Abstract
Objective
Test the efficacy of SmartLoss℠, a smartphone-based weight loss intervention, in a pilot study.
Design and Methods
A 12-week randomized controlled trial. Adults (25<BMI<35 kg/m2) were randomized to SmartLoss (n=20) or an attention-matched Health Education control group (n=20). SmartLoss participants were prescribed a 1200-1400 kcal/d diet and were provided with a smartphone, body weight scale, and accelerometer that wirelessly transmitted body weight and step data to a website. In the SmartLoss Group, mathematical models were used to quantify dietary adherence based on body weight and counselors remotely delivered treatment recommendations based on these objective data. The Health Education group received health tips via smartphone. A mixed model determined if change in weight and other endpoints differed between the groups (baseline was a covariate).
Results
The sample was 82.5% female. Mean±SD baseline age, weight (kg), and BMI were −4.4±11.8 years, 80.0±11.2 kg, and 29.8±2.9 kg/m2, respectively. One participant was lost to follow-up in each group before week 4. Weight loss was significantly (P<.001) larger in the SmartLoss (Least Squares Mean±SEM: −9.4±0.5%) compared to the Health Education group (−0.6±0.5%).
Conclusions
SmartLoss efficaciously promote clinically meaningful weight loss compared to an attention-matched control group. Smartphone-based interventions might prove useful in intervention dissemination.
Keywords: weight loss, smartphone, weight graph, SmartLoss, mHealth
Introduction
Over half (68.5%) of the adult population in the United States (U.S.) is overweight or obese(1) and a large proportion of the U.S. qualifies for weight loss treatment based on treatment guidelines(2). Behavioral interventions delivered in clinic settings that target diet and exercise effectively promote weight loss (3), yet the ability to affordably and widely disseminate these interventions is a limitation. Internet interventions that include counselor support and tailored recommendations produce weight loss in the range of 4.4 to 7.5 kg (4-7), yet it is unclear to what extent these intensive approaches can be disseminated widely. Commercial websites that have wide dissemination frequently rely on automated treatment delivery via email or text messages and produce minimal weight loss (8), for example, 0.8 kg over 12 months (9). Additionally, utilization of Internet-based weight management programs decreases over time (10, 11) and maintaining engagement for a number of months is difficult, possibly because more of our Internet activity now occurs via smartphones (12)
Mobile health (mHealth) weight loss interventions incorporate smartphones and other technology and are easily disseminated. Further, mHealth approaches provide the ability to collect objective ecologically valid data in real-time; thus, providing a platform to provide near real-time feedback about behavior (13). This platform important as behavior change theories, e.g., learning theory, (14) postulate that temporally contiguous, data-driven feedback results in superior behavior change and fosters participants’ engagement in treatment.
The purpose of this pilot study was to test the efficacy of SmartLoss℠, a smartphone-based weight loss intervention, at reducing body weight and secondary endpoints (e.g., waist circumference) in a randomized controlled trial.
Methods
Participants
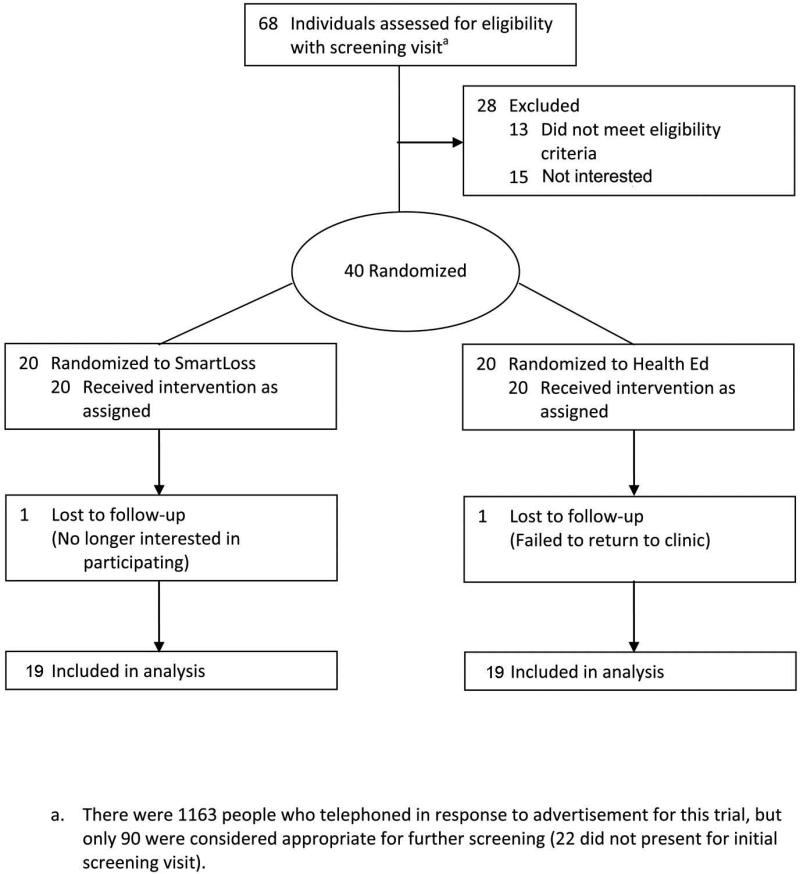
Forty overweight and obese adults (25 < Body Mass Index < 35 kg/m2) age 18-65 years enrolled in the trial. Details about recruitment and study flow are depicted in Figure 1. Exclusion criteria included current dieting; ±2 kg weight change in the past 60 days assessed by self-report; inability to engage in moderate intensity exercise; diagnosis of diabetes, cancer, or thyroid condition, or other conditions that affect body weight, appetite, or metabolism; use of prescription or over-the-counter medications that affect appetite, body weight, or metabolism (including diuretics); hypertension; and for females current or planned pregnancy.
Figure 1.
Diagram describing recruitment and study flow.
This study was conducted at the Pennington Biomedical Research Center, Baton Rouge, Louisiana. All applicable institutional and government regulations concerning the ethical use of human volunteers were followed. The research was approved by the Institutional Review Board of PBRC and all participants provided written informed consent prior to the initiation of any study procedures.
Protocol
After eligibility was established, participants were randomly assigned to the SmartLoss condition or the Heath Education condition for the 12 week trial (n=20 in each group). Randomization followed the minimization allocation method and was stratified by gender and weight status (“low BMI”: 25.0–29.9 kg/m2; “high BMI”: 30.0–35.0 kg/m2). The study Principle Investigator and measurement staff were blind to randomization; the counselors delivering the intervention were not blind.
Description of the Interventions
Participants in both groups received information from the Center remotely via smartphone while they lived in their natural environment. Participants presented to the clinic only for the measurement of outcome variables at Weeks 0 (baseline), 4, 8, and 12 (participants in the SmartLoss group received equipment from their counselor at the Week 0/baseline visit, as detailed herein). After baseline, all communication between participants in both groups and their counselors occurred via smartphone in the form of text messages, emails, or phone calls. Efforts were made to equate the number of contacts with study staff between groups to control for the effects of attention on the outcome variables.
The SmartLoss Intervention
SmartLoss provides the ability to deliver intensive behavioral weight loss interventions, consistent with treatment guidelines,(2) remotely. The platform provides remote monitoring of progress and the delivery of personalize treatment recommendations and lesson material via the multimedia capabilities of smartphones. In this pilot study, SmartLoss participants were prescribed a diet consistent with the American Heart Association's recommendations, e.g., less than 10% kcal from saturated fat, 55% carbohydrates, and protein derived from low-fat sources, such as fish and poultry. The caloric prescription for females and males was 1200 kcal/d (5024 kJ/d) and 1400 kcal/d (5862 kJ/d), respectively. SmartLoss participants received guidance on gradually increasing physical activity, with a goal of achieving 10,000 steps/day, consistent with the guidelines of national organizations(15)to achieve this goal.
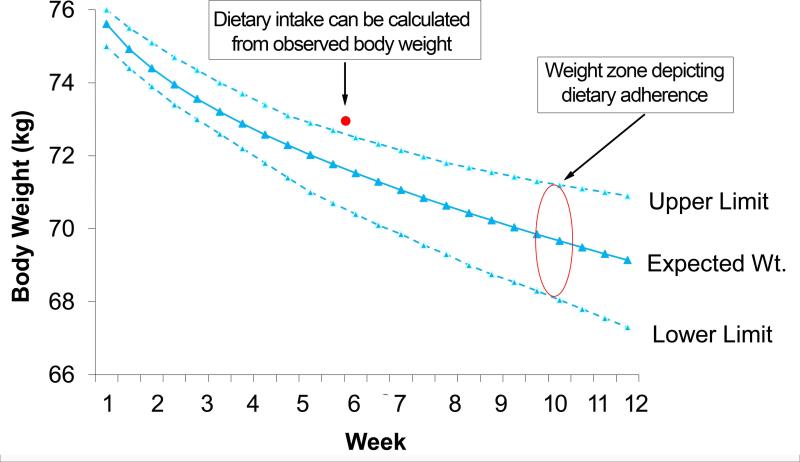
On the first day of the intervention, each participant's individual baseline data (age, sex, height, weight) and calorie prescription were entered into a dynamic energy balance equation to calculate the amount of weight each participant should lose over time if they were adherent to their calorie prescription. The output of the equation was displayed on a graph (i.e., a weight loss nomogram) that illustrated the participant's expected weight loss over time. The energy balance equations have been validated for predicting individual weight change and dietary adherence over time in response to an energy restricted diet in combination with the exercise levels promoted in this study, (16-19) and are also validated to calculate energy intake over time based on observed body weight (20). The graphs/weight loss nomograms include a “zone” of adherence, which was created by fitting an upper and lower curve through the mean absolute error obtained from validation of the differential equations as described in Thomas et al.(19). Participants were considered adherent over time if their body weight was within this zone and if they were losing weight at the expected rate (Figure 2). Being out of the zone is a sensitive diagnostic and predictor of weight loss over one year (21) and this weight graph approach has been used to quantify dietary adherence objectively and to guide treatment delivery during in-person interventions(16, 22). The report herein is, to our knowledge, the first use of this individualized weight graph approach in a remotely delivered intervention.
Figure 2.
The weight graph or nomogram. Participants are considered adherent to the caloric prescription if their body weight is within the “zone” depicted on the weight graph. Additionally, mean energy intake over time can be accurately calculated based on observed body weight.
At baseline, counselors educated each participant that the weight graph was used to objectively quantify adherence to the calorie prescription and to guide counseling and treatment recommendations. Further, the participant was instructed to weigh daily on a bathroom scale provided to them (A&D Engineering, Inc., Wellness Connected Wireless™ Precision Scale UC-324THX; San Jose, CA, USA) that automatically and wirelessly transmitted their data from the scale to a transceiver on an Internet-enabled computer, which then transmitted the data to a website that was accessible by their counselor. These body weight data were plotted onto participant's individual weight graph to evaluate adherence. Participants were trained how to use the scale when they received the equipment and they were also loaned a smartphone (Blackberry ® Curve 8320; Waterloo, Ontario, Canada). They received their weight graph via email on the smartphone and their counselor provided feedback and treatment recommendations via the smartphone by communicating via email, text, or phoning the participant. This feedback was based on the objective adherence data from the weight graph and participants received feedback at least once per week. Participants were educated on how the weight graph was used to gauge dietary adherence and that counselors would provide additional treatment recommendations when: 1) requested by the participant, 2) weight was above the zone, 3) weight was in the zone but no longer trending down, such that it would soon be out of the zone, or 4) weight was below the zone indicating that the rate of weight loss was too rapid. Additionally, if body weight was in the zone and/or continuing to trend down, counselors sent reinforcing feedback. When participants required additional assistance, we followed a toolbox approach, which has been previously published and described (23, 24), and involved working with the participant to problem-solve and identify strategies to foster adherence to the caloric prescription. Toolbox options included: portion-controlled foods, reducing eating out, portion control, reduced intake of sugar-sweetened beverages and fried foods, stimulus control, enlisting social support, differential reinforcement of other behavior, and the remote food photography method(25, 26). The success of the chosen strategy was evaluated over the following weeks and alternative techniques were employed if weight change was not decreasing at the expected rate.
To track activity (steps/day) remotely, participants were loaned an activity monitor (A&D Engineering, Inc., Wellness Connected Wireless™ Activity Monitor XL-20; San Jose, CA, USA) that wirelessly and automatically uploaded data to a website via the transceiver connected to an Internet enabled computer. Participants were instructed to wear the activity monitor at all times (the activity monitor fit on participants’ shoe) and participant's counselors used these objective step data to determine if the participant was increasing activity and meeting their individual goal. Counselors sent feedback to participants via the smartphone at least once per week.
The Health Education Group
Participants in the Health Education control group (n=20) received health information via text messages or emails delivered to the smartphone during the study. Topics included suggestions for stress management, healthy eating, exercise, and sleep hygiene. The topics and content of these health promotion messages were obtained from similar health information control groups from our earlier web-based intervention studies (27-30). To control for attention effects between the SmartLoss and Health Education groups, the number of text messages or emails that participants in the Health Education group received was similar to the number of contacts that participants in the SmartLoss group received from their counselor. This was accomplished by increasing or decreasing the number of contacts that each Health Education participant received based on the number of contacts that a matched SmartLoss group participant received.
Outcome Measures
The outcome variables were change in body weight (percent of original body weight and kg), waist circumference (cm), and systolic and diastolic blood pressure (mmHg). These variables were measured at the initial screening visit and Weeks 0/baseline, 4, 8, and 12. Height was measured at screening.
At Week 12, SmartLoss participants completed the SmartLoss Satisfaction Questionnaire, which assessed user satisfaction with SmartLoss.
Statistical Analyses
Analyses were carried out for all randomized participants who had at least one follow up visit using a modified intent to-treat approach. A mixed model analysis of covariance (ANCOVA) for repeated measures was performed to investigate treatment effects on changes from baseline (at week 4, 8, and 12) in weight (kg and %), waist circumference, and blood pressure. The model included factors with fixed effects (treatment group, time, and treatment group by time interaction), in addition to the random effects of subjects within time. Time was the repeated factor and the covariance matrix was modeled as autoregressive. Data were analyzed with and without adjustment for baseline values in the models. Findings are reported with baseline value adjusted since results from both approaches were similar. In addition, Fisher's exact test was used to compare group differences on proportions of 5% or 10% weight loss at week 12. Alpha was set at .05 for all analyses.
Ratings from the SmartLoss Satisfaction Questionnaire were summarized with descriptive statistics. Percent of days on which body weight and exercise data were successfully sent wirelessly from SmartLoss participants’ homes to the website/counselor were summarized with descriptive statistics. Lastly, SmartLoss participant's weight loss nomograms were normalized to reflect the distance in or out of the zone and illustrated in figures at the individual and group level.
All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
Results
Forty participants were enrolled and 38 participants completed the trial (one SmartLoss and one Health Education participant voluntarily withdrew before week 4). Participants were predominantly female (82.5%) and Caucasian (72.5%), and mean±SD BMI was 29.8±2.9 kg/m2 (Table 1). Baseline values did not differ significantly between the groups.
Table 1.
Baseline characteristics of study participants [Mean (standard error of the mean or SEM)]. Differences between the groups at baseline were evaluated with two-sample t-tests.
| Total Sample (n=40) | Health Education (n=20) | SmartLoss (n=20) | P value | |
|---|---|---|---|---|
| Age | 44.4 (1.86) | 43.3 (2.63) | 45.6 (2.67) | .55 |
| Height (cm) | 163.8 (1.20) | 165.1 (1.73) | 162.5 (1.65) | .29 |
| Body weight (kg) | 80.3 (1.82) | 80.6 (2.91) | 80.0 (2.28) | .87 |
| BMI (kg/m2) | 29.8 (0.47) | 29.5 (3.24) | 30.2 (2.66) | .41 |
| Waist circumference (cm) | 93.8 (1.49) | 94.5 (2.05) | 93.2 (2.19) | .66 |
| Systolic blood pressure (mm Hg) | 118.8 (2.08) | 117.8 (3.07) | 119.8 (2.86) | .64 |
| Diastolic blood pressure (mm Hg) | 75.1 (1.51) | 74.8 (2.16) | 75.4 (2.16) | .85 |
| N (%) | n (%) | n (%) | P (X2) | |
|---|---|---|---|---|
| Sex | ||||
| Female | 33 (82.5) | 17 (85.0) | 16 (80.0) | .68 |
| Male | 7 (17.5) | 3 (15.0) | 4 (20.0) | |
| Race | ||||
| Minority, primarily African-American | 11 (27.5) | 4 (20.0) | 7 (35.0) | .29 |
| Caucasian | 29 (72.5) | 16 (80.0) | 13 (65.0) |
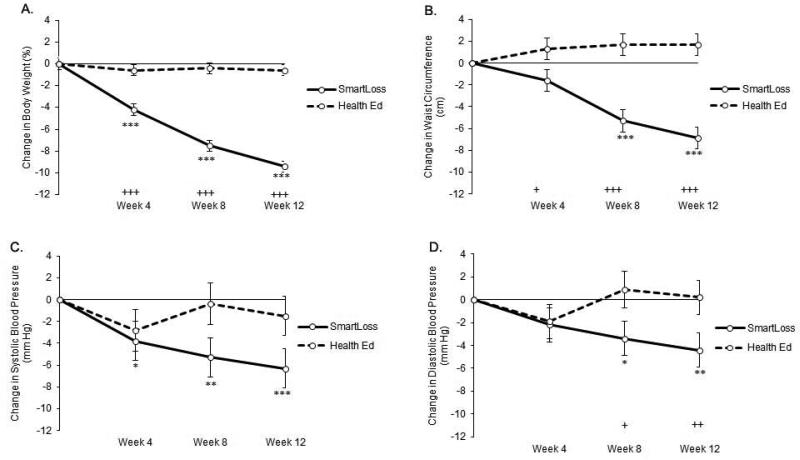
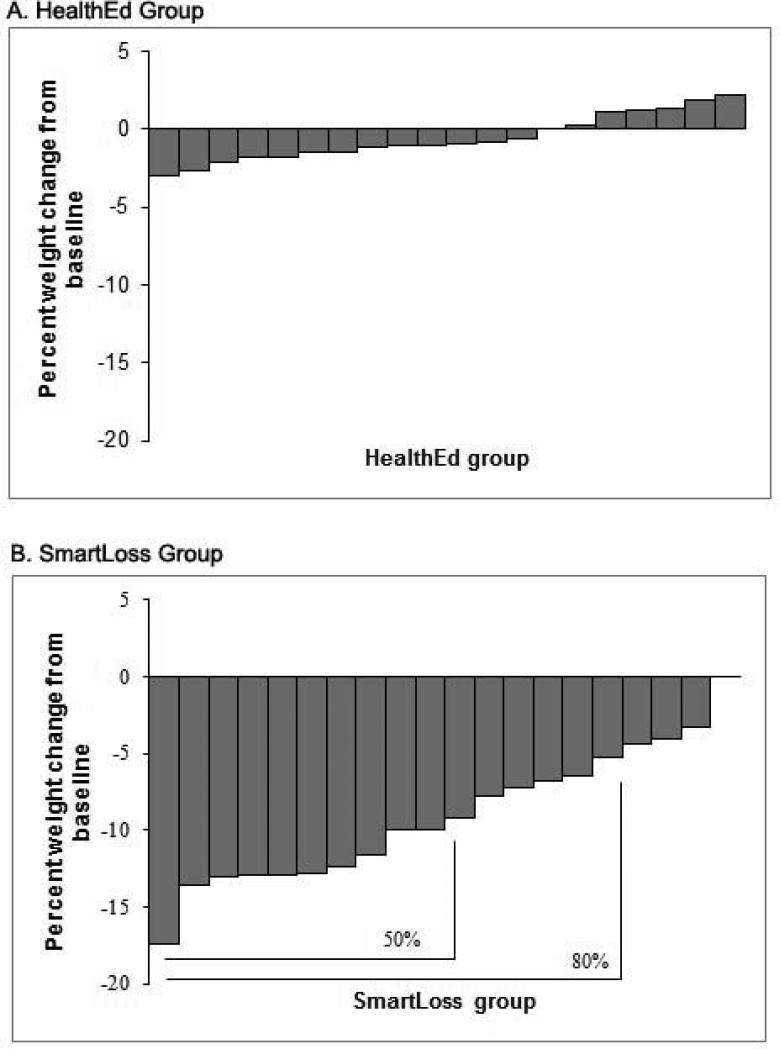
The SmartLoss group experienced significantly greater weight loss (percent of initial weight) than the Health Education group, F(1,35)=100.62, P<.001, with significant differences occurring between the groups at Weeks 4, 8, and 12 (Figure 3). A significantly greater proportion of SmartLoss participants lost a clinically meaningful amount of weight compared to the Health Education group. By Week 12, 80% and 50% of SmartLoss participants lost >5% and >10% of their body weight, respectively (Figure 4); no participants in the Health Education condition met the 5% criterion, Χ2(1,40) = 26.67, P < .001, or the 10% criterion, Χ2(1,40) = 13.33, P < .001). At week 12, the participants in the SmartLoss and Health Education groups lost 9.4±0.5% and 0.6±0.5% (Least Squares Mean±SEM) of weight, respectively. Weight loss expressed in kg also differed significantly between groups, F(1,35)=75.98, P<.001. Mean±SEM weight loss for the SmartLoss group was −3.5±0.46, −6.2±0.47, and −7.8±0.46 kg at weeks 4, 8, and 12, respectively. Mean±SEM weight loss in the Health Education groups was −0.5±0.47, −0.4±0.47, and −0.6±0.46 kg at weeks 4, 8, and 12, respectively.
Figure 3.
Change from baseline (least square means) for the primary outcome variables (error bars represent standard errors of the mean). The solid line and dashed lines represent the SmartLoss and Health Education Control groups, respectively. Asterisks indicate significant within group change from baseline (*P<.05, **P<.01, ***P<.001). Plus signs indicate significant differences between groups on change from baseline (+P<.05, ++P<.01, +++P<.001).
Figure 4.
Weight change over 12 weeks per individual in the Health Education (Panel A) and SmartLoss (Panel B) group.
Participants in the SmartLoss group also had significant improvements compared to Health Education on waist circumferences at all timepoints (P's<.05) (Figure 3). Mean±SEM waist circumference change for the SmartLoss group was −1.6±1.00, −5.3±1.01, and −6.9±1.00 cm at weeks 4, 8, and 12, respectively. Waist circumference change in the Health Education groups was 1.3±1.04, 1.7±1.04, and 1.7±1.00 cm at weeks 4, 8, and 12, respectively.
SmartLoss participants had significantly larger reductions in systolic blood pressure compared to the Health Education group, F(1,40.3)=4.17, P<.05, though no group comparisons at individual timepionts were significant (P-values > .06, Figure 3). By Week 12, systolic blood pressure change in the SmartLoss and Health Education groups was −6.3±1.77 and −1.5±1.78 mm Hg, respectively. The comparison between groups on change in diastolic blood pressure did not reach statistical significance, F(1,41.5)=3.51, P>.06; therefore, the differences between groups at Weeks 8 and 12 are not interpreted (Figure 3). At Week 12, diastolic blood pressure change in the SmartLoss and Health Education groups was −4.4±1.50 and 0.2±1.50 mm Hg, respectively.
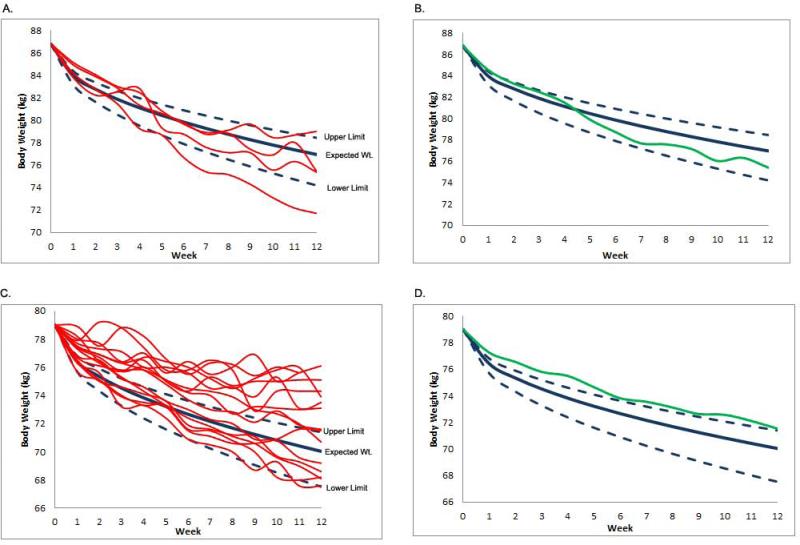
Figure 5 provides the weight loss nomograms for men (Panel A) and women (Panel B) in the SmartLoss group. These figures illustrate the weight change of men and women in relation to the nomogram and zone of adherence.
Figure 5.
Weight loss nomograms that are normalized to reflect the distance in or out of the zone for men and women. Panels A and B include the individual and group level data for men, respectively. Panels C and D include the individual and group level data for women, respectively. As illustrated, men were, as a group, more frequently in the zone compared to women.
The frequency and mode (email, text, phone) of counselor contact with participants is provided in Table 2. The number of contacts each month, overall, and averaged by week did not differ between the groups. By design, mode of contact differed between the groups; Health Education participants received more texts and less phone calls and emails than SmartLoss participants.
Table 2.
Frequency [Mean ( SEM)] of counselor contact for SmartLoss and Health Education participants by month (top panel) and weekly means by mode of contact* (phone, text, email) (bottom panel). Differences between the groups were evaluated with two-sample t-tests.
| Health Education (n=20) | SmartLoss (n=20) | P value | |
|---|---|---|---|
| Month 1 | 7.4 (1.2) | 9.5 (1.1) | .18 |
| Month 2 | 7.2 (1.1) | 9.6 (1.1) | .15 |
| Month 3 | 7.8 (1.3) | 8.0 (0.7) | .89 |
| All 3 months | 22.4 (3.5) | 27.1 (2.7) | .29 |
| Telephone | 1.0 (0.6) | 8.8 (1.6) | <.001 |
| Text message | 21.3 (3.5) | 6.8 (1.6) | .001 |
| 0.2 (0.2) | 11.5 (1.9) | <.001 | |
| All modes of communication | 1.9 (0.3) | 2.3 (0.2) | .29 |
Notes.
Per the study design, significant differences on mode of contact were expected between the groups.
Satisfaction with SmartLoss and reliability of wireless data transfer
SmartLoss participants’ satisfaction ratings are provided in Table 3 and, in brief, indicate that participants rated the intervention as convenient and helpful in facilitating weight loss.
Table 3.
SmartLoss participants' satisfaction ratings for the intervention. The Likert ratings ranged from 1 (e.g., Extremely Inconvenient, etc.) to 6 (e.g., Extremely Convenient, etc.). Question 3 asks about the activity sensor bothering subjects; thus, lower ratings reflect better ratings. The number (and percent in parentheses) of subjects endorsing each rating per question is provided in the table. Eighteen SmartLoss participants completed the satisfaction questionnaire.
| Likert rating | ||||||
|---|---|---|---|---|---|---|
| Question | 1 | 2 | 3 | 4 | 5 | 6 |
| 1. How convenient was it that you did not need to visit the center to return food diaries and activity logs (because this information was sent over the Blackberry)? | 0 (0) | 0 (0) | 1 (5.6) | 0 (0) | 0 (0) | 17 (94.4) |
| 2. How much did wearing the activity sensor remind you to exercise daily? | 0 (0) | 0 (0) | 2 (11.1) | 4 (22.2) | 4 (22.2) | 8 (44.4) |
| 3. How much did wearing the activity sensor bother you? | 12 (66.7) | 2 (11.1) | 2 (11.1) | 1 (5.6) | 1 (5.6) | 0 (0) |
| 4. How helpful were the step count goals and other specific suggestions that you received? | 0 (0) | 1 (5.6) | 0 (0) | 2 (11.1) | 3 (16.7) | 12 (66.7) |
| 5. Overall, how much did the e-Health intervention (the remote food photography method, activity sensor, and daily weighing) help you lose weight? | 0 (0) | 0 (0) | 0 (0) | 1 (5.6) | 1 (5.6) | 16 (88.9) |
Weight data were successfully wirelessly transmitted to counselors on 66 days (79% of the days in the study). Activity/step data were wirelessly transmitted on 54 days (64% of the days in the study). In case of transmission failure, participants occasionally self-reported body weight data and when these data are considered, weight data were received by counselors on 69 days (82% of the days in the study). On average, participants weighed 5.75 times per week.
Discussion
SmartLoss promoted clinically meaningful weight loss compared to a Health Education control group over 12 weeks in this pilot study. The weight loss observed in the SmartLoss group was sizeable and similar to the amount of weight loss observed in intensive lifestyle interventions delivered through in-person clinic-based programs (3) and via the Internet. (4-7) SmartLoss promoted significant weight loss with an intervention that was less intense and less didactic than lifestyle change programs, such as the Diabetes Prevention Program or Look AHEAD, that provide lessons and educational material through face-to-face meetings. SmartLoss relied on individualized weight graphs to quantify dietary adherence and a toolbox approach was used to problem-solve barriers to adherence. The effectiveness of strategies to promote adherence were then evaluated through change in body weight in relation to the zone of adherence, and, if ineffective, alternative strategies were employed and evaluated. Use of the weight graph allowed counselors and participants to easily and quickly determine if adherence to the caloric prescription was a problem. This approach has been used in other interventions that include a calorie restricted diet (22), and the results of this study support its efficacy and use in remotely delivered interventions. Participants and counselors reported that the weight graph approach was easy to utilize and eliminated the uncertainty of determining dietary adherence based on food records or other self-report data.
The results of this trial provide important empirical data on the efficacy of smartphone-based weight loss interventions. Despite the preponderance of weight management apps and enthusiasm over their promise, (13) (31) only 15% of 2014 apps reviewed included 5 or more of the evidence-based practices for weight management,(32) and evidence of the efficacy of these apps is minimal (33) (34) (35). The SmartLoss approach incorporates learning theory and a systematic approach to behavior modification, e.g., a toolbox, that has been used successfully in other studies (22-24). The amount of weight loss in this study was very similar to Thomas et al. (36), who found that a behavioral intervention that relied on smartphones to promote self-monitoring, provide feedback to participants, and to promote skills training resulted in weight loss of 9% and 11% of initial body weight at weeks 12 and 24, respectively. A study by Steinberg and colleagues (37) reported weight loss of 6.6% and their intervention relied on an Internet-connected scale and weekly emails with tailored feedback and weight management information.
Minimal contact interventions, however, produce less weight loss. For example, delivering health recommendations via podcast for 6 months and using an app to promote self-monitoring of diet and activity resulted in weight loss of 2.7% (38), and a 12-month text-messaging intervention produced weight loss of 3.6 pounds (39). Together, these studies suggest that smartphone-based interventions that promote self-regulatory behavior (e.g., self-monitoring, frequent weighing, and charting of body weight) can be efficacious at promoting clinically meaningful weight loss and are more effective than passive minimal contact interventions. It is likely that these self-regulatory behaviors facilitate behavior change in their own right and also provide participants with important objective data that better enables them to link their behavior to weight change and empowers them to make behavioral changes.
The SmartLoss group experienced significant improvements on several endpoints other than weight, which is expected given the amount of weight lost in the SmartLoss group. Notably, the improvements in blood pressure are important since this sample, although overweight, was reasonably healthy (mean blood pressure was 119/75 mm Hg at baseline). Additionally, waist circumference decreased by ~7 cm in the SmartLoss group. The improvements in blood pressure and waist circumference have important implications for disease risk and highlight the benefits of the weight loss induced by the SmartLoss intervention.
SmartLoss participants favorably rated the intervention and indicated that it helped them effectively lose weight. These satisfaction data are important since continued use of such interventions is likely necessary to effectively promote weight loss and long-term weight loss maintenance. Additionally, the technology used in this study reliably transferred body weight and exercise data from participants’ homes to the websites where counselors accessed data for provision of clinical services. This is a positive result since the technology has advanced since these data were collected and wireless automated data transfer is becoming more reliable, efficient, and commonplace.
The pilot study reported herein has its limitations. First, the study was only 12 weeks in duration and the study sample was small. Nonetheless, this randomized controlled study found convincing evidence that the SmartLoss intervention was feasible and efficacious over the short-term. It is acknowledged that longer term weight loss and weight loss maintenance studies are needed. Second, the scalability of SmartLoss was not formally evaluated nor was a cost-effectiveness analysis performed in this study, though this and similar approaches offer the ability to provide participants with automated feedback as well as feedback from a counselor, thus improving cost-effectiveness. Additionally, counselors reported that service delivery was very efficient and that they could provide services to many more SmartLoss patients per unit of time compared to traditional clinic-based interventions. Lastly, the SmartLoss intervention was not housed in a professionally programed smartphone-based application or “app” when this study was conducted, though such an app has since been developed.
In conclusion, SmartLoss promoted clinically meaningful weight loss over 12 weeks compared to an attention-matched control group, and user satisfaction with SmartLoss was favorable. These results suggest that SmartLoss and similar smartphone-based weight loss interventions might provide effective and scalable methods to remotely deliver weight loss treatment to large segments of the population, including people with limited access to healthcare.
What is Already Known About this Subject
- Intensive Internet-based weight loss treatments that include counselor support and tailored recommendations produce clinically significant weight loss, yet dissemination and utilization of such programs is limited
- Smartphone applications (apps) offer the ability to more easily and widely disseminate mobile health weight loss interventions, yet very few incorporate behavior change theory
- There is little empirical evidence on the efficacy of smartphone apps for weight loss and no reports of any apps that rely on mathematical models to predict weight loss and foster dietary adherence
What this Study Adds
- This is among the first studies to report the efficacy of a smartphone-based weight loss program in healthy adults
- The study reported herein is the first to utilize mathematical models to predict weight loss and foster dietary adherence in a remotely delivered smartphone intervention
- The positive results of this study indicate that smartphone-based weight loss interventions can produce clinically meaningful weight loss that is similar to weight loss achieved during clinic-based interventions; thus, providing support for the continued development and evaluation of smartphone-based interventions
Acknowledgments
Funding/Support: This study was supported by National Institutes of Health (NIDDK) grants R03 DK083533A and P30 DK072476. The NIH had no role in the design and conduct of the study.
Footnotes
Trial registration: ClinicalTrials.gov identifier: NCT00883350. http://clinicaltrials.gov/ct2/show/NCT00883350
Conflicts of Interest: TC, CC, HH, and AM report no conflicts of interest. SmartLoss is a registered trademark of the Louisiana State University System, with the trademarked approach having been developed by Drs. Martin, Thomas, and Leanne Redman.
Author Contributions: CM and DT designed the study. Data was collected by AM, analyzed by HH, and interpreted by CM, DT, AM, HH, TC, and CC. All authors participated in writing and revising the manuscript.
Additional Contributions: We thank all of the volunteers whose participation made the study possible.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Childhood and Adult Obesity in the United States 2011-2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2013 Nov 12; PubMed PMID: 24222017. Epub 2013/11/14. Eng. [Google Scholar]
- 3.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004 Dec;12(Suppl):151S–62S. doi: 10.1038/oby.2004.282. PubMed PMID: 15687411. [DOI] [PubMed] [Google Scholar]
- 4.Harvey-Berino J, West D, Krukowski R, Prewitt E, VanBiervliet A, Ashikaga T, et al. Internet delivered behavioral obesity treatment. Preventive Medicine. 2010;51(2):123–8. doi: 10.1016/j.ypmed.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krukowski RA, Harvey-Berino J, Ashikaga T, Thomas CS, Micco N. Internet-based weight control: The relationship between web features and weight loss. Telemedicine and e-Health. 2007;14(8):775–82. doi: 10.1089/tmj.2007.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tate DF, Jackvony EH, Wing RR. Effects of Internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: a randomized trial. Journal of American Medical Association. 2003 Apr 9;289(14):1833–6. doi: 10.1001/jama.289.14.1833. PubMed PMID: 12684363. [DOI] [PubMed] [Google Scholar]
- 7.Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an Internet weight loss program. Arch Intern Med. 2006 Aug 14-28;166(15):1620–5. doi: 10.1001/archinte.166.15.1620. PubMed PMID: 16908795. [DOI] [PubMed] [Google Scholar]
- 8.Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med. 2005 Jan 4;142(1):56–66. doi: 10.7326/0003-4819-142-1-200501040-00012. PubMed PMID: 15630109. [DOI] [PubMed] [Google Scholar]
- 9.Womble LG, Wadden TA, McGuckin BG, Sargent SL, Rothman RA, Krauthamer-Ewing ES. A randomized controlled trial of a commercial internet weight loss program. Obes Res. 2004 Jun;12(6):1011–8. doi: 10.1038/oby.2004.124. PubMed PMID: 15229342. [DOI] [PubMed] [Google Scholar]
- 10.Williamson DA, Copeland AL, Anton SD, Champagne C, Han H, Lewis L, et al. Wise Mind Project: A school-based environmental approach for preventing weight gain in children. Obesity. 2007;15(4):906–17. doi: 10.1038/oby.2007.597. [DOI] [PubMed] [Google Scholar]
- 11.Stewart T, Han H, Allen RH, Bathalon GC, Ryan DH, Newton RL, et al. H.E.A.L.T.H.: Efficacy of an internet/population-based behavioral weight management program for the U. S. Army. J Diabetes Sci Technol. 2011;5(1):178–87. doi: 10.1177/193229681100500125. PubMed PMID: 21303642. Epub 2011/02/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horrigan J. Wireless Internet Use. [January 25, 2010];Pew Internet & American Life Project. [Google Scholar]
- 13.Ayers JW, Althouse BM, Dredze MD. Could BehavioralMedicine Lead theWeb Data Revolution? JAMA. 2014;311(14):1399–400. doi: 10.1001/jama.2014.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. PubMed PMID: 16318590. Epub 2005/12/02. eng. [DOI] [PubMed] [Google Scholar]
- 15.Physical activity and cardiovascular health. NIH Consensus Development Panel on Physical Activity and Cardiovascular Health. Jama. 1996 Jul 17;276(3):241–6. PubMed PMID: 8667571. [PubMed] [Google Scholar]
- 16.Pieper C, Redman LM, Bapkar M, Roberts SB, Racette SB, Rochon J, et al. Development of adherence metrics for caloric restriction interventions. Clinical Trials: Journal of the Society for Clinical Trials. 2011 Apr;8(2):155–64. doi: 10.1177/1740774511398369. Epub 2011 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas DM, Ciesla A, Levine JA, Stevens JG, Martin CK. A mathematical model of weight change with adaptation. Math Biosci Eng. 2009 Oct;6(4):873–87. doi: 10.3934/mbe.2009.6.873. PubMed PMID: 19835433. Epub 2009/10/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas DM, Das SK, Levine JA, Martin CK, Mayer L, McDougall A, et al. New fat free mass - fat mass model for use in physiological energy balance equations. Nutrition and Metabolism. 2010;7(39) doi: 10.1186/1743-7075-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas DM, Martin CK, Heymsfield S, Redman LM, Schoeller DA, Levine JA. A simple model predicting individual weight change in humans. Journal of Biological Dynamics. 2011;fFirst:1–21. doi: 10.1080/17513758.2010.508541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas DM, Schoeller DA, Redman LA, Martin CK, Levine JA, Heymsfield SB. A computational model to determine energy intake during weight loss. American Journal of Clinical Nutrition. 2010;92(6):1326–31. doi: 10.3945/ajcn.2010.29687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas DM, Martin CK, Heymsfield SB, Marshall K, Bodrato VE, Williams DA, et al. Predicting successful long-term weight loss from short term weight loss outcomes: New insights from a dynamic energy balance model (The POUNDS LOST Study). American Journal of Clinical Nutrition. doi: 10.3945/ajcn.114.091520. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rickman AD, Williamson DA, Martin CK, Gilhooly CH, Stein RI, Bales CW, et al. The CALERIE Study: Design and methods of an innovative 25% caloric restriction intervention. Contemporary Clinical Trials. 2011;32:874–81. doi: 10.1016/j.cct.2011.07.002. Epub July 8, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LookAHEAD Research Group The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006 May;14(5):737–52. doi: 10.1038/oby.2006.84. PubMed PMID: 16855180. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diabetes Prevention Program (DPP) Research Group The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002 Dec;25(12):2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin CK, Correa JB, Han H, Allen HR, Rood J, Champagne CM, et al. Validity of the Remote Food Photography Method (RFPM) for estimating energy and nutrient intake in near real-time. Obesity. 2012 Apr;20(4):891–9. doi: 10.1038/oby.2011.344. Epub 2011 Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin CK, Han H, Coulon SM, Allen HR, Champagne CM, Anton SD. A novel method to remotely measure food intake of free-living individuals in real time: the remote food photography method. The British journal of nutrition. 2009 Feb;101(3):446–56. doi: 10.1017/S0007114508027438. PubMed PMID: 18616837. Pubmed Central PMCID: 2626133. Epub 2008/07/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart T, May S, Allen HR, Bathalon GP, Lavergne G, Sigrist L, et al. Development of an internet/population-based weight management program for the U.S. Army. Journal of Diabetes Science and Technology. 2008;2(1):116–26. doi: 10.1177/193229680800200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williamson DA, Champagne CM, Harsha D, Han H, Martin CK, Newton R, Jr., et al. Louisiana (LA) Health: Design and methods for a childhood obesity prevention program in rural schools. Contemp Clin Trials. 2008 Mar 26;29:783–95. doi: 10.1016/j.cct.2008.03.004. PubMed PMID: 18448393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williamson DA, Copeland AL, Anton SD, Champagne C, Han H, Lewis L, et al. Wise Mind project: a school-based environmental approach for preventing weight gain in children. Obesity. 2007 Apr;15(4):906–17. doi: 10.1038/oby.2007.597. PubMed PMID: 17426326. eng. [DOI] [PubMed] [Google Scholar]
- 30.Williamson DA, Walden HM, White MA, York-Crowe E, Newton RL, Jr., Alfonso A, et al. Two-year internet-based randomized controlled trial for weight loss in African-American girls. Obesity (Silver Spring) 2006 Jul;14(7):1231–43. doi: 10.1038/oby.2006.140. PubMed PMID: 16899804. Epub 2006/08/11. eng. [DOI] [PubMed] [Google Scholar]
- 31.Rodgers GP, Collins FS. The next generation of obesity research: no time to waste. JAMA : the journal of the American Medical Association. 2012 Sep 19;308(11):1095–6. doi: 10.1001/2012.jama.11853. PubMed PMID: 22990265. Epub 2012/09/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breton ER, Fuemmeler BF, Abroms LC. Weight loss—there is an app for that! But does it adhere to evidence-informed practices? Transl Behav Med. 2011;1(4):523–9. doi: 10.1007/s13142-011-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomlinson M, Rotheram-Borus MJ, Swartz L, Tsai AC. Scaling up mHealth: where is the evidence? PLoS medicine. 2013;10(2):e1001382. doi: 10.1371/journal.pmed.1001382. PubMed PMID: 23424286. Pubmed Central PMCID: 3570540. Epub 2013/02/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas JG, Bond DS. Review of innovations in digital health technology to promote weight control. Curr Diab Rep. 2014 May;14(5):485. doi: 10.1007/s11892-014-0485-1. PubMed PMID: 24664797. Epub 2014/03/26. eng. [DOI] [PubMed] [Google Scholar]
- 35.Powell AC, Landman AB, Bates DW. In Search of a Few Good Apps. JAMA. 2014;311(18):1851–2. doi: 10.1001/jama.2014.2564. [DOI] [PubMed] [Google Scholar]
- 36.Thomas JG, Wing RR. Health-e-call, a smartphone-assisted behavioral obesity treatment: pilot study. JMIR mHealth and uHealth. 2013;1(1):e3. doi: 10.2196/mhealth.2164. PubMed PMID: 25100672. Pubmed Central PMCID: 4114436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel-Hodge C, Ward DS. The efficacy of a daily self-weighing weight loss intervention using smart scales and e-mail. Obesity. 2013 Sep;21(9):1789–97. doi: 10.1002/oby.20396. PubMed PMID: 23512320. Pubmed Central PMCID: 3788086. Epub 2013/03/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner-McGrievy G, Tate D. Tweets, Apps, and Pods: Results of the 6-month Mobile Pounds Off Digitally (Mobile POD) randomized weight-loss intervention among adults. J Med Internet Res. 2011;13(4):e120. doi: 10.2196/jmir.1841. PubMed PMID: 22186428. Pubmed Central PMCID: 3278106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shapiro JR, Koro T, Doran N, Thompson S, Sallis JF, Calfas K, et al. Text4Diet: a randomized controlled study using text messaging for weight loss behaviors. Prev Med. 2012 Nov;55(5):412–7. doi: 10.1016/j.ypmed.2012.08.011. PubMed PMID: 22944150. [DOI] [PubMed] [Google Scholar]