Abstract
We propose a simple two-disease epidemic model where one disease exhibits only a drug-sensitive strain, while the other exhibits both drug-sensitive and drug-resistant strains. Treatment for the first disease may select for resistance in the other. We model antibiotic use as a mathematical game through the study of individual incentives and community welfare. The basic reproduction number is derived and the existence and local stability of the model equilibria are analyzed. When the force of infection of each disease is unaffected by the presence of the other, we find that there is a conflict of interest between individual and community, known as a tragedy of the commons, under targeted treatment towards persons infected by the single strain disease, but there is no conflict under mass treatment. However, we numerically show that individual and social incentive to use antibiotics may show disaccord under mass treatment if the restriction on the transmission ability of the dually infected people is removed, or drug resistant infection is worse than drug sensitive infection, or the uninfected state has a comparative disutility over the infected states.
Keywords: drug-resistance, game theory, two diseases, tragedy of the commons, mass treatment, targeted treatment
1 Introduction
In recent years, the problem of bacterial antibiotic resistance has led to suggestions that antibiotics are overprescribed [8, 27], and that decreasing the use of antibiotics could benefit society as a whole by minimizing the emergence of drug-resistance [36, 12]. It has been argued that antibiotic efficacy should be considered as a common good [7], and that collective action may be needed to preserve this common good against overuse. Some authors believe that individual incentives may drive overuse of antibiotics, leading to a “tragedy of the commons” [1]. The concept of the tragedy of the commons originated from an example on population control proposed by Lloyd [29] in 1833 and later developed by Hardin [18] in the 1960s. One example of this is seen in simple mathematical models of drug resistance, where increasing treatment of mild or early disease may benefit the individual, despite the fact that such an outcome may lead to an increase in drug-resistant bacteria and thus a decrease in the overall efficacy of antibiotic treatment [38].
An important example of how drug-resistance occurs is the use of broad spectrum antibiotics [20]. In this case, treatment of one infection or disease may select for resistance in other organisms which are present [50]. This phenomenon has been observed during the use of mass azithromycin to eliminate trachoma due to Chlamydia trachomatis, a leading cause of infectious blindness in the world [52]. The World Health Organization promotes antibiotic treatment for trachoma control, using mass administration of single-dose oral azithromycin [44, 52]. While Chlamydia trachomatis has never exhibited epidemiologically important drug resistance [49, 21], the emergence of macrolide-resistant pneumococcus due to mass administration of azythromycin has been observed [28, 47], though such resistance has declined after cessation of treatment [47, 19]. Fears of increased mortality have proven unfounded, e.g. [39, 17, 24, 25].
In this paper, based on the pattern seen for pneumococcus and Chlamydia trachomatis, we propose and analyze a simple model of coinfection and cotransmission of two infectious agents in order to determine whether antibiotic resistance is a tragedy of the commons in a two diseases setting. For one infectious agent, we assume that both sensitive and resistant strains are possible, while for the other, only drug sensitive strains are present. Both are modeled as simple SIS (susceptible-infectious-susceptible) processes [23]. Coinfection by multiple pathogens or diseases is a global challenge for public health. It has attracted increasing attention in the field of mathematical epidemiology since the pioneering works by Dietz [14], Bremermann and Thieme [5], and others. For example, a number of mathematical models for HIV/TB coninfection [32, 45, 2, 43], HIV/malaria coinfection [34], HIV/gonorrhea coinfection [35], malaria and meningitis coinfection [26], and CA-MRSA/HA-MRSA co-colonization [11, 40] have been developed in recent years.
Our model assumes that treatment is targeted to the agent that only has sensitive strains, but can select for resistance in the other infectious agent (as a type of “collateral damage”). We will assume that the population as a whole has a particular treatment rate, which gives rise to an equilibrium prevalence of both infections. A single individual in the population who changes her or his treatment rate will then experience either more or less infection. When increasing infection rate for an individual causes that individual to spend less time infected, that individual has an incentive to increase treatment. However, if increasing the population rate of treatment causes a higher population cost, a tragedy of the commons results. Previously we used this method to analyze the tragedy of the commons resulting from incentives to treat early or mild disease ([38]; see [42] for a general exposition), and in this paper we will apply the same method to a simple model of cotransmission.
2 The model
We recently analyzed a simple SIS model (susceptible-infective-susceptible) of drug resistance [38] for one disease, denoted by P. The state of a single individual may be completely susceptible, infected with drug-susceptible organisms only, or infected with drug-resistant organisms only. We consider a single individual in a large population, subject to constant forces of infection. Let X(i), , and denote the probability the individual is susceptible, infected with the sensitive strain, or infected with the resistant strain, respectively. Treatment, occurring at rate , may lead to a new clinical appearance of drug resistance with probability δ ∈ (0, 1). Let ρP denote the recovery rate. Denoting the force of infection (only dependent on the number of individuals infected, and independent of individual choice of treatment) due to sensitive strains by λS and due to resistant strains by λR, a single individual follows the Markov chain
which is Model 1 in the previous paper ([38]).
We now extend this model to include a second disease, denoted by C. Motivated by the example of Chlamydia, we again assume a simple SIS process for the second disease, and assume no drug resistance is possible for this second disease. Nevertheless, treating individuals with this disease may select for drug resistance in the first disease (since this first disease might be present). We now denote the recovery rate for the second disease by ρC, and the force of infection by λC. Let X(i) denote the probability that a single individual in a large population is infected with no infectious agent, the probability the individual is infected by the drug-sensitive strain of the first agent (and not infected with the second agent), the probability the individual is infected by the drug-resistant strain of the first agent (and not infected with the second agent), the probability the individual is infected by the second agent (and not infected with either strain of the first agent), the probability the individual is infected by the drug-sensitive strain of the first agent and also by the second agent, and finally the probability the individual is infected by the drug-resistant strain of the first agent and also by the second agent. The treatment rate for individuals infected by the second infectious agent only is ; the treatment rate for individuals infected simultaneously by both agents is denoted . Here, a susceptible individual who contacts a dually infected person could become infected only with the first, the second or both agents as a result of the single contact; we thus have infection rates of λSC→S, λSC→C and λSC→SC (or λRC→R, λRC→C and λRC→RC), correspondingly. Then, we have
| (2.1) |
We omit human migration, birth, natural and disease-induced death; the total probability satisfies . For any given set of forces of infection, these linear equations can be solved for the equilibrium values of the probabilities of being in each state. We denote the equilibrium by .
As indicated above, we wish to consider a particular individual who chooses treatment rates or , faced by a unanimous choice θP, θC or θPC made by all other individuals. The following system of equations describing the corresponding community-level transmission dynamics:
| (2.2) |
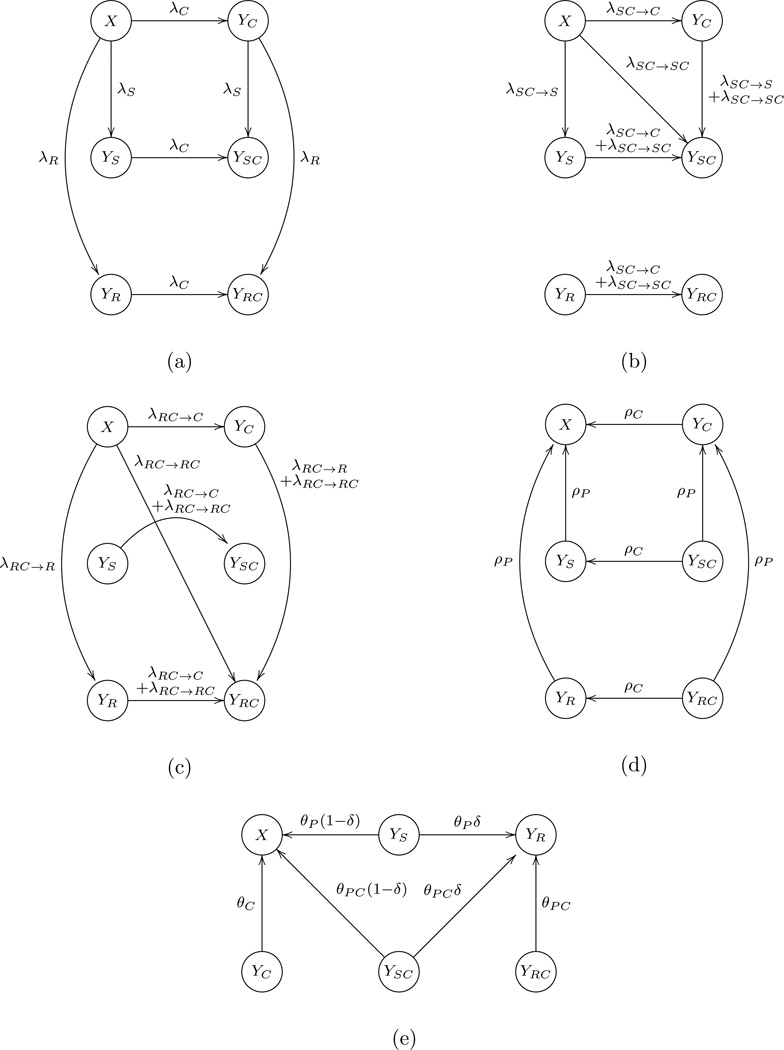
where X, YS, YR, YC, YSC, and YRC are the proportion of each disease state in the whole population; X + YS + YR + YC + YSC + YRC = 1. A state transition diagram for the disease transmission is shown in Figure 1. We assume that the forces of infection are proportional to the prevalence fractions and the force of infection of one agent is not affected by the presence of the other:
-
(A1)
λS = βSYS, λR = βRYR where βR < βS; λC = βCYC;
-
(A2)
λSC→SC = β11YSC, λSC→S = β10YSC, λSC→C = β01YSC,
;
-
(A3)
β11 + β10 = βS and β11 + β01 = βC, and .
Figure 1.
Flow diagram of the model. Infection process: (a) encounter YS, YR and YC, respectively, and get infected; (b) encounter YSC and get infected; (c) encounter YRC and get infected. (d) Recovery process. (e) Treatment process.
Here βS, βR, and βC are transmission coefficients of the drug-sensitive strain of the first agent, drug-resistant strain of the first agent, and the second agent, respectively. Note that an individual in a large population is subject to constant exogenous forces of infection (unaffected by the decision of that single individual), while the forces of infection at the population level are determined by the overall disease prevalence in the community. In many cases the emergence of drug resistance is indeed associated with a fitness cost [31], i.e., βR < βS, but a fitness cost of resistance may not be universally exhibited (e.g. [13]). Also, we do not assume that both infections in dually infected people are simply transmitted independently. Cotransmission from dually infected people is assumed possible, as a single infectious contact could contain a sufficient dose of both infectious agents. It is assumed that all model parameters are positive, with the exception of treatment rates , θP, θC, θPC and parts of transmission coefficients β11, β10, β01, which can be zero.
In general, we let DP and DC be an average health state disutility of infection or colonization by the first or second agent, respectively; we assume no difference in disutility between drug susceptible and drug resistant strains. Rather, individuals who are infected with a drug resistant strain are at a disadvantage because we assume treatment will be less effective (leading to longer mean durations of infection). We also assume no interaction between the agents, so that the disutility of being infected by both agents is the sum of the separate disutilities. Thus, for a single individual we wish to minimize
where and represent the probabilities being infected by the first or second agent, respectively, and is the stable equilibrium of the individual equations. Analogously, for a community we can define its average disutility as
where JP = ȲS + ȲR + ȲSC + ȲRC and JC = ȲC + ȲSC + ȲRC represent the fractions being infected by the first or second agent, respectively, and Ē = (X̄, ȲS, ȲR, ȲC, ȲSC, ȲRC) is the stable steady state of the community equations.
We will examine these equations under two treatment strategies: (a) mass treatment, and (b) treatment targeted towards persons infected by disease C. In the first case, we assume θP = θC = θPC = θ, so that all individuals are equally likely to be treated, regardless of their infection status, as would be the case during mass administration of azithromycin to eliminate trachoma. In the second case, we assume θP = 0 and θC = θPC = θ. Here, targeting infectives with the second agent may select for drug resistance in the first agent. Similarly, we assume , and and , respectively, for an individual under the two treatment strategies.
The disutility to each individual is determined not only by that individual’s choice of treatment, but also by all other individuals’ average choice. We followed standard methods ([42, 38]) to calculate the disutility of an individual, and determined whether individual incentives always parallel to community outcomes. To analyze individual incentives for treatment, we first determined the equilibrium dynamics of infection. We then examined a single individual whose forces of infection (for each agent that is circulating) are determined by the overall treatment rate in the entire population, and determined the expected amount of time that would be spent infected in each disease if this individual chose a different treatment rate rather than the population as a whole.
3 Main results
In this section, we derive the basic reproduction number for the population-level model, and then study the existence and local stability of feasible equilibria. The possibility of the occurrence of a tragedy of the commons under mass treatment or targeted treatment is analytically investigated.
3.1 The basic reproduction number
We consider the community equations (2.2) in which the forces of infection are not exogenous, but determined by the disease prevalence. It is clear that E0 = (1, 0, 0, 0, 0, 0) is the unique disease free equilibrium of system (2.2). Following the method and notations of van den Driessche and Watmough [51], we find
and
The basic reproduction number ℛ0 of model (2.2) is defined as the spectral radius of the next generation matrix F · V−1, i.e., where
For i = 1, …, 5, ℛi0 is the reproduction number corresponding to epidemiological classes YS, YR, YC, YSC, and YRC, respectively. In case of mass treatment or targeted treatment, we know ℛ40 < min{ℛ10, ℛ30}, ℛ50 < min{ℛ20, ℛ30}, and hence . Moreover, E0 is locally asymptotically stable if ℛ0 < 1 and unstable if otherwise.
3.2 The equilibria
For an individual subject to constant exogenous forces of infection (unaffected by the treatment strategy that person chooses), the coefficient matrix of its individual equations (2.1), denoted by A = (aij)6×6, is (or can be reduced to) a constant irreducible matrix (or submatrix) with nonnegative off-diagonal entries and zero column sums. It follows from Corollary 4.3.2 in Smith [48] or Lemma 1 in Cosner et al. [10] that (2.1) has a unique nonnegative equilibrium which is globally stable in the hyperplane
Direct computations find that the community model equations (2.2) can have up to four types of steady states as follows. The detailed derivation and stability analysis appear in Appendix A.
Theorem 3.1
Let ℜ(z) be the real part of a complex number z. For system (2.2) under mass treatment or targeted treatment, we have
The no-disease or disease free equilibrium E0 = (1, 0, 0, 0, 0, 0) always exists and it is stable if ℛ0 < 1 and unstable otherwise.
- One-strain equilibrium:
- exists if and only if ℛ10 > 1 and θP = 0 (targeted treatment). E1 is stable if and only if ℛ10 > max{1, ℛ20, ℛ50} and ℛ30 < 1.
- exists if and only if ℛ20 > 1. E2 is stable if and only if ℛ20 > max{1, ℛ10, ℛ40} and ℛ30 < 1.
- exists if and only if ℛ30 > 1. Targeted treatment: E3 is stable if and only if ℛ30 > 1, ℛ20 < 1, and . Mass treatment: E3 is stable if and only if ℛ10 < 1, ℛ20 < 1, and ℛ30 > 1. Here denote the roots of λ2 + M1λ + M0 = 0 with
- Two-strain equilibrium:
- exists if and only if ℛ10 > ℛ20, ℛ10 > 1, and θP > 0. E12 is stable if and only if ℛ30 < 1. Here
- exists if and only ℛ10 > 1, ℛ30 > 1 and θP = θC = θPC = 0 (no treatment). E13 is stable if and only if βS > βR. Here X13 ∈ [1/(ℛ10ℛ30), min{1/ℛ10, 1/ℛ30}) is the smaller root to
if β11 > 0 and equals 1/(ℛ10ℛ30) if β11 = 0. - exists if and only if ℛ20 > 1 and ℛ30 > 1. Mass treatment: E23 is stable if and only if ℛ10 < ℛ20. Targeted treatment: E23 is stable if and only if and . Here X23 ∈ [1/(ℛ20ℛ30), min{1/ℛ20, 1/ℛ30}) is the smaller root to
if and equals 1/(ℛ20ℛ30) if . Here are solutions to λ2 + H1λ + H0 = 0 with
Coexistence equilibrium of the form Ẽ = (X̃, ỸS, ỸR, ỸC, ỸSC, ỸRC) in which all the components are positive. Ẽ exists only if ℛ10 > 1, ℛ30 > 1, ℛ10 > ℛ20 and θC > 0.
Remark 3.2
Note that if ℛ10 < 1 and ℛ30 > 1 then M1 > 0 and M0 > 0. Thus E3 is stable if ℛ30 > 1, ℛ20 < 1 and ℛ10 < 1. In particular, when θ = 0, E3 is stable if and only if ℛ30 > 1, ℛ20 < 1 and ℛ10 < 1. However, for a non-zero targeted treatment rate θ, E3 can remain stable even if ℛ10 > 1. For example, given βS = 1.1, βR = 0.5, βC = 3, ρP = 1, ρC = 0.5, θ = 1, β11 = 0.5, we have ℛ30 = 2 > 1, ℛ20 = 0.5 < 1, ℛ10 = 1.1 > 1, but M1 = 3.65 and M0 = 1.675. In addition, if β11 = 0 then M0 > 0 implies M1 > 0.
Remark 3.3
Under targeted treatment, E23 is stable if ℛ10 < ℛ20. However, it is possible that E23 remains stable even if ℛ10 > ℛ20. For example, given βS = 2, βR = 1.5, βC = 3, ρP = 1, ρC = 1, θ = 1, β11 = 0, , we have ℛ10 = 2 > ℛ20 = 1.5 > 1 and ℛ30 = 1.5 > 1, but H1 = 11/3 and H0 = 1/9. In addition, if then H0 > 0 implies H1 > 0.
Remark 3.4
By comparing the existence and stability condition of equilibria, we find that there exists at most one stable equilibrium under mass treatment, or under targeted treatment if ℛ10 < 1 or ℛ10 < ℛ20. In particular, when the coexistence equilibrium exists under mass treatment, it is the only possibly stable equilibrium.
Moreover, numerical calculations suggest that there exists exactly one stable equilibrium for any parameter setting and the coexistence equilibrium is stable whenever it exists.
3.3 The tragedy of the commons
Given a set of parameter values, we first solve for the stationary solutions of (2.2) and substitute the stable solution into the exogenous forces of infection of the individual model (2.1), and then find the proportion in each infected state. From these proportions, we can then compute the disutility of an individual. An individual has an incentive to increase antibiotic treatment if the individual disutility J(i) is decreasing in terms of θ(i), i.e., more treatment produces less disutility. A community benefits from treatment if the community disutility J is decreasing in θ. Locally, a tragedy of the commons occurs when the goal of the individual conflicts with that of the community. Mathematically, this means that for a fixed parameter set, we have
Let and . Recall that under mass treatment, we assume all infected people are treated at the same rate (θP = θPC = θC = θ and ), while under targeted treatment, that individuals with C are treated, whether or not they exhibit the other infection (θP = 0, θPC = θC = θ and ). The proof of the following is postponed to Appendix B.
Theorem 3.5
Modeling a single individual according to (2.1) implies
under mass treatment, and
under mass treatment or targeted treatment, respectively.
The community model (2.2) implies that
under mass treatment, and
under mass treatment or targeted treatment, respectively.
In addition, under targeted treatment, there exist some parameter sets such that (or JP) is increasing in θ(i) (or θ).
Thus, under mass treatment, both and J = DPJP + DCJC are decreasing in θ(i) and θ, respectively, for any DP > 0 and DC > 0. This indicates that increasing mass treatment increases the utility of both the individual and community, and there is no tragedy of the commons in two diseases setting under mass treatment.
However, J = DPJP + DCJC can increase in θ for some DP > 0 and DC > 0 (e.g. DP ≫ DC) under targeted treatment. For this reason, the incentives for the individual and community do not always coincide, and a tragedy of the commons may occur provided that only infectives with the second agent receive treatment.
Theorem 3.6
For system (2.2) under targeted treatment, assume that there always exists a globally stable equilibrium. If ℛ10 > 1, ℛ10 > ℛ20 and ℛ30 > 1 at θ = 0, then JP < 1 − 1/ℛ10 for θ ∈ (0, βC − ρC), and JP = 1 − 1/ℛ10 for θ ∈ {0} ∪ [βC − ρC, ∞).
Proof
No treatment (θ = 0): E13 is the unique stable equilibrium and JP = 1 − 1/ℛ10.
Small treatment (0 < θ < βC − ρC): one of E3, E23 and Ẽ is stable. If E3 is stable, then JP = 0; else if E23 is stable, then JP = 1 − (X23 + 1/ℛ20 − X23) = 1 − 1/ℛ20 < 1 − 1/ℛ10; else if Ẽ is stable then, JP < 1 − 1/ℛ10 due to (ρP − βS(X̃ + ỸC))(ỸS + ỸSC) = −θỸSC < 0.
High values for the treatment rate (θ ≥ βC − ρC): disease C disappears. E1 is stable and JP = 1 − 1/ℛ10.
Remark 3.7
Under targeted treatment, we define Q(θ) = θȲSC/(ȲS + ȲSC) as the treatment rate for the sensitive strain of disease P at a stable equilibrium Ē (θ). Assume that there always exists a globally stable equilibrium, it follows from the proof of Theorem 3.6 that
which implies that maxθ≥0 Q(θ) = minθ≥0 JP, namely, the fraction of population being infected with the first agent is minimized (or maximized) whenever the treatment rate for the first infectious agent reaches its maximum (or minimum).
However, if there is no cotransmission from YRC, i.e., , then is decreasing in θ(i), which implies that J(i) is decreasing in θ(i) for any DP > 0 and DC > 0 (see Appendix B). In addition, if disease C has higher disutility than disease P, i.e., DC ≥ DP, then J(i) is constantly decreasing in θ(i). We omit the straightforward but tedious proof.
4 Numerical simulations
The above analysis shows that a tragedy of the commons does not exist under mass treatment. However, as defined above, a tragedy of the commons can appear for the case of targeted treatment. In this case, individuals who choose treatment rates which are larger than those adopted by the community achieve lower disutility, even though the entire community will experience more disease if everyone increases their treatment rates in the same way. But in this case—targeted treatment—the treatment rates apply only to dual infection; as the treatment rate increases, eventually the second disease is completely eliminated, and with it, all opportunity to treat the first infection. In a sense, this is not a classical tragedy of the commons, because it is removable after a change of treatment strategy from targeted treatment to mass treatment. However, the same two-disease model can exhibit a conflict of interest between individual and society under mass treatment if (i) assumption (A3) is not required, namely, the force of infection of dually infected hosts is different from singly infected hosts, or (ii) drug sensitive and drug resistant strains of the first disease have different disutility, or (iii) the uninfected has significant disutility.
In general, the relationship between antimicrobial resistance and virulence is not straight-forward (e.g. [6]). While in some cases, a clear fitness cost of resistance is believed to apply, it cannot be assumed that drug resistant strains are less virulent (e.g. [13], but see [15]). In some cases, drug resistance genes are present on a plasmid which also includes virulence factors (e.g. [33]), but in other cases evolution of drug resistance may simply alter expression of virulence factors [22]. To explore this possibility in our model, we assume the resistant infection could have higher disutility than the sensitive infection. One disadvantage of the use of broad-spectrum antibiotics in general, including azithromycin, is the disruptive effect such treatments have on the normal microbiome, which may permit the overgrowth of other harmful organisms [3]. This is particularly true in the case of Clostridium difficile in the gastrointestinal tract for example (e.g. [37]). Could colonization (though of course not infection) by pneumococcus have benefits in preventing the growth of other organisms? While some literature supports the notion that pneumococcal colonization is not beneficial [46], we explore the consequences of assuming that individuals in the susceptible state for pneumococcus have a comparative disutility over the colonized (infectious) states. In these two cases, we can define the individual and community disutility as
and
respectively, where DU, DS, DR and DC are, respectively, the average disutility of the uninfected, the sensitive and resistant infections of the first disease, and the infections of the second disease. In what follows, we will give numerical examples to consolidate our analytical arguments.
Example 4.1
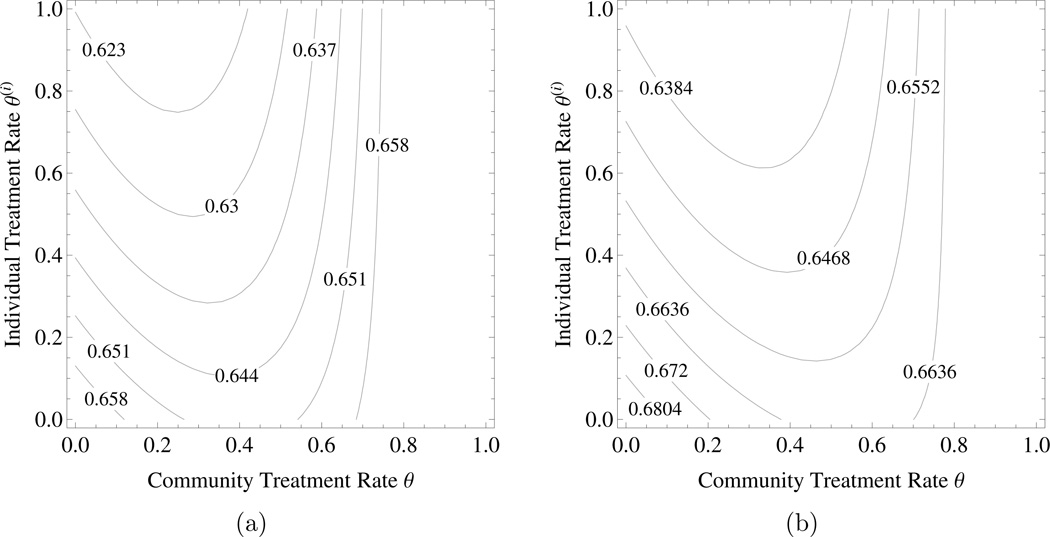
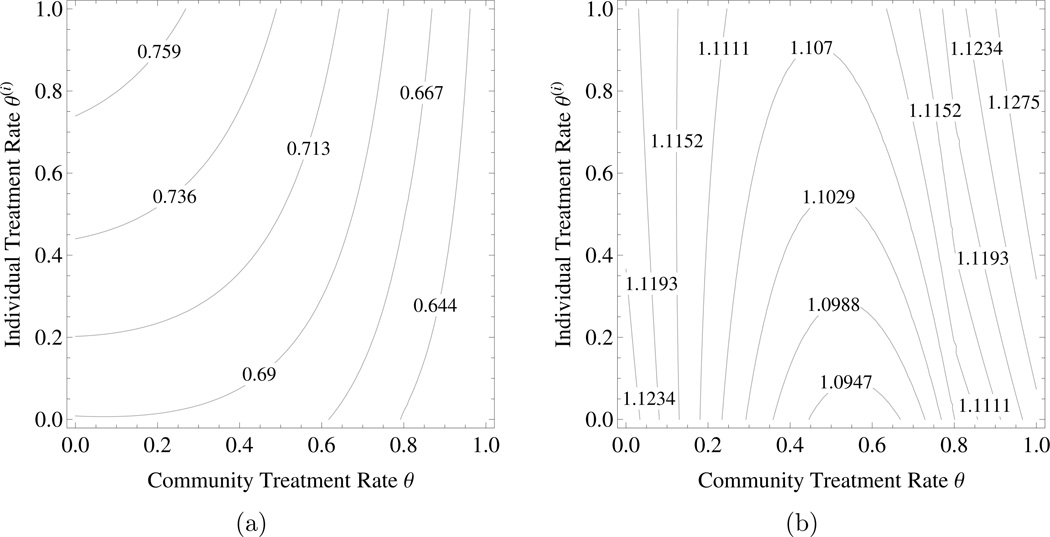
The tragedy of the commons under targeted treatment. Consider community model (2.2) with βS = 3, βR = 1.2, βC = 1.8, β11 = 1, , δ = 0.3, ρP = 1, ρC = 1, and θ ∈ [0, 1]. β10, β01, can be determined accordingly by (A3). Figures 2a shows the probability of an individual being infected by agent P and Figure 2b represents the individual disutility under targeted treatment with DP = 1 and DC = 0.05. Here an individual gets better if s/he departs from the community strategy and treats more, but things are worse if everyone does that. For the same parameter values, the tragedy of the commons under targeted treatment remains even if there is no cotransmission, i.e., . However, the tragedy of the commons disappears when the ratio DP: DC decreases below a certain threshold value.
Figure 2.
The tragedy of the commons under targeted treatment. a) The probability of an individual being infected by agent , b) the individual disutility due to both diseases – . The parameter values are as in the text. The horizontal axis is the community level of targeted treatment rate and the vertical axis is the level of targeted treatment rate chosen by an individual within the community.
Example 4.2
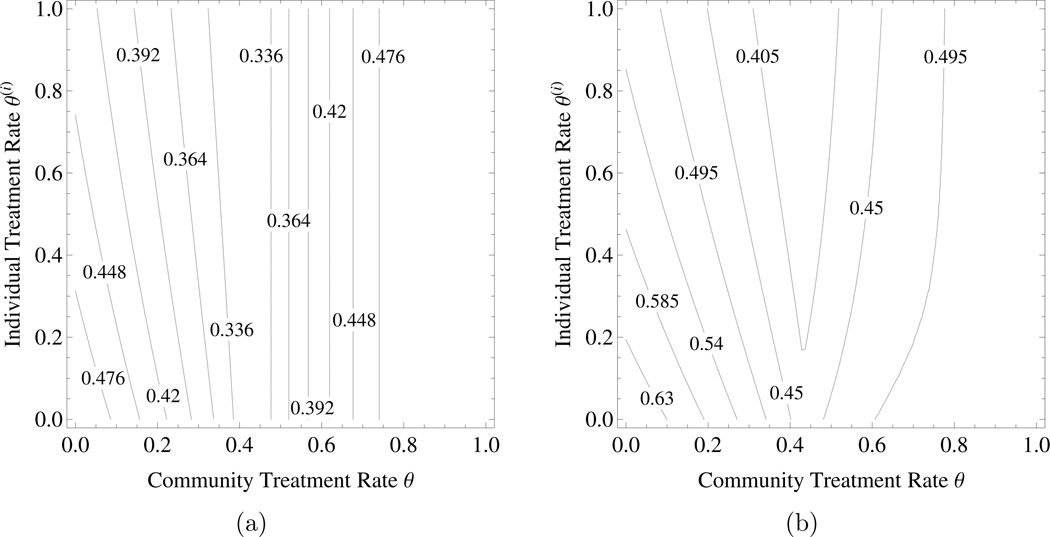
The tragedy of the commons under mass treatment without (A3). The values of parameters are βS = 2.1, βR = 2, βC = 1.2, β11 = 0.6, β10 = 1.4, β01 = 0.6, , δ = 0.5, ρP = 1, ρC = 0.4, and θ ∈ [0, 1]. Figures 3a shows the probability of an individual being infected by agent P and Figure 3b represents the individual disutility under mass treatment with DP = 1 and DC = 0.25. Here disease C is assumed to differentially suppresse the resistant strain of disease P, namely, the transmission of the resistant strain of disease P from YRC is much lower than the transmission of the sensitive strain of disease P from . Note that the formulae for and are the same as the case of mass treatment with assumption (A3) (see Theorem 3.5). As θ > 0.43, the sensitive strain of disease P goes extinct in the community which leads to . Thus and it is independent of personal choice of treatment when θ > 0.43. In this scenario, a higher treatment rate for the sensitive strain of the first infectious agent, Q(θ) = θ, could cause a larger proportion of people being infected with the first agent, JP.
Figure 3.
The tragedy of the commons under mass treatment without assumption (A3). a) The probability of an individual being infected by agent , b) the individual disutility due to both diseases – . Parameters are as in the text. The horizontal axis is community level of mass treatment rate and the vertical axis is the level of mass treatment rate chosen by an individual within the community.
Example 4.3
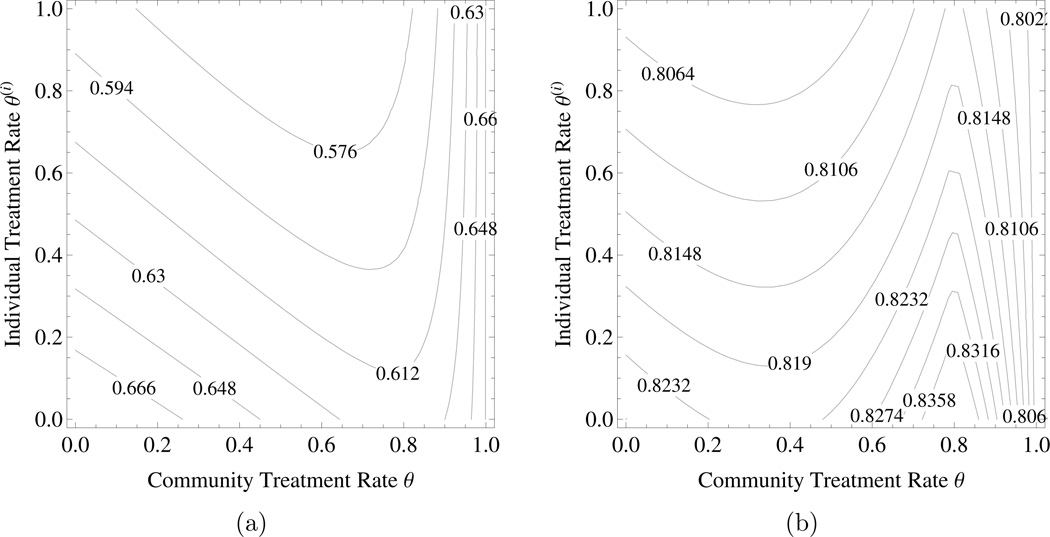
The tragedy of the commons under mass treatment with DS ≠ DR or DU > 0. Choose the same parameter as in Example 4.1 except that βR = 1.5, δ = 0.1, and the average disutility of infection/noninfection are different. Figures 4a and 4b represent the individual disutility under mass treatment with DU = 0, DS = 1 < DR = 2 and DC = 0.05, and DU = 0.7, DS = DR = 1 and DC = 0.05, respectively. In both cases there is a conflict of interest between individual and society: good for individual but bad for community.
Figure 4.
The tragedy of the commons under mass treatment with DS ≠ DR or DU > 0. a) the individual disutility J(i) when resistant infection has higher disutility than sensitive infection, b) the individual disutility J(i) when the uninfected has significant disutility. Parameters are as in the text. The horizontal axis is community level of mass treatment rate and the vertical axis is the level of mass treatment rate chosen by an individual within the community.
It is worth noting that under certain circumstance individual incentives may favor under-treatment while increasing treatment will benefit community. Again choose the same parameter as in Example 4.1 except the average disutility of infection/noninfection. Figures 5a and 5b represent the individual disutility under mass treatment with DU = 0, DS = 1 < DR = 2.5 and DC = 0.05, and DU = 1.2, DS = DR = 1 and DC = 0.5, respectively. In both cases an increasing in treatment rate could be bad for individual but good for community.
Figure 5.
Individual incentives which may favor undertreatment in case of mass treatment with DS ≠ DR or DU > 0. a) the individual disutility J(i) when resistant infection has higher disutility than sensitive infection, b) the individual disutility J(i) when the uninfected state has a lower utility than the infected states. Parameters are as in the text. The horizontal axis is community level of mass treatment rate and the vertical axis is the level of mass treatment rate chosen by an individual within the community.
5 Discussion
Rational antibiotic policy must consider the possibility that individual incentives to use antibiotics may drive overtreatment. Such overtreatment may lead to increasing drug resistance in other organisms, yielding a tragedy of the commons [1]. In a previous paper [38], we studied two single disease models of drug resistance: a simple SIS model and a two-stage (mild and severe) model, and found that a conflict of interest between individual and society does not occur for the former but is possible for the later under certain circumstances—individual incentives can favor overtreatment of mild infection leading to a worse outcome for society. However, mass administration of azithromycin during trachoma control provides a possible example of second mechanism for a conflict of interest between individual and society: treatment of one disease can lead to drug resistance in another organism.
In this paper, we extend previous game theory models of antibiotic policy to a setting of two infectious diseases which are cocirculating. In this model, treatment of one disease selects for resistance in the other, mimicking the behavior of induced resistance in pneumococcus caused by treatment of chlamydia. Mass antibiotic distributions for trachoma elimination are known to select for macrolide resistance in pneumococcus [47], although the prevalence of such resistance has been seen to rapidly decline after cessation of mass distribution [19]. Our model was designed to reflect specific features of chlamydia and pneumoccus in this setting, but we did not restrict the analysis to parameters reflecting the biology of pneumococcus and chlamydia. In this specific setting, for a base case scenario, we assumed that the two infections do not interact competitively (the presence of one organism does not reduce transmission of the other). In our model, treatment induces drug resistance in one organism (pneumococcus) but not in the other. We examined two scenarios: mass treatment, in which individual treatment is not based on knowledge of chlamydial infection status, and targeted treatment, in which individuals without chlamydia are not treated. The occurrence of a tragedy of the commons resulting from individual incentives to be treated is strongly influenced by the choice of mass versus targeted treatment, as well as by the health state utility of the various epidemiological states of individuals.
More specifically, we find that the model can imply conflicting individual and social incentives to use antibiotics. Such discord arises for a given population rate of treatment when individuals who diverge from it do better (or worse), while if all individuals make the same choice, all do worse (or better). Moreover, for a given parameter set, such discord may arise for some treatment values but not others. Also, individual incentives can favor underuse as well as overuse. We identified four different examples of discord between the individual and the community in our model.
First, suppose that infection or colonization by drug resistant pneumococcus is worse than infection or colonization by drug sensitive pneumococcus (the health state utility is lower for drug resistant infection or colonization). For specific parameter values, it is possible that individuals who choose rates higher than the population lower their health state utility because of the acquisition of drug resistance during treatment. Yet if the entire population chooses this new, higher treatment rate, the overall prevalence of infection is lower and the population benefits.
However, this example is far from the only way that individual incentives can lead to socially undesirable outcomes. Suppose now that the uninfected state has a lower utility than the infected states, because pneumococcal colonization is protecting the individual against infection by a third organism (whose presence is not explicitly modeled). Numerical scenarios reveal that for low treatment rates, the individual and community incentives can favor increased treatment, due to the benefits of curing chlamydia. But for higher treatment rates, individuals who choose higher rates than the community begin to experience worse outcomes, because the assumed disadvantage of curing pneumococcal colonization outweighs the benefits of clearing chlamydial infection. It is possible for individual incentives to favor lower treatment rates despite the fact that the population as a whole could continue to benefit from higher treatment, leading to another example of individual incentives leading to underuse of antibiotics.
These examples aside, in our model, a true tragedy of the commons can arise in which individual incentives can drive overuse of antibiotics. In our base case scenario, we assumed that for pneumococcus, drug susceptible and drug resistant infection (or colonization) have the same health state utility, and that it is better to be uninfected or uncolonized. While pneumococcus and chlamydia themselves are unlikely to interact competitively—each is unlikely to reduce the transmission of the other—infectious agents of course can be in competition, and we considered the following example in which the drug resistant strain of pneumococcus is less fit in the presence of chlamydia than the drug sensitive strain of pneumococcus. In other words, a fitness cost of drug resistance in pneumococcus is manifested by reduced ability to spread in the presence of chlamydia. Under these assumptions, under mass treatment, a tragedy of the commons may arise. Population level treatment rates can be found for which individuals do better if they exceed the population treatment rate. If the entire population, however, chooses a higher rate of treatment, the resulting reduction in chlamydia leads to an overcompensating degree of drug resistant pneumococcus, because (in this hypothetical scenario) chlamydia is no longer inhibiting drug resistant pneumococcus to the same degree. We found that for mass treatment, such a tragedy of the commons is impossible if the two organisms do not interact competitively.
If we depart from the assumption of mass treatment and allow targeted treatment, individual incentives can again drive socially disadvantageous treatment rates, but for a different reason. Under this assumption, individuals are only treated if they show signs of chlamydia (unlike in a mass administration campaign). Pneumococcus is only treated for individuals who are coinfected with chlamydia, so that the effective rate of pneumococcal treatment becomes smaller as the prevalence of chlamydia drops. Even when we assume identical health utility of drug sensitive and drug resistant pneumococcal colonization and infection, that either is worse than being uncolonized or uninfected, and that chlamydia and pneumococcus do not interact competitively, a kind of tragedy of the commons arises. Here, individual incentives favor increased treatment, but if the population as a whole chooses a larger rate of treatment, the declining prevalence of chlamydia reduces opportunities to treat pneumococcus. Individual incentives drive overuse, but this mathematical tragedy of the commons is unrelated to drug resistance and can be avoided by a different choice of antibiotic policy.
Our model does not reflect all features of trachoma mass drug administration in practice. Specifically, we did not include cocirculation of multiple pneumococcal strains, the role of strain-specific immunity in pneumococcus [9], the presence of pneumococcal vaccination, or the timing of mass drug administration. We only included a single organism for which resistance can be induced, and we have ignored age structure, demography, latency, multiple chlamydial strains, chlamydial cross immunity, and network effects. We also observe that the mathematical analysis of the current model has not revealed explicit criteria for the existence, uniqueness, and stability of the coexistence equilibrium. Numerical simulations suggest that under the base scenario there always exists exactly one (globally) stable equilibrium and the coexistence equilibrium is (globally) stable whenever it exists. Finally, the relationship between antibiotic use and drug resistance may be more complex than simple selection models would imply [30]. We cannot conclude that a tragedy of the commons is impossible in a more general setting.
Recent recommendations to improve antibiotic stewardship include efforts to avoid overuse of broad spectrum antibiotics (e.g. [4]), in part because of a belief that broad spectrum antibiotic use promotes drug resistance [41]. Simple game theory models as we present here can be our first step in understanding the forces which shape the epidemiology of drug resistance. If antibiotic use exceeds the socially optimal level, it is important to understand whether such excessive antibiotic use really benefits the individuals who use them. Overuse of antibiotics based on a mistaken belief that they are helpful does not reflect a true conflict of interest between the individual and society, but a true conflict of interest does arise if individuals have genuine health incentives to use antibiotics at a level exceeding the socially optimal value. This work suggests that a tragedy of the commons does not arise in simple models of trachoma control through the use of mass treatment. More realistic models of the population biology of drug resistant strains may provide examples of the tragedy of the commons due to treatment of unrelated organisms.
Highlights.
A two-disease epidemic model with drug-resistance is proposed.
Treatment for one disease may select for resistance in the other.
Antibiotic use is modeled as a mathematical game between individual and society.
The tragedy of the commons for mass treatment and targeted treatment are discussed.
A conflict of interest between individual and society can occur in several cases.
Acknowledgement
The work was partially supported the Models of Infectious Disease Agent Study (MIDAS) (NIGMS U01GM087728) and the TANA study (NEI U10EY016214). We thank our colleagues for helpful comments and discussions.
Appendix
A. Proof of Theorem 3.1
The calculation of boundary equilibria for the community equations (2.2) is straightforward and simple except that of E13 and E23 which can be found in Gao et al. [16]. So we will only focus on the stability analysis. By substituting X = 1 − (YS + YR + YC + YSC + YRC) into the last five equations of (2.2), we obtain a qualitatively equivalent 5-dimensional ODEs system, denoted by (2.2)′, with respect to YS, YR, YC, YSC, and YRC. We represent the equilibria corresponding to the reduced system by , and Ẽ′, where the first component of E0, E1, E2, E3, E12, E13, E23, and Ẽ is removed, respectively. The Jacobian matrix of (2.2)′ at an equilibrium and that of (2.2) at the corresponding equilibrium E ∈ {E0, E1, E2, E3, E12, E13, E23, Ẽ} have the same set of nonzero eigenvalues.
Local stability of E0
The Jacobian matrix of (2.2)′ at is and the set of its eigenvalues is
The no-disease equilibrium E0 is stable if ℛ0 < 1 and unstable otherwise.
Local stability of E1
The Jacobian matrix of system (2.2)′ at is and the set of its eigenvalues is
under targeted treatment. Recall that (or E1) exists if and only if ℛ10 > 1 and θP = 0 which means that (or E1) does not exist under mass treatment.
Targeted treatment: since ℛ10 > 1 and ℛ10 > ℛ40, the first and fourth eigenvalues of are negative. E1 is stable if and only if ℛ10 > max{1, ℛ20, ℛ50} and ℛ30 < 1.
Local stability of E2
The Jacobian matrix of system (2.2)′ at is and the set of its eigenvalues is
under either targeted treatment or mass treatment.
Since ℛ20 > 1 and ℛ20 > ℛ50, the second and last eigenvalues of are negative. E2 is stable if and only if ℛ20 > max{1, ℛ10, ℛ40} and ℛ30 < 1.
Local stability of E3
The Jacobian matrix of system (2.2)′ at is J(E3) and the set of its eigenvalues is
under targeted treatment, where and denote the roots of λ2 + M1λ + M0 = 0, or
under mass treatment.
Targeted treatment: E3 is stable if and only if ℛ30 > 1, ℛ20 < 1, and .
Mass treatment: E3 is stable if and only if ℛ10 < 1, ℛ20 < 1, and ℛ30 > 1.
Local stability of E12
Recall that E12 exists if and only if ℛ10 > ℛ20, ℛ10 > 1, and θP > 0 (mass treatment). The Jacobian matrix of system (2.2)′ at is and the set of its eigenvalues is
where and are solutions to
respectively. Here
which imply that E12 is stable if and only if ℛ30 < 1.
Local stability of E13
Recall that E13 exists if and only if ℛ10 > 1, ℛ30 > 1 and θ = 0 (no treatment). The Jacobian matrix of system (2.2)′ at is and the set of its eigenvalues is
It follows from X13 < min{ρP/βS, ρC/βC} and βS > βR that E13 is stable when it exists.
Local stability of E23
Recall that E23 exists if and only if ℛ20 > 1 and ℛ30 > 1. The Jacobian matrix of system (2.2)′ at is and the set of its eigenvalues is
under mass treatment or
under targeted treatment where are solutions to λ2 + H1λ + H0 = 0.
Mass treatment: E23 is stable if and only if ℛ10 < ℛ20.
Targeted treatment: E23 is stable if and only if .
Existence of Ẽ
It follows from (2.2) that the equilibrium Ẽ satisfies
under mass treatment or
under targeted treatment. Hence, in both cases, a necessary condition for the existence of Ẽ is that: ℛ10 > 1, ℛ30 > 1, ℛ10 > ℛ20 and θ > 0. For a simple case: under mass treatment, we can solve the equilibrium equations by substitution and rigorously prove that there exists at most one positive equilibrium. In addition, ρC + θ − βC is an eigenvalue of the Jacobian J(Ẽ′) (or J(Ẽ)).
B. Proof of Theorem 3.5
Denote the k-th equilibrium equation of the individual equations (2.1) and community equations (2.2) as eqk and EQk, respectively. Let and .
Community model under mass treatment (θP = θPC = θC = θ)
The sum of EQ4, EQ5 and EQ6 gives
Since X̄ + ȲS + ȲR + ȲC + ȲSC + ȲRC = 1, we have
The sums of EQ2 and EQ5, and, EQ3 and EQ6 give
Direct calculations yield
| (5.1) |
and hence
Community model under targeted treatment (θP = 0, θPC = θC = θ)
The derivation of JC for community model under targeted treatment is exactly the same as that for community model under mass treatment. JP is not always a decreasing function of community treatment rate θ. For example, given a parameter set under targeted treatment: βS = 3, βR = 1.2, βC = 1.8, β11 = 1, , δ = 0.3, ρP = 1, ρC = 1, we have ∂JP/∂θ ≈ 0.121952 > 0 at θ = 0.7. For the same parameter values except that , we still have ∂JP/∂θ ≈ 0.113367 > 0 at θ = 0.7.
Individual model under mass treatment
The sum of eq4, eq5 and eq6 gives
Since , we have
The sums of eq2 and eq5, and, eq3 and eq6 give
Again since , we get three linear equations with respect to and . Direct calculations yield
and hence
Moreover, it follows (5.1) that and can be explicitly written in terms of model parameters and we can study an individual’s disutility in community treatment rate.
Individual model under targeted treatment
The derivation of for individual model under targeted treatment is exactly the same as that for individual model under mass treatment.
Now we give an outline of the proof to the statement: is not necessarily decreasing in θ(i). First, we solve the individual equations and simplify the derivative of with respect to θ(i). We find that the sign of is the same as a polynomial in θ(i) of the form
where
and . Here c0, c1, c20, c21, c30, c31 and c4 are the addition of some positive terms. Furthermore, we find that c20 > c21 and c30 > c31 which imply that and c3 > 0. In particular, if cotransmission of R and C is rare, i.e., , then and h(θ(i)) < 0 for any θ(i).
Nevertheless, it is possible that h(θ(i)) > 0 for some θ(i) when . To construct such a counterexample, we observe that λSC→SC, λSC→C, λRC→C, λC, ρC do not appear in but in other coefficients. Let λSC→SC = λSC→C = λRC→C = λC = ρC = 0 and then h(θ(i)) takes the form
where
Here ĉ0, ĉ1, ĉ20, ĉ3 are polynomials. Thus, can dominate the sign of ĥ(θ(i)) as λRC→RC → 0. For example, given a parameter set under targeted treatment: λSC→SC = 0.0001, λSC→C = 0.0001, λSC→S = 1, λRC→RC = 0.001, λRC→C = 0.0001, λRC→R = 1, λS = 1, λR = 1, λC = 0.0001, ρP = 1, ρC = 0.0001, δ = 0.5, we have at θ(i) = 1.
However, if DC ≥ DP, then we find that ∂J(i)/∂θ(i) is constantly negative and hence an individual always benefits from increasing his/her treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baquero F, Campos J. The tragedy of the commons in antimicrobial chemotherapy. Rev Esp Quimioter. 2003;16(1):11–13. [PubMed] [Google Scholar]
- 2.Bhunu C, Garira W, Mukandavire Z. Modeling HIV/AIDS and tuberculosis coinfection. Bulletin of Mathematical Biology. 2009;71(7):1745–1780. doi: 10.1007/s11538-009-9423-9. [DOI] [PubMed] [Google Scholar]
- 3.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nature Reviews Microbiology. 2009;7(12):887–894. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL, Mace SE, McCracken GH, Moore MR, St Peter SD, Stockwell JA, Swanson JT. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clinical Infectious Diseases. 2011;53(7):e25–e76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bremermann HJ, Thieme HR. A competitive exclusion principle for pathogen virulence. Journal of Mathematical Biology. 1989;27(2):179–190. doi: 10.1007/BF00276102. [DOI] [PubMed] [Google Scholar]
- 6.Cameron DR, Howden BP, Peleg AY. The interface between antibiotic resistance and virulence in Staphylococcus aureus and its impact upon clinical outcomes. Clinical Infectious Diseases. 2011;53(6):576–582. doi: 10.1093/cid/cir473. [DOI] [PubMed] [Google Scholar]
- 7.Cars O, Högberg LD, Murray M, Nordberg O, Sivaraman S, Lundborg CS, So AD, Tomson G. Meeting the challenge of antibiotic resistance. BMJ. 2008;337:a1438. doi: 10.1136/bmj.a1438. [DOI] [PubMed] [Google Scholar]
- 8.CDC. Mission critical: Preventing antibiotic resistance. 2012 [Google Scholar]
- 9.Cobey S, Lipsitch M. Niche and neutral effects of acquired immunity permit coexistence of pneumococcal serotypes. Science. 2012;335(6074):1376–1380. doi: 10.1126/science.1215947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosner C, Beier JC, Cantrell RS, Impoinvil D, Kapitanski L, Potts MD, Troyo A, Ruan S. The effects of human movement on the persistence of vector-borne diseases. Journal of Theoretical Biology. 2009;258(4):550–560. doi: 10.1016/j.jtbi.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Agata EMC, Webb GF, Pressley J. Rapid emergence of co-colonization with community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus strains in the hospital setting. Mathematical Modelling of Natural Phenomena. 2010;5(03):76–93. doi: 10.1051/mmnp/20105306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies SC. Annual Report of the Chief Medical Officer, Volume Two, 2011, infections and the rise of antimicrobial resistance. 2013 doi: 10.1016/S0140-6736(13)60604-2. [DOI] [PubMed] [Google Scholar]
- 13.Dharan NJ, Gubareva LV, Meyer JJ, Okomo-Adhiambo M, McClinton RC, Marshall SA, George KS, Epperson S, Brammer L, Klimov AI, Bresee JS, Fry AM for the Oseltamivir-Resistance Working Group. Infections with oseltamivir-resistant influenza (a(h1n1)united states [Google Scholar]
- 14.Dietz K. Epidemiologic interference of virus populations. Journal of Mathematical Biology. 1979;8(3):291–300. doi: 10.1007/BF00276314. [DOI] [PubMed] [Google Scholar]
- 15.Duan S, Boltz DA, Seiler P, Li J, Bragstad K, Nielsen LP, Webby RJ, Webster RG, Govorkova EA. Oseltamivir–resistant pandemic H1N1/2009 influenza virus possesses lower transmissibility and fitness in ferrets. PLoS Pathogens. 2010;6(7):e1001022. doi: 10.1371/journal.ppat.1001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao D, Porco TC, Ruan S. Coinfection dynamics of two diseases in a single host population. doi: 10.1016/j.jmaa.2016.04.039. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebre T, Ayele B, Zerihun M, House JI, Stoller NE, Zhou Z, Ray KJ, Gaynor BD, Porco TC, Emerson PM, Lietman TM, Keenan JD. Latrine promotion for trachoma: assessment of mortality from a cluster-randomized trial in Ethiopia. The American Journal of Tropical Medicine and Hygiene. 2011;85(3):518. doi: 10.4269/ajtmh.2011.10-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardin G. The tragedy of the commons. Science. 1968;162(5364):1243–1248. [PubMed] [Google Scholar]
- 19.Haug S, Lakew T, Habtemariam G, Alemayehu W, Cevallos V, Zhou Z, House J, Ray K, Porco T, Rutar T, Keenan J, Lietman T, Gaynor B. The decline of pneumococcal resistance after cessation of mass antibiotic distributions for trachoma. Clinical Infectious Diseases. 2010;51(5):571–574. doi: 10.1086/655697. [DOI] [PubMed] [Google Scholar]
- 20.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128(6):1053–1061. doi: 10.1542/peds.2011-1337. [DOI] [PubMed] [Google Scholar]
- 21.Hong KC, Schachter J, Moncada J, Zhou Z, House J, Lietman TM. Lack of macrolide resistance in chlamydia trachomatis after mass azithromycin distributions for trachoma. Emerging Infectious Diseases. 2009;15(7):1088–1090. doi: 10.3201/eid1507.081563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howden BP, Peleg AY, Stinear TP. The evolution of vancomycin intermediate Staphylococcus aureus (VISA) and heterogenous-VISA. Infection, Genetics and Evolution. 2014;21(0):575–582. doi: 10.1016/j.meegid.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 23.Keeling MJ, Rohani P. Modeling Infectious Diseases in Humans and Animals. Princeton: Princeton University; 2008. [Google Scholar]
- 24.Keenan JD, Ayele B, Gebre T, Zerihun M, Zhou Z, House JI, Gaynor BD, Porco TC, Emerson PM, Lietman TM. Childhood mortality in a cohort treated with mass azithromycin for trachoma. Clinical Infectious Diseases. 2011;52(7):883–888. doi: 10.1093/cid/cir069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keenan JD, Emerson PM, Gaynor BD, Porco TC, Lietman TM. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Internal Medicine. 2013;173(9):821–823. doi: 10.1001/jamainternmed.2013.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawi G, Mugisha J, Omolo-Ongati N. Mathematical model for malaria and meningitis co-infection among children. Applied Mathematical Sciences. 2011;5(47):2337–2359. [Google Scholar]
- 27.Laxminarayan R, Duse A, Wattal C, Wattal AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, et al. Antibiotic resistancethe need for global solutions. The Lancet Infectious Diseases. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 28.Leach AJ, Shelby-James TM, Mayo M, Gratten M, Laming AC, Currie BJ, Mathews JD. A prospective study of the impact of community-based azithromycin treatment of trachoma on carriage and resistance of streptococcus pneumoniae. Clinical Infectious Diseases. 1997;24(3):356–362. doi: 10.1093/clinids/24.3.356. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd WF. Two Lectures on the Checks to Population. Oxford, England: Oxford University Press; 1833. [Google Scholar]
- 30.MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clinical Microbiology Reviews. 2005;18(4):638–656. doi: 10.1128/CMR.18.4.638-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maher MC, Alemayehu W, Lakew T, Gaynor BD, Haug S, Cevallos V, Keenan JD, Lietman TM, Porco TC. The fitness cost of antibiotic resistance in Streptococcus pneumoniae: insights from the field. PLoS One. 2012;7(1):e29407. doi: 10.1371/journal.pone.0029407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massad E, Burattini MN, Coutinho FAB, Yang HM, Raimundo SM. Modeling the interaction between AIDS and tuberculosis. Mathematical and Computer Modelling. 1993;17(9):7–21. [Google Scholar]
- 33.McCarthy AJ, Lindsay JA. The distribution of plasmids that carry virulence and resistance genes in staphylococcus aureus is lineage associated. BMC Microbiology. 2012;12(1):104. doi: 10.1186/1471-2180-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukandavire Z, Gumel A, Garira W, Tchuenche J. Mathematical analysis of a model for HIV-malaria co-infection. Math. Biosci. Eng. 2009;6(2):333–359. doi: 10.3934/mbe.2009.6.333. [DOI] [PubMed] [Google Scholar]
- 35.Mushayabasa S, Tchuenche J, Bhunu C, Ngarakana-Gwasira E. Modeling gonorrhea and HIV co-interaction. BioSystems. 2011;103(1):27–37. doi: 10.1016/j.biosystems.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Neu HC. The crisis in antibiotic resistance. Science. 1992;257(5073):1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor KA, Kingston M, O’Donovan M, Cryan B, Twomey C, O’Mahony D. Antibiotic prescribing policy and Clostridium difficile diarrhoea. QJM. 2004;97(7):423–429. doi: 10.1093/qjmed/hch076. [DOI] [PubMed] [Google Scholar]
- 38.Porco TC, Gao D, Scott JC, Shim E, Enanoria WT, Galvani AP, Lietman TM. When does overuse of antibiotics become a tragedy of the commons? PloS ONE. 2012;7(12):e46505. doi: 10.1371/journal.pone.0046505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porco TC, Gebre T, Ayele B, House J, Keenan J, Zhou Z, Hong KC, Stoller N, Ray KJ, Emerson P, Gaynor BD, Lietman TM. Effect of mass distribution of azithromycin for trachoma control on overall mortality in Ethiopian children: a randomized trial. JAMA. 2009;302(9):962–968. doi: 10.1001/jama.2009.1266. [DOI] [PubMed] [Google Scholar]
- 40.Pressley J, D’Agata EMC, Webb GF. The effect of co-colonization with community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus strains on competitive exclusion. Journal of Theoretical Biology. 2010;264(3):645–656. doi: 10.1016/j.jtbi.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pulcini C, Gyssens IC. How to educate prescribers in antimicrobial stewardship practices. Virulence. 2013;4(2):192–202. doi: 10.4161/viru.23706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reluga TC, Galvani AP. A general approach for population games with application to vaccination. Mathematical Biosciences. 2011;230(2):67–78. doi: 10.1016/j.mbs.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roeger L, Feng Z, Castillo-Chavez C. Modeling TB and HIV co-infections. Math. Biosci. Eng. 2009;6(4):815–837. doi: 10.3934/mbe.2009.6.815. [DOI] [PubMed] [Google Scholar]
- 44.Schachter J, West SK, Mabey D, Dawson CR, Bobo L, Bailey R, Vitale S, Quinn TC, Sheta A, Sallam S, Mkocha H, Mabey D, Faal H. Azithromycin in control of trachoma. Lancet. 1999;354(9179):630–635. doi: 10.1016/S0140-6736(98)12387-5. [DOI] [PubMed] [Google Scholar]
- 45.Sharomi O, Podder C, Gumel A, Song B. Mathematical analysis of the transmission dynamics of HIV/TB coinfection in the presence of treatment. Math. Biosci. Eng. 2008;5(1):145. doi: 10.3934/mbe.2008.5.145. [DOI] [PubMed] [Google Scholar]
- 46.Simell B, Auranen K, Käyhty H, Goldblatt D, Dagan R, O’Brien KL. The fundamental link between pneumococcal carriage and disease. Expert Review of Vaccines. 2012;11(7):841–855. doi: 10.1586/erv.12.53. [DOI] [PubMed] [Google Scholar]
- 47.Skalet A, Cevallos V, Ayele B, Gebre T, Zhou Z, Jorgensen J, Zerihun M, Habte D, Assefa Y, Emerson P, Gaynor B, Porco T, Lietman T, Keenan J. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7(12):e1000377. doi: 10.1371/journal.pmed.1000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith HL. Monotone Dynamical Systems: An Introduction to the Theory of Competitive and Cooperative Systems. Vol. 41. Providence, RI: American Mathematical Society; 1995. [Google Scholar]
- 49.Solomon AW, Mohammed Z, Massae PA, Shao JF, Foster A, Mabey DCW, Peeling RW. Impact of mass distribution of azithromycin on the antibiotic susceptibilities of ocular chlamydia trachomatis. Antimicrobial Agents and Chemotherapy. 2005;49(11):4804–4806. doi: 10.1128/AAC.49.11.4804-4806.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spellberg B, Blaser M, Guidos RJ, Boucher HW, Bradley JS, Eisenstein BI, Gerding D, Lynfield R, Reller LB, Rex J, Schwartz D, Septimus E, Tenover FC, Gilbert DN. Combating antimicrobial resistance: Policy recommendations to save lives. Clinical Infectious Diseases. 2011;52(supp. 5):S397–S428. doi: 10.1093/cid/cir153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Driessche P, Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Mathematical Biosciences. 2002;180(2):29–48. doi: 10.1016/s0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 52.WHO. Progress report on elimination of trachoma, 2010. Weekly Epidemiological Record. 2012;87:161–168. [Google Scholar]