Abstract
Purpose
To report interim cosmetic results and toxicity from a prospective study evaluating accelerated partial-breast irradiation (APBI) administered using a highly conformal external beam approach.
Methods and Materials
We enrolled breast cancer patients in an institutional review board–approved prospective study of APBI using beamlet intensity-modulated radiotherapy (IMRT) at deep-inspiration breath-hold. Patients received 38.5 Gy in 3.85 Gy fractions twice daily. Dosimetric parameters in patients who maintained acceptable cosmesis were compared with those in patients developing unacceptable cosmesis in follow-up, using t-tests.
Results
Thirty-four patients were enrolled; 2 were excluded from analysis because of fair baseline cosmesis. With a median follow-up of 2.5 years, new unacceptable cosmesis developed in 7 patients, leading to early study closure. We compared patients with new unacceptable cosmesis with those with consistently acceptable cosmesis. Retrospective analysis demonstrated that all but one plan adhered to the dosimetric requirements of the national APBI trial. The mean proportion of a whole-breast reference volume receiving 19.25 Gy (V50) was lower in patients with acceptable cosmesis than in those with unacceptable cosmesis (34.6% vs. 46.1%; p = 0.02). The mean percentage of this reference volume receiving 38.5 Gy (V100) was also lower in patients with acceptable cosmesis (15.5% vs. 23.0%; p = 0.02).
Conclusions
The hypofractionated schedule and parameters commonly used for external beam APBI and prescribed by the ongoing national trial may be suboptimal, at least when highly conformal techniques such as IMRT with management of breathing motion are used. The V50 and V100 of the breast reference volume seem correlated with cosmetic outcome, and stricter limits may be appropriate in this setting.
Keywords: Breast cancer, Accelerated partial-breast irradiation, Radiotherapy, Cosmesis, IMRT
INTRODUCTION
In recent years, interest in accelerated partial-breast irradiation (APBI) has increased, largely owing to the desire to minimize the expense, inconvenience, and/or toxicity associated with the administration of conventional whole-breast radiotherapy after breast-conserving surgery. After all, although standard whole-breast radiation has been established to yield high rates of local control for patients with early-stage breast cancer and modest improvements in survival for at least those facing higher baseline risks of locoregional recurrence (1), whole-breast treatment can expose adjacent normal tissues in some patients to potentially harmful radiation and must be administered over several weeks, even when hypofractionated regimens are used (2, 3).
In light of evidence suggesting that the majority of in-breast tumor recurrences occur in the vicinity of the lumpectomy cavity (4–6), it has been hypothesized that radiation treatment targeting a smaller volume of tissue may be equally as efficacious as whole-breast irradiation. Still, other evidence suggests that recurrences may occur outside even a generous volume beyond the primary tumor (7) and that microscopic disease may extend far from the original primary site (8–10). Therefore, the efficacy of partialbreast irradiation is being investigated further in the setting of a nationwide randomized trial, the ongoing Radiation Therapy Oncology Group (RTOG) 0413/National Surgical Adjuvant Breast and Bowel Project (NSABP) B-39 study (11).
Studies are necessary not only to evaluate the efficacy of partial-breast irradiation but also to assess the safety and toxicity of various techniques of treatment and dosing schedules, because partial-breast irradiation may be accomplished in a wide variety of ways. Whereas most initial experiences with APBI used brachytherapy (12–15), improvements in target localization and dosimetric planning have led to an increasing interest in the possibility of accomplishing partial-breast irradiation noninvasively, using external beam radiotherapy. Potential advantages of external beam treatment include the ability to offer the treatment after full pathologic information is available without subjecting the patient to a second invasive procedure or anesthesia, decreased operator-dependence, and less financial expense (16).
Although increased homogeneity of dose with external beam therapy might also reduce complications from fat necrosis seen in brachytherapy series, determining appropriate dose for tumor control by extrapolating from doses used in the brachytherapy studies has been difficult precisely because of the large differences in dose homogeneity between these techniques (17). Moreover, improved target coverage with external beam techniques comes at the cost of a higher integral dose to the remaining normal breast (18). Ensuring appropriate targeting, including appropriate accounting for breathing motion (19, 20) and setup accuracy, has been an additional challenge with this approach (21). Indeed, because the dose homogeneity and volumes irradiated with external beam techniques are substantially different from those when brachytherapy is used, it is necessary to accumulate further experience before deeming the most common fractionation schedule in current use—3.85 Gy b.i.d. for 5 days, as prescribed by the ongoing national trial (11)—to be acceptable, both in terms of efficacy and in terms of toxicity.
In this study, we report the interim cosmetic results and toxicity observed in a prospective study initiated at our institution to evaluate APBI administered using a highly conformal external beam approach.
METHODS AND MATERIALS
We enrolled selected Stage 0–I breast cancer patients in a prospective study of APBI on an institutional review board–approved protocol at the University of Michigan beginning in 2004. All patients had pathologically node-negative, unifocal, and histologically proven breast cancer, excised with negative margins ≥3 mm. Ineligibility criteria included the presence of lobular histology, diffuse calcifications, multifocal or multicentric disease, lymphovascular invasion, extensive lobular carcinoma in situ or extensive ductal carcinoma in situ (DCIS), systemic disease, prior radiation to the ipsilateral breast or chest wall, a deleterious BRCA1/2 mutation, high risk for hereditary breast cancer due to strong family history, history of scleroderma or lupus, mammographically occult disease, and pregnancy. Patients were required to be ≥40 years old with life expectancy of at least 5 years and Karnofsky performance status of at least 70. Only patients who could provide informed consent, use a mouthpiece with an active breathing control (ABC) system, and tolerate a breath hold of at least 20 s while in the treatment planning position were eligible to participate. Patients were not allowed to have received neoadjuvant or concurrent chemotherapy. The patients were permitted to receive systemic chemotherapy or hormonal therapy after completion of the radiation treatment.
The study was designed as a Phase I–II feasibility study. Enrolled subjects underwent a free-breathing CT scan with 5-mm cuts in the treatment position, on a carbon fiber breast board (Sinmed; Reeuwijk, The Netherlands), with both arms extended above their heads, to develop a standard two-field tangent plan. Radio-opaque catheters were placed in the locations expected for the field borders of standard tangent fields before scanning. The free-breathing CT scan was followed by a second CT scan using the ABC device, to suspend breathing motion at deep inspiration. The ABC device (vMax; Sensormedics, Yorba Linda, CA) used a spirometry system to suspend the patient’s breath-hold at a specific deep inspiration breath-hold state (65–80%) determined to be comfortable for the patient.
For planning, the lumpectomy cavity was drawn by the treating physician. Both breasts, lungs, and heart were contoured by a dosimetrist and approved by the treating physician. The contoured breast excluded the pectoral musculature, excluded 5mmof skin and tissue from the body surface, and extended to 5 mm inside the medial and lateral catheters denoting the borders of the tangent fields. Margins of 1.5 cm in three dimensions were added to the delineated lumpectomy cavity to create a planning target volume (PTV) (1.0 cm to account for subclinical microscopic tumor extension, not extending beyond the contoured breast volume, and an additional 0.5 cm to account for set-up variation). The final PTV was edited to ensure that it did not extend to within 5 mm of the skin surface.
An in-house-developed inverse planning system was then used to develop the best, optimized, beamlet intensity-modulated radiotherapy (IMRT) plan by which to administer treatment to the PTV (22). The IMRT plan was developed using the CT scan of the patient at deep-inspiration breath-hold. The number of beams ranged from three to five, and photon energy was 6 MV; beam angles were chosen to avoid dose to the contralateral breast or lung. The cost function highly prioritized minimizing dose to the heart and creating a homogeneous dose to the target volume.
Once the IMRT plan was considered to be optimal the patient proceeded with treatment. Treatment began approximately 2 weeks after simulation scanning, with the PTV receiving 38.5 Gy in 3.85 Gy per fraction b.i.d., at least 6 hours apart, over 5 consecutive days, using the ABC device at the same breath-hold state as for the planning scan for each treatment. Daily imaging of the treatment fields was obtained at the correct breath-hold state, and positioning was verified using a lead wire on the incision scar as a reference mark, in addition to anatomic features.
Follow-up visits were conducted by the treating radiation oncologist 1 week and 1 month after completion of radiotherapy. Patients were then again seen at 6 months from completion of radiotherapy and again every 3–6 months for the first 2 years after completion of treatment. After completing 2 years of follow-up, patients are seen every 6 months until reaching 5 years from completion of treatment.
Toxicities were assessed using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE, version 3.0) (23). Cosmesis was defined on a four-point scale by the treating physician, using the criteria articulated on the cosmetic assessment form of the national study (24). The size, shape, and texture of the treated breast were compared with the untreated breast or original appearance of the breast. Excellent cosmesis was defined as “minimal or no difference”; good cosmesis was defined as “a slight difference”; fair cosmesis was defined as “obvious differences … [involving] a quarter or less of the breast”; and poor cosmesis was defined as “marked change … involving more than a quarter of the breast tissue.” Patients were also asked to consent to photographs to document their ultimate cosmetic outcomes. Decision rules were constructed to stop the trial early if accumulating trial evidence suggested that the probability of Grade 3+ toxicity or that the probability of an unsatisfactory cosmesis assessment was >20%, as estimated with 90% confidence.
For comparison to the NSABP B-39/RTOG 0413, a breast reference volume (BRV) was created retrospectively to include the region encompassed by traditional tangential portals (11). This BRV, as well as the structures contoured for treatment planning, are depicted in Fig. 1.
Fig. 1.
Volumes contoured on an axial image of a sample patient. The lumpectomy cavity is contoured in red. Margins were added as described in the text to create a planning target volume, as depicted in green. The contoured breast is depicted in pink and excludes the pectoral musculature, excludes 5 mm of skin and tissue from the body surface, and extends to 5 mm inside the medial and lateral catheters denoting the borders of the tangent fields. For comparison with the Radiation Therapy Oncology Group (RTOG) 0413/National Surgical Adjuvant Breast and Bowel Project (NSABP) B-39 study (11), a breast reference volume was created retrospectively to include the region encompassed by traditional tangential portals; the large yellow contour corresponds to this volume.
The analysis considered only patients with acceptable (excellent or good) cosmesis at baseline. Dosimetric parameters and other characteristics of patients who subsequently developed unacceptable (fair or poor) cosmesis at baseline were compared with those among patients who maintained consistently acceptable cosmesis in follow-up. For continuous dosimetric measurements, all comparisons were conducted using t tests and nonparametrically using the Wilcoxon rank–sum test. Results were consistent between the methods, and are therefore reported for the t tests alone. For categoric endpoints (e.g., chemotherapy receipt), statistical inference was based on Fisher’s exact test. All statistical analyses were performed using SAS statistical software, version 9.2 (SAS Insitute, Cary, NC); p values <0.05 were considered significant.
RESULTS
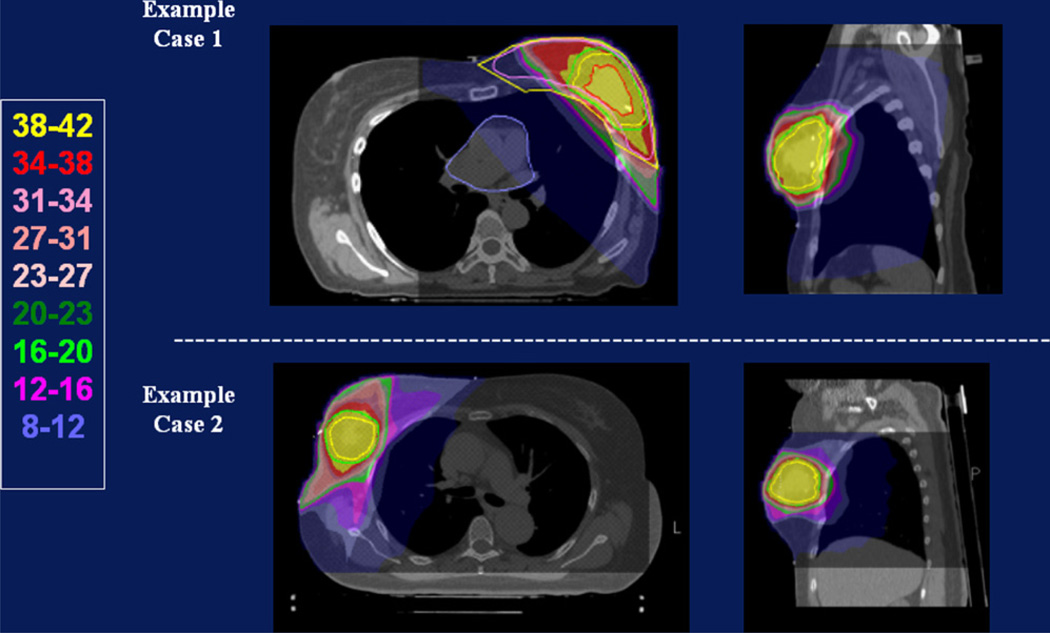
Between 2004 and 2007, 34 patients were enrolled on the study protocol. Median patient age was 56 years (range, 40–80 years). The vast majority of patients (32 of 34) were white. Median tumor size was 0.9 cm (range, 0.1–2.0 cm). All patients met the strict eligibility criteria articulated in Methods and Materials, including nonlobular histology, negative lymph nodes, and resection with negative margins. Table 1 details other important characteristics of the patient population. Figure 2 shows the isodose distributions of two typical cases treated on this protocol.
Table 1.
Characteristics of 34 enrolled patients
| Laterality | |
|---|---|
| Left | 12 (35.3) |
| Right | 22 (64.7) |
| Histology | |
| Invasive ductal | 18 (52.9) |
| DCIS | 16 (47.1) |
| Chemotherapy | |
| Yes | 6 (17.7) |
| No | 28 (82.3) |
| Hormonal Therapy | |
| Yes | 12 (35.3) |
| No | 22 (64.7) |
| Re-excision | |
| Yes | 19 (55.9) |
| No | 15 (44.1) |
| Excision cavity (GTV) (cm3) | 40.3 (10.1–102.3) |
| PTV (cm3) | 185.8 (59.8–382.0) |
| V100 to BRV (%) | 17.5 (6.8–29.5) |
| V50 to BRV (%) | 37.7 (16.6–64.5) |
| V100 to contoured breast (%) | 27.2 (11.7–48.8) |
| V50 to contoured breast (%) | 47.9 (22.7–79.1) |
| Maximum dose to 1% (%) | 40.0 (38.9–42.0) |
| Separation (cm) | 21.1 (16.2–26.0) |
Abbreviations: DCIS = ductal carcinoma in situ; GTV = gross tumor volume; PTV = planning target volume; BRV = breast reference volume.
Values are number (percentage) or mean (range).
Fig. 2.
Isodose distributions achieved in 2 patients. Both cases illustrate the conformality of the treatment. The cases differ with respect to the volume of the lumpectomy cavity and the proportion of normal breast irradiated.
Median follow-up from time of completion of radiotherapy was 2.5 years (range, 1.5–3.7 years). Table 2 details the toxicities observed in the treated population. No local failures have been observed to date.
Table 2.
Toxicities*
| Toxicity | Worst Grade* | No. experiencing as acute toxicity† (of 34 patients enrolled) |
No. experiencing as late toxicity‡ (of 34 patients enrolled) |
|---|---|---|---|
| Pain | 1 | 6 | 3 |
| 2 | 0 | 1 | |
| Fatigue | 1 | 17 | 7 |
| Burn | 1 | 3 | 0 |
| Dry skin | 1 | 10 | 5 |
| 2 | 2 | 0 | |
| Hyperpigmentation | 1 | 19 | 6 |
| 2 | 1 | 0 | |
| Hypopigmentation | 1 | 1 | 2 |
| Induration/fibrosis | 1 | 11 | 19 |
| 2 | 1 | 3 | |
| Pruritus | 1 | 2 | 0 |
| Rash | 1 | 7 | 0 |
| Nipple/areolar defect | 1 | 10 | 11 |
| 2 | 3 | 10 | |
| 3 | 0 | 1 | |
| Volume loss/hypoplasia | 1 | 14 | 14 |
| 2 | 3 | 12 | |
| 3 | 0 | 1 | |
| Edema | 1 | 1 | 0 |
| Fibrosis of deep connective tissue | 1 | 2 | 2 |
| Anorexia and nausea | 1 | 1 | 0 |
| Telangiectasia | 1 | 0 | 5 |
| 2 | 0 | 1 | |
| 3 | 0 | 1 | |
| Atrophy (skin) | 1 | 0 | 1 |
| Atrophy (subcutaneous fat) | 2 | 0 | 1 |
| Wound complication | 1 | 0 | 1 |
| Striae | 1 | 0 | 1 |
| Dyspnea | 1 | 0 | 1 |
Toxicities scored on the National Cancer Institute’s form for Common Toxicity Criteria for Adverse Events (CTCAE), version 3.0.
Acute toxicities are defined as those noted on the CTCAE forms completed while the patient was on treatment, or at the 1-week or 1-month follow-up visits.
Late toxicities are defined as those noted on the CTCAE forms completed at the 6-month or subsequent follow-up visits. One patient developing Class I congestive heart failure deemed secondary to trastuzumab use developed Grade 1 dyspnea and Grade 1 fatigue (included in the late toxicities above).
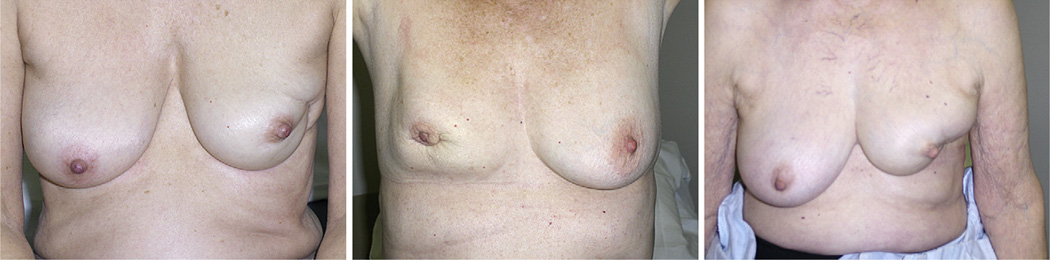
Two of the patients treated on study have been excluded from the remaining analyses owing to fair baseline cosmesis. All patients were evaluated after at least 2 years of follow-up except 1, who had only reached the 18-month assessment. At time of writing, new unacceptable cosmesis had developed in 7 patients. Figure 3 depicts the nature of the fair cosmesis in 3 cases. On most recent follow-up, of the 7 patients with overall unacceptable cosmesis, all had volume loss affecting cosmesis, 6 had retraction or contour defect affecting cosmesis, and 1 had telangiectasia affecting cosmesis. In addition, among these 7 patients, 6 had fibrosis that did not affect cosmesis, 1 had telangiectasia that did not affect cosmesis, 1 had retraction or contour defect that did not affect cosmesis, 2 had fat necrosis that did not affect cosmesis, and 1 had pigment change that did not affect cosmesis. The frequency of unacceptable cosmetic outcomes ultimately led to the premature closure of the study.
Fig. 3.
Visible impairment in cosmesis observed in 3 patients deemed to have unacceptable cosmesis after treatment.
To determine whether we could identify systematic differences in dosimetric or patient characteristics between those who developed unacceptable cosmesis and those who did not, we compared the 7 patients with newly developed unacceptable cosmesis with the 25 patients who have had consistently acceptable cosmesis. Retrospective analysis demonstrated that all but 1 patient’s plan adhered to the national study’s requirement that <60% of the breast reference volume receive ≥50% of the prescribed dose, and <35% receive the prescribed dose (11). However, the mean proportion of the BRV receiving 19.25 Gy (V50) was lower (p = 0.02) in patients with acceptable cosmesis (34.6%; range, 16.6–49.2%) than in those who developed unacceptable cosmesis (46.1%; range, 31.3–64.5%). The mean percentage of the BRV receiving 38.5 Gy (V100) was also lower (p = 0.02) in patients with acceptable cosmesis (15.5%; range, 6.8–25.4%) than in those who developed unacceptable cosmesis (23.0%; range, 14.0–29.5%).
The mean proportion of the contoured breast receiving 38.5 Gy was 25.3% (range, 11.7–39.7%) in patients with acceptable cosmesis and 33.0% (range, 21.0–48.8%) in those who developed unacceptable cosmesis (p = 0.16). The mean proportion of the contoured breast receiving 19.25 Gy was 45.0% (range, 22.7–69.2%) in patients with acceptable cosmesis and 56.5% (range, 38.1–79.1%) in those who developed unacceptable cosmesis (p = 0.07). The mean percentage of normal breast (contoured breast minus PTV) receiving at least 19.25 Gy was 31.1% (range, 14.4–58.4%) among patients with acceptable cosmesis and 38.3% (range, 22.0–68.3%) among those developing unacceptable cosmesis (p = 0.29). The mean maximum dose to 1% of the PTV was 39.9 Gy (range, 38.9–41.5 Gy) in patients with acceptable cosmesis and 40.3 Gy (range, 39.0–42.0%) in patients with unacceptable cosmesis (p = 0.39).
Mean lumpectomy bed size was 36.5 cm3 (range, 10.1–89.5 cm3) in patients with acceptable cosmesis and 58.3 cm3 (range, 15.7–102.3 cm3) in those who developed unacceptable cosmesis (p = 0.22). There was no significant difference in the proportion of patients in each group undergoing re-excision procedures (p = 0.99). Of the patients with acceptable cosmesis, 14 of 25 (56%) underwent re-excision procedures (8 had excisional biopsy, without the intent of obtaining negative margins, followed by re-excision; 6 had re-excision after a lumpectomy intended to yield negative margins). Of the patients with unacceptable cosmesis, 4 of 7 (57.1%) underwent re-excision (2 had re-excision after excisional biopsy, and 2 had re-excision after lumpectomy).
Adriamycin-based chemotherapy was received by 3 of 25 (12.0%) of the patients with acceptable cosmesis and 2 of 7 (28.6%) of those who developed unacceptable cosmesis (p = 0.30). Hormonal therapy was received by 18 of 25 (72.0%) of the patients with acceptable cosmesis and 2 of 7 (28.6%) of those who developed unacceptable cosmesis (p = 0.07).
The primary tumor was located in the outer breast of 19 patients (76%) with acceptable cosmesis and in the inner or central breast of the remaining 6 (24.0%). The primary tumor was located in the outer breast of 3 patients (42.9%) with unacceptable cosmesis and in the inner or central breast of the remaining 4 (57.1%). This difference was not statistically significant (p = 0.17).
All analyses were repeated after excluding patients receiving chemotherapy, to determine whether inclusion of patients receiving chemotherapy affected our results. The same dosimetric parameters were significantly different between the groups (V50 and V100 to the BRV). Specifically, in patients not receiving chemotherapy who had unacceptable cosmesis, the mean V50 to the breast reference volume was 45.4%, compared with 35.5% in those with consistently acceptable cosmesis (p = 0.002); the mean V100 was 23.5% in those with unacceptable cosmesis, compared with 16.0% in those with consistently acceptable cosmesis (p = 0.02). In addition, when chemotherapy patients were excluded, tumor location was significantly different (p = 0.047) between patients with unacceptable cosmesis (80% with inner/central tumors) and those with acceptable cosmesis (27.3% with inner/central tumors).
Multivariate analysis was not conducted owing to the small number of events.
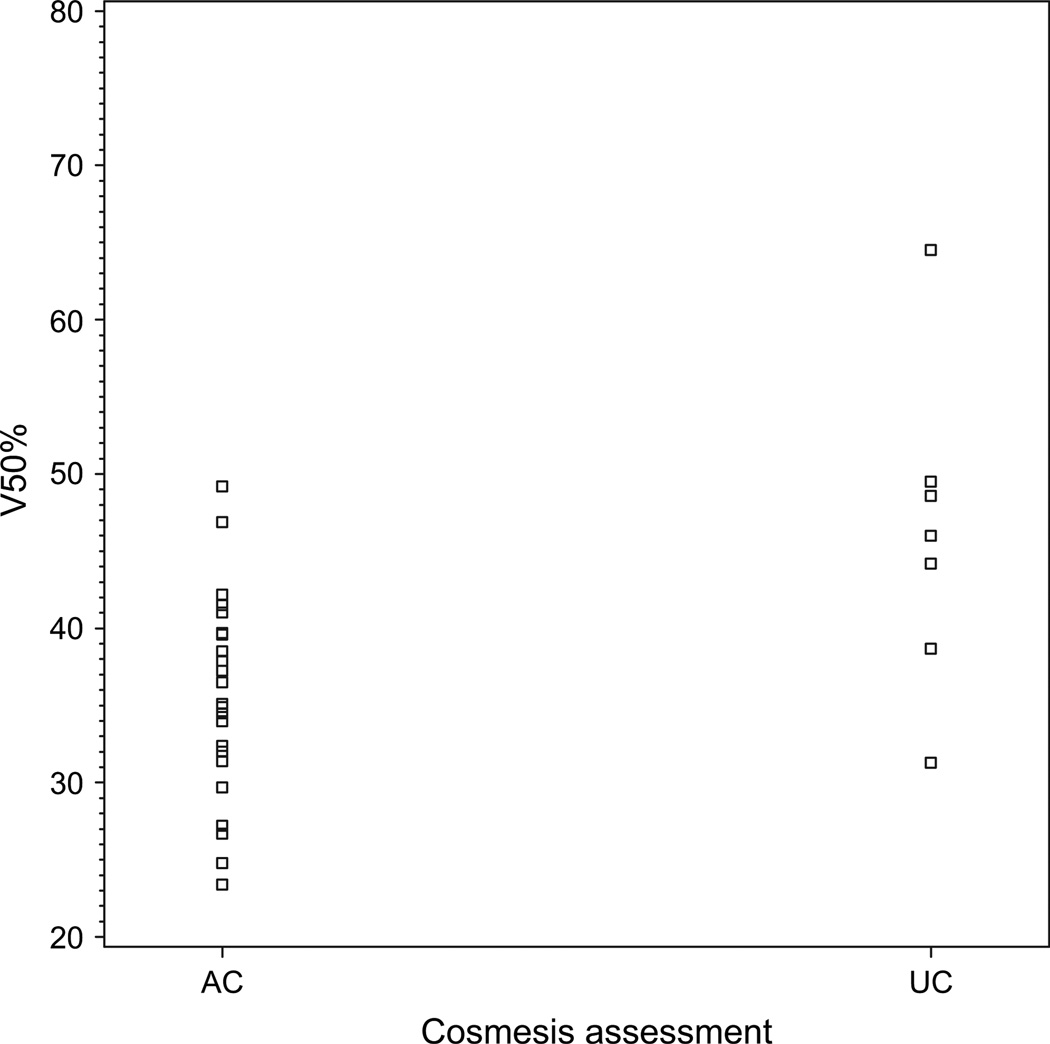
Finally, although small numbers precluded more definitive assessment of dosimetric cutpoints using multiple comparison corrections, we did conduct an exploratory analysis to determine whether there might be a threshold value for V50 above which unacceptable cosmesis was substantially likely. As the scatterplot in Fig. 4 depicts, there seems to be a possible threshold at 40%, with 5 of 10 patients (50%) with V50 exceeding 40% experiencing unacceptable cosmesis and only 2 of 22 (9%) below that threshold experiencing it (p = 0.02).
Fig. 4.
Distribution of the proportion of the breast reference volume in each case receiving 50% of prescribed dose (V50), by cosmetic outcome, among patients with good or excellent cosmesis at baseline.
DISCUSSION
As growing numbers of radiation treatment facilities begin to offer APBI to patients, and increasing media attention and advertising lead many patients to inquire about this form of treatment, it becomes particularly important to ensure that the radiation oncology community share its experiences with different treatment techniques, to identify the areas in which our understanding of the consequences of this relatively new form of treatment still needs to advance. In our institutional experience with APBI, using an external beam technique of beamlet IMRT with active breathing control, more than 20% of treated patients developed new unacceptable cosmesis after their radiotherapy. This was an unexpected outcome and one that contrasts sharply with the cosmetic outcomes observed when treating with conventional whole-breast irradiation at this institution (25). This finding suggests that the optimal dose, fractionation, and dosimetric parameters should be reconsidered when such highly conformal techniques are used.
Only a few others to date have reported the clinical outcomes of APBI using external beam techniques, and the limited existing evidence has involved regimens of varying dose and fractionation. In an early study, Christie Hospital’s Holt Radium Institute randomly assigned 708 patients with breast cancers of ≤4 cm in diameter to limited-field vs. wide-field radiotherapy (26). The limited-field radiation was administered using electrons of energies between 8 and 14 MeV (prescribed to 100%), to a total dose of 40–42.5 Gy in 8 fractions over 10 days, with an average field size of 8 × 6 cm. Wide-field radiation was administered with 4-MV photons to a dose of 40 Gy in 15 fractions over 21 days, using standard tangent fields matched to a single field treating the ipsilateral axilla, supraclavicular, and infraclavicular regions. After a median of 65 months of follow-up (27), overall survival was similar between the two groups. The 7-year actuarial rate of breast recurrence as a first event was 19.6% in the limited-field group, compared with 11.0% in the wide-field group (p = 0.0008), with a smaller difference when patients with lobular histology were excluded. A larger percentage of patients treated with limited vs. wide fields had marked telangiectasias (33% vs. 12%) or marked fibrosis (14% vs. 5%).
Subsequent single-institutional studies have generally used stricter selection criteria, more cautious dosing schedules, and more advanced planning techniques. Formenti et al. at the University of Southern California and New York University (NYU) have developed a technique of three-dimensional conformal external beam APBI in the prone position (28, 29). The NYU group has administered 30 Gy to the tumor bed plus a 1.5–2-cm margin in 5 fractions within 10 days, noting that a biologically effective dose calculation for tumor effect with an α/β ratio of 4 suggests equivalence of this dose to a regimen of 50 Gy in 25 fractions, whereas a calculation for fibrosis using an α/β of 2 suggests equivalence to a dose of 60 Gy in 30 fractions (30). Their predominant technique involves a pair of parallel-opposed mini-tangents. In their early experience with the first 47 patients, the mean V100 of the ipsilateral breast was 26% (range, 10–45%), and the mean V50 was 47% (range, 23–75%), strikingly similar to the results in our patients who maintained consistently acceptable cosmesis. The mean surgical cavity size was 52 cm3 (range, 7–379 cm3). With a median of 18 months of follow-up, they report no local recurrences and limited acute toxicity, mainly Grade 1–2 erythema, with 21 late toxicities occurring in 14 patients (all Grade 1). Cosmetic results were rated as fair in 2 patients at 12 and 18 months of follow-up.
Investigators at the William Beaumont Hospital have described a technique of three-dimensional conformal external beam APBI performed in the supine position and their clinical experience with this form of treatment (19). In contrast to the parallel-opposed mini-tangents generally used by the NYU group, the Beaumont group has used four to five noncoplanar photon beams. The dose prescribed was 34 Gy to the tumor bed plus expansion in the first 6 patients and 38.5 Gy in the remainder, all in 10 fractions. The investigators note that the latter fractionation yields a radiobiologically equivalent tumor dose of approximately 45 Gy in 25 fractions, assuming an α/β ratio of 10. In an initial report considering the experience of the first 31 patients, with a median follow-up of only 10 months, acute toxicities were minimal (31). The mean V100 of the breast was 23% (range, 14–39%), and the mean V50 was 47% (range, 34–60%), similar to the figures reported by NYU and to those derived from the treatment plans of patients with consistently acceptable cosmesis in our series. In a recent update, among 21 patients with a cosmetic assessment with a minimum follow-up of 24 months, 91% received a rating of excellent or good (32).
In recent years, other investigators have begun considering even more sophisticated external beam modalities. At the Massachusetts General Hospital in Boston, proton therapy is being investigated as a highly conformal means of treatment (33, 34). Other groups have begun investigating IMRT in the administration of partial-breast irradiation (35–38). We therefore find it particularly important to report the unexpected experience with cosmetic outcomes in our institution’s study of IMRT with active breathing control.
In this study, unacceptable cosmesis was so frequent that per stopping rules, the study was closed prematurely. The adverse cosmetic outcomes we observed may have been due to overly aggressive surgical resection, radiation dose and fractionation, breath-hold techniques, or an interplay between the these factors. We find it unlikely that surgical technique alone was responsible, however. Margin requirements for this protocol were the same as those historically required at our institution for patients with DCIS, and patients in this study were treated by the same complement of surgeons as patients in our previously reported series, in which 94% of patients had good or excellent cosmetic outcomes after breast-conserving surgery and standard whole-breast radiation (25). Thus, we believe that caution is warranted when considering the administration of APBI using the dosimetric parameters and highly conformal radiation techniques used in our study.
Of note, the dose and fractionation schedule used in this study are identical to those prescribed by the ongoing national RTOG 0413/NSABP B-39 randomized trial. The treatment plans in our study also generally conformed to the dosimetric requirements specified by the national study. The national study only allows three-dimensional conformal techniques if the treating physician wishes to use an external beam approach of partial-breast treatment. However, with the rapid accrual and recent closure of the study to low-risk patients (those older than 50 years with hormone receptor–positive, node-negative tumors, or DCIS) (39), practitioners wishing to offer APBI as an option to low-risk patients while awaiting maturation of the trial results may now increasingly begin to do so off protocol and may consider using more highly conformal techniques, such as the IMRT technique used in our study. We caution the community that our findings suggest that the hypofractionated schedule and parameters commonly used for external beam APBI and prescribed by the national study may be suboptimal, at least when highly conformal techniques such as IMRT with management of breathing motion are used. The V50 and V100 of the BRV were correlated with cosmetic outcome, and stricter limits may be appropriate in this setting. Although differences in the volume of the contoured breast and normal breast were not statistically significant between the groups of patients with unacceptable cosmesis and those with consistently acceptable cosmesis, the small numbers in this series may have made it impossible to detect a true difference. We continue to believe that the contoured breast and normal breast are important metrics to consider when determining the acceptability of partial-breast treatment plans. Particular caution may be warranted in patients with central or medial tumors. Future studies to develop panels of genetic markers that identify individuals at high risk for radiation sensitivity may also be useful in guiding appropriate patient selection and dosing (40, 41). The exploratory analysis conducted to identify a potential dosimetric threshold in this study, although by no means definitive, suggests that limiting the proportion of the BRV receiving 50% of prescribed dose to <40% may be worthy of further study.
Clearly, APBI may prove to be an exciting alternative to whole-breast radiotherapy and in appropriately selected patients may be equally efficacious. However, our experience highlights the importance of investigating this new approach in the context of carefully monitored studies that can identify unexpected outcomes, such as the compromised cosmesis observed in this study. As this study demonstrates, the optimal dose, fractionation, and dosimetric parameters for highly conformal methods of external beam APBI—not only in regard to efficacy but also tolerability—have yet to be determined.
Acknowledgments
Supported in part by Grant No. P01 CA598 27 from the National Institute of Health.
Footnotes
Presented in part at the Annual Meeting of the American Society for Therapeutic Radiology and Oncology, September 21–25, 2008, Boston, MA.
Conflict of interest: none.
REFERENCES
- 1.Clarke M, Collins R, Darby S. The Early Breast Cancer Trialists Collaborative Group. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 2.Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94:1143–1150. doi: 10.1093/jnci/94.15.1143. [DOI] [PubMed] [Google Scholar]
- 3.The START Trialists Group. The UK Standardization of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol. doi: 10.1016/S1470-2045(08)70077-9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark RM, McCulloch PB, Levine MN, et al. Randomized clinical trial to assess the effectiveness of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer. J Natl Cancer Inst. 1992;84:683–689. doi: 10.1093/jnci/84.9.683. [DOI] [PubMed] [Google Scholar]
- 5.Vicini FA, Kestin LL, Goldstein NS. Defining the clinical target volume for patients with early-stage breast cancer treated with lumpectomy and accelerated partial breast irradiation: A pathologic analysis. Int J Radiat Oncol Biol Phys. 2004;60:722–730. doi: 10.1016/j.ijrobp.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Vicini FA, Kestin L, Chen P, et al. Limited field radiation therapy in the management of early-stage breast cancer. J Natl Cancer Inst. 2003;95:1205–1211. doi: 10.1093/jnci/djg023. [DOI] [PubMed] [Google Scholar]
- 7.Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: Longterm results of a randomized trial. Ann Oncol. 2001;12:997–1003. doi: 10.1023/a:1011136326943. [DOI] [PubMed] [Google Scholar]
- 8.Holland R, Veling S, Mravunac M, et al. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast conserving therapy. Cancer. 1985;56:979–990. doi: 10.1002/1097-0142(19850901)56:5<979::aid-cncr2820560502>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Vaidya JS, Vyas JJ, Chinoy RF, et al. Multicentricity of breast cancer: Whole organ analysis and clinical implications. Br J Cancer. 1996;74:820–824. doi: 10.1038/bjc.1996.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morimoto T, Okazaki K, Komaki K, et al. Cancerous residue in breast-conserving surgery. J Surg Oncol. 1993;52:71–76. doi: 10.1002/jso.2930520203. [DOI] [PubMed] [Google Scholar]
- 11.Radiation Therapy Oncology Group. RTOG 0413/NSABP B-39 Study Protocol. [Accessed May 8, 2008]; Available at: http://www.rtog.org/members/protocols/0413/0413.pdf. [Google Scholar]
- 12.Benitez PR, Chen PY, Vicini FA, et al. Partial breast irradiation in breast-conserving therapy by way of interstitial brachytherapy. Am J Surg. 2004;188:355–364. doi: 10.1016/j.amjsurg.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Kuske RR, Bolton JS. Radiation Therapy Oncology Group publication no. 1055. Philadelphia: RTOG; 1995. A phase I/II trial to evaluate brachytherapy as the sole method of radiation therapy for stage I and II breast carcinoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perera F, Chisela F, Engle J, et al. Method of localization and implantation of the lumpectomy site for high dose rate brachytherapy after conservative surgery for T1 and T2 breast cancer. Int J Radiat Oncol Biol Phys. 1995;31:959–965. doi: 10.1016/0360-3016(94)00576-1. [DOI] [PubMed] [Google Scholar]
- 15.Polgar C, Sulyok Z, Fodor J, et al. Sole brachytherapy of the tumor bed after conservative surgery for T1 breast cancer: Five-year results of a phase I-II study and initial findings of a randomized phase III trial. J Surg Oncol. 2002;80:121–128. doi: 10.1002/jso.10110. [DOI] [PubMed] [Google Scholar]
- 16.Formenti SC. External-beam partial breast irradiation. Semin Radiat Oncol. 2005;15:92–99. doi: 10.1016/j.semradonc.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Cuttino LW, Todor D, Pacyna L, et al. Three dimensional conformal external beam radiotherapy for accelerated partial breast irradiation: What is the correct prescription dose? Am J Clin Oncol. 2006;29:474–478. doi: 10.1097/01.coc.0000225409.99284.f2. [DOI] [PubMed] [Google Scholar]
- 18.Weed DW, Edmundson GK, Vicini FA, et al. Accelerated partial breast irradiation: A dosimetric comparison of three different techniques. Brachytherapy. 2005;4:121–129. doi: 10.1016/j.brachy.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Baglan KL, Sharpe MB, Jaffray D, et al. Accelerated partial breast irradiation using 3D conformal radiation therapy (3D-CRT) Int J Radiat Oncol Biol Phys. 2003;55:302–311. doi: 10.1016/s0360-3016(02)03811-7. [DOI] [PubMed] [Google Scholar]
- 20.Frazier RC, Vicini F, Sharpe MB. The impact of respiration on whole breast radiotherapy: A dosimetric analysis using active breathing control (Abstr.) Int J Radiat Oncol Biol Phys. 2000;48:200. doi: 10.1016/j.ijrobp.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Weed DW, Yan D, Martinez AA, et al. The validity of surgical clips as a radiographic surrogate for the lumpectomy cavity in image-guided accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2004;60:484–492. doi: 10.1016/j.ijrobp.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Kessler ML, McShan DL, Epelman MA, et al. Costlets: A generalized approach to cost functions for automated optimization of IMRT treatment plans. Optimization Engineering. 2005;6:421–448. [Google Scholar]
- 23.National Cancer Institute. Common toxicity criteria for adverse events. [Accessed May 8, 2008]; Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 24.Radiation Therapy Oncology Group. Form COS (06-28-2005) [Accessed May 15, 2008]; Available at: http://www.rtog.org/members/protocols/0413/0413.pdf. [Google Scholar]
- 25.Ben-David MA, Sturtz DE, Griffith KA, et al. Long-term results of conservative surgery and radiotherapy for ductal carcinoma in situ using lung density correction: The University of Michigan experience. Breast J. 2007;13:392–400. doi: 10.1111/j.1524-4741.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro GG, Dunn G, Swindell R, et al. Conservation of the breast using two different radiotherapy techniques: Interim report of a clinical trial. Clin Oncol. 1990;2:27–34. doi: 10.1016/s0936-6555(05)80215-8. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro GG, Magee B, Swindell R, et al. The Christie Hospital breast conservation trial: An update at 8 years from inception. Clin Oncol. 1993;5:278–283. doi: 10.1016/s0936-6555(05)80900-8. [DOI] [PubMed] [Google Scholar]
- 28.Formenti SC, Rosenstein B, Skinner KA, et al. T1 stage breast cancer: Adjuvant hypofractionated conformal radiation therapy to the tumor bed in selected postmenopausal breast cancer patients—pilot feasibility study. Radiology. 2002;222:171–178. doi: 10.1148/radiol.2221010769. [DOI] [PubMed] [Google Scholar]
- 29.Truong M, Hirsch A, Formenti S. Novel approaches to post operative radiation therapy as part of breast conserving therapy for early stage breast cancer. Clin Breast Cancer. 2003;4:253–263. doi: 10.3816/cbc.2003.n.030. [DOI] [PubMed] [Google Scholar]
- 30.Formenti SC, Truong MT, Goldberg JD, et al. Prone accelerated partial breast radiation after breast conserving surgery: preliminary clinical results and dose volume histogram analysis. Int J Radiat Oncol Biol Phys. 2004;60:493–504. doi: 10.1016/j.ijrobp.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 31.Vicini FA, Remouchamps V, Wallace M, et al. Ongoing clinical experience utilizing 3D conformal external beam radiotherapy to deliver partial-breast irradiation in patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2003;57:1247–1253. doi: 10.1016/s0360-3016(03)01573-6. [DOI] [PubMed] [Google Scholar]
- 32.Vicini FA, Chen P, Wallace M, et al. Interim cosmetic results and toxicity using 3D conformal external beam radiotherapy to deliver accelerated partial breast irradiation in patients with early-stage breast cancer treated with breast-conserving surgery. Int J Radiat Oncol Biol Phys. 2007;69:1124–1130. doi: 10.1016/j.ijrobp.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 33.Kozak KR, Katz A, Adams J, et al. Dosimetric comparison of proton and photon three-dimensional, conformal, external beam accelerated partial breast irradiation techniques. Int J Radiat Oncol Biol Phys. 2006;65:1572–1578. doi: 10.1016/j.ijrobp.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 34.Kozak KR, Smith BL, Adams J, et al. Accelerated partial breast irradiation using proton beams: Initial clinical experience. Int J Radiat Oncol Biol Phys. 2006;66:691–698. doi: 10.1016/j.ijrobp.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 35.Lacombe MA, McMahon J, Al-Najjar W, et al. Accelerated partial breast irradiation using intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60(1) Suppl. 1:S237–S244. [Google Scholar]
- 36.Rusthoven KE, Carter DL, Howell K, et al. Accelerated partialbreast intensity-modulated radiotherapy results in improved dose distribution when compared with three-dimensional treatment- planning techniques. Int J Radiat Oncol Biol Phys. 2008;70:296–302. doi: 10.1016/j.ijrobp.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 37.Livi L, Paiar F, Buonamici FB, et al. Accelerated intensity-modulated external radiotherapy as a new technical approach to treat the index quadrant after conserving surgery in early breast cancer: A preliminary study. Tumori. 2005;91:227–232. doi: 10.1177/030089160509100303. [DOI] [PubMed] [Google Scholar]
- 38.Oliver M, Chen J, Wong E, et al. A treatment planning study comparing whole breast radiation therapy against conformal, IMRT and tomotherapy for accelerated partial breast irradiation. Radiother Oncol. 2007;82:317–323. doi: 10.1016/j.radonc.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 39.National Surgical Adjuvant Breast and Bowel Project. [Accessed February 6, 2007];Memorandum to NSABP principal investigators and program coordinators from Judy Langer, Regulatory Specialist, NSABP Operations Center. 2006 Dec 21; Re: NSABP B-39/RTOG 0413 impending closure of accrual to specific populations (second notice). Available at: http://www.rtog.org/members/protocols/0413/B-39%20L-R%20Group%20Closure%20Notice%20Reminder_12-21-06.pdf. [Google Scholar]
- 40.Li C, Wilson P, Levine E. TGF-beta levels in pre-treatment plasma identify breast cancer patients at risk of developing post-radiotherapy fibrosis. Int J Cancer. 1999;84:155–159. doi: 10.1002/(sici)1097-0215(19990420)84:2<155::aid-ijc11>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 41.Quarmby S, Fakhoury H, Levine E, et al. Association of transforming growth factor beta-1 single nucleotide polymorphisms with radiation-induced damage to normal tissues in breast cancer patients. Int J Radiat Biol. 2003;79:137–143. [PubMed] [Google Scholar]