This study demonstrates that small molecule STF-31 effectively eliminates undifferentiated human pluripotent stem cells (hPSCs) across a broad range of cell culture conditions with important advantages over previously described methods that target metabolic processes. The results demonstrate the importance of an NAD+ salvage pathway in hPSC biology and describe how inhibition of nicotinamide phosphoribosyltransferase can eliminate hPSCs from culture, advancing development of safe, clinically relevant hPSC-derived cell-based therapies.
Keywords: Selective toxicity, NAD, Metabolism, Salvage pathway, Human pluripotent stem cells
Abstract
The tumorigenic potential of human pluripotent stem cells (hPSCs) is a major limitation to the widespread use of hPSC derivatives in the clinic. Here, we demonstrate that the small molecule STF-31 is effective at eliminating undifferentiated hPSCs across a broad range of cell culture conditions with important advantages over previously described methods that target metabolic processes. Although STF-31 was originally described as an inhibitor of glucose transporter 1, these data support the reclassification of STF-31 as a specific NAD+ salvage pathway inhibitor through the inhibition of nicotinamide phosphoribosyltransferase (NAMPT). These findings demonstrate the importance of an NAD+ salvage pathway in hPSC biology and describe how inhibition of NAMPT can effectively eliminate hPSCs from culture. These results will advance and accelerate the development of safe, clinically relevant hPSC-derived cell-based therapies.
Significance
The tumorigenic potential of human pluripotent stem cells (hPSCs) is a major limitation to the widespread use of hPSC derivatives in the clinic. This study provides detailed analyses of cellular metabolic flux to define an efficient strategy for selective hPSC elimination that is effective across many culture conditions and does not have cytotoxic effects on hPSC-derived progeny. Of broad significance to the stem cell and regenerative medicine fields, this study also highlights the importance of examining the effect of in vitro culturing parameters when evaluating the efficacy of hPSC-elimination strategies, especially those that target metabolic processes.
Introduction
Human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs), collectively termed human pluripotent stem cells (hPSCs), can differentiate into almost any human cell type. Although significant progress has been made in developing effective strategies for the differentiation of hPSCs to progeny useful for drug toxicity testing and human disease modeling [1–4], continued safety issues preclude their broad use for human therapeutics. Cell heterogeneity, purity, and mode of transplantation are technically problematic, but the potential formation of teratomas at the site of transplantation represents a significant obstacle for clinical applications. Teratoma formation has been reported in animal models following transplantation of low numbers of mouse and human pluripotent stem cells [5, 6] and after injection of hPSC-derived cells [7–10]. Preclinical testing of hESC-derived neural progenitor cells in the Geron trial also resulted in cyst formation in the spines of mice, suggesting additional limitations for transplantation of hESC products in human subjects [11]. Therefore, the elimination of potentially tumorigenic pluripotent stem cells prior to transplantation is required before hPSC-based therapies can become widely available.
Three major strategies have been used to eliminate the tumorigenic potential of hPSC-derived cells for therapy: the use of suicide genes, cell selection or sorting techniques, and metabolic approaches. Although promising, each approach has limitations. Genomic modifications are required for suicide genes targeting hPSCs and for reporter genes for cell type-specific selection [12, 13]. Cell sorting strategies using markers to positively or negatively select hPSCs often fail to yield pure populations, particularly when the markers are not cell type restricted. Moreover, cell sorting is not always amenable to tissue transplantation because the sample must be dissociated to the single cell level [14, 15]. The use of metabolite-deficient medium can be effective but is not broadly applicable. For example, medium depleted of glucose and supplemented with lactate is relevant only to cardiomyocyte cultures [16]. Finally, small molecules that inhibit oleate synthesis (PluriSIn #1; hereafter referred to as PluriSIn) [17, 18] or glucose transporters (WZB117) [19] have been recently reported to selectively eliminate hPSCs, but it remains unclear whether these methods are toxic under diverse culture conditions or exhibit adverse effects on the functionality of hPSC progeny.
Here, we have extended our previous analysis of glucose transporter 1 (GLUT1) inhibitors for selective toxicity of hPSCs [19]. We report that WZB117 eliminates hPSCs through inhibition of GLUT1 but that the putative GLUT1 inhibitor, STF-31, does not function in this manner. STF-31 removes hPSCs through inhibition of the NAD+ salvage pathway. Based on these findings, we developed a novel strategy for using STF-31 to effectively eliminate hPSCs across a range of culture conditions by a process that spares differentiated progeny. These results provide an important advancement toward the development of clinically safe hPSC-derived progeny for human stem cell-based therapies.
Materials and Methods
Cell Culture and Reagents
hiPSCs (DF6-9-9T and KB3 [19, 20]) and hESCs (H1, H7, and H9 [21]) were cultivated in monolayer culture on Matrigel (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) in E8 or mTeSR medium as previously described [19]. For all experiments, hPSCs were plated at 1.5 × 105 total cells per 2 cm2, unless otherwise specified, as plating an explicit number of cells during routine passaging enhances reproducibility among experiments and maintains high quality monolayer cultures of pluripotent cells. Treatment with small molecule inhibitors was initiated between 24 and 96 hours postplating. These time points represent the times at which cells are typically 15%–20% and 100% confluent (Fig. 1E), respectively. hiPSC-derived cardiomyocytes were differentiated and maintained as previously described [22]. hESC-derived neuronal progenitors and an epithelial cell line were cultured as described in the supplemental online data. For hESC colonies that were grown on a feeder cell layer, H1 colonies were cultured on mitotically inactivated human fibroblast feeders in E8 medium, and colonies were passaged with collagenase IV for 40 minutes at 37°C [23]. Human fibroblasts were cultured as previously described [19]. Small molecules STF-31 (4-[[[[4-(1,1-dimethylethyl)phenyl]sulfonyl]amino]methyl]-N-3-pyridinylbenzamide; Tocris Bioscience, Bristol, U.K., http://www.tocris.com), WZB117 (EMD Millipore, Billerica, MA, http://www.millipore.com), PluriSIn (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), nicotinic acid (Sigma-Aldrich), and nicotinamide (Sigma-Aldrich) were used to treat cells. For glucose deprivation, E8 medium was prepared as described [19] with the modification that Dulbecco’s modified Eagle’s medium (DMEM) F-12 without glucose (United States Biological, Swampscott, MA, http://www.usbio.net) was used. For glucose deprivation control medium, 17.5 mM glucose (Sigma-Aldrich) was added into glucose-free E8 medium.
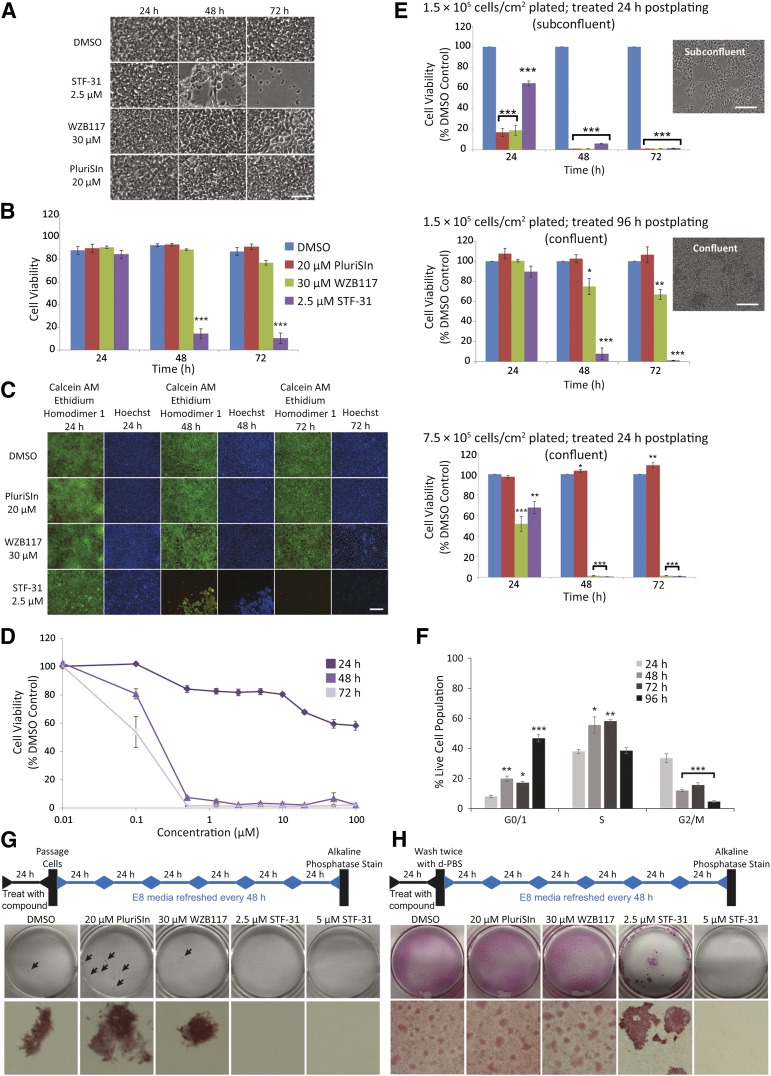
Figure 1.
Human pluripotent stem cells treated with small molecules. (A): Representative brightfield images of confluent human induced pluripotent stem cells (hiPSCs) (DF6-9-9T) after treatment with 2.5 μM STF-31, 30 μM WZB117, and 20 μM PluriSIn for 24–72 hours. Scale bar = 50 μm. (B): Cell viability as measured by SYTOX Green assay in hiPSCs treated with 20 μM PluriSIn, 30 μM WZB117, and 2.5 μM STF-31 (n = 3). (C): Representative images of live (calcein AM, green) or dead staining (ethidium homodimer 1, red) in hiPSCs after treatment with 20 μM PluriSIn, 30 μM WZB117, and 2.5 μM STF-31. Scale bar = 100 μm. (D): Cell viability with STF-31 titration for 24–72 hours in confluent hiPSCs determined by neutral red assay (n = 3). (E): Cell viability of varying densities of hiPSCs after treatment with 20 μM PluriSIn, 30 μM WZB117, and 2.5 μM STF-31 determined by neutral red assay (n = 3). Representative images of cell morphology and density at 24 and 96 hours postplating are coupled with respective bar graphs. Scale bars = 200 μm. (F): Percentage of live hiPSCs in each phase of the cell cycle at 24–96 hours postplating (n = 3). (G, H): Schematic and results of colony forming assay in which cells were passaged (G) or washed (H) prior to continued culture for 6 days prior to alkaline phosphatase staining. Arrows indicate individual colonies, which were imaged on Nikon Stereoscope SMZ1500 with ×1 objective. Bar graphs representing average number of colonies per plate for each treatment scheme are shown in supplemental online Figure 4A and 4B. The data are represented as means ± SEM. ∗, p ≤ .05; ∗∗, p ≤ .01; ∗∗∗, p ≤ .001 compared with DMSO control. See also supplemental online Figures 1, 2, and 4. Abbreviations: AM, acetomethoxy; DMSO, dimethyl sulfoxide; d-PBS, Dulbecco’s phosphate-buffered saline; h, hours.
In Vitro Toxicity Assays
Treatment with small molecules or glucose deprivation was initiated in hESCs or hiPSCs at 24 and 96 hours postplating, and in vitro toxicity assays were performed at specified treatment endpoints 24–96 hours after treatment initiation. For pulsed treatment, hiPSCs were treated with STF-31 for 24 hours, washed twice with Dulbecco’s phosphate-buffered saline (d-PBS), and cultured in medium for an additional 48 hours. Neutral red assays for cell viability were performed as previously described [24]. In vitro cell death was determined using SYTOX Green nucleic acid stain (Life Technologies, Rockville, MD, http://www.lifetech.com). Briefly, cells were incubated in 5 μM SYTOX Green for 30 minutes at 37°C in a humidified cell incubator with 5% CO2. The percentage of cell viability was determined by normalizing to replicate cells incubated with 120 μM digitonin (Sigma-Aldrich) and 5 μM SYTOX Green. Imaging of cell viability was performed using a Live/Dead Viability/Cytotoxicity Kit for mammalian cells (Life Technologies). The cells were stained for 20 minutes at room temperature with 4 μM calcein acetomethoxy (AM) to detect viable cells and 2 μM ethidium homodimer 1 to detect cells with compromised membranes. Representative images of alterations in cell morphology were acquired on confluent hPSCs 24–72 hours after treatment initiation. Imaging was performed with a Nikon (Tokyo, Japan, http://www.nikon.com) Ti-U inverted microscope.
BrdU Incorporation and Flow Cytometry
Cells were plated at 7.5 × 105 cells per 9.6 cm2, and 10 μM 5-bromo-2-deoxyuridine (BrdU) was incorporated in hiPSCs 24–96 hours postplating for 1 hour at 37°C in a humidified cell incubator with 5% CO2. After incorporation, cells were collected and stained using the fluorescein isothiocyanate BrdU Flow Kit per the manufacturer’s guidelines (BD Biosciences). Cell viability was determined using Fixable Viability Dye eFluor 450 (eBiosciences, San Diego, CA, http://www.ebioscience.com). Analyses were performed on 30,000 events acquired on a BD LSRII flow cytometer (BD Biosciences), using FCSExpress V3 (DeNovo Software, Glendale, CA, http://www.denovosoftware.com). The percentage of cells in each phase of the cell cycle was determined by gating on populations within each phase in the live cell population.
Colony Formation Assay
The colony formation assay was performed in three different variations on confluent hiPSCs that were treated with 2.5 and 5 μM STF-31, 30 μM WZB117, and 20 μM PluriSIn for 24 hours. The first iteration was adapted from a previously described method [25], after 24 hours of treatment, hiPSCs were detached with Accutase (StemCell Technologies, Vancouver, BC, Canada, http://www.stemcell.com), resuspended in E8 medium, passed through a 12 × 75-mm tube with cell strainer cap (Falcon), and plated at 5 × 104 live cells per 2 cm2. The medium was changed every 48 hours, and cells were cultured for 6 days, at which time staining for alkaline phosphatase was performed with leukocyte alkaline phosphatase kit (Sigma-Aldrich) as described [26], and plates were visually inspected for phosphatase-positive colonies. This strategy represents the most common implementation of the colony formation assay. However, concerns regarding the cell death normally encountered during passaging prompted another approach for stricter assessment. In the second iteration, cells were treated for 24 hours and washed twice with 0.5 ml of d-PBS, and the medium was refreshed with E8. Thereafter, the media were replaced daily for 6 days, at which point staining for alkaline phosphatase activity was performed. In this version, cells do not undergo the stress of passaging, which may result in erroneously low viability that is not a direct result of the compound; thus, this modified assay is considered a more stringent assessment of hPSC elimination than the former. Elimination of hiPSCs from cocultures with differentiated cells was performed with human fibroblasts and day 10 hiPSC-derived cardiomyocytes. hiPSCs (DF6-9-9T) were plated at concentrations ranging from 100 to 10,000 live cells per well with human fibroblasts (1.75 × 105 cells) or day 10 hiPSC-derived cardiomyocytes (3.75 × 105 cells) in E8 and Y-27632 per 9.6 cm2. After 24 hours, treatment was performed with 5 μM STF-31 for 24 or 48 hours, cells were washed twice, and medium was refreshed daily with E8. Six days after plating, staining for alkaline phosphatase activity was performed. For elimination of hESC colonies grown on mitotically inactivated human fibroblast feeders, colonies were treated for 96 hours with 2.5 μM STF-31, at which point cells were imaged with brightfield microscopy and stained for alkaline phosphatase activity or colonies were passaged with collagenase IV (2 mg/ml for 40 minutes at 37°C), cultured for an additional 72 hours, and stained for alkaline phosphatase activity. Imaging of wells was performed with a Cybershot DSC-TX30 camera (Sony, Tokyo, Japan, http://www.sony.com) and individual colonies with a Nikon stereoscope.
Characterization of Cardiomyocytes
For all experiments, a 24-hour pulse treatment with 5 μM STF-31 was performed on day 10 hiPSC-derived cardiomyocytes (DF6-9-9T). Flow cytometry was performed 72 hours after initiation of STF-31 treatment for TNNI3 (troponin I type 3) and IRX4 (Iroquois class homeodomain protein) as previously described [22]. Quantitative real-time polymerase chain reaction (qPCR) for TNNI3, TNNT2 (troponin T2), and NKX2-5 (homeobox protein Nkx-2.5) was performed using TaqMan assays (Life Technologies) 24–72 hours after initiation of STF-31 treatment as previously described [19, 22]. For immunofluorescent detection of TNNT2, cardiomyocytes were passaged onto coverslips 72 hours after initiation of STF-31 treatment and cultured for an additional 48 hours. Cells were then fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100 incubated with primary antibody for TNNT2 (catalog no. ab8295; Abcam, Cambridge, U.K., http://www.abcam.com) overnight at 4°C. The cells were then incubated in secondary antibody goat anti-mouse IgG1-Alexa 568 (catalog no. A-21124; Life Technologies), and nuclei were detected with Hoechst 33342 (catalog no. H21492; Life Technologies). Cells were imaged with a Nikon Ti-U inverted microscope and Nikon Eclipse 90i confocal microscope.
Extracellular Flux Analysis
hiPSCs were plated at 6 × 104 cells per well on a specialized microplate (Seahorse Bioscience, North Billerica, MA; http://www.seahorsebio.com) as previously described [27] with several exceptions. Extracellular flux analysis was performed on hiPSCs 48 hours postplating using Seahorse Bioscience XF24 Analyzer. Cells were treated with 2.5 μM STF-31, 30 μM WZB117, 20 mM 2-deoxyglucose (2-DG; Sigma-Aldrich), vehicle control, or control medium for 5.5 hours, washed twice with 750 μl of assay medium, and placed in 750 μl of assay medium containing the appropriate treatment. The microplate was equilibrated in non-CO2 incubator for 1.5 hours and analyzed for 16 hours in Seahorse Bioscience XF24 Analyzer. Assay medium consists of basal E8 reagents with the following exceptions: basal DMEM F-12 without phenol red (Gibco, Life Technologies), no sodium bicarbonate, 2.5 mM GlutaMAX (Gibco), and 15 mM Hepes (Gibco). Assay medium was adjusted to pH 7.4 at 37°C prior to use. The extracellular acidification rate (ECAR) and basal oxygen consumption rate (OCR) were collected approximately every 60 minutes. Treatments were normalized to baseline control medium value and are represented as averages of three biological replicates.
Glucose Uptake Assay
Subconfluent hiPSCs (24 hours postplating) were treated with 2.5 μM STF-31, 30 μM WZB117, 20 μM cytochalasin B (Sigma-Aldrich), and vehicle control for 1, 15, 18, and 24 hours. For 1 hour of treatment, cells were placed in Krebs-Ringer Hepes (KRH) buffer containing treatment at 37°C in a humidified cell incubator with 5% CO2. For 15, 18, and 24 hours treatment, cells were treated in E8 medium for 14, 17, and 23 hours, respectively, at which point the medium was changed to KRH buffer containing specific treatment for 1 hour. After specified treatment time, glucose uptake was performed with 0.5 μCi of [3H]2-deoxyglucose for 5 minutes at 37°C as previously described [28]. Uptake data were normalized to protein concentration measured with Lowry method.
Immunoblot Analysis
Nonadherent cells were collected by centrifugation; adherent cells were washed once with d-PBS and lysed in Laemmli buffer, combined with nonadherent cell pellet, and heated at 95°C for 5 minutes. Protein concentration was measured using the Qubit protein assay (Life Technologies). Twenty-five micrograms of total protein was separated by SDS-polymerase chain reaction, transferred to nitrocellulose membrane (GE Healthcare Life Sciences, Little Chalfont, U.K., http://www.gehealthcare.com), and blocked according to the manufacturer’s instructions. Membranes were incubated overnight at 4°C with the following dilutions of primary antibodies: rabbit anti-cleaved caspase-3 (1:500), rabbit anti-caspase-9 (1:1,000), rabbit anti-phospho-AMPK (1:1,000), and rabbit anti-AMPK (1:1,000) from Cell Signaling Technology (Beverly, MA, http://www.cellsignal.com), and mouse anti-GAPDH (1:10,000) from Life Technologies. Membranes were then incubated with secondary antibodies for 45 minutes at the following concentrations: donkey anti-mouse horseradish peroxidase (1:5,000) and donkey anti-rabbit horseradish peroxidase (1:7,000) from Jackson Immunoresearch Laboratories (West Grove, PA, http://www.jacksonimmuno.com), followed by detection using enhanced chemiluminescence [29].
Cleaved Caspase-3/7 Fluorescence-Based Assay
Treatment with 2.5 μM STF-31, 30 μM WZB117, or glucose deprivation was initiated in subconfluent hiPSCs (24 hours postplating) for 6, 12, 18, and 24 hours, at which time cleaved caspase-3/7 activity was measured as previously described [30, 31] with the following exceptions: cells were cultured in 500 μl E8 medium, and 250 μl of 3× caspase buffer was added to the medium at endpoint.
Nucleotide Pool Measurements
ATP, ADP, AMP, and NAD+ were extracted using perchloric acid precipitation and analyzed using high performance liquid chromatography, following a previously published method [32]. ATP, ADP, AMP, and NAD+ peaks were measured for each sample, compared with the standards, and normalized to protein levels.
Statistical Analysis
All experiments were performed in a minimum of three biological replicates. The data are represented as means with standard error of the mean for N biological replicates. Statistical analysis was performed using one-way analysis of variance with Tukey post hoc test.
Results
STF-31 Toxicity Is Independent of Cell Density and Proliferation Rate
STF-31- and WZB117-mediated toxicity in hPSCs were directly compared with PluriSIn, a previously reported small molecule used for hPSC elimination. Treatment with STF-31 did not modify the cell morphology following 24 hours of incubation (Fig. 1A). However, patches of diminished, nonadherent cells and reduction in overall cell number were present following 48 hours of treatment (Fig. 1A), and only a limited number of cells remained after 72 hours. In contrast to STF-31, 72 hours of treatment with WZB117 were required to cause cell swelling, whereas there were minimal alterations in the morphology of PluriSIn-treated cells. These morphological changes are consistent with assays of cell viability. STF-31 reduced cell viability to 10% following 72 hours treatment as determined by the SYTOX Green nucleic acid stain (Fig. 1B). In contrast, there was no significant reduction in the viability of PluriSIn- or WZB117-treated cells. Because the SYTOX assay relies on the presence of dead cells to quantify viability, we also performed live/dead staining by calcein AM and ethidium homodimer 1 (Fig. 1C). Following 48 hours of STF-31 treatment, small patches of live cells remained that were not visible by 72 hours of treatment. In contrast, WZB117 and PluriSIn were not toxic to confluent hPSCs (Fig. 1C). Because STF-31 appeared to be the most robust small molecule for the elimination of hPSCs cultured under these conditions, we determined the median lethal dose (LD50) of STF-31 for hiPSCs to be 182 nM STF-31 48 hours after continuous treatment (Fig. 1D). Increasing STF-31 concentrations to 100 μM did not accelerate the time at which significant toxicity was observed by neutral red assay (Fig. 1D; supplemental online Fig. 1A).
Because we were unable to fully reproduce the previously reported toxic effects of PluriSIn on hPSCs, we tested alternative culture conditions. Subconfluent and confluent cells were generated either by plating cells at the same density and allowing them to grow for different lengths of time (24 vs. 96 hours) or by plating the cells at two different densities (1.5 × 105 vs. 7.5 × 105 cells per cm2) and allowing them to grow for 24 hours (Fig. 1E). For each cell density condition, cells were treated with STF-31, PluriSin, or WZB117 for 72 hours. In these comparisons, the time required to detect STF-31-mediated toxicity was similar for both confluent and subconfluent cells; with fewer than 2% viable cells remaining 72 hours after start of treatment. In contrast, toxicity of WZB117 and PluriSIn varied among the conditions (Fig. 1E). WZB117 was toxic to subconfluent cells and confluent cells that were treated 24 hours postplating but was significantly less toxic to confluent cells that were treated 96 hours postplating. PluriSIn was only effective on subconfluent cells and did not induce significant toxicity in confluent cells. Moreover, increasing concentrations of PluriSIn did not result in cell death in confluent cells (supplemental online Figure 2A, 2B). Importantly, cell death in response to each of the compounds was similar among three hESC (H1, H7, and H9) (supplemental online Figure 1B–1D; data not shown) and two hiPSC (KB3 and DF6-9-9T) lines (Fig. 1; data not shown), as well as between hPSCs cultured in either E8 or mTeSR medium compositions (supplemental online Fig. 2C, 2D). Additionally, STF-31 was toxic to hESC colonies grown on human fibroblast feeder cells with toxicity occurring between 48 and 72 hours. Following 96 hours of treatment, toxicity was evaluated with brightfield microscopy and staining for alkaline phosphatase activity. Colonies were not detected with alkaline phosphatase staining, although positive staining consisting of nonadherent cells and acellular debris was observed (supplemental online Fig. 1E, top). Brightfield microscopy confirmed an absence of colonies (supplemental online Fig. 1E, middle). To confirm elimination of hESCs, colonies were passaged 96 hours after treatment initiation and cultured for an additional 72 hours. No colony growth or alkaline phosphatase activity was detected in STF-31-treated cells (supplemental online Fig. 1E, bottom), indicating elimination of hESCs from feeder-based cultures.
Cell density is an important variable for many in vitro differentiation protocols used to generate tissue-specific progeny from hPSCs (e.g., neuronal cells, hepatocytes, and cardiomyocytes [19, 22, 33, 34]). Additionally, cell proliferation rates can also vary among common hPSC culture conditions and hPSC lines. To verify that STF-31 is toxic at various cell proliferation rates, hiPSCs were incubated with BrdU (10 μM) 24–96 hours postplating, and the cell cycle was examined (Fig. 1F; supplemental online Fig. 1F). At 24 hours postplating (low densities), 8% of cells are in the G0/G1 phase, whereas at 96 hours postplating (high densities) 47% of the cells were in the G0/G1 phase. Therefore, although all methods of elimination tested here were toxic to rapidly proliferating hPSCs, STF-31was the only agent toxic to hPSCs across a broad range of hPSC densities and rates of proliferation. Thus, STF-31-mediated toxicity may be advantageous in confluent culture systems in which hPSCs may have altered proliferation rates.
STF-31 Is Effective for hPSC Elimination
The effectiveness of STF-31 for the prevention of hPSC self-renewal was evaluated in vitro with a colony formation assays alongside WZB117 and PluriSIn. We determined that 24 hours was the minimal time required to achieve complete toxicity of hPSCs with STF-31 (data not shown). Using a 24-hour pulse treatment scheme, we found that STF-31 prevented the formation of alkaline phosphatase-positive colonies from confluent hiPSCs, whereas PluriSIn and WZB117 did not prevent colony growth (Fig. 1G). As a more stringent examination of the effectiveness of STF-31 for the removal of hiPSCs, a colony formation assay was performed without cell passaging to eliminate exaggerated cell death caused by stress during the dissociation and replating of cells (Fig. 1H). In this strategy, STF-31, WZB117, and PluriSIn were applied for 24 hours to confluent hiPSCs, and then the medium was replaced daily for 6 days. Similar levels of alkaline phosphatase-positive cells were observed in the DMSO-, WZB117-, and PluriSIn-treated hiPSCs. In contrast, a limited number of colonies formed in hiPSCs treated with 2.5 μM STF-31, and no colonies were observed in the hiPSCs treated with 5 μM STF-31. These findings demonstrate that for confluent hiPSCs, 24-hour treatment with STF-31 prevents reformation of alkaline phosphatase-positive colonies, whereas PluriSIn and WZB117 are ineffective against a confluent monolayer of hPSCs.
STF-31 Treatment Affords Selective Toxicity
To further evaluate the utility of STF-31, we examined the effects of a 24-hour pulse treatment of STF-31 on subconfluent and confluent hiPSCs, as well as differentiated cells. The LD50 of STF-31 was determined to be 1.35 and 1.78 μM when subconfluent or confluent cells were treated, respectively (Fig. 2A). Consistent with the data in Figure 1, 24-hour treatment with WZB117 and PluriSIn eliminated subconfluent hiPSCs, but not confluent hiPSCs (Fig. 2B). STF-31 had limited toxicity toward human fibroblasts, in which a decrease in viability was only observed at the highest concentrations and was limited to a reduction in viability by 30% at 100 μM. The LD50 of STF-31 on hiPSC-derived cardiomyocyte cultures was 40 μM, a 22-fold higher concentration than the LD50 for hiPSCs (Fig. 2A). Moreover, this 24-hour pulse treatment was not toxic to retinal pigmented epithelial cells (ARPE-19 line) (supplemental online Fig. 5A) or hESC-derived paired box protein Pax6 (PAX6)-positive neural progenitors (supplemental online Fig. 5B, 5D) 48 hours after treatment initiation. Morphology of hESC-derived PAX6-positive neural progenitors was not affected after 24 hours of treatment, as visualized by neuron-specific class III β-tubulin (TUJ1) and PAX6 staining (supplemental online Fig. 5D).We found that extending the pulse treatment to a 72-hour continuous treatment did not modify the cell morphology or viability of ARPE-19 cells or fibroblasts (supplemental online Fig. 5C; data not shown), which is consistent with our previous report [19]. However, hPSC-derived cardiomyocytes and PAX6-positive neural progenitor cells displayed a reduction in viability after 72 hours with continuous treatment (supplemental online Fig. 5E; data not shown). These data indicate that toxicity to hPSC derivatives at early stages of differentiation may be avoided with a shorter pulse treatment.
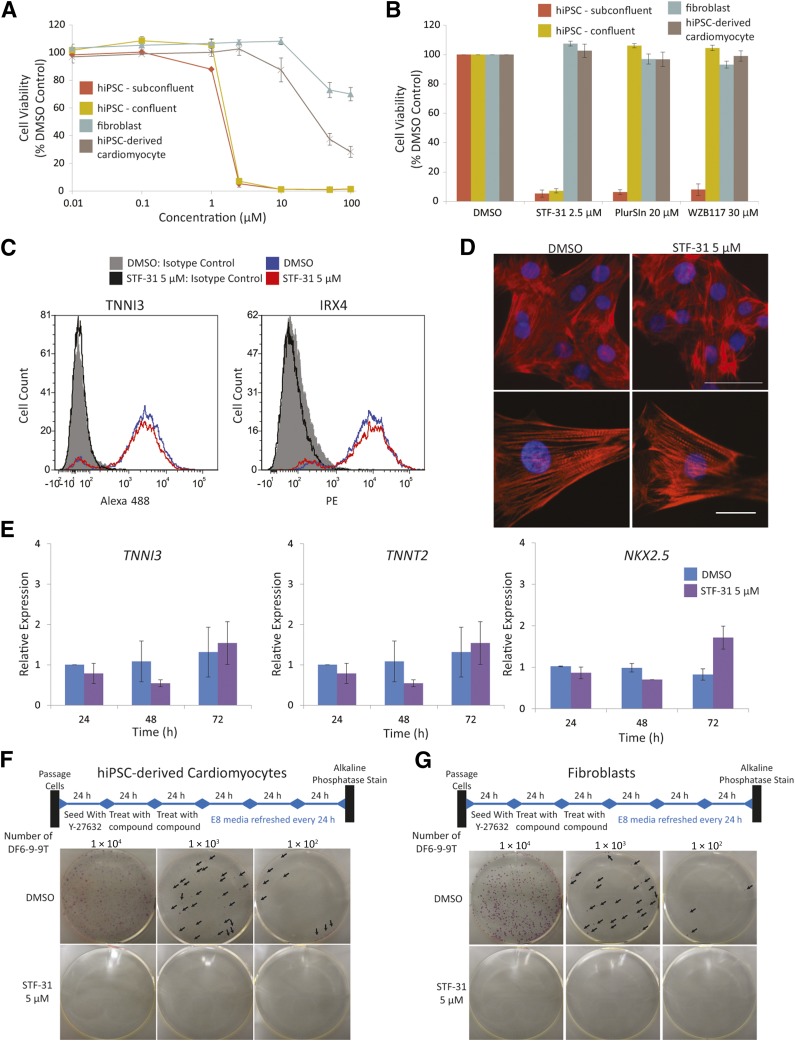
Figure 2.
Twenty-four-hour pulse treatment for selective in vitro elimination of hiPSCs. (A, B): Titration (A) and comparison of small molecules (B) with 24 hours of treatment with STF-31 (0.01–100 μM), 30 μM WZB117, and 20 μM PluriSIn in subconfluent and confluent hiPSCs (DF6-9-9T), fibroblasts, and DF6-9-9T hiPSC-derived cardiomyocytes. Average viability was measured with neutral red assay 72 hours after treatment initiation (n = 3). (C): Representative flow cytometry histograms of TNNI3 and IRX4 abundance in hiPSC-derived cardiomyoyctes 72 hours after initiation of a 24 hours treatment with 5 μM STF-31 (n = 3). (D): Representative images for immunofluorescent detection of TNNT2 organization in day 15 hiPSC-derived cardiomyocytes following a 24-hour pulse treatment with 5 μM STF-31 on day 10 of differentiation. Top: cell clusters imaged at ×40. Bottom: individual cells showing structural organization imaged at ×100 magnification. Scale bars in top panel = 50 μm, in bottom panel = 20 μm (n = 3). (E): Bar graphs for qPCR of TNNI3, TNNT2, and NKX2.5 24–72 hours after initiation of a pulse treatment with 5 μM STF-31 in day 10 hiPSC-derived cardiomyocytes. (F, G): Representative images of alkaline phosphatase staining to detect DF6-9-9T hiPSC colonies in cocultures with day 10 hiPSC-derived cardiomyocytes (F) and human fibroblasts (G). DF6-9-9T were plated between 1 × 102 and 1 × 104 live cells and treated for 48 hours with 5 μM STF-31 24 hours after plating (n = 3 for cardiomyocytes and n = 5 for human fibroblasts). Bar graphs representing the average numbers of colonies per well for treatment of DF6-9-9T coculture with fibroblasts are shown in supplemental online Figure 4. Abbreviations: DMSO, dimethyl sulfoxide; h, hours; hiPSC, human induced pluripotent stem cells.
The conditions established for hPSC elimination (5 μM STF-31 treatment for 24 hours) were tested for effects on day 10 hiPSC-derived cardiomyocyte function and markers. In this strategy, STF-31 treatment did not affect cardiomyocyte spontaneous contractility (supplemental online Videos 1, 2); abundance of cardiac proteins TNNI3 and IRX4 as measured by flow cytometry (Fig. 2C); the structural organization of TNNT2, which is found in cardiac sarcomeres, (Fig. 2D); or expression of cardiac genes as measured by qPCR (Fig. 2E).
To confirm selective toxicity in the presence of differentiated cells, colony formation assays were performed by titrating amounts of hiPSCs with either day 10 hiPSC-derived cardiomyocytes (Fig. 2F) or fibroblasts (Fig. 2G). Initially, a 24-hour pulse treatment was used and resulted in elimination of a majority of hiPSC colonies from day 10 hiPSC-derived cardiomyocytes; however, a minimal number of colonies remained after treatment (supplemental online Fig. 1G). Increasing treatment time to 48 hours with 5 μM STF-31 was found to completely eliminate hiPSCs from fibroblasts and hiPSC-derived cardiomyocytes. Both the fibroblasts and cardiomyocytes remained viable after treatment, and spontaneous contraction was observed in the cardiomyocytes at the time of alkaline phosphatase staining (supplemental online Videos 3, 4) and up to at least 10 days after treatment initiation (data not shown). Altogether, these findings demonstrate that a 24–48-hour pulse treatment with STF-31 is an effective strategy for selective elimination of hPSCs from hPSC-derived progeny or terminally differentiated cells.
STF-31 Inhibits an NAD+ Salvage Pathway
In cancer cells, STF-31 was initially described as a GLUT1 inhibitor that induces necrosis [35]. However, because of the differences in timing and efficacy that we observe between STF-31 and WZB117 (an irreversible GLUT1 inhibitor), we set out to further explore the mechanism of action of STF-31 in hPSCs. In these studies, glucose deprivation was used for comparison because it has been previously reported as a method for hPSC elimination [16]. Similar to the results observed for WZB117, glucose deprivation was toxic to subconfluent cells, but toxicity was both delayed and decreased in confluent cells (Fig. 3A). Using extracellular flux analysis, the ECAR was measured to provide information on glycolysis, and the OCR was monitored to probe mitochondrial respiration. Treatment of hiPSCs with the GLUT1 inhibitor WZB117 led to a decrease in ECAR by the earliest time point measured (Fig. 3B), acting in a manner similar to that observed with 2-DG, an inhibitor of glucose metabolism (supplemental online Fig. 3A). In contrast, the decrease in ECAR following STF-31 treatment was significantly delayed by up to 15 hours as compared with the inhibitors of glucose oxidation (Fig. 3B). The results for OCR are similar to ECAR, because hiPSCs treated with STF-31 required 15 hours before reductions in OCR were observed. This finding is in contrast to the effects of WZB117, which decreased OCR by the first time point examined (8 hours) (Fig. 3B).
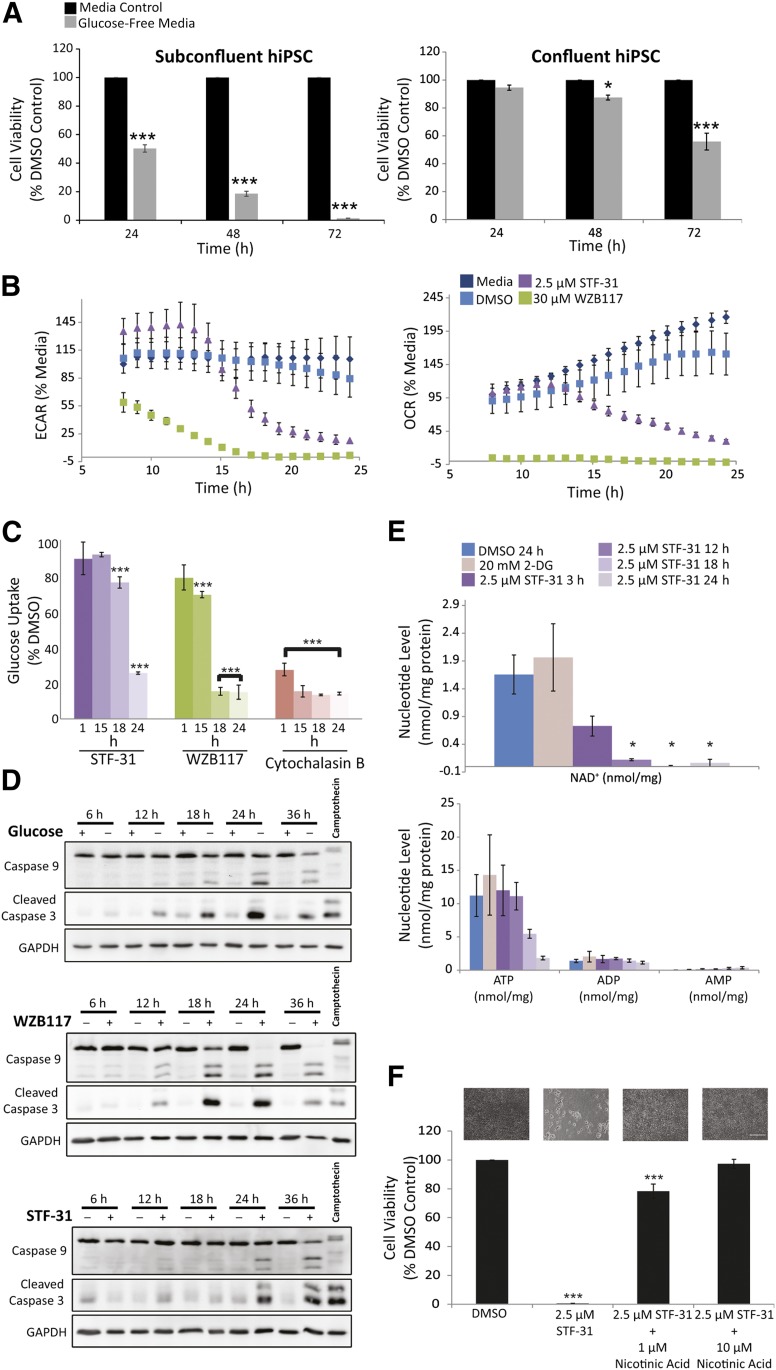
Figure 3.
STF-31 inhibits an NAD+ salvage pathway. (A): Effect of glucose deprivation on hiPSC (DF6-9-9T) viability as measured by neutral red assay in subconfluent cells and confluent cells (n = 3). (B): ECAR and OCR in hiPSCs after treatment with 2.5 μM STF-31 and 30 μM WZB117 (n = 3). (C): Bar graphs representing glucose uptake as a percentage of vehicle control for hiPSCs treated for 1, 15, 18, and 24 hours with 2.5 μM STF-31, 30 μM WZB117, and 20 μM cytochalasin B in E8 medium (n = 3). (D): Representative immunoblots for total and cleaved caspase-9, cleaved caspase-3, and GAPDH in subconfluent hiPSCs treated with glucose deprivation, 30 μM WZB117, or 2.5 μM STF-31. hiPSCs were treated for 3 hours with 1 μM camptothecin as a positive control for immunoblotting. Densitometry measurements are presented in supplemental online Figure 3. (E): Nucleotide levels in hiPSCs after treatment with 20 mM 2-DG for 24 hours or 2.5 μM STF-31 for 3–24 hours (n = 3). (F): hiPSC viability as measured by neutral red assay in cells treated with STF-31 alone or in the presence of 1 or 10 μM nicotinic acid (n = 3). Above each bar are representative brightfield images illustrating cell density and morphology after 48 hours treatment in each condition. Scale bar = 200 μm. The data are represented as means ± SEM. ∗, p ≤ .05; ∗∗∗, p ≤ .001 compared with medium or DMSO control. See also supplemental online Figure 3. Abbreviations: 2-DG, 2-deoxyglucose; DMSO, dimethyl sulfoxide; ECAR, extracellular acidification rate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; h, hours; hiPSC, human induced pluripotent stem cells; OCR, oxygen consumption rate.
Consistent with the effects on glucose metabolism, STF-31 did not alter glucose uptake in hiPSCs following 1 hour of treatment, whereas 1 hour of treatment with WZB117 resulted in a 20% decrease in uptake (Fig. 3C). The first time point at which STF-31 caused a decrease in glucose uptake was 18 hours after treatment, which was 3 hours after the decrease in ECAR. Thus, the significant decrease in glucose uptake that is consistent with results previously reported in cancer cells [35] occurred after STF-31-mediated inhibition of glycolysis and mitochondrial oxidative metabolism. The delayed effects of STF-31 on glucose uptake were unexpected for an inhibitor affecting either glucose transport or GLUT1 biogenesis and are not consistent with the reported mechanism of action for STF-31 as a GLUT1 inhibitor.
WZB117 and STF-31 have been reported to induce necrosis in cancer cells based on annexin staining at 24–48 and 24–72 hours of treatment, respectively, but other reports have associated a decrease in ATP caused by glucose starvation with apoptosis [36–38]. To determine how WZB117 and STF-31 promote hiPSC death, energy sensing pathways were examined by measuring phosphorylation of AMP-activated protein kinase (p-AMPK) in subconfluent cells. In response to WZB117 treatment and glucose deprivation, p-AMPK was detected as early as 12 hours post-treatment, and the total levels of AMPK decreased by 24 hours following WZB117 treatment of hiPSCs (supplemental online Fig. 3B). In response to STF-31 treatment, there was an increase in p-AMPK at 24 hours, approximately 8 hours after the decrease in ECAR and OCR. The timing of AMPK activation was different between STF-31 and both glucose deprivation and WZB117 treatment. Moreover, in WZB117-treated and glucose-deprived cells, cleaved caspase-3 and -9 were observed following a 12-hour incubation and remained elevated until 36 hours, at which time decreases in cleaved caspase-3 and -9 levels are observed (Fig. 3D). Induction of caspase-3/7 activity was also observed as early as 12 hours (supplemental online Fig. 3C). In contrast, STF-31 caused caspase-3 and -9 cleavage significantly later (Fig. 3D) and this coincided with caspase-3/7 activity at 24 hours after initiation of treatment (supplemental online Fig. 3C). Thus, WZB117 and STF-31 appear to act on hiPSCs, although activation of caspase mediated cell death pathways; yet the temporal induction of caspase activation and p-AMPK differs between agents.
The temporal discordance among metabolism, glucose uptake, and caspase activation (Figs. 3A–3D, 4) prompted us to measure adenine and pyridine nucleotide levels because they are known to affect both glycolytic and oxidative metabolism and cell viability [32]. These analyses revealed that with STF-31 treatment, NAD+ levels are reduced to ∼50% by 3 hours and to <1% by 18 hours. ATP levels do not decrease until 18 hours (Fig. 3E). These data suggest that STF-31 leads to an inhibition of the NAD+ de novo synthesis or salvage pathways. To investigate which NAD+ pathway is being targeted, rescue experiments were performed using metabolites added in the presence of STF-31. The addition of 1 and 10 μM nicotinic acid restored hPSC viability to 78% and 97%, respectively (Fig. 3F). Nicotinic acid is normally not present in hPSC culturing conditions and can be used by nicotinic acid phosphoribosyltransferase in one of the three NAD+ salvage pathways. Alternatively, the addition of 10 and 100 μM nicotinamide, the substrate for nicotinamide phosphoribosyltransferase (NAMPT), failed to rescue STF-31-mediated toxicity (data not shown). Altogether, these data suggest that STF-31 inhibits the NAMPT-dependent NAD+ salvage pathway.
Figure 4.
Diagram representing temporal effects after treatment of human pluripotent stem cells with STF-31 or glycolytic inhibition.
Discussion
This study establishes and defines an effective strategy for selective toxicity of hPSCs. Pluripotent stem cell metabolism is characterized by aerobic glycolysis, decreased mitochondrial membrane potential, and increased reliance on anabolic processes [39]. Enhanced expression of GLUT1 and reliance on glycolytic metabolism make glucose deprivation [16] or inhibition of GLUT1 promising strategies for elimination of hPSCs, as we previously described [19]. However, in our culture system, we found that glucose deprivation, WZB117, and PluriSIn had limited to no toxicity on confluent monolayers of hPSCs, an observation that was consistent among five hPSC lines and two different media compositions. Therefore, these strategies may not be ideal for applications that require confluent cell monolayers of hPSCs such as cardiomyocytes, hepatocytes, and neuronal cultures [19, 22, 33, 34]. We show that hPSCs can be selectively targeted using STF-31, with ideal toxicity characteristics for culture systems that require or produce confluent monolayers of cells with varying proliferation rates. STF-31 offers an advantage because it exhibits toxicity in hPSCs after a pulse treatment, which provided a means for selective elimination of the hPSCs from hPSC-derived cardiomyocytes or terminally differentiated fibroblasts. Regenerative medicine strategies that require tissues, scaffolds, or other three-dimensional cell products could therefore benefit from these findings.
STF-31 has been reported to inhibit GLUT1 in cancer cells [35] and is currently marketed as a GLUT1 inhibitor. However, our data for STF-31-mediated toxicity and effects on metabolic flux do not support this mechanism of action. Rather, these data show that the effects on glycolytic metabolism are due to depletion of NAD+. STF-31-mediated temporal effects on metabolism (glycolysis and oxidative phosphorylation) and cell death differ from both glucose deprivation and WZB117. Additionally, the failure to block glucose uptake prior to inhibition of glycolytic flux demonstrates that STF-31-mediated toxicity in hPSCs is not due to GLUT1 inhibition. As a result of these findings, we set out to define the mechanism of action of STF-31. Our data indicate that STF-31 targets NAMPT, the enzyme that catalyzes the rate-limiting step in the conversion of nicotinamide to NAD+ in one of the NAD+ salvage pathways. Further support of this mechanism is found in two separate studies that were published while our manuscript was under review [40, 41]. Dragovich et al. [41] demonstrated that compound 51, which has the same structure as STF-31, inhibited NAMPT (IC50 = 19 nM) and was shown to bind in the ligand-binding pocket by x-ray crystallography. Adams et al. [40] demonstrated that STF-31 mediates NAMPT inhibition and that STF-31 mediated toxicity is consistent with other NAMPT inhibitors. Altogether, these data provide evidence that STF-31-mediated toxicity is due to the inhibition of the NAMPT-mediated NAD+ salvage pathway. Additionally, our study provides evidence that the toxic effects mediated by STF-31 in hPSCs can be attributed to depletion of NAD+, not inhibition of glucose transport. This discovery provides fundamental insight into the basic metabolic profile of pluripotency, because these cells have a diminished capacity to compensate for a loss of NAMPT activity compared with their differentiated progeny. The reliance on NAD+ salvage pathways shown here is consistent with a recent report that used the NAMPT inhibitor FK866 to provide evidence that adequate NAD+ levels are required to establish and maintain pluripotency during reprogramming [42]. However, the report by Son et al. [42] did not demonstrate complete cell death of hPSCs in the conditions tested. Going forward, more potent and water-soluble inhibitors of NAMPT should be important reagents for the preparation of clinically relevant cells and tissues derived from hPSCs.
Conclusion
This study establishes that targeting the NAD+ salvage pathway mediated by NAMPT represents an effective and efficient strategy for selective hPSC toxicity. Our detailed analyses of the cellular metabolic events support the use of NAMPT inhibitors over glucose starvation or GLUT1 inhibition for the elimination of hPSCs in culture. When using STF-31 to inhibit NAMPT in a 24- or 48-hour pulse treatment scheme, hPSC elimination can be achieved across many culture conditions without cytotoxic effects on the terminally differentiated cells and hPSC-derived progeny tested in this study. Of broad significance to the stem cell and regenerative medicine fields, this study also highlights the importance of examining the effect of in vitro culturing parameters when evaluating the efficacy of hPSC-elimination strategies, especially those that target metabolic processes.
Supplementary Material
Acknowledgments
This research was supported by NIH Grant 4R00HL094708 and Institutional Research Grant 86-004-26 from the American Cancer Society (to R.L.G.), NIH Grants R01DK52194 and R01DK44458 (to J.A.C.), American Heart Association Grant 13POST16940076 (to K.A.B.), American Heart Association Grant 14PRE20380585 to (to B.J.O.), American Heart Association Grant 14PRE18580022 (to A.C.C.), NIH Grant R01HL58012 (to D.S.), Health and Medical Research Fund Project 02131456 and Research Grants Council of Hong Kong Theme-Based Research Scheme T13-706/11 (to K.R.B). E.M.K. is a member of the Medical College of Wisconsin Medical Scientist Training Program, which is partially supported by T32 Grant GM080202 from National Institute of General Medical Sciences, NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Dr. Sridhar Rao for providing reagents and thoughtful discussion, Sandra Chuppa (Medical College of Wisconsin) for technical assistance, Dr. Claudius Mahr (University of Washington) for careful review of the manuscript, and Dr. Paul Burridge (Stanford University) for critical review of the data and manuscript and conceptual guidance during study design.
Author Contributions
E.M.K.: conception and design, collection of data, data analysis and interpretation, manuscript writing, final approval of manuscript; B.J.O., K.A.B., S.B., A.C.C., A.R.D., Q.H., D.S., and N.H.: collection of data, data analysis and interpretation, final approval of manuscript; K.R.B.: conception and design, collection of data, data analysis and interpretation, and manuscript writing, final approval of manuscript; J.A.C.: conception and design, data analysis and interpretation, and manuscript writing final approval of manuscript; R.L.G.: conception and design, data analysis and interpretation, financial support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
A.R.D.’s spouse has compensated employment and compensated stock from Seahorse Bioscience. The other authors indicated no potential conflicts of interest.
References
- 1.Chong JJ, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebert AD, Liang P, Wu JC. Induced pluripotent stem cells as a disease modeling and drug screening platform. J Cardiovasc Pharmacol. 2012;60:408–416. doi: 10.1097/FJC.0b013e318247f642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grskovic M, Javaherian A, Strulovici B, et al. Induced pluripotent stem cells: Opportunities for disease modelling and drug discovery. Nat Rev Drug Discov. 2011;10:915–929. doi: 10.1038/nrd3577. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140:2457–2461. doi: 10.1242/dev.092551. [DOI] [PubMed] [Google Scholar]
- 5.Hentze H, Soong PL, Wang ST, et al. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res (Amst) 2009;2:198–210. doi: 10.1016/j.scr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Lawrenz B, Schiller H, Willbold E, et al. Highly sensitive biosafety model for stem-cell-derived grafts. Cytotherapy. 2004;6:212–222. doi: 10.1080/14653240410006031. [DOI] [PubMed] [Google Scholar]
- 7.Cui L, Guan Y, Qu Z, et al. WNT signaling determines tumorigenicity and function of ESC-derived retinal progenitors. J Clin Invest. 2013;123:1647–1661. doi: 10.1172/JCI65048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doi D, Morizane A, Kikuchi T, et al. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson’s disease. Stem Cells. 2012;30:935–945. doi: 10.1002/stem.1060. [DOI] [PubMed] [Google Scholar]
- 9.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 10.Lee AS, Tang C, Rao MS, et al. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeFrancesco L. Fits and starts for Geron. Nat Biotechnol. 2009:303. doi: 10.1038/nbt0409-303. [DOI] [PubMed] [Google Scholar]
- 12.Cao F, Drukker M, Lin S, et al. Molecular imaging of embryonic stem cell misbehavior and suicide gene ablation. Cloning Stem Cells. 2007;9:107–117. doi: 10.1089/clo.2006.0E16. [DOI] [PubMed] [Google Scholar]
- 13.Rong Z, Fu X, Wang M, et al. A scalable approach to prevent teratoma formation of human embryonic stem cells. J Biol Chem. 2012;287:32338–32345. doi: 10.1074/jbc.M112.383810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-David U, Nudel N, Benvenisty N. Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat Commun. 2013;4:1992. doi: 10.1038/ncomms2992. [DOI] [PubMed] [Google Scholar]
- 15.Tang C, Lee AS, Volkmer JP, et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011;29:829–834. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tohyama S, Hattori F, Sano M, et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12:127–137. doi: 10.1016/j.stem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Ben-David U, Benvenisty N. Chemical ablation of tumor-initiating human pluripotent stem cells. Nat Protoc. 2014;9:729–740. doi: 10.1038/nprot.2014.050. [DOI] [PubMed] [Google Scholar]
- 18.Ben-David U, Gan QF, Golan-Lev T, et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013;12:167–179. doi: 10.1016/j.stem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Boheler KR, Bhattacharya S, Kropp EM, et al. A human pluripotent stem cell surface N-glycoproteome resource reveals markers, extracellular epitopes, and drug targets. Stem Cell Reports. 2014;3:185–203. doi: 10.1016/j.stemcr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharya S, Burridge PW, Kropp EM, et al. High efficiency differentiation of human pluripotent stem cells to cardiomyocytes and characterization by flow cytometry. J Vis Exp. 2014:52010. doi: 10.3791/52010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amit M, Itskovitz-Eldor J. Morphology of human embryonic and induced pluripotent stem cell colonies cultured with feeders. In: Amit M, Itskovitz-Eldor J, eds, editors. Atlas of Human Pluripotent Stem Cells. New York, NY: Humana Press; 2012. pp. 15–39. [Google Scholar]
- 24.Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3:1125–1131. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor MD, Kardel MD, Eaves CJ. Functional assays for human embryonic stem cell pluripotency. Methods Mol Biol. 2011;690:67–80. doi: 10.1007/978-1-60761-962-8_4. [DOI] [PubMed] [Google Scholar]
- 26.Rao S, Zhen S, Roumiantsev S, et al. Differential roles of Sall4 isoforms in embryonic stem cell pluripotency. Mol Cell Biol. 2010;30:5364–5380. doi: 10.1128/MCB.00419-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Nuebel E, Wisidagama DR, et al. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat Protoc. 2012;7:1068–1085. doi: 10.1038/nprot.2012.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto N, Ueda M, Sato T, et al. Curr Protoc Pharmacol 2011;Chapter 12:Unit 12.14. Measurement of glucose uptake in cultured cells. pp. 1–22. [DOI] [PubMed] [Google Scholar]
- 29.Khan P, Idrees D, Moxley MA, et al. Luminol-based chemiluminescent signals: Clinical and non-clinical application and future uses. Appl Biochem Biotechnol. 2014;173:333–355. doi: 10.1007/s12010-014-0850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meares GP, Hughes KJ, Jaimes KF, et al. AMP-activated protein kinase attenuates nitric oxide-induced beta-cell death. J Biol Chem. 2010;285:3191–3200. doi: 10.1074/jbc.M109.047365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrasco RA, Stamm NB, Patel BK. One-step cellular caspase-3/7 assay. Biotechniques. 2003;34:1064–1067. doi: 10.2144/03345dd02. [DOI] [PubMed] [Google Scholar]
- 32.Broniowska KA, Diers AR, Corbett JA, et al. Effect of nitric oxide on naphthoquinone toxicity in endothelial cells: Role of bioenergetic dysfunction and poly (ADP-ribose) polymerase activation. Biochemistry. 2013;52:4364–4372. doi: 10.1021/bi400342t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallanna SK, Duncan SA. Differentiation of hepatocytes from pluripotent stem cells. Curr Protoc Stem Cell Biol. 2013;26:Unit 1G.4. doi: 10.1002/9780470151808.sc01g04s26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y, Kirwan P, Smith J, et al. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477–486. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan DA, Sutphin PD, Nguyen P, et al. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94ra70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altman BJ, Rathmell JC. Metabolic stress in autophagy and cell death pathways. Cold Spring Harb Perspect Biol. 2012;4:a008763. doi: 10.1101/cshperspect.a008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coloff JL, Mason EF, Altman BJ, et al. Akt requires glucose metabolism to suppress puma expression and prevent apoptosis of leukemic T cells. J Biol Chem. 2011;286:5921–5933. doi: 10.1074/jbc.M110.179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Coloff JL, Ferguson EC, et al. Glucose metabolism attenuates p53 and Puma-dependent cell death upon growth factor deprivation. J Biol Chem. 2008;283:36344–36353. doi: 10.1074/jbc.M803580200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Folmes CD, Nelson TJ, Dzeja PP, et al. Energy metabolism plasticity enables stemness programs. Ann N Y Acad Sci. 2012;1254:82–89. doi: 10.1111/j.1749-6632.2012.06487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams DJ, Ito D, Rees MG, et al. NAMPT is the cellular target of STF-31-like small-molecule probes. ACS Chem Biol. 2014;9:2247–2254. doi: 10.1021/cb500347p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dragovich PS, Zhao G, Baumeister T, et al. Fragment-based design of 3-aminopyridine-derived amides as potent inhibitors of human nicotinamide phosphoribosyltransferase (NAMPT) Bioorg Med Chem Lett. 2014;24:954–962. doi: 10.1016/j.bmcl.2013.12.062. [DOI] [PubMed] [Google Scholar]
- 42.Son MJ, Son MY, Seol B, et al. Nicotinamide overcomes pluripotency deficits and reprogramming barriers. Stem Cells. 2013;31:1121–1135. doi: 10.1002/stem.1368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.