31). Previous studies have implicated aberrant elastin deposition and organization in scleroderma, as well as murine models of fibrosis (32–34). To examine the role of FnEDA in elastic fiber accumulation, we examined lesional skin from wild-type and FnEDA-null mice by Verhoeff–Van Gieson staining. In wild-type mice, short wavy stretches of elastic fibers were seen throughout the dermis scattered randomly between adjacent collagen fibers (fig. S2, C and D). Development of dermal fibrosis was accompanied by an increase in the number of elastic fibers, and of elongated fibers, in wild-type mice, but not in FnEDA-null mice.
To further evaluate the role of FnEDA, we induced cutaneous fibrosis in wild-type and FnEDA-null mice in parallel by subcutaneous injections of adenovirus expressing constitutively active TGF-β1 (AdTGF-β1). Mice were sacrificed 28 days after the second subcutaneous injection, and lesional skin was analyzed. In contrast to wild-type mice that showed a ~50% increase in dermal thickness (P = 0.02), in FnEDA-null mice, AdTGF-β1 elicited only a 15% increase (P = 0.20) (Fig. 2D). Moreover, collagen fiber deposition in the lesional dermis was notably attenuated. Results from these complementary animal models together indicate that FnEDA plays a fundamental role in the development of experimentally induced cutaneous fibrosis.
Stimulation of collagen synthesis and myofibroblast differentiation by FnEDA
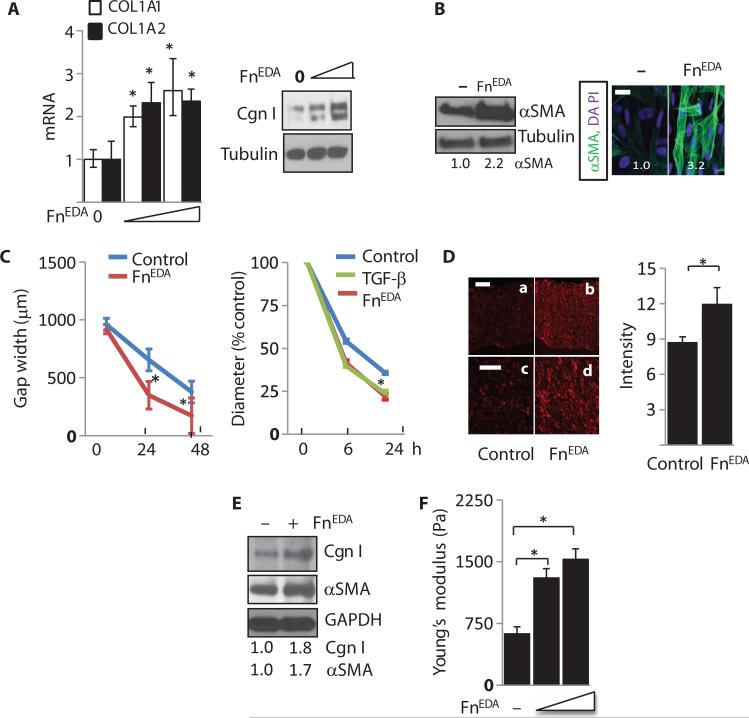
To investigate the regulation of fibrotic responses by FnEDA, we seeded normal dermal fibroblasts in plastic dishes, and confluent mono-layers were incubated with FnEDA (5 or 10 μg/ml) for up to 72 hours. Real-time qPCR and Western analysis demonstrated a dose-dependent increase in COL1A1 mRNA expression and type I collagen levels (Fig. 3A). Comparable stimulation of collagen gene expression was noted in scleroderma skin fibroblasts stimulated with FnEDA (fig. S3A). Furthermore, treatment with FnEDA resulted in substantially enhanced αSMA expression and stress fiber incorporation (Fig. 3B). In stark contrast to FnEDA, fibronectin had no effect on collagen and αSMA expression under similar experimental conditions (fig. S3E). Furthermore, preincubation of the cultures with a neutralizing antibody specific for the EDA domain abrogated the profibrotic effects, indicating the essential role of the EDA domain in triggering these responses (fig. S3E).
Fig. 3. FnEDA elicits profibrotic responses in normal fibroblasts.
(A to C) Normal skin fibroblasts were incubated in medium with FnEDA (5 and 10 μg/ml) for 72 hours. (A) Left panel: mRNA levels were determined by real-time qPCR. Results, normalized with GAPDH, are the means ± SD of triplicate determinations from three independent experiments (P < 0.01, one-way ANOVA followed by Bonferroni's multiple comparison test). Open bars, COL1A1; closed bars, αSMA. Right panel: Whole-cell lysates were examined by Western analysis. Representative immunoblots. Cgn I, type I collagen. (B) Left panel: Representative immunoblots. Values below indicate fold induction (means from three independent experiments corrected for tubulin in each lane). Right panel: Slides were immunostained with antibodies to αSMA (green). Nuclei are identified by DAPI (blue). Representative images. Scale bar, 50 μm. Values indicate fold induction (means from three independent experiments). (C) Left panel: Fibroblasts were seeded on plates coated with FnEDA or left untreated, and at confluence, scratch wounds were created. Fibroblast migration was determined by measuring gap width at 24 and 48 hours. Results are means ± SD of triplicate determinations at three randomly selected locations from triplicate determinations (at 24 hours, P = 0.0043; at 48 hours, P = 0.0450, Mann-Whitney U test). Right panel: Collagen gel contraction assays. Fibroblasts were seeded in type I collagen gels and were incubated in medium in the presence or absence of TGF-β and FnEDA (10 μg/ml). At 6 and 24 hours, gel diameters were determined. Results, expressed as percentage of gel area compared to controls (time 0), are the means ± SD of triplicate determinations (at 24 hours: control versus TGF-β, P < 0.0001; control versus FnEDA, P < 0.0001, one-way ANOVA followed by Bonferroni's multiple comparison test). (D) 3D human skin equivalents constructed without or with FnEDA (10 μg/ml) were seeded with fibroblasts. After 18 days, rafts were stained with Picrosirius Red. Left panel: Representative images. Scale bars, 25 μm (a and b) or 10 μm (c and d). Right panel: Immunofluorescence intensity. Bars represent the means ± SD from six randomly selected hpf per raft from three independent raft experiments (P = 0.022, Mann-Whitney U test). (E) Total protein from dermal compartments was analyzed. Representative Western blot. Numbers below indicate relative band intensities (means from two independent experiments corrected for GAPDH in each lane). (F) Stiffness of the dermal compartment was determined as described in Materials and Methods. The results represent the means ± SD from three independent rafts (P < 0.0001, control versus FnEDA low; P < 0.0001, control versus FnEDA high, oneway ANOVA followed by Bonferroni's multiple comparison test).
Cell migration and matrix contraction are fibroblast responses required for effective wound healing, and their deregulation is implicated in pathological fibrogenesis (35, 36). To evaluate the effect of FnEDA on in vitro wound healing, we used a pipette tip to create a linear scratch in confluent fibroblast monolayers plated on dishes coated with or without FnEDA (10 μg/ml), and cell migration into the wounded area was monitored for up to 48 hours. As shown in Fig. 3C (left panel), fibroblasts grown on FnEDA-coated plastic showed significantly accelerated migration compared to fibroblasts on uncoated plastic. Moreover, fibroblast contractility in collagen gels was enhanced when FnEDA (10 μg/ml) was incorporated in the gels (Fig. 3C, right panel).
Substrate stiffness has profound influence on fibrogenesis by modulating fibroblast morphology, migration, differentiation, and survival (37). Plastic dishes traditionally used for cell culture studies present an excessively rigid substrate with stiffness approaching 2 GPa, potentially confounding the interpretation of the results (38). To dissociate the effect of FnEDA on fibroblasts from that of mechanical forces imparted by the stiff substrate, we took an alternate experimental approach using human fibroblasts embedded in three-dimensional (3D) organotypic skin raft cultures that spontaneously organize a “dermal compartment” with mechanical stiffness approximating that of normal dermis (39). Normal fibroblasts were embedded in skin rafts made with or without FnEDA for 14 days, followed by harvesting and analysis of the dermal compartment. Inclusion of FnEDA in the rafts was associated with a significant increase in deposition of cross-linked “mature” collagen (Fig. 3D). Western analysis confirmed increased collagen (1.8-fold) and αSMA (~1.7-fold) accumulation in the dermal compartment when FnEDA was included (Fig. 3E). We next examined the effect of FnEDA on substrate stiffness in the 3D skin equivalents. Organotypic human skin equivalents constructed using collagen only elaborated a matrix that had physiological stiffness (~600 Pa), whereas incorporation of FnEDA into the rafts resulted in a concentration-dependent increase in stiffness of the dermal compartment (Fig. 3F). Stiff substrates directly promote fibrogenesis by eliciting fibrotic responses such as type I collagen production in explanted fibroblasts (37, 39). These complementary ex vivo models together demonstrate that FnEDA exerts potent profibrotic effect on fibroblasts in both 2D monolayers and 3D skin organotypic human skin equivalents.
FnEDA, an endogenous TLR4 ligand
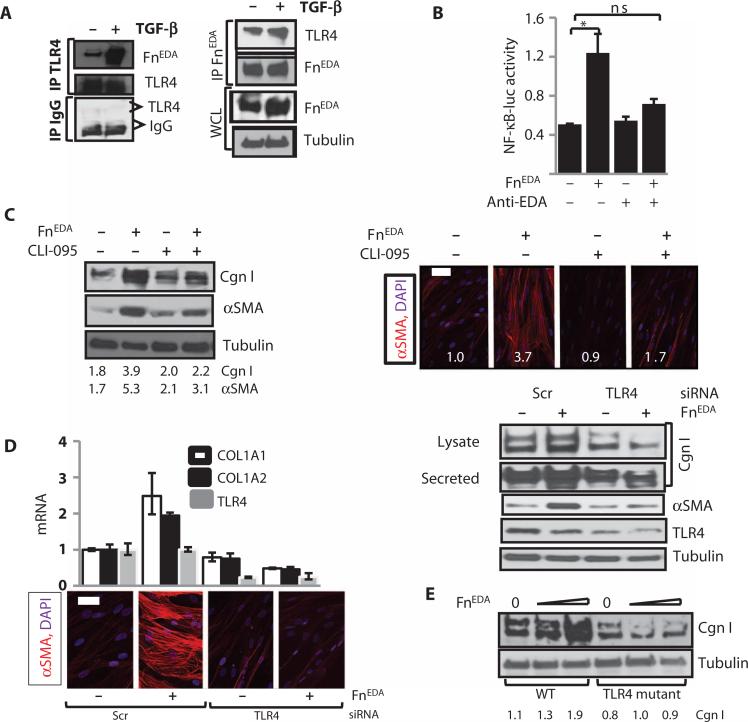
In murine mast cells and macrophages, FnEDA binds to and activates TLR4 (25, 26). Whole-cell lysates from fibroblasts incubated with or without TGF-β for 24 hours were immunoprecipitated sequentially with TLR4, FnEDA, or control immunoglobulin G (IgG) and immuno-blotted with antibodies to TLR4 or FnEDA to examine whether FnEDA functions as a TLR4 ligand in skin fibroblasts. The results revealed direct interaction of cellular TLR4 with FnEDA in unstimulated fibro-blasts, which was increased in TGF-β–treated fibroblasts (Fig. 4A).
Fig. 4. FnEDA elicits TLR4-dependent fibroblast responses.
(A) FnEDA interacts directly with TLR4. Whole-cell lysates (WCL) from normal skin fibroblasts incubated in the presence or absence of TGF-β for 24 hours were immunoprecipitated (IP) with antibodies against TLR4 or FnEDA or IgG and immuno-blotted using antibodies to FnEDA or TLR4. Representative immunoblots. (B) Fibroblasts transiently transfected with NF-κB-luc were incubated in medium with endotoxin-stripped FnEDA in the presence or absence of neutralizing antibodies to FnEDA (anti-EDA) for 24 hours. Whole-cell lysates were assayed for their luciferase activities. Results are means ± SD from three independent experiments. *P = 0.002, control versus FnEDA; *P = 0.04, FnEDA versus anti-EDA–treated, Sidak's multiple comparison test. ns, not significant. (C to E) Human skin fibroblasts (C and D) or skin fibroblasts (E) from TLR4-mutant and WT mouse were incubated in medium with FnEDA (10 μg/ml) in the presence or absence of CLI-095 for 72 hours. (C) Left panels: Whole-cell lysates were subjected to Western analysis. Numbers below indicate relative band intensities corrected for tubulin (means from three independent experiments). Right panels: Fibroblasts were immunostained with antibodies to αSMA. Representative immunofluorescence confocal images. Scale bar, 50 μm. Numbers represent fluorescence intensity determined in four randomly selected locations per hpf for each sample (means from three independent experiments). (D) Human skin fibroblasts were transfected with TLR4-specific siRNA or scrambled (Scr) siRNA, followed by incubation in medium with FnEDA for 72 hours. Left upper panels: mRNA levels were determined by real-time qPCR. Results, normalized with GAPDH mRNA, are means ± SD of triplicate determinations from an experiment representative of two independent experiments. Left lower panels: Immunofluorescence using antibodies to αSMA (red). Representative images. Scale bar, 50 μm. Right panels: Whole-cell lysates were examined by Western analysis. Representative immunoblots. (E) Skin fibroblasts from WT and TLR4-mutant mice in parallel were incubated in medium with FnEDA for 72 hours. Whole-cell lysates were examined by Western analysis. Representative immunoblots. Numbers below indicate relative band intensities corrected for tubulin (means from two independent experiments).
To evaluate how FnEDA modulates TLR4 signaling in fibroblasts, we first examined TLR4-dependent inflammatory responses. A series of experiments demonstrated that incubation of fibroblasts with FnEDA elicited a range of classic inflammatory TLR4 responses, including marked stimulation of interleukin-6 (IL-6) secretion and NF-κB-luc activity (Fig. 4B and fig. S3). The stimulatory effect of FnEDA was attenuated when cultures were pretreated with neutralizing antibodies specific for the EDA domain (Fig. 4B). Furthermore, the stimulatory effects of FnEDA were unaffected by pretreatment with polymyxin B in contrast to lipopolysaccharide (LPS), excluding a role for potential endotoxin contamination of the FnEDA preparations (fig. S3C).
FnEDA-mediated fibrotic responses via TLR4
Having demonstrated a direct interaction of FnEDA with TLR4 in fibroblasts, three complementary loss-of-function approaches were pursued to assess the role of TLR4 in mediating the profibrotic effect of FnEDA. First, we showed that pretreatment of fibroblasts with CLI-095, a small molecule that selectively blocks TLR4 responses by binding to its intracellular signaling domain (40), completely abrogated FnEDA-induced stimulation of type I collagen accumulation and αSMA synthesis (Fig. 4C). Second, selective small interfering RNA (siRNA) knockdown of TLR4 was accompanied by substantially reduced FnEDA stimulation of collagen gene expression and myofibroblast differentiation (Fig. 4D). To further confirm the essential role of TLR4 in the FnEDA response, we pursued a genetic loss-of-function approach using TLR4-mutant C3H/HeJ mice that harbor a TLR4 missense mutation, resulting in LPS hyporesponsiveness (41). Primary skin fibroblasts ex-planted from C3H/HeJ mice and wild-type C3H/HeOuJ mice were grown to confluence in parallel and incubated with FnEDA for 72 hours. Western analysis showed that in contrast to wild-type control fibro-blasts, in TLR4-mutant fibroblasts, FnEDA failed to stimulate collagen synthesis (Fig. 4E). Endotoxin contamination could not account for the stimulatory responses because preincubation of the cultures with polymyxin B did not abrogate the profibrotic effect of FnEDA while effectively blocking LPS-induced cytokine as well as COL1A1 induction (fig. S3D). Moreover, stripping the FnEDA preparation of LPS also failed to abrogate the stimulatory effects (Fig. 4B and fig. S3F). Together, these pharmacological, RNA interference, and genetic approaches in combination unequivocally establish the sufficient and necessary role for TLR4 in mediating profibrotic responses elicited by FnEDA.
To gain insight into the cellular mechanisms underlying the TLR4-dependent profibrotic FnEDA responses, we examined the potential role of miR29, a microRNA of particular interest in light of its emerging role as master regulator of fibrotic responses implicated in scleroderma (15, 42, 43). Incubation of normal fibroblasts with FnEDA caused suppression of miR29a and miR29b, with a maximal ~60% decrease at 72 hours (fig. S4A). The inhibitory response was abrogated by CLI-095, indicating its dependence on TLR4 (fig. S4B). Significantly, pre-miR29a abrogated the collagen stimulatory effect of FnEDA, whereas miR29-specific antagomirs caused an increase in basal levels of COL1A1 mRNA and further enhanced the stimulatory response elicited by FnEDA (fig. S4, C and D). These observations suggest that down-regulation of miR29 might be a mechanism underlying the profibrotic responses elicited by FnEDA.
Essential role of TLR4 for the development of cutaneous fibrosis
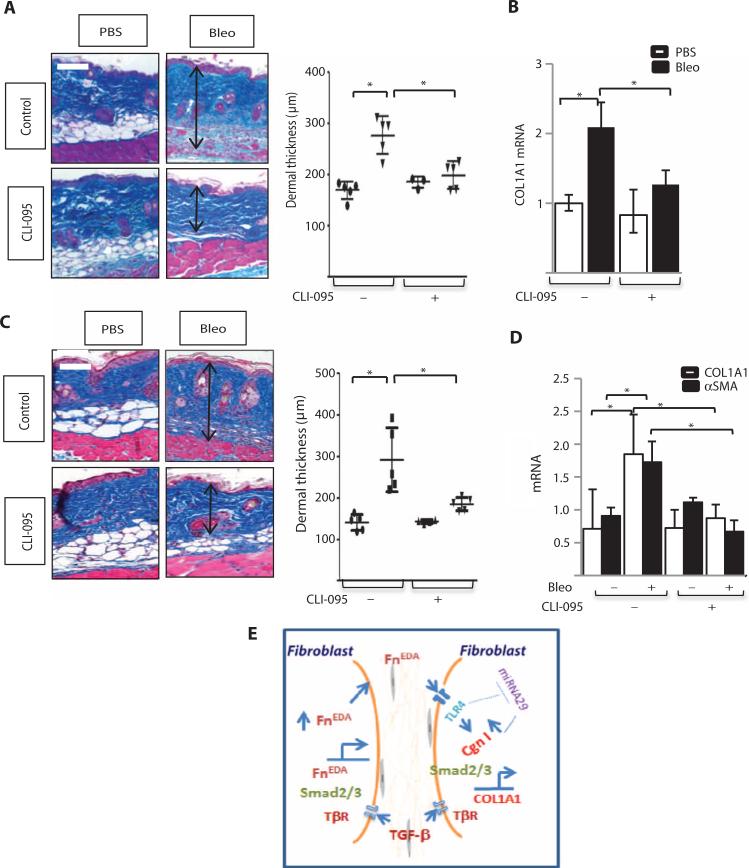
Because our in vitro studies implicated FnEDA-TLR4 signaling in fibrosis, we sought to evaluate the pathogenetic role of TLR4 in vivo using pharmacological TLR4 inhibition. Mice were given daily injections of sub-cutaneous bleomycin alone or concurrently with the TLR4 inhibitor CLI-095. There was no weight loss or other evidence of toxicity in mice treated with CLI-095. Lesional skin from mice treated with bleomycin showed a substantial increase in dermal thickness, accumulation of densely packed collagen bundles accompanied by loss of subcutaneous adipose, and marked necrosis of the subjacent skeletal muscle (Fig. 5A). Mice treated with CLI-095 showed a significantly attenuated increase in dermal thickness (P < 0.001) and collagen deposition (24.0 to 11.4%, P = 0.034). Moreover, bleomycin-induced muscle necrosis was virtually absent. Real-time qPCR showed that CLI-095 treatment mitigated the up-regulation of COL1A1 and αSMA mRNA in the skin (Fig. 5B). Additional experiments showed that TLR4 blockade reversed cutaneous fibrosis, with reduced dermal thickness, collagen accumulation (18.6 to 11.4%, P = 0.011), and collagen and αSMA mRNA expression, when mice were treated with CLI-095 starting at day 15 (Fig. 5, C and D). These results demonstrate that pharmacological blockade of TLR4 using a well-tolerated small molecule both prevented and promoted regression of skin fibrosis, suggesting an essential role for TLR4 signaling in its development and persistence.
Fig. 5. Pharmacological TLR4 blockade both prevents and reverses bleomycin-induced skin fibrosis.
(A to D) C57BL/6J mice received daily subcutaneous injections of bleomycin or PBS. Mice also received CLI-095 (2 mg/kg) starting on day 0 (A and B) or on day 15 (C and D) and sacrificed at day 24 (A and B) or day 28 (C and D), and lesional skin was harvested for analysis. (A and C) Masson's trichrome stain. Representative images (left panels). Scale bars, 100 μm. Right panel: Dermal thickness, determined in four random locations per hpf, is shown as means ± SD from five mice per group. Ovals, PBS; inverted triangles, bleomycin. *P < 0.0001, PBS versus bleomycin; *P < 0.001, bleomycin versus CLI-095, one-way ANOVA followed by Bonferroni's multiple comparison test for (A). *P = 0.0005, PBS versus bleomycin; *P = 0.007, bleomycin versus CLI-095, Sidak's multiple comparison test for (C). (B and D) Levels of mRNA were determined by real-time qPCR. The results, normalized for GAPDH, represent the means ± SD of triplicate determinations from four (A and B) or five (C and D) mice per group. *P = 0.002, WT versus bleomycin; *P = 0.014, bleomycin versus bleomycin + CLI-095, one-way ANOVA followed by Bonferroni's multiple comparison test. (E) Cartoon depicting contribution of TLR4-mediated fibroblast activation elicited by FnEDA to persistent fibrogenesis. By triggering fibroblast TLR4 signaling, FnEDA serves as a switch converting self-limited tissue repair into sustained fibrogenesis. Persistent injury leads to fibroblast activation with generation and extracellular accumulation of FnEDA that in turn triggers TLR4-dependent cellular signaling, resulting in sustained fibroblast activation with production of FnEDA and other ECM molecules. A self-amplifying vicious cycle of fibrogenesis ensues.
DISCUSSION
In scleroderma, the tightly regulated and self-limited repair process that normally leads to tissue regeneration in the skin and multiple organs is subverted, and the ensuing persistent fibroblast activation underlies pathological fibrosis (6, 11–14). We now show that an alternately spliced variant of fibronectin that is normally absent in adult tissue but up-regulated transiently upon injury was constitutively raised in both the serum and skin biopsies from patients with scleroderma, and its expression in normal fibroblasts was stimulated preferentially by TGF-β. Serving as a bona fide endogenous TLR4 ligand, FnEDA elicited potent TLR-dependent fi brotic responses in monolayer fibroblast cultures and in organotypic human skin equivalents. Moreover, in mice, both genetic deletion of FnEDA and pharmacologic blockade of TLR4 mitigated cutaneous fibrosis. Together, these observations identify an FnEDA-TLR4 axis as a pathway fundamental for maintaining sustained fibroblast activation implicated in scleroderma.
The FnEDA splice variant is prominently expressed in embryogenesis but becomes undetectable in adults (17). Physiologic tissue remodeling is associated with transient expression of FnEDA, whereas cancer and chronic inflammation are associated with sustained elevation (44–49). Circulating levels of FnEDA show marked increase after trauma, stroke, and ischemic heart disease (20), and elevated circulating FnEDA predicts liver fibrosis in patients with chronic hepatitis C patients (50). We found elevated FnEDA levels in the serum as well as in skin biopsies from patients with scleroderma. Previous studies demonstrated aberrant FnEDA deposition in scleroderma lesional skin (51), and in lungs from patients with idiopathic pulmonary fibrosis (24), suggesting persistent tissue accumulation of FnEDA as a hallmark of fibrosis. Autocrine TGF-β signaling is thought to underlie the constitutively activated fibroblast phenotype in scleroderma (52). We found that in normal fibroblasts, TGF-β caused a marked stimulation of FnEDA, but only modest change in total fibronectin, a selective response consistent with previous reports (53, 54). Another splice variant of fibronectin, FnEDB, was undetected in scleroderma skin biopsies. We speculate that autocrine TGF-β stimula-tory loops in the scleroderma lesion underlie the persistence of FnEDA expression, but the mechanisms regulating alternative splicing of fibronectin in scleroderma fibro-blasts remain to be uncovered.
Myofibroblast differentiation is an essential step in the formation of fibrosis, and FnEDA is indispensable for the process (19). Our results demonstrate that in ex-planted skin fibroblasts in monolayers and fibroblasts embedded in 3D organotypic human skin equivalents, FnEDA elicited a range of fibrotic responses (39). Previous studies indicated that recombinant FnEDA interacts with and signals through TLR4 (25, 26, 28). Our present results confirm that FnEDA is an endogenous TLR4 ligand in skin fibroblasts. It has been shown that unfolding of FnEDA caused by tensional forces in the fibrotic microenvironment exposes the EDA domain (55). Thus, in the stiff scleroderma skin, TGF-β–driven FnEDA synthesis, combined with exposure of its EDA domain caused by unfolding due to rigid matrix, is likely to increase FnEDA bioavailability as an endogenous TLR4 ligands. The present results indicate that in addition to signaling via α4β1, α4β7, and α9β1 integrins (56–59), FnEDA also uses TLR4 to elicit cellular responses.
A fundamental role of FnEDA in fibrosis is underscored by our results showing that FnEDA-null mice were protected from cutaneous fibrosis. Previous studies demonstrated aberrant wound healing and attenuated lung fibrosis in mice lacking FnEDA (21, 24, 55). Inhibition of TLR4 was shown to attenuate FnEDA-mediated ischemic brain injury, highlighting the importance of the FnEDA-TLR4 axis in this context (60). We now show that pretreatment with a TLR4 inhibitor prevented cutaneous fibrosis, collagen deposition, and myofibroblast accumulation and caused regression of established fibrosis when given therapeutically. Micro-RNAs play fundamental roles in a variety of cellular responses. In particular, miR29 is thought to be important in controlling fibro-genesis and shows reduced expression in fibrotic conditions including scleroderma (61–64). In the present studies with normal fibroblasts, FnEDA suppressed miR29a expression in a TLR4-dependent manner. Rescuing miR29a expression in FnEDA -treated fibroblasts abrogated the stimulation of collagen gene expression, whereas suppressing miR29a using an antagomir enhanced the response, implicating miR29 as a possible mediator of the TLR4-driven profibrotic effects of FnEDA (42, 65).
There are limitations to the current study. The cohort evaluated for serum FnEDA levels was relatively small, precluding firm conclusions regarding the utility of serum FnEDA as a biomarker in scleroderma or as a marker of organ involvement or disease activity. It should be informative to compare serum FnEDA levels in chronic autoimmune and fibrotic diseases. Although our results demonstrate marked FnEDA accumulation in lesional skin, it remains to be determined whether it is FnEDA or other endogenous TLR4 ligands that are primarily responsible for maintaining the activated fibroblast phenotype in scleroderma lesions. Indeed, several such damage-associated endogenous TLR4 ligands have been shown to be elevated in scleroderma, including hyaluronan and biglycan. In addition, although the present results clearly implicate the FnEDA-TLR4 axis in cutaneous fibrosis induced by bleomycin or TGF-β, it will be of great interest to explore the pathogenetic role of the FnEDA TLR4 axis in other forms of cutaneous and extracutaneous organ fibrosis.
On the basis of our in vitro and in vivo observations, we propose a model for persistent cutaneous fibrosis (Fig. 5), where activated fibro-blasts exposed to extracellular FnEDA within the fibrotic milieu acquire a TLR4-dependent fibrotic phenotype with further stimulation of the production and deposition of ECM molecules including FnEDA, thereby exacerbating and sustaining fibrogenesis. In this way, fibroblast innate immune signaling triggered by DAMPs might represent one of the cellular pathways responsible for converting self-limited regenerative repair into an intractable fibrotic process. Accordingly, we propose that disrupting sustained TLR4 signaling in the skin lesion represents a potential strategy for breaking the vicious cycle of fibrosis in scleroderma.
MATERIALS AND METHODS
Study design
This was an observational study designed to identify differences among scleroderma patients and healthy controls. Serum levels of FnEDA were determined in 48 scleroderma patients evaluated at a single center and 16 healthy controls in parallel. All patients met American College of Rheumatology criteria for the classification of systemic sclerosis (SSc) (66) and were sub-classified as limited or diffuse cutaneous disease using published criteria (67). Duration of disease was defined as the interval from the onset of the first non-Raynaud disease manifestation to the time of the serum collection, and patients were subdivided into early (<24 months) and late (>24 months) subgroups. Pulmonary function testing was performed within 6 months of the time of serum collection (68). To assess FnEDA accumulation in lesional tissue, skin biopsies from the clinically involved forearm of 13 SSc patients and 7 healthy controls were processed in parallel for immunofluorescence. RNA was isolated from skin biopsies of 19 scleroderma patients and 9 healthy controls and processed for real-time qPCR.
Animal experiments were performed according to institutionally approved protocols and in compliance with the guidelines of the Northwestern University Animal Care and Use Committee. In selected experiments, mice were randomly assigned to treatment groups, and treatment groups were blinded during experimental procedures and raw data analysis. No animals or potential outliers were excluded from the data sets presented in this study. All in vitro studies were performed in replicates (n = 3, unless otherwise specified).
Cell culture and reagents
Primary human fibroblast cultures were established by explantations from neonatal foreskin or from skin biopsies of the clinically affected forearms of patients with scleroderma and the forearm of healthy adult volunteers (15). Biopsies were performed with written informed consent and in accordance with protocols approved by the Institutional Review Board for Human Studies at Northwestern University. Primary fibroblasts at low passage grown in monolayers in plastic dishes were studied at early confluence or were embedded into “dermal” collagen plugs used to create 3D organotypic human skin equivalents (15, 69, 70). Skin fibroblast cultures were also established from 8-week-old C3H/HeJ (TLR4-mutant) and C3H/HeOuJ (wild-type) mice (The Jackson Laboratory) and studied in parallel. Cultures were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (Gibco BRL), 1% vitamin solutions, and 2 mM l-glutamine. All other tissue culture re-agents were from Lonza. Cultures were placed in serum-free medium containing 0.1% bovine serum albumin (BSA) for 24 hours before addition of TGF-β (PeproTech) or FnEDA. To generate FnEDA, conditioned media were collected from human embryonic IMR90 fibroblasts incubated for 48 hours and purified with affinity chromatography (24). The purity of FnEDA was confirmed by Coomassie staining and by Western analysis (fig. S3A, left panel)). Endotoxin was quantitated in the FnEDA preparations with Pierce LAL Chromogenic Endotoxin Quantitation Kit (Thermo Fisher Scientific). In selected experiments, cultures were incubated in medium with FnEDA preparations that had been subjected to endotoxin removal with Pierce High Capacity Endotoxin Removal Resin (Thermo Fisher Scientific). The TLR4 inhibitor CLI-095 (InvivoGen) or neutralizing antibodies specific for the EDA domain (IST9, Abcam) were added to the cultures 60 min before FnEDA. Levels of IL-6 secreted in the medium were determined by ELISA (R&D Systems).
FnEDA effects in 3D organotypic human skin equivalents
To evaluate the effects of FnEDA in 3D organotypic human skin equivalents, foreskin fibroblasts (300,000 cells/ml) were resuspended in rat tail type I collagen (4 mg/ml, from BD Biosciences) with or without FnEDA (10 μg/ml) and seeded in 12-well plates (69). Briefly, foreskin fibroblasts (300,000 cells/ml) were resuspended in a buffered solution of rat tail type I collagen (4 mg/ml, from BD Biosciences) with or without FnEDA (10 μg/ml) and seeded in 12-well cell culture plates (1.5 ml per well). The dermal collagen plugs were allowed to polymerize for 30 min and then supplemented with 10% DMEM overnight. Primary human epidermal keratinocytes (200,000 cells/ml) isolated from a pool (n = 3) of neonatal foreskins were seeded on top of the dermal collagen plug in E medium [3:1 of DMEM/Ham's F-12 containing 180 μM adenine, insulin (5 μg/ml), transferrin (5 μg/ml), T3 (5 μg/ml), hydrocortisone (0.4 ng/ml), and cholera toxin (10 mg/ml)] supplemented with epidermal growth factor (5 ng/ml) as previously described (70). After 48 hours of incubation, the skin equivalents were transferred to the top of a metal grid raised above the surface of a 60-mm tissue culture dish and exposed to air to promote epidermal stratification. Culture media were replaced every other day for up to 12 days, and rafts were harvested and lysed for Western analysis, fixed in 10% neutral buffered formalin, and embedded in paraffin for staining or processed for determination of stiffness. Picrosirius Red staining was used to evaluate collagen deposition and fiber alignment in the dermal compartment.
To determine the effect of FnEDA on substrate stiffness in organotypic raft cultures, a parallel-plate rheometer (Oscillatory Shear Paar Physica MCR Rheometer, Anton Paar GmbH) was used. Circular samples (20-mm diameter) of control and FnEDA-containing rafts were placed between parallel plates, and plate distance of 0.2 mm was preset to compress the raft. Frequency (F = 0.1 to 10 Hz) sweep tests under oscillatory conditions were conducted at a constant temperature of 37°C, with controlled shear deformation γ = 0.5% and 15 measuring points, which led to an average test duration of 16 min. Results, expressed as Young's modulus, were calculated with US-200 software (Anton Paar Co.).
In vitro cell migration and collagen gel contraction assays
The effects of FnEDA on modulating fibroblast function were further evaluated by in vitro wound healing and collagen gel contraction assays (36). Briefly, human skin fibroblasts were seeded on FnEDA-coated plates, and confluent monolayers in serum-free DMEM were mechanically wounded with standard p1000 pipette tips. Cultures were monitored for up to 48 hours by phase-contrast microscopy, and pictures were taken at ×25 magnification. The wound gap width (micrometers) was determined at six randomly selected sites per slide at indicated intervals. Collagen gel contraction assays were performed with fibro-blasts seeded in type I collagen gels as described (36). After incubation of the gels in medium containing FnEDA (10 μg/ml) for the indicated intervals, gel diameters were determined with ImageJ software [National Institutes of Health (NIH), Bethesda, MD]. All experiments were performed in triplicate.
Isolation and analysis of RNA and microRNA
At the end of the experiments, total RNA was isolated from explanted fibroblasts, skin biopsies, or organotypic skin rafts and reverse-transcribed to complementary DNA (cDNA) with SuperMix as described (cDNA Synthesis SuperMix, Quanta BioSciences) (15). The products (50 ng) were amplified with SYBR Green PCR Master Mix (Applied Biosystems) on an Applied Biosystems 7500 Prism Sequence Detection System. The sequence of the primers is shown in Table 1. MicroRNA was isolated from confluent fibroblasts with the mirVana miRNA Isolation Kit (Ambion/Applied Biosystems) and amplified with TaqMan probes (Applied Biosystems). Levels of miRNA were determined by qPCR with Applied Biosystems 7500 Prism Sequence Detection System (15). Data were normalized to GAPDH RNA, and fold change in samples was calculated as 2−ΔΔCt {2−[(Ct target − Ct GAPDH) treatment − (Ct target − Ct GAPDH) nontreatment]}.
Western and immunoprecipitation/immunoblot analysis
At the end of the experiments, fibroblasts were harvested, whole-cell lysates were prepared, and equal amounts of proteins (20 to 50 μg per lane) were subjected to Western analysis with primary antibodies specific for type I collagen (Southern Biotechnology), FnEDA (Sigma-Aldrich), and tubulin (Sigma-Aldrich) as described (15). Membranes were then incubated with appropriate secondary antibodies and subjected to enhanced chemiluminescence detection with ECL Reagent (Pierce). In other experiments, whole-cell lysates (~600 μg) were immunoprecipitated and subjected to immunoblot analysis with antibodies to FnEDA (Sigma-Aldrich) and TLR4 (Santa Cruz Biotechnology). Band intensities were quantitated with ImageJ software and corrected for tubulin in each lane.
Transient transfection assays
Subconfluent skin fibroblasts in serum-free medium were transfected with NF-κB-luc with SuperFect reagent (Qiagen), followed by incubation in medium containing FnEDA (10 μg/ml) in the presence or absence of neutralizing EDA domain–specific antibodies (IST9) and polymyxin B for 24 hours. Cultures were harvested, and whole-cell ly-sates were assayed for their luciferase activities. In each experiment, fibroblasts were cotransfected with Renilla luciferase pRL-TK plasmids (Promega) as controls for transfection efficiency. Experiments were performed in triplicate and repeated at least twice with consistent results.
Immunofluorescence confocal microscopy
To assess the modulation of fibroblast responses by FnEDA by immunocytochemistry, fibroblasts on eight-well Lab-Tek II chamber glass slides (Nalgene Nunc International) were incubated in serum-free DMEM supplemented with 0.1% BSA containing FnEDA (10 μg/ml) for up to 72 hours. In selected experiments, fibroblasts were transfected with TLR4-specific siRNA or scrambled control siRNA (Dharmacon) for 24 hours before the addition of Fn-EDA. Cells were then fixed, permeabilized, and incubated with antibodies to type I collagen or αSMA (Sigma) at 1:100 or 1:500 dilution, followed by Alexa Fluor– labeled secondary antibodies (Invitrogen). Nuclei were identified with DAPI. Subcellular distribution of immunofluorescence was evaluated under an immunofluorescence microscope or Zeiss UV Meta 510 confocal microscope (Carl Zeiss Inc.) (15).
Serum FnEDA determinations by ELISAs
To determine serum levels of FnEDA, we developed a specific ELISA. Briefly, 96-well plates were coated with primary antibody to FnEDA (3E2, Sigma) (1.4 μg/ml in 0.05 M carbonate-bicarbonate buffer, pH 9.6) for 60 min at 37°C followed by blocking with 5% BSA. Serum samples in triplicate were incubated with peroxidase-conjugated goat anti-human fibronectin IgG (1:8000; MP Biomedicals, LLC; catalog no. 55240) for 30 min and washed four times with PBS. Substrate solution (50 μl) was added, and after sufficient color development, reactions were terminated with 0.5 M sulfuric acid and absorbance at 490 nm was measured with an automated plate reader. The sample concentrations were interpolated from standard curves prepared with serial dilutions of known FnEDA concentrations (detection range, 0.8 to 50.0 μg/ml). To ensure specificity, all assays were run with plasma fibronectin as a negative control.
FnEDA expression in scleroderma skin biopsies
Paraffin-embedded sections (4 μm) were incubated with primary antibodies against FnEDA (Sigma, 1:50), followed by mouse Alexa Fluor secondary antibodies (Invitrogen) or DAPI. Slides were mounted, and immunofluorescence was evaluated under a Zeiss UV Meta 510 confocal microscope. Immunopositivity was specific for FnEDA because substitution of the primary antibody resulted in the absence of staining and Western blot using the antibody showed a single band. Computer-generated 3D image plots of fluorescence intensity were analyzed with ImageJ software (NIH).
Experimental animal models of cutaneous fibrosis
To evaluate the role of FnEDA in fibrogenesis, we studied cutaneous fibrosis induced by bleomycin or by AdTGF-β1 in FnEDA-null mice (21). Eight-week-old female FnEDA-null mice and wild-type C57BL/6J mice (The Jackson Laboratory) in parallel were given daily subcutaneous injections of bleomycin (10 mg/kg per day) or PBS for 10 days (15). In other experiments, FnEDA-null mice and wild-type mice in parallel received two subcutaneous injections of AdTGF-β1 (1 × 109 plaque-forming units) or empty vector administered 14 days apart. Mice were sacrificed at day 28, and lesional skin was harvested for analysis. Four-micrometer-thick sections of paraffin-embedded tissues were stained with hematoxylin and eosin, or Masson's trichrome for visualizing collagen. Collagen deposition in the dermis was assessed in Masson's trichrome–stained slides by quantitating blue pixels with ImageJ software. Immunofluorescence analyses were performed by incubating tissues with primary rabbit antibodies against αSMA (Sigma, 1:500), FnEDA (Abcam, 1:50), or phospho-Smad2 (Cell Signaling Technology, 1:100) followed by Alexa Fluor–labeled rabbit secondary antibodies. Nuclei were detected with DAPI. Slides were mounted, and immunofluorescence was evaluated under a Zeiss UV Meta 510 confocal microscope. Each experimental group consisted of at least five mice. Elastic fibers in the skin were visualized with Verhoeff's stain followed by Van Gieson's counterstain (71). Accumulation of elastic fibers was quantitated by determining the percentage of positive pixels (elastin stained) from five randomly selected hpf per slide with Adobe Photoshop CS5 (Adobe Systems) (71).
To investigate the preventive and therapeutic effects of pharmacological TLR4 blockade on cutaneous fibrosis in vivo, C57BL/6J mice were given daily subcutaneous injections of bleomycin (10 mg/kg) or PBS for 15 days. One group of mice was given CLI-095 (2 mg/kg) (InvivoGen) by daily intraperitoneal injections starting concurrently with PBS or bleomycin, and sacrificed on day 24, whereas in another group of mice, daily CLI-095 injections were started at day 15 and mice were sacrificed on day 28. Each experimental group consisted of five mice.
Statistical analysis
Data are presented as means ± SD. Two-tailed Student's t test or Mann-Whitney U test was used for comparisons between two groups, and P < 0.05 was considered significant. Spearman's rank correlation coefficient was used to analyze the relationship between serum FnEDA levels and clinical parameters. Comparisons among three or more groups were performed with ANOVA followed by Bonferroni correction or Sidak's multiple comparison test. Data were analyzed with GraphPad Prism (version 5 or 6; GraphPad Software).
Study approval
Animal studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the Northwestern University. Studies involving human subjects were approved by the Institutional Review Board of the Northwestern University, and all the participants provided written informed consent.
Supplementary Material
Acknowledgments
We are grateful to R. P. Schleimer, A. Dreffs, and A. Booth for reagents, and W. Tourtellotte, S. Hussain, and members of the Varga and Shea laboratories and the staff of the Northwestern University Imaging Core for helpful discussions and excellent technical support. The Skin Diseases Research Core assisted with construction of human skin equivalents. Funding: Supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR42309 and AR057216).
Footnotes
Author contributions: S.B. and J.V. conceived and designed the experiments. W.W., Z.T., S.B., and P.H. performed the experiments. S.B. and Z.T. analyzed the data. E.S.W., S.G., and M.H. contributed reagents/materials/analysis tools. S.B. and J.V. prepared the manuscript.
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/6/232/232ra50/DC1
Fig. S1. FnEDA expression is elevated in bleomycin-treated mice.
Fig. S2. Attenuated Smad2 activation and elastin accumulation in FnEDA-null mice.
Fig. S3. FnEDA elicits TLR4-dependent proinflammatory and fibrotic responses.
Fig. S4. miR29 mediates fibroblast responses elicited by FnEDA.
Table S1. Primers for real-time qPCR.
Table S2. Comparison of patients with low versus high serum levels of FnEDA.
Citation: S. Bhattacharyya, Z. Tamaki, W. Wang, M. Hinchcliff, P. Hoover, S. Getsios, E. S. White, J. Varga, FibronectinEDA promotes chronic cutaneous fibrosis through Toll-like receptor signaling. Sci. Transl. Med. 6, 232ra50 (2014).
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Denton CP, Ong VH. Targeted therapies for systemic sclerosis. Nat. Rev. Rheumatol. 2013;9:451–464. doi: 10.1038/nrrheum.2013.46. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbloom J, Castro SV, Jimenez SA. Narrative review: Fibrotic diseases: Cellular and molecular mechanisms and novel therapies. Ann. Intern. Med. 2010;152:159–166. doi: 10.7326/0003-4819-152-3-201002020-00007. [DOI] [PubMed] [Google Scholar]
- 3.Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor β (TGFβ) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem. J. 1987;247:597–604. doi: 10.1042/bj2470597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varga J, Abraham D. Systemic sclerosis: A prototypic multisystem fibrotic disorder. J. Clin. Invest. 2007;117:557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: Paradigms for connective tissue remodeling. Am. J. Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharyya S, Wei J, Varga J. Understanding fibrosis in systemic sclerosis: Shifting paradigms, emerging opportunities. Nat. Rev. Rheumatol. 2012;8:42–54. doi: 10.1038/nrrheum.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010;2010:672395. doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beutler B. Neo-ligands for innate immune receptors and the etiology of sterile inflammatory disease. Immunol. Rev. 2007;220:113–128. doi: 10.1111/j.1600-065X.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- 10.Tolle LB, Standiford TJ. Danger-associated molecular patterns (DAMPs) in acute lung injury. J. Pathol. 2013;229:145–156. doi: 10.1002/path.4124. [DOI] [PubMed] [Google Scholar]
- 11.Huebener P, Schwabe RF. Regulation of wound healing and organ fibrosis by toll-like receptors. Biochim. Biophys. Acta. 2013;1832:1005–1017. doi: 10.1016/j.bbadis.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat. Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 13.Pulskens WP, Rampanelli E, Teske GJ, Butter LM, Claessen N, Luirink IK, van der Poll T, Florquin S, Leemans JC. TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J. Am. Soc. Nephrol. 2010;21:1299–1308. doi: 10.1681/ASN.2009070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell MT, Hile KL, Zhang H, Asanuma H, Vanderbrink BA, Rink RR, Meldrum KK. Toll-like receptor 4: A novel signaling pathway during renal fibrogenesis. J. Surg. Res. 2011;168:e61–e69. doi: 10.1016/j.jss.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharyya S, Kelley K, Melichian DS, Tamaki Z, Fang F, Su Y, Feng G, Pope RM, Budinger GR, Mutlu GM, Lafyatis R, Radstake T, Feghali-Bostwick C, Varga J. Toll-like receptor 4 signaling augments transforming growth factor-β responses: A novel mechanism for maintaining and amplifying fibrosis in scleroderma. Am. J. Pathol. 2013;182:192–205. doi: 10.1016/j.ajpath.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciechomska M, Cant R, Finnigan J, van Laar JM, O'Reilly S. Role of toll-like receptors in systemic sclerosis. Expert Rev. Mol. Med. 2013;15:e9. doi: 10.1017/erm.2013.10. [DOI] [PubMed] [Google Scholar]
- 17.White ES, Baralle FE, Muro AF. New insights into form and function of fibronectin splice variants. J. Pathol. 2008;216:1–14. doi: 10.1002/path.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muro AF, Caputi M, Pariyarath R, Pagani F, Buratti E, Baralle FE. Regulation of fibronectin EDA exon alternative splicing: Possible role of RNA secondary structure for enhancer display. Mol. Cell. Biol. 1999;19:2657–2671. doi: 10.1128/mcb.19.4.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-β1. J. Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.To WS, Midwood KS. Plasma and cellular fibronectin: Distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair. 2011;4:21. doi: 10.1186/1755-1536-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J. Cell Biol. 2003;162:149–160. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am. J. Pathol. 1991;138:1257–1265. [PMC free article] [PubMed] [Google Scholar]
- 23.Hernnäs J, Nettelbladt O, Bjermer L, Särnstrand B, Malmström A, Hällgren R. Alveolar accumulation of fibronectin and hyaluronan precedes bleomycin-induced pulmonary fibrosis in the rat. Eur. Respir. J. 1992;5:404–410. [PubMed] [Google Scholar]
- 24.Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, Baralle FE, Toews GB, White ES. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008;177:638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., III The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 26.Gondokaryono SP, Ushio H, Niyonsaba F, Hara M, Takenaka H, Jayawardana ST, Ikeda S, Okumura K, Ogawa H. The extra domain A of fibronectin stimulates murine mast cells via Toll-like receptor 4. J. Leukocyte Biol. 2007;82:657–665. doi: 10.1189/jlb.1206730. [DOI] [PubMed] [Google Scholar]
- 27.Sofat N, Robertson SD, Wait R. Fibronectin III 13-14 domains induce joint damage via Toll-like receptor 4 activation and synergize with interleukin-1 and tumour necrosis factor. J. Innate Immun. 2012;4:69–79. doi: 10.1159/000329632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefebvre JS, Lévesque T, Picard S, Paré G, Gravel A, Flamand L, Borgeat P. Extra domain A of fibronectin primes leukotriene biosynthesis and stimulates neutrophil migration through activation of Toll-like receptor 4. Arthritis Rheum. 2011;63:1527–1533. doi: 10.1002/art.30308. [DOI] [PubMed] [Google Scholar]
- 29.Hubmacher D, Apte SS. The biology of the extracellular matrix: Novel insights. Curr. Opin. Rheumatol. 2013;25:65–70. doi: 10.1097/BOR.0b013e32835b137b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turino GM, Ma S, Lin YY, Cantor JO, Luisetti M. Matrix elastin: A promising biomarker for chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2011;184:637–641. doi: 10.1164/rccm.201103-0450PP. [DOI] [PubMed] [Google Scholar]
- 31.Klingberg F, Hinz B, White ES. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013;229:298–309. doi: 10.1002/path.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatterjee S, Mark ME, Wooley PH, Lawrence WD, Mayes MD. Increased dermal elastic fibers in the tight skin mouse. Clin. Exp. Rheumatol. 2004;22:617–620. [PubMed] [Google Scholar]
- 33.Walters R, Pulitzer M, Kamino H. Elastic fiber pattern in scleroderma/morphea. J. Cutan. Pathol. 2009;36:952–957. doi: 10.1111/j.1600-0560.2009.01201.x. [DOI] [PubMed] [Google Scholar]
- 34.Lemaire R, Korn JH, Schiemann WP, Lafyatis R. Fibulin-2 and fibulin-5 alterations in Tsk mice associated with disorganized hypodermal elastic fibers and skin tethering. J. Invest. Dermatol. 2004;123:1063–1069. doi: 10.1111/j.0022-202X.2004.23471.x. [DOI] [PubMed] [Google Scholar]
- 35.Rhee S. Fibroblasts in three dimensional matrices: Cell migration and matrix remodeling. Exp. Mol. Med. 2009;41:858–865. doi: 10.3858/emm.2009.41.12.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu M, Melichian DS, de la Garza M, Gruner K, Bhattacharyya S, Barr L, Nair A, Shahrara S, Sporn PH, Mustoe TA, Tourtellotte WG, Varga J. Essential roles for early growth response transcription factor Egr-1 in tissue fibrosis and wound healing. Am. J. Pathol. 2009;175:1041–1055. doi: 10.2353/ajpath.2009.090241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 38.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr. Biol. 2012;4:410–421. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- 39.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur. J. Pharmacol. 2008;584:40–48. doi: 10.1016/j.ejphar.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 41.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 42.Maurer B, Stanczyk J, Jüngel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, Gay S, Distler O. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 43.Schulz MH, Pandit KV, Lino Cardenas CL, Ambalavanan N, Kaminski N, Bar-Joseph Z. Reconstructing dynamic microRNA-regulated interaction networks. Proc. Natl. Acad. Sci. U.S.A. 2013;110:15686–15691. doi: 10.1073/pnas.1303236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rybak JN, Roesli C, Kaspar M, Villa A, Neri D. The extra-domain A of fibronectin is a vascular marker of solid tumors and metastases. Cancer Res. 2007;67:10948–10957. doi: 10.1158/0008-5472.CAN-07-1436. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto E, Yoshida T, Kawarada Y, Sakakura T. Expression of fibronectin isoforms in human breast tissue: Production of extra domain A+/extra domain B+ by cancer cells and extra domain A+ by stromal cells. Jpn. J. Cancer Res. 1999;90:320–325. doi: 10.1111/j.1349-7006.1999.tb00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H, Chen B, Zardi L, Ramos DM. Soluble fibronectin promotes migration of oral squamous-cell carcinoma cells. Int. J. Cancer. 1998;78:261–267. doi: 10.1002/(sici)1097-0215(19981005)78:2<261::aid-ijc22>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 47.Flanagan M, Liang H, Norton PA. Alternative splicing of fibronectin mRNAs in chondrosarcoma cells: Role of far upstream intron sequences. J. Cell. Biochem. 2003;90:709–718. doi: 10.1002/jcb.10687. [DOI] [PubMed] [Google Scholar]
- 48.Kriegsmann J, Berndt A, Hansen T, Borsi L, Zardi L, Bräuer R, Petrow PK, Otto M, Kirkpatrick CJ, Gay S, Kosmehl H. Expression of fibronectin splice variants and oncofetal glycosylated fibronectin in the synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Rheumatol. Int. 2004;24:25–33. doi: 10.1007/s00296-003-0316-1. [DOI] [PubMed] [Google Scholar]
- 49.Claudepierre P, Allanore Y, Belec L, Larget-Piet B, Zardi L, Chevalier X. Increased Ed-B fibronectin plasma levels in spondyloarthropathies: Comparison with rheumatoid arthritis patients and a healthy population. Rheumatology. 1999;38:1099–1103. doi: 10.1093/rheumatology/38.11.1099. [DOI] [PubMed] [Google Scholar]
- 50.Hackl NJ, Bersch C, Feick P, Antoni C, Franke A, Singer MV, Nakchbandi IA. Circulating fibronectin isoforms predict the degree of fibrosis in chronic hepatitis C. Scand. J. Gastroenterol. 2010;45:349–356. doi: 10.3109/00365520903490606. [DOI] [PubMed] [Google Scholar]
- 51.Rajkumar VS, Howell K, Csiszar K, Denton CP, Black CM, Abraham DJ. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res. Ther. 2005;7:R1113–R1123. doi: 10.1186/ar1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ihn H. Autocrine TGF-β signaling in the pathogenesis of systemic sclerosis. J. Dermatol. Sci. 2008;49:103–113. doi: 10.1016/j.jdermsci.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 53.Balza E, Borsi L, Allemanni G, Zardi L. Transforming growth factor β regulates the levels of different fibronectin isoforms in normal human cultured fibroblasts. FEBS Lett. 1988;228:42–44. doi: 10.1016/0014-5793(88)80580-5. [DOI] [PubMed] [Google Scholar]
- 54.White ES, Sagana RL, Booth AJ, Yan M, Cornett AM, Bloomheart CA, Tsui JL, Wilke CA, Moore BB, Ritzenthaler JD, Roman J, Muro AF. Control of fibroblast fibronectin expression and alternative splicing via the PI3K/Akt/mTOR pathway. Exp. Cell Res. 2010;316:2644–2653. doi: 10.1016/j.yexcr.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, Peters JH, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ. Res. 2011;108:582–592. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- 56.Shinde AV, Bystroff C, Wang C, Vogelezang MG, Vincent PA, Hynes RO, Van De Water L. Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin α9β1-dependent cellular activities. J. Biol. Chem. 2008;283:2858–2870. doi: 10.1074/jbc.M708306200. [DOI] [PubMed] [Google Scholar]
- 57.Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. The EIIIA segment of fibronectin is a ligand for integrins α9β1 and α4β1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J. Biol. Chem. 2002;277:14467–14474. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- 58.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-β1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J. Biol. Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 59.Fernandes DJ, Bonacci JV, Stewart AG. Extracellular matrix, integrins, and mesenchymal cell function in the airways. Curr. Drug Targets. 2006;7:567–577. doi: 10.2174/138945006776818700. [DOI] [PubMed] [Google Scholar]
- 60.Khan MM, Gandhi C, Chauhan N, Stevens JW, Motto DG, Lentz SR, Chauhan AK. Alternatively-spliced extra domain A of fibronectin promotes acute inflammation and brain injury after cerebral ischemia in mice. Stroke. 2012;43:1376–1382. doi: 10.1161/STROKEAHA.111.635516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kogure T, Costinean S, Yan I, Braconi C, Croce C, Patel T. Hepatic miR-29ab1 expression modulates chronic hepatic injury. J. Cell. Mol. Med. 2012;16:2647–2654. doi: 10.1111/j.1582-4934.2012.01578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhong X, Chung AC, Chen HY, Meng XM, Lan HY. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J. Am. Soc. Nephrol. 2011;22:1668–1681. doi: 10.1681/ASN.2010111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M, Tacke F, Trautwein C, Luedde T. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 64.Pandit KV, Milosevic J, Kaminski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl. Res. 2011;157:191–199. doi: 10.1016/j.trsl.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Yang T, Liang Y, Lin Q, Liu J, Luo F, Li X, Zhou H, Zhuang S, Zhang H. MiR-29 mediates TGFβ1-induced extracellular matrix synthesis through activation of PI3K-AKT pathway in human lung fibroblasts. J. Cell Biochem. 2013;114:1336–1342. doi: 10.1002/jcb.24474. [DOI] [PubMed] [Google Scholar]
- 66.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 67.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr., Rowell N, Wollheim F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 68.Hinchcliff M, Huang CC, Wood TA, Mahoney JM, Martyanov V, Bhattacharyya S, Tamaki Z, Carns M, Podlusky S, Sirajuddin A, Shah SJ, Lee J, Chang RW, Lafyatis R, Varga J, Whitfield ML. Molecular signatures in skin associated with clinical improvement during mycophenolate treatment in systemic sclerosis. J. Invest. Dermatol. 2013;133:1979–1989. doi: 10.1038/jid.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Getsios S, Simpson CL, Kojima S, Harmon R, Sheu LJ, Dusek RL, Cornwell M, Green KJ. Desmoglein 1–dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J. Cell Biol. 2009;185:1243–1258. doi: 10.1083/jcb.200809044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simpson CL, Kojima S, Getsios S. RNA interference in keratinocytes and an organotypic model of human epidermis. Methods Mol. Biol. 2010;585:127–146. doi: 10.1007/978-1-60761-380-0_10. [DOI] [PubMed] [Google Scholar]
- 71.Pellicoro A, Aucott RL, Ramachandran P, Robson AJ, Fallowfield JA, Snowdon VK, Hartland SN, Vernon M, Duffield JS, Benyon RC, Forbes SJ, Iredale JP. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology. 2012;55:1965–1975. doi: 10.1002/hep.25567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.