Abstract
In celebration of the 50th anniversary of the discovery of B cells, I take a look back at the history of T cell help to B cells, which was discovered 47 years ago. In addition, I summarize and categorize the distinct molecules that are expressed by CD4+ T cells that constitute ‘help’ to B cells, and particularly the molecules expressed by T follicular helper (TFH) cells, which are the specialized providers of help to B cells.
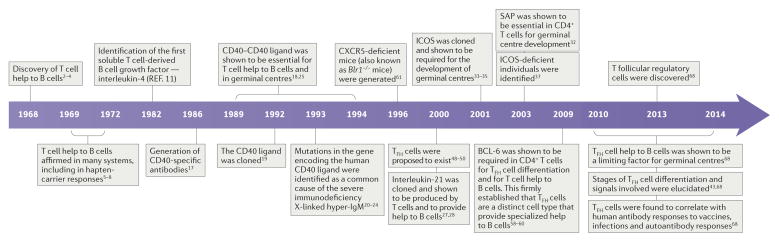
A timeline of B cell help discoveriess
Providing help to B cells was one of the earliest discovered functions of T cells, resulting in the coining of the term ‘T helper (TH) cell’. The first indications came from Claman and colleagues in 1966 (REF. 1), but an unambiguous demonstration of a role for thymus-derived helper cells in antibody responses was made in a trio of back-to-back papers by Miller and Mitchell in 1968 (REFS 2–4) (FIG. 1). Using cell transfer experiments, they showed that transfer of neither thymus (T) cells nor bone marrow (B) cells to irradiated mice was sufficient to result in the development of an antibody response after immunization of mice with sheep erythrocytes. However, co-transfer of both bone marrow-derived and thymus-derived cells led to robust antibody responses2,3. These experiments showed that the cells from the thymus were necessary for the antibody response to the immunogen but that the thymus-derived cells did not produce the antibodies themselves. Thus, two different cell types — B cells and T cells — were required to collaborate to induce an antibody response. The T cells were recognized as a form of supporting cell type and termed ‘antigen-reactive cells’ by the authors2. The definitive nature of these papers resulted from a series of careful and clever controls — including using T cell-depleting antiserum, thymectomies and chromosomal markers2–4. In one experiment, Miller and Mitchell transferred thoracic duct cells from CBA mice crossed with C57BL/6 mice (consisting of predominantly mature T cells obtained by cannulation) into adult thymectomized and irradiated CBA mice that had been reconstituted for 2 weeks with CBA bone marrow and then immunized. They made use of strain-specific antiserum (H2-specific serum) to deplete CBA or C57BL/6 cells in vitro from spleen cell preparations from the immunized mice. Splenocyte preparations depleted of C57BL/6-derived cells (eliminating the thoracic duct-derived transferred cells but not the bone marrow-derived cells) did not lose antibody-secreting cells, whereas splenocyte preparations depleted of CBA-derived cells (in which the thoracic duct-derived cells and bone marrow-derived cells were eliminated) lost 97% of all antibody-secreting cells3.
Figure 1. A timeline of discoveries about T cell help to B cells.
T cell help to B cells was discovered only a few years after the discovery of B cells. Subsequent discoveries lead to coining of the term T follicular helper (TFH) cells ~30 years later. BCL-6, B cell lymphoma 6; CXCR5, CXC-chemokine receptor 5; ICOS, inducible T cell co-stimulator; SAP, SLAM-associated protein.
A rapid flurry of confirmatory studies were published showing the requirement of T cell help for antibody responses against many types of antigens in a plethora of experimental systems5, including the important hapten-carrier systems that enabled B cell and T cell antigens to be distinguished at the molecular level5,6. One compelling experimental approach made use of T cell-depleting antiserum (θ-specific serum) to eliminate T cells7 and thereby to prevent T cell help to B cells and antibody responses to immunogens8. However, of note, T cell help was not required for antibody responses to Salmonella adelaide flagellin, which is the antigen that is used in the seminal and brilliant 1958 ‘one cell — one antibody specificity’ paper by Nossal and Lederberg9. By 1972, the term ‘helper T cells’ was widely used to describe the thymus-educated cells that provide help to B cells5,8.
Discovery of interleukin-4
The nature of the ‘help’ was not immediately apparent5. Indeed, even today we are still trying to understand the process of T cell help to B cells. One early model was that helper T cells may secrete one or more cytokines that are the molecular embodiment of the ‘help’ to B cells. In 1982, interleukin-4 (IL-4) was discovered as the first B cell help factor10,11 (FIG. 1). The role of IL-4 was identified on the basis of its secretion from the mouse thymoma EL4 cell line and the in vitro ability of IL-4 in combination with B cell receptor (BCR) signalling to increase the number of B cells. With the development of the TH1 cell–TH2 cell paradigm in 1986 (REF. 12), it was generally inferred that as there were two types of CD4+ T cells and only TH2 cells expressed IL-4, these must be the CD4+ T cells that help B cells. Although the initial TH1 cell–TH2 cell paper had more refined conclusions, the simple interpretation that TH2 cells are the providers of B cell help became the standard interpretation, ingrained in textbooks and scientific papers alike. That deduction based on in vitro data was erroneous, but it was many years before the correct CD4+ T cell type would be identified. Along the way, there were sporadic publications showing that deletion of TH2-associated genes did not result in a loss of germinal centres in vivo13,14; these studies revealed a major gap in the understanding of T cell help to B cells. Germinal centres are microanatomical structures within the B cell regions of the lymph nodes and the spleen. The germinal centres are the active sites of large-scale antigen-specific B cell proliferation and mutation. It is primarily in germinal centres that B cells evolve high-affinity BCRs via mutation and selection by CD4+ T cells, and it is almost mainly via germinal centres that B cells develop memory in the form of long-lived plasma cells and memory B cells15. Germinal centres depend on CD4+ T cells for their development and maintenance, and for the production of plasma cells. Of particular note, germline deletion of Il4 in mice resulted in no significant reduction of germinal centres or of total IgG in response to immunizations; the effects of loss of Il4 were generally restricted to loss of IgE and IgG1, and a bias in the ratios of different IgG subtypes13,16. These results indicated that IL-4 uniquely contributes to IgE class-switch recombination but that most other aspects of T cell help to B cells primarily depend on other molecules.
Discovery of CD40
In the meantime, the importance of CD40 and CD40 ligand (CD40L) was discovered, creating interest in the help to B cells that occurs via direct interactions between CD4+ T cells and B cells, in addition to the role of the secretion of cytokines (which may act at a distance). The first step to recognizing the role of CD40 came in 1986 with the generation of an antibody specific for human CD40, which induced B cell proliferation when combined with BCR signals17. Furthermore, stimulation of human germinal centre B cells with a CD40-specific antibody prevented apoptosis18. Clearly, CD40 was an important molecule on the surface of B cells, but what was the ligand? CD40L was cloned in 1992 and was found to be highly expressed by activated CD4+ T cells19. Strikingly, treatment of naive mouse or human B cells with a CD40L–Fc fusion protein could induce B cell proliferation in the absence of any additional co-stimulation, which indicates that CD40L signalling to CD40 is a dominant mechanism of T cell help to B cells19. Shortly thereafter, it was determined by several research groups that the severe human genetic immunodeficiency X-linked hyper-IgM syndrome is frequently caused by mutations in CD40LG (the gene encoding CD40L)20–24, which reinforced the concept that CD40L signals from CD4+ T cells are a primary component of T cell help to B cells. Individuals with X-linked hyper-IgM syndrome who had mutations in CD40LG lacked germinal centres. In 1994, it was shown that a CD40L-specific monoclonal antibody could prevent the formation of germinal centres in mice25. In addition, germinal centres were known to contain CD4+ T cells25,26, which implied that CD4+ T cells provide help to germinal centre B cells, and at least one component of that T cell help was contact-dependent CD40L.
Discovery of the role of IL-21
In 2000, the cytokine IL-21 was cloned and shown to help B cell proliferation27,28. IL-21 has since been shown to be the most potent cytokine for stimulating plasma cell differentiation29,30. Mice that are deficient for both IL-4 and the IL-21 receptor (Il4−/−Il21r−/− mice) were found to have severe defects in antibody production, class-switch recombination and germinal centres, which indicated that the combination of these two cytokines was important for help to B cells31. However, the source of the IL-4 and IL-21 remained unclear.
Contact dependency
At this time, the evidence for additional contact-dependent help functions of T cells to B cells was also accumulating. Both inducible T cell co-stimulator (ICOS) and SLAM-associated protein (SAP; also known as SH2D1A) are expressed by CD4+ T cells and deletion of the corresponding genes (Icos or Sh2d1a, respectively) results in severe defects in germinal centres and B cell memory32–35. Mutations in SH2D1A36 (which result in the clinical disorder X-linked lymphoproliferative disease)or ICOSL (which encodes ICOS ligand)37 result in immunodeficiency. A mutation in SH2D1A frequently leads to child mortality as a result of increased susceptibility to certain infections36. As SAP binds cytoplasmic tails of signalling lymphocytic activation molecule (SLAM) family receptors, it was proposed that SAP was involved in adhesion and/or co-stimulation of B cells by T cells38. Nevertheless, discovering the importance of ICOS and SAP in T cell help to B cells raised more questions than answers about B cell–T cell interactions. The crucial importance of colocalization of CD4+ T cells and B cells for T cell help was highlighted when technological advances in microscopy enabled intravital microscopy imaging. Intravital microscopy studies revealed extensive cognate interactions between CD4+ T cells and B cells in the border region between the T cell zone and the B cell follicle early during antigen-specific immune responses39–41 and later in germinal centres42. Later, it would become clear that ICOS has roles in T follicular helper (TFH) cell differentiation43, migration44 and cytokine production45.
Defining TFH cells
Although the TH1 cell–TH2 cell paradigm held sway for many years, cracks in that oversimplification emerged over time and, ultimately, discoveries showed that the catalogue of CD4+ T cell types included many more than just TH1 cells and TH2 cells. This started with the firm establishment of regulatory T (TReg) cells in 2000–2003 — catalysed by the discovery of forkhead box P3 (FOXP3)46. The catalogue of CD4+ TH cell types then expanded to include TH17 cells in 2005–2006 (REF. 47). These revelations opened the door for serious consideration that there may be a subset of CD4+ T cells that are specialized in B cell help. TFH cells were first proposed in 2000 and 2001 (REFS 48–50) (FIG. 1). However, that proposal was mainly ignored as shown by the lack of mention of TFH cells in almost all CD4+ T cell reviews and textbook chapters in the years thereafter. Nevertheless, some savvy scientists recognized the importance of the TFH cell concept and forded key areas51–57. TFH cells were not widely accepted until 2009 when the transcriptional repressor B cell lymphoma 6 (BCL-6) was identified as a lineage-defining transcription factor of TFH cells58–60. A range of experiments — including the use of Bcl6−/− CD4+ T cells, constitutive expression of BCL-6 in antigen-specific CD4+ T cells and manipulation of the expression of B lymphocyte-induced maturation protein 1 (BLIMP1; a potent antagonist of BCL-6) in CD4+ T cells — showed that the expression of BCL-6 by CD4+ T cells is necessary for TFH cell differentiation and that TFH cells are the unique providers of T cell help to B cells for the development of germinal centres and for the generation of most class-switched antibodies58–60. A central marker of TFH cells is CXC-chemokine receptor 5 (CXCR5), which was shown a decade earlier to be required by B cells for entry into follicles61 and therefore it was logical that TFH cells would need to express the same chemokine receptor. It was also determined that TFH cells express IL-21 (REF. 50) and that TFH cells are the primary producers of IL-4 in lymphoid tissue62,63. The regulation of Il4 differs between TFH cells and TH2 cells — in TFH cells Il4 is regulated by SAP and protein kinase Cθ (PKCθ), and in TH2 cells it is regulated by GATA-binding protein 3 (GATA3) — and it is the IL-4 secreted from TFH cells that is necessary for class-switch recombination64–67.
Following the identification of BCL-6, the study of TFH cells and T cell help to B cells has markedly increased. Stages of TFH cell differentiation, inductive signals, migration patterns, memory, associations with human autoimmune diseases, and BCL-6+ TReg cells (also known as T follicular regulatory (TFR) cells) have since been discovered and have recently been reviewed68. Briefly, TFH cell differentiation is independent of the differentiation of TH1 cells, TH2 cells or TH17 cells54, and induction of BCL-6 expression and TFH cell differentiation can occur within the first 48 hours of CD4+ T cell priming, by the second cell division43,69–71, by dendritic cells or by other myeloid antigen-presenting cells43,72. Furthermore, our understanding of the nature of T cell help to B cells has become more refined.
What is T cell help?
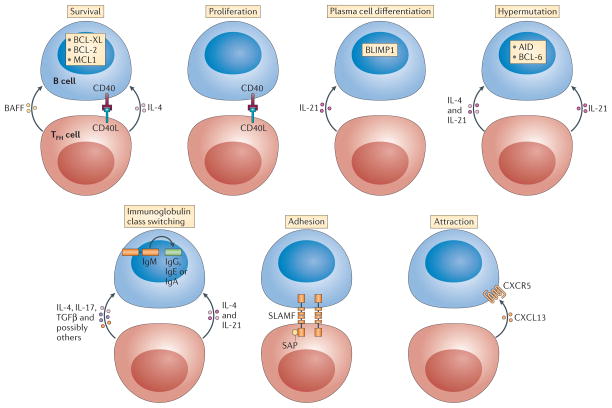
‘Help’ to B cells is not a single product of TFH cells and not even a single process. T cell help to B cells can be divided into seven distinct functions, as illustrated in FIG. 2: proliferation, survival, plasma cell differentiation, somatic hypermutation, class-switch recombination, adhesion and attraction. These seven different forms of help are all contributors to TFH cell–B cell interactions, and each process consists of multiple pathways, with only a minority shown in FIG. 2 for simplicity. Furthermore, some molecules have a role in several different forms of help.
Figure 2. Categories of T cell help to B cells.
Help can come in many different forms and can have different consequences for different processes. T follicular helper (TFH) cells provide seven main forms of T cell help to B cells: signals that promote survival, proliferation, plasma cell differentiation, hypermutation, class-switch recombination, adhesion and chemoattraction (cell migration). For simplicity, only a few examples of factors that are important for each process are shown, although many more molecules are involved in the regulation of the processes. Several of these pathways are reviewed in detail elsewhere64,73,85. Some molecules have pleiotropic effects, resulting in combinatorial possibilities and functional redundancies between molecules. AID, activation-induced cytidine deaminase; BAFF, B cell-activating factor; BCL, B cell lymphoma; BLIMP1, B lymphocyte-induced maturation protein 1; CD40L, CD40 ligand; CXCL13, CXC-chemokine ligand 13; CXCR5, CXC-chemokine receptor 5; IL, interleukin; MCL1, myeloid cell leukaemia 1; SAP, SLAM-associated protein; SLAMF, signalling lymphocytic activation molecule F; TGFβ, transforming growth factor-β.
The simplest B cell help function that is provided by TFH cells is the induction of B cell proliferation. CD40L is the most prominent protein expressed by TFH cells that contributes to pro-mitotic signalling in B cells64. Survival signals from TFH cells are also crucial, as germinal centre B cells are exquisitely pro-apoptotic73. IL-4 produced by TFH cells triggers pro-survival signals to germinal centre B cells via the IL-4 receptor complex64. Somatic hypermutation is central to germinal centre biology and the primary purpose of germinal centres is to facilitate affinity maturation of B cells via sequential rounds of immunoglobulin gene mutation and selection68,74,75. The enzyme activation-induced cytidine deaminase (AID; which is encoded by Aicda) induces the DNA damage in the immunoglobulin genes that is then converted into mutations by DNA repair enzymes73. BCL-6 must be co-expressed with AID by the germinal centre B cell to repress the DNA damage response programme that would otherwise trigger self-destruction of the cell76. The signals that induce AID and BCL-6 expression by B cells are not entirely defined, but CD40L, IL-4 and IL-21 contribute77. Indeed, the combination of CD40L, IL-4 and IL-21 in different ratios seems to be the primary mix of T cell help signals that control B cell proliferation, somatic hypermutation and differentiation. Class-switch recombination can also be induced by instructive signals from TFH cells to B cells. AID is necessary for class-switch recombination, but the specific target of the heavy chain constant region gene recombination depends on additional factors that are selectively activated by different cytokines, which predominantly, but not exclusively, come from CD4+ T cells. Human IgM to IgG class-switch recombination is most efficiently induced by IL-21, whereas IgE recombination is induced by a high IL-4 to IL-21 ratio78,79.
B cell help crucially depends on cell contact, probably because of a mixture of cell-surface co-stimulatory ligand interactions and directional cytokine production during cognate interactions. Therefore, adhesion molecules expressed by TFH cells and B cells (FIG. 2) are necessary components of T cell help to B cells, as they regulate the overall duration of the ‘pas de deux’. The most dramatic example of this requirement is SAP, which is described above. SAP binds to the intracellular domains of SLAM family surface receptors, which are involved in cell–cell adhesion. In the absence of SAP, the duration of B cell–T cell adhesion is short and inadequate for the TFH cell to provide sufficient help signals to the B cell. This leads to a general defect in SAP-dependent T cell help to B cells and thus a loss of antigen-specific B cell proliferation and survival, as well as a complete loss of germinal centres and of most memory B cells and long-lived plasma cells32,57,80.
Finally, chemoattraction is another component of T cell help to B cells (FIG. 2). CXC-chemokine ligand 13 (CXCL13) is the ligand for CXCR5 and human germinal centre TFH cells constitutively secrete copious quantities of CXCL13 (REFS 81,82), which probably recruits B cells to colocalize with the TFH cells and to facilitate confinement of the B cells to the germinal centre. Notably, CXCL13 signalling via CXCR5 also modifies B cell adhesion and lymphotoxin synthesis, which shows that CXCL13 also has cytokine-type functions83,84. Thus, chemoattraction is another form of T cell help to B cells.
Conclusions and perspectives
T cell help to B cells is a complex interplay of many factors and processes. Particularly in the germinal centre, many signals (both stimulatory and inhibitory) are exchanged between TFH cells and germinal centre B cells (and other cells in the microenvironment) in an iterative manner, over many rounds of rapid B cell division, mutation and selection. These integrated interactions remain poorly understood at the molecular and temporal levels, as the features of the cells change and the availability of antigen becomes more and more limiting. Furthermore, fundamental gaps remain in defining the signals that induce or that inhibit TFH cell differentiation68. Finally, it is important to better understand TFH cells in humans who have been immunized with vaccines to learn how to better boost vaccine responses, and an increased understanding of TFH cells in individuals with autoantibody-associated autoimmune diseases or allergies is important for learning how to ameliorate or how to block these TFH cell responses. There is much to be learned in the next 47 years about T cell help to B cells!
Footnotes
Competing interests statement
The author declares no competing interests.
References
- 1.Claman HN, Chaperon EA, Triplett RF. Thymus-marrow cell combinations. Synergism in antibody production. Proc Soc Exp Biol Med. 1966;122:1167–1171. doi: 10.3181/00379727-122-31353. [DOI] [PubMed] [Google Scholar]
- 2.Miller JF, Mitchell GF. Cell to cell interaction in the immune response. I. Hemolysin-forming cells in neonatally thymectomized mice reconstituted with thymus or thoracic duct lymphocytes. J Exp Med. 1968;128:801–820. doi: 10.1084/jem.128.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell GF, Miller JF. Cell to cell interaction in the immune response. II. The source of hemolysin-forming cells in irradiated mice given bone marrow and thymus or thoracic duct lymphocytes. J Exp Med. 1968;128:821–837. doi: 10.1084/jem.128.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nossal GJ, Cunningham A, Mitchell GF, Miller JF. Cell to cell interaction in the immune response. 3. Chromosomal marker analysis of single antibody-forming cells in reconstituted, irradiated, or thymectomized mice. J Exp Med. 1968;128:839–853. doi: 10.1084/jem.128.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz DH, Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- 6.Rajewsky K, Schirrmacher V, Nase S, Jerne NK. The requirement of more than one antigenic determinant for immunogenicity. J Exp Med. 1969;129:1131–1143. doi: 10.1084/jem.129.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raff M. θ-isoantigen as a marker of thymus-derived lymphocytes in mice. Nature. 1969;224:378–379. doi: 10.1038/224378a0. [DOI] [PubMed] [Google Scholar]
- 8.Raff MC. Role of thymus-derived lymphocytes in the secondary humoral immune response in mice. Nature. 1970;226:1257–1258. doi: 10.1038/2261257a0. [DOI] [PubMed] [Google Scholar]
- 9.Nossal GJ, Lederberg J. Antibody production by single cells. Nature. 1958;181:1419–1420. [PubMed] [Google Scholar]
- 10.Paul WE, Ohara J. B-cell stimulatory factor-1/interleukin 4. Annu Rev Immunol. 1987;5:429–459. doi: 10.1146/annurev.iy.05.040187.002241. [DOI] [PubMed] [Google Scholar]
- 11.Howard M, et al. Identification of a T cell-derived B cell growth factor distinct from interleukin 2. J Exp Med. 1982;155:914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 13.Kopf M, Le Gros G, Coyle AJ, Kosco-Vilbois M, Brombacher F. Immune responses of IL-4, IL-5, IL-6 deficient mice. Immunol Rev. 1995;148:45–69. doi: 10.1111/j.1600-065x.1995.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 14.Grusby MJ. Stat4- and Stat6-deficient mice as models for manipulating T helper cell responses. Biochem Soc Trans. 1997;25:359–360. doi: 10.1042/bst0250359. [DOI] [PubMed] [Google Scholar]
- 15.Kurosaki T, Kometani K, Ise W. Memory B cells. Nature Rev Immunol. 2015;15:149–159. doi: 10.1038/nri3802. [DOI] [PubMed] [Google Scholar]
- 16.Thorbecke GJ, Tsiagbe VK. The Biology of Germinal Centers in Lymphoid Tissue. Springer Verlag; 1998. [DOI] [PubMed] [Google Scholar]
- 17.Clark EA, Ledbetter JA. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci USA. 1986;83:4494–4498. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu YJ, et al. Mechanism of antigen-driven selection in germinal centres. Nature. 1989;342:929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- 19.Armitage RJ, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 20.Allen RC, et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993;259:990–993. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 21.Aruffo A, et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993;72:291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- 22.Korthäuer U, et al. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993;361:539–541. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- 23.DiSanto JP, Bonnefoy JY, Gauchat JF, Fischer A, de Saint Basile G. CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature. 1993;361:541–543. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- 24.Fuleihan R, et al. Defective expression of the CD40 ligand in X chromosome-linked immunoglobulin deficiency with normal or elevated IgM. Proc Natl Acad Sci USA. 1993;90:2170–2173. doi: 10.1073/pnas.90.6.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foy TM, et al. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994;180:157–163. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu YJ, Johnson GD, Gordon J, MacLennan IC. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992;13:17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- 27.Parrish-Novak J, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 28.Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci USA. 2000;97:11439–11444. doi: 10.1073/pnas.200360997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ettinger R, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 30.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 31.Ozaki K, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 32.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 33.McAdam AJ, et al. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 34.Tafuri A, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 35.Dong C, Temann UA, Flavell RA. Cutting edge: critical role of inducible costimulator in germinal center reactions. J Immunol. 2001;166:3659–3662. doi: 10.4049/jimmunol.166.6.3659. [DOI] [PubMed] [Google Scholar]
- 36.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 37.Grimbacher B, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nature Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 38.Cannons JL, et al. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okada T, et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 41.Okada T, Cyster JG. B cell migration and interactions in the early phase of antibody responses. Curr Opin Immunol. 2006;18:278–285. doi: 10.1016/j.coi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Allen CDC, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi YS, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu H, et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496:523–527. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- 45.Morita R, et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng MH, Anderson MS. Monogenic autoimmunity. Annu Rev Immunol. 2012;30:393–427. doi: 10.1146/annurev-immunol-020711-074953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nature Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaerli P, et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim CH, et al. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breitfeld D, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chtanova T, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-TH1/ TH2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 52.Vinuesa CG, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 53.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 54.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haynes NM, et al. Role of CXCR5 and CCR7 in follicular TH cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- 56.Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nature Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu D, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 61.Förster R, et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 62.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yusuf I, et al. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) J Immunol. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crotty S. Follicular helper CD4+ T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 65.Vijayanand P, et al. Interleukin-4 production by follicular helper T cells requires the conserved Il4 enhancer hypersensitivity site V. Immunity. 2012;36:175–187. doi: 10.1016/j.immuni.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harada Y, et al. The 3′ enhancer CNS2 is a critical regulator of interleukin-4-mediated humoral immunity in follicular helper T cells. Immunity. 2012;36:188–200. doi: 10.1016/j.immuni.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 67.Liang HE, et al. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nature Immunol. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crotty ST. Follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi YS, Eto D, Yang JA, Lao C, Crotty S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J Immunol. 2013;190:3049–3053. doi: 10.4049/jimmunol.1203032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi YS, et al. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J Immunol. 2013;190:4014–4026. doi: 10.4049/jimmunol.1202963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baumjohann D, Okada T, Ansel KM. Cutting edge: Distinct waves of BCL6 expression during T follicular helper cell development. J Immunol. 2011;187:2089–2092. doi: 10.4049/jimmunol.1101393. [DOI] [PubMed] [Google Scholar]
- 72.Goenka R, et al. Cutting edge: dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J Immunol. 2011;187:1091–1095. doi: 10.4049/jimmunol.1100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 74.Victora GD, Mesin L. Clonal and cellular dynamics in germinal centers. Curr Opin Immunol. 2014;28:90–96. doi: 10.1016/j.coi.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nature Rev Immunol. 2015;15:137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nature Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nojima T, et al. In-vitro derived germinal centre B cells differentially generate memory B or plasma cells in vivo. Nature Commun. 2011;2:465. doi: 10.1038/ncomms1475. [DOI] [PubMed] [Google Scholar]
- 78.Avery DT, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. 2010;207:155–171. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suto A, et al. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line Cε transcription of IL-4-stimulated B cells. Blood. 2002;100:4565–4573. doi: 10.1182/blood-2002-04-1115. [DOI] [PubMed] [Google Scholar]
- 80.Kageyama R, et al. The receptor Ly108 functions as a SAP adaptor-dependent on-off switch for T cell help to B cells and NKT cell development. Immunity. 2012;36:986–1002. doi: 10.1016/j.immuni.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kroenke MA, et al. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol. 2012;188:3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rasheed AU, Rahn HP, Sallusto F, Lipp M, Müller G. Follicular B helper T cell activity is confined to CXCR5hiICOShi CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 83.Sáez de Guinoa J, Barrio L, Mellado M, Carrasco YR. CXCL13/CXCR5 signaling enhances BCR-triggered B-cell activation by shaping cell dynamics. Blood. 2011;118:1560–1569. doi: 10.1182/blood-2011-01-332106. [DOI] [PubMed] [Google Scholar]
- 84.Ansel KM, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 85.Vinuesa CG, Cyster JG. How T cells earn the follicular rite of passage. Immunity. 2011;35:671–680. doi: 10.1016/j.immuni.2011.11.001. [DOI] [PubMed] [Google Scholar]