Abstract
The synthesis of several novel substituted (Z)-2-amino-5-(1-benzyl-1H-indol-3-yl)methylene-1-methyl-1H-imidazol-4(5H)-ones structurally related to aplysinopsin have been carried out under microwave irradiation and conventional heating methods. The analogs 3a, 3b, 3d–3g, and 3k and 3l were evaluated for their in vitro cytotoxic activity against an NCI 60 human tumor cell line panel. Compound 3f exhibited good growth inhibitory properties against all but four of the human cancer cell lines examined, and afforded LC50 values <10 mM for 30% of the cell lines in the panel. Compound 3e was an effective inhibitor of leukemia, CNS, melanoma, and breast cancer cell growth, but generally less effective as a cytotoxic agent. Thus, the aplysinopsin analog 3f was regarded as a useful lead compound for further structural optimization.
Keywords: N-benzyl indole-3-carboxaldehydes, creatinine, in vitro cytotoxicity
In the past several decades researchers have been challenged by the task of identifying effective clinical agents to treat cancer, which is the second leading cause of death in the United States.1 The World Cancer Congress (WCC) has released a report stating that 8 million people died from cancer in 2008, and 12 million people were suffering from cancer during the same time period. Anticancer drugs such as cisplatin, 5-fluorouracil, paclitaxel and docetaxel, are some of the major chemotherapeutic agents currently being used to treat cancer.2 However, research is still needed to discover newer, more effective anticancer agents. Indole-derived aplysinopsin analogs (Fig 1. structure 1) have been reported to be potent and selective cytotoxic agents against cancer cells.3 Li et al.4 have synthesized and studied a series of structurally related N-heterocyclic indolylglyoxylamides (Fig 1. structure 2) and found that such compounds possess interesting activity against several cancer cell lines, including multidrug resistance (MDR) cell lines. David et al.5 also reported structure-activity relationship (SAR) studies on a series of N-benzylindole and indolizine glyoxylamides (Fig 1. structure 3) that exhibit substantial in vitro anti-proliferative activity against various cancer cell lines, including hematologic and solid tumor cell lines (i.e., leukemia, breast, colon, and uterine). As the part of a drug discovery program to discover and develop small molecules as potential anticancer agents, we identified (Z)-2-(N-benzylindol-3-ylmethylidene)quinuclidin-3-ol and (Z)-(±)-2-[N-(4-chlorobenzyl)indole-3-yl-methyl-idene]quinuclidin-3-ol as potent thermal-sensitizers capable of lowering the threshold for Hsf1 activation and thermal sensitivity. These compounds were considered as potential thermal radiosensitization agents.6
Figure 1.
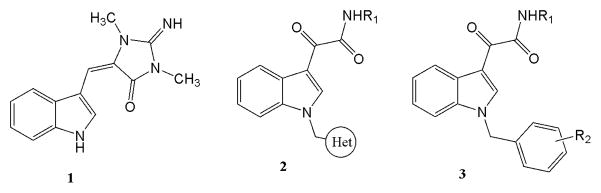
Cytotoxic indole-derived aplysinopsin analogs (1–3)
In continuation of our work on the design and synthesis of substituted (Z)-5-(N-benzyl-1H-indol-3-yl)methylene derivatives we focused on a series of novel (Z)-2-amino-5-(1-benzyl-1H-indol-3-yl)methylene-1-methyl-1H-imidazol-4(5H)ones structurally related to aplysinopsin that incorporated electron donating and electron withdrawing substituents in both the indolic ring and the phenyl ring of the N-benzyl moiety.
The aromatic substituted N-benzylindole-3-carboxaldehydes were synthesized in 85–90% yield by treating the appropriately substituted indole-3-carboxaldehyde with various substituted benzyl halides under phase-transfer catalytic (PTC) conditions utilizing triethylbenzyl ammonium chloride (TEBA) and a mixture of dichloromethane in 50% w/v aqueous NaOH solution (Scheme 1). Aldol condensation of the appropriate N-benzylindole-3-carboxaldehyde with creatinine, in the presence of CH3COOH/sodium acetate utilizing either conventional heating or microwave irradiation methodologies (Scheme 1) afforded a series of novel substituted (Z)-2-amino-5-(1-benzyl-1H-indol-3-yl)-methylene-1-methyl-1H-imidazol-4(5H)-one analogs. The microwave irradiation method was found to be more advantageous than conventional heating, and afforded product yields in the range of 85–91% compared to 70–83% for the latter method (Table 1). In addition, reaction times were very short with microwave irradiation (30–60 sec) compared to conventional heating (7–10 h). All the synthesized compounds were fully characterized by 1H-NMR, 13C-NMR and mass spectral analysis.8
Scheme 1.
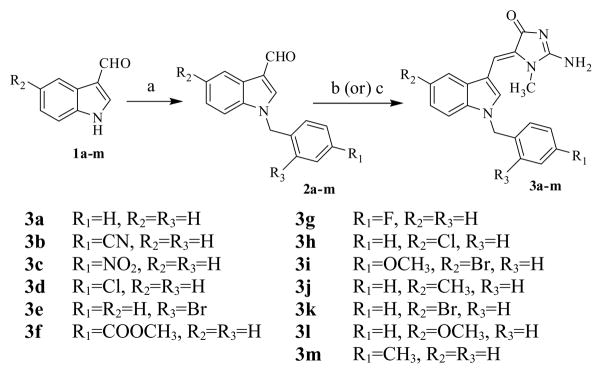
Synthesis of (Z)-2-amino-5-(1-benzyl-1H-indol-3-yl)methylene-1-methyl-1H-imidazol-4(5H)-one analogs: Reagents and conditions (a) appropriate benzyl halide, aqueous NaOH solution, triethylbenzyl ammonium chloride, DCM, RT; (b) creatinine (1.1 mol. eq), NaOAc (1.2 mol. eq), AcOH, MWI, 30–60 sec; (c) creatinine (1.1 mol. eq), NaOAc (1.2 mol. eq), AcOH, reflux, 7–10 h.
Table 1.
Reaction times and yields of novel substituted (Z)-2-amino-5-(1-benzyl-1H-indol-3-yl)methylene-1-methyl-1H imidazol-4(5H)-ones
| Compds | Method A | Method B | ||
|---|---|---|---|---|
|
| ||||
| Yield (%) | Time (Sec) | Yield (%) | Time (h) | |
| (Microwave Condition) | (Conventional heating) | |||
| 3a | 90 | 40 | 79 | 7 |
| 3b | 85 | 60 | 71 | 8 |
| 3c | 88 | 60 | 83 | 10 |
| 3d | 86 | 30 | 73 | 9 |
| 3e | 91 | 40 | 82 | 7 |
| 3f | 87 | 60 | 75 | 10 |
| 3g | 86 | 30 | 70 | 7 |
| 3h | 89 | 40 | 77 | 9 |
| 3i | 87 | 60 | 81 | 10 |
| 3j | 88 | 50 | 78 | 8 |
| 3k | 86 | 60 | 80 | 10 |
| 3l | 87 | 50 | 74 | 9 |
| 3m | 89 | 60 | 72 | 10 |
The in vitro screening studies involved a two-stage process with preliminary evaluation of compounds 3a, 3b, 3d–3g, and 3k and 3l against a 60 human tumor cell line panel at a single dose of 10μM, according to the procedures described by Rubinstein et al.7 The human tumor cell line panel included leukemia, non-small cell lung, colon, CNS, melanoma, ovarian, renal, prostate, and breast cancer cell lines. Only compounds which showed more than 60% growth inhibition in at least 8 of the 60 tumor cell lines were selected for further dose-response studies; the remaining compounds were not further investigated.
The two most active compounds (3e and 3f) from the preliminary 60 cell screen were subsequently evaluated in five dose-response studies for their in vitro cytotoxic effects on growth parameters against each of the 60 human tumor cell lines. Dose-response curves were created by plotting cytotoxic effect against the log10 of the drug concentration for each cell line. Cytotoxic effects of each compound were determined as GI50 and LC50 values, which represent the molar drug concentration required to cause 50% growth inhibition, and the concentration that kills 50% of the cells, respectively. The results are presented in Table 2. Except for EVVX (non-small cell lung), UACC-257 (melanoma), A498 (renal) and CAKI-1 (renal) cell lines, compound 3f afforded GI50 values in the range 1.46–9.46 μM against all the other cell lines utilized, with 85% of these GI50 values falling in the range 1.46–2.93 μM. Of particular interest was the effect of 3f on cell lines HCC-2998 (colon; GI50=2.99 μM; LC50=4.71 μM), SF-539 and SNB-75 (CNS; GI50=1.54 μM and 1.46 μM, respectively; LC50=5.56 μM and 5.47 μM, respectively), SK-MEL-28 and SK-MEL-5 (melanoma; GI50=1.78 μM and 1.69 μM, respectively; LC50=6.14 μM and 5.75 μM, respectively), 786-0 and ACHN (renal; GI50=1.75 μM and 1.76 μM, respectively; LC50=5.85 μM and 6.14 μM, respectively), and T-47D and MDA-MB-468 (breast; GI50=1.68 μM and 1.69 μM, respectively; LC50=5.74 μM and 6.78 μM, respectively). Compound 3f exhibited generally poor LC50 values against leukemia and non-small cell lung cancer cell lines. With the exception of the NCI/ADR-RES ovarian cancer cell line, compound 3e exhibited growth inhibitory effects against all the cell lines tested, with GI50 values ranging from 1.19–82.1 μM, and with 77% of the cells affording GI50 values falling in the range 1.19–4.57 μM. Good growth inhibitory activity was observed against leukemia (GI50=1.19–4.57 μM), CNS (GI50=1.58–5.38 μM), and breast (GI50=1.68–4.07 μM) cell line sub-panels. Generally, 3e exhibited poorer LC50 values compared to those obtained for 3f. Most notable were the effects of 3e against HT29 (colon; GI50=2.18 μM; LC50=6.94 μM), SF-539 (CNS; GI50=1.73 μM; LC50=6.94 μM), OVCAR-3 (ovarian; GI50=1.95 μM; LC50=7.35 μM), and MDA-MB-468 (breast; GI50=1.69 μM; LC50=6.90 μM) cell lines.
Table 2.
Antitumor growth inhibitory activity (GI50/μM)a and cytotoxicity (LC50/μM)b data for compounds 3e and 3f in 5 dose studies against an NCI 60-cancer cell line panel.
| Panel/cell line | Compound 3e | Compound 3f | ||
|---|---|---|---|---|
| GI50 | LC50 | GI50 | LC50 | |
| Leukemia | 2.54 | 36.2 | 2.91 | >100 |
| CCRF-CEM | ||||
| HL-60(TB) | 1.85 | 21.0 | 2.67 | >100 |
| K-562 | 2.45 | 77.4 | 2.23 | >100 |
| MOLT-4 | 4.57 | >100 | 2.42 | >100 |
| RPMI-8226 | 1.19 | 65.9 | 3.25 | >100 |
| SR | 1.96 | 72.7 | 2.48 | >100 |
| Non-Small Cell Lung | 28.7 | >100 | 5.05 | >100 |
| A549/ATCC | ||||
| EKVX | 58.1 | >100 | >100 | >100 |
| HOP-62 | 8.78 | 51.2 | 1.99 | 33.0 |
| HOP-92 | 2.98 | 44.5 | 1.51 | 7.75 |
| NCI-H226 | 1.37 | 69.9 | 2.26 | >100 |
| NCI-H23 | 8.07 | 54.8 | 3.23 | 58.8 |
| NCI-H322M | 11.0 | >100 | 2.56 | >100 |
| NCI-H460 | 3.95 | 56.8 | 2.55 | >100 |
| NCI-H522 | 5.38 | >100 | 1.93 | >100 |
| Colon | 2.21 | 12.3 | 1.91 | nd |
| COLO 205 | ||||
| HCC-2998 | 6.95 | 47.2 | 2.99 | 4.71 |
| HCT-116 | 2.42 | 30.9 | 1.79 | 7.70 |
| HCT-15 | 19.8 | >100 | 2.27 | >100 |
| HT29 | 2.18 | 6.94 | 2.17 | nd |
| KM12 | 5.71 | 50.8 | 3.18 | >100 |
| SW-620 | 3.68 | 43.3 | 2.20 | >100 |
| CNS | 5.38 | 59.8 | 2.56 | >100 |
| SF-268 | ||||
| SF-295 | 4.96 | >100 | 2.36 | >100 |
| SF-539 | 1.73 | 6.94 | 1.54 | 5.56 |
| SNB-19 | 4.50 | 43.8 | 2.39 | 31.0 |
| SNB-75 | 1.58 | 18.6 | 1.46 | 5.47 |
| U251 | 3.01 | 66.2 | 1.81 | 7.72 |
| Melanoma | 1.97 | 9.37 | 1.89 | 8.66 |
| LOX IMVI | ||||
| MALME-3M | 3.30 | 73.8 | 1.70 | 8.33 |
| M14 | 4.46 | 47.8 | 1.90 | 7.25 |
| MDA-MB-435 | 4.97 | 68.6 | 1.93 | nd |
| SK-MEL-2 | 5.73 | >100 | 2.70 | >100 |
| SK-MEL-28 | 4.14 | 38.4 | 1.78 | 6.14 |
| SK-MEL-5 | 3.38 | 42.3 | 1.69 | 5.75 |
| UACC-257 | 12.9 | 66.9 | 17.9 | >100 |
| Ovarian | 20.0 | >100 | 5.91 | >100 |
| IGR-OV1 | ||||
| OVCAR-3 | 1.95 | 7.35 | 2.29 | 7.98 |
| OVCAR-4 | 2.41 | 25.2 | 2.18 | >100 |
| OVCAR-5 | 2.12 | 29.0 | 1.90 | 18.5 |
| OVCAR-8 | 10.1 | >100 | 3.45 | >100 |
| NCI/ADR-RES | >100 | >100 | 9.46 | >100 |
| SK-OV-3 | 11.9 | 49.9 | 1.98 | 8.68 |
| Renal | 6.15 | 46.2 | 1.75 | 5.85 |
| 786-0 | ||||
| A498 | 13.9 | 53.1 | 14.1 | 53.2 |
| ACHN | 11.8 | 59.0 | 1.76 | 6.14 |
| CAKI-1 | 68.1 | >100 | 35.1 | >100 |
| RXF 393 | 2.77 | 53.7 | 1.95 | 8.71 |
| SN12C | 6.07 | 76.3 | 1.74 | 8.59 |
| TK-10 | 6.84 | >100 | 3.16 | >100 |
| UO-31 | 7.95 | >100 | 3.20 | >100 |
| Prostate | 10.4 | 48.1 | 6.27 | >100 |
| PC-3 | ||||
| DU-145 | 82.1 | 68.8 | 2.25 | 14.8 |
| Breast | 2.25 | 48.1 | 2.45 | 77.8 |
| MCF7 | ||||
| MDA-MB-231/ATCC | 4.07 | 46.7 | 2.01 | 14.3 |
| HS 578T | 3.97 | >100 | 2.17 | >100 |
| BT-549 | 2.05 | >100 | 1.73 | >100 |
| T-47D | 1.68 | 13.1 | 1.50 | 5.74 |
| MDA-MB-468 | 1.69 | 6.90 | 1.63 | 6.78 |
GI50: 50% Growth inhibition, concentration of drug resulting in a 50% reduction in net protein increase compared with control cells.
LC50: Lethal concentration, concentration of drug lethal to 50% of cells.
nd: Not determined
In summary, a series of novel substituted (Z)-2-amino-5-(1-benzyl-1H-indol-3-yl)methylene-1-methyl-1H-imidazol-4(5H)-one analogs have been synthesized and evaluated for anticancer activity against a panel of 60 human cancer cell lines. Compounds 3e and 3f were identified as molecules of interest from a single dose assay, and were then evaluated for dose-dependent growth inhibition and cytotoxicity in all 60 human cancer cell lines. Compound 3f exhibited good growth inhibitory properties against all but four of the human cancer cell lines examined, and afforded LC50 values <10 μM for 30% of the cell lines in the panel. Compound 3e was an effective inhibitor of leukemia, CNS, melanoma, and breast cancer cell growth, but was generally less effective as a cytotoxic agent. Thus, the aplysinopsin analog 3f was regarded as a useful lead compound for further structural optimization in the search for anticancer agents with clinical potential.
Acknowledgments
We are grateful to the NCI/NIH for their financial support under grant number PO1 CA104457 and to the NCI Developmental Therapeutic Program (DTP) for screening data.
References and notes
- 1.De M, Jessica K, Boger DL. Drugs of the Future. 2008;33(11):969–979. [Google Scholar]
- 2.Curran WJ. Oncology. 2002;63(2):29–38. doi: 10.1159/000067145. [DOI] [PubMed] [Google Scholar]
- 3.Hollenbeak KH, Schmitz FJ. Lloydia. 1977;40:479–81. [PubMed] [Google Scholar]
- 4.Li WT, Hwang DR, Chen CP, Shen CW, Huang CL, Chen TW, Lin CH, Chang YL, Lo YK, Tseng HY, Lin CC, Song JS, Chen HC, Chen SJ, Wu SH, Chen CT. J Med Chem. 2003;46:1706. doi: 10.1021/jm020471r. [DOI] [PubMed] [Google Scholar]
- 5.James David A, Koya Keizo, Li Hao, Liang Guiqing, Xia Zhiqiang, Ying Weiwen, Wu Yaming, Sun Lijun. Bioorg Med Chem Lett. 2008;18:1784–1787. doi: 10.1016/j.bmcl.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Sonar VN, Thirupathi Reddy Y, Sekhar KR, Sasi S, Freeman ML, Crooks PA. Bioorg Med Chem Lett. 2007;17(24):6821–6824. doi: 10.1016/j.bmcl.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubinstein LV, Shoemaker RH, Paull KD, Simo RM, Tosini S, Skehan P, Scudiero PA, Monks A, Boyd MRJ. Natl Cancer lnst. 1990;82:1113. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- 8.Analytical data for compound 3e. 1H NMR (DMSO-d6): δ 3.28 (s, 3H, N-CH3), 5.53 (s, 2H, CH2), 6.53 (s, 1H, CH), 6.76–6.79 (d, 1H, J=6.9 Hz C4-H), 7.17–7.27 (m, 4H, Ar-H), 7.45–7.47 (t, 1H, J=7.5 Hz C5-H), 7.68–7.70 (t, 1H, J=6 Hz, C6-H), 7.71 (bs, 2H, NH2), 7.95–7.98 (d, 1H, J=8.1 Hz, C7-H), 9.12 (s, 1H, C2-H) ppm. 13C NMR (DMSO-d6): δ 27.81, 49.59, 103.49, 109.08, 110.16, 118.30, 119.80, 121.97, 122.10, 127.93, 128.11, 128.61, 129.49, 131.19, 131.42, 132.62, 135.49, 136.17, 164.97, 175.11 ppm. ES-API LC-MS m/z 409.8 and 410.8 (MH+).Analytical data for compound 3f. 1H NMR (DMSO-d6): δ 3.49 (s, 3H, N-CH3), 3.81 (s, 3H, OCH3), 5.70 (s, 2H, CH2), 7.20 (s, 1H, CH), 7.23–7.26 (m, 2H, C4-H and C7-H), 7.30–7.32 (d, 2H, J=8.4 Hz, Ar-H), 7.52–7.55 (t, 1H, J=9.0 Hz, C5-H), 7.90–7.93 (d, 2H, J=8.1 Hz, Ar-H), 8.09–8.12 (t, 1H, J=9.3 Hz, C6-H), 9.03 (s, 1H, C2-H). 9.31 (bs, 2H, NH2) ppm. 13C NMR (DMSO-d6): δ 28.74, 49.36, 52.16, 108.23, 110.93, 113.56, 118.75, 120.96, 123.67, 127.09, 128.25, 128.76, 129.48, 133.59, 135.64, 142.55, 151.73, 161.55, 165.65 ppm. ES-API LC-MS m/z 388.90 (MH+).


