This study aimed to investigate the therapeutic effects of Atsttrin, a novel tumor necrosis factor α (TNFα) blocker, on osteoarthritis (OA) treatment. Our results indicated that suppression of TNFα activity is an effective strategy for OA treatment and that intra-articular injection of mesenchymal stem cells that express recombinant Atsttrin could be a promising therapeutic modality.
Keywords: Osteoarthritis, TNFα, Mesenchymal stem cells, Atsttrin, Coculture
Abstract
Osteoarthritis (OA) remains an intractable clinical challenge. Few drugs are available for reversing this degenerative disease, although some promising candidates have performed well in preclinical studies. Tumor necrosis factor α (TNFα) has been identified as a crucial effector modulating OA pathogenesis. This study aimed to investigate the therapeutic effects of Atsttrin, a novel TNFα blocker, on OA treatment. We developed genetically modified mesenchymal stem cells (MSCs) that expressed recombinant Atsttrin (named as MSC-Atsttrin). Expression levels of ADAMTS-5, MMP13, and iNOS of human chondrocytes were analyzed when cocultured with MSC-GFP/Atsttrin. OA animal models were induced by anterior cruciate ligament transection, and MSC-GFP/Atsttrin were injected into the articular cavity 1 week postsurgery. The results showed that MSC-Atsttrin significantly suppressed TNFα-driven up-regulation of matrix proteases and inflammatory factors. Intra-articular injection of MSC-Atsttrin prevented the progression of degenerative changes in the surgically induced OA mouse model. Additionally, levels of detrimental matrix hydrolases were significantly diminished. Compared with nontreated OA samples at 8 weeks postsurgery, the percentages of MMP13- and ADAMTS-5-positive cells were significantly reduced from 91.33% ± 9.87% to 24.33% ± 5.7% (p < .001) and from 91.33% ± 7.1% to 16.67% ± 3.1% (p < .001), respectively. Our results thus indicated that suppression of TNFα activity is an effective strategy for OA treatment and that intra-articular injection of MSCs-Atsttrin could be a promising therapeutic modality.
Significance
The main novelty of this study is the finding of Atsttrin modified mesenchymal stem cells (MSCs-Atsttrin) for blocking osteoarthritis (OA) development within an in vivo mouse surgically induced osteoarthritis model. Because MSCs have already been widely used in the treatment of patients and have demonstrated good efficacy and safety, MSC-based Atsttrin gene therapy could be a promising modality for the treatment of OA patients.
Introduction
Osteoarthritis (OA) is a painful chronic inflammatory disease that is characterized by progressive loss of cartilage matrix and disturbance of the dynamic equilibrium between the anabolic and catabolic activities of resident chondrocytes [1]. OA is more prevalent than any other musculoskeletal system disability in the elderly population [2, 3]. The incidence of symptomatic knee OA is ∼13% among people aged 60 and older and increases to 40% in those older than 70 years [4]. To date, few safe and effective medications are available for OA treatment, and current therapeutic approaches mainly focus on mitigating disease symptoms without sufficiently slowing the progression of tissue structural degeneration [1, 3–5]. Confronted with such an enormous health care burden, there is a dire need for the discovery and development of novel therapeutic strategies to ameliorate OA cartilage degeneration [1, 6].
Although the etiology and pathogenesis underlying osteoarthritis are poorly understood, a growing body of scientific evidence indicates that a combination of various genetic, metabolic, biochemical, and inflammatory factors are probably involved [3, 5]. In particular, secreted inflammatory molecules, especially proinflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-15, IL-17, IL-18, IL-21, and tumor necrosis factor α (TNFα), play critical roles in the disturbance of metabolic homeostasis within OA cartilage. Previous studies have demonstrated that TNFα could drive inflammatory damage in OA and induce the degradation of articular cartilage matrix [1, 4, 6–8], which in turn makes it a promising therapeutic target. Furthermore, the level of TNFα is elevated in either circulating or local synovial fluid, which is positively associated with the severity of knee OA [9, 10]. This disease feature, coupled with recent clinical approval of anti-TNFα therapeutics in either rheumatoid arthritis (RA) or psoriatic arthritis [11, 12], strengthens the option of TNF-α as a potential drug target.
TNFα antagonists blocks TNFα-related pathways by preventing this cytokine from binding to its cognate receptors (TNFα receptor 1 and 2), which in turn prevents initiation of the inflammatory response within OA joints [1, 6]. Monoclonal anti-TNFα antibodies and soluble TNFα receptors have been extensively investigated as potential inhibitors of TNFα activity in OA. Four anti-TNFα monoclonal antibodies (infliximab, adalimumab, golimumab, and certolizumab pegol) and one TNFα receptor fusion protein (etanercept) are currently approved as anti-TNFα therapeutics by the Food and Drug Administration [13]. These new pharmacological agents have revolutionized the treatment of RA and other autoimmune diseases with acceptable clinical efficacy [14]. Nevertheless, efforts to achieve similar results in the treatment of OA with these new drugs have yielded rather poor clinical efficacy and disappointing results [1]. Moreover, it must be noted that these TNFα antagonists have fairly short half-lives and also increase the risk of opportunistic infection by compromising the immune system [1]. Therefore, the development of novel rational TNFα blocker, which exhibits better performance in OA, still remains a challenge.
Progranulin (PGRN), containing seven and a half repeats of a cysteine-rich motif (CX5-6CX5CCX8CCX6CCXDX2HCCPX4CX5-6C, where X indicates any amino acid) in the order of PGFBACDE (where A–G are full repeats, and P is the half-motif), is expressed in a variety of cells including cycling epithelial cells, leukocytes, neurons, chondrocytes, and cancer cells [11, 15, 16]. It is well-known that PGRN plays a crucial role in cartilage development and can retard inflammatory degradation processes through binding with the TNF receptor (TNFR) [11, 17, 18].
Atsttrin (antagonist of TNF-TNFR signaling via targeting to TNFR) is an engineered protein derived from PGRN, and it is constituted of half-units of granulins A, C, and F plus linkers P3, P4, and P5 [11]. Three TNFR superfamily members, TNFR1, TNFR2, and TNFRSF25 (DR3), can directly bind with Atsttrin [11, 19]. Compared with recombinant human PGRN (rhPGRN), Atsttrin has a substantially longer half-life (∼120 hours) than PRGN (∼40 hours), and administration of Atsttrin has been shown to be better at delaying the onset and attenuating the severity of inflammatory arthritis in the collagen-antibody induced model. Overall, Atsttrin treatment yielded higher efficacy than either rhPGRN or etanercept in the collagen-antibody induced model of inflammatory arthritis [11]. However, to ensure complete prevention of inflammatory arthritis, a dose of 0.5 mg/kg body weight once a week or a higher dose of 10 mg/kg per 3 weeks was required [11, 20], which may be disadvantaged by high costs and patient discomfort.
Mesenchymal stem cells (MSCs), originating from bone marrow or other adult tissues, possess self-renewal capacity, as well as potency to differentiate into multiple lineages including chondrocytes, osteoblasts, and adipocytes [21, 22]. MSCs have been extracted from either healthy or diseased cartilage and retain the potential to regenerate cartilage in vivo [23, 24]. A pioneering clinical research study demonstrated that intra-articular injection of 1.0 × 108 adipose-derived MSCs into the OA knee improved knee joint function and relieved pain without causing any adverse side effects [25]. More recently, a novel strategy of using genetically modified MSCs in OA therapy has been developed [26]. By exploiting its self-renewal property, MSCs can be used as delivery vehicles for exogenous expression of specific genes that can contribute to alleviation of OA symptoms [27].
In this study, we developed genetically modified mesenchymal stem cells, which express Atsttrin under transcriptional control of a tetracycline-inducible promoter. We measured the TNFα expression in normal and OA samples by immunohistochemistry and analyzed the mRNA expression levels of MMP13, ADAMTS-5, iNOS, and NF-κb (P65) in human chondrocytes after treatment with TNFα, or coculture with MSC-GFP/Atsttrin. Furthermore, intra-articular injection of MSC-Atsttrin was carried out for in vivo OA therapy. The efficacy of cartilage regeneration was evaluated and analyzed using standard clinical parameters.
Materials and Methods
Vector Construction and Lentivirus Production
The PGRN gene was cloned from the cDNA of A549 cells (a lung adenocarcinoma cell line) with primer Ppgrn-F (ATGTGGACCCTGGTGAGCTGGGTG) and Ppgrn-R (TCACAGCAGCTGTCTCAAGG). Subsequently, the gene of Atsttrin [11] was subcloned from PGRN by splicing over extension (SOE) polymerase chain reaction (PCR) as our previous study [28, 29]. Firstly, the three fragments (FP3, P4A, and P5C) were amplified from PGRN with three sets of primers (F1 and R1, F2 and R2, and F3 and R3). The primer sequences were as follows: F1, ATGCCCCAGGCTTCCTGCTGTG; R1, CTCCTTGGACGAGCTGGACAAGGCCACTGCCCT; F2, TGTCCAGCTCGTCCAAGGAGGACGCTACCACGGACCT; R2, CCTGTTCACACCAGGCCCCCGACTGTAGAC; F3, CGGGGGCCTGGTGTGAACAGGGGCCCCACCAGGTG; and R3, TTATGGGATTGGACAGCAGCCCCACTC. The bold letters indicate the complementary regions designed for subsequence SOE. Secondly, Atsttrin was generated by SOE with primers F1 and R3 using equal amount of the three fragments as templates. Then the nucleotide sequence of PGRN signal peptide and that of hemagglutinin (HA) were fused to the 5′- and 3′-ends of the gene of Atsttrin by PCR with primer F4 (GAATTCATGTGGACCCTGGTGAGCTGGGTGGCCTTAACAGCAGGGCTGGTGGCTGGAACGCGGTGCCCCCAGGCTTCCTGCTGTGAAGA) and R4 (GAATTCTTAAGCGTAATCTGGAACATCGTATGGGTATGGGATTGGACAGCAGCCCCACTC).
The Atsttrin gene containing signal peptides and HA was subcloned into FUW tetracycline-inducible lentiviral vector [30] (provided by Dr. Marius Wernig, Stanford University). A third generation self-inactivating lentivirus vector contains a tetracycline-responsive promoter upstream of the cloning restriction site. Additionally, green fluorescent protein (GFP) containing the same signal sequence was used as the control to discount any change in gene expression profile that may result from the delivery method. Four plasmids comprising the constructed lentiviral vector and another three package vectors were transfected into 293FT cells (a cell line established from primary embryonic human kidney; Invitrogen, Carlsbad, CA, http://www.invitrogen.com) with Lipofectamine (Invitrogen) according to the manufacturer’s instructions. The medium was replaced 16 hours after transfection. The virus-containing medium was pooled 48 hours later, passed through a 0.45-μm filter to remove cell debris, and subsequently used to infect target cells or frozen at −80°C.
Cell Culture and Lentivirus Infection
Human healthy primary chondrocytes were isolated from the articular cartilages of the discarded specimens of knee replacement surgery with no history of OA. Human OA cartilage samples were obtained from OA patients undergoing total knee replacement surgery. Briefly, the harvested cartilage samples, which were stripped of adjacent tissues, were immersed in phosphate-buffered saline containing penicillin (100 units/ml) and streptomycin (100 μg/ml) (Gibco, Grand Island, NY, http://www.invitrogen.com) for 10 minutes and then cut into small pieces followed by digestion with 0.2% (wt/vol) mixed collagenase (Gibco) at 37°C in a humidified atmosphere of 5% CO2 and 95% air. An equal volume of Dulbecco’s modified Eagle’s medium (DMEM) containing 10% (vol/vol) fetal bovine serum (FBS; Gibco) was added 4 hours later, and the mixture was digested overnight. The tissue residues were then discarded, and the cells were collected by centrifugation at 1,200 rpm. The pooled cells were maintained in DMEM containing 10% (vol/vol) FBS with medium being changed every other day. When the confluence of cultured cells reached 80%, they were detached by treatment with 0.25% (wt/vol) trypsin and 0.1% (wt/vol) ethylenediaminetetraacetic acid (Gibco) and reseeded at a density of 1 × 104 cells per cm2. Cultured cells before passage 2 were used for experiments.
C3H10T1/2 cell (ATCC CCL-226), a cell line of mouse mesenchymal stem cells cultured in the same condition with chondrocytes, were transfected with lentivirus (containing Atsttrin or GFP) when cells grew to 30%–50% confluence at a multiplicity of infection of 200. More than 95% of the cells were still viable 12 hours after infection, and then the medium was changed. Three days later, all infected cells (labeled as MSC-Atsttrin or MSC-GFP hereafter) were treated with 4 μg/ml doxycycline (Sigma, St. Louis, MO, https://www.sigmaaldrich.com/) in the medium to activate tetracycline-inducible gene expression and were selected with Zeocin (Invitrogen) at 200 μg/ml for 10 days. Transduced cells before passage 5 after selection were used for functional studies.
Cell Coculture
Noncontacting coculture systems were used to investigate the biological activity of engineered MSCs on chondrocytes. The 6.5-mm Transwell with an 8.0-μm pore polycarbonate membrane insert (Corning Enterprises, Corning, NY, http://www.corning.com) was used in this assay. Human articular chondrocytes were seeded on the bottom of 24-well plates, and MSC-Atsttrin or MSC-GFP was seeded onto the membrane of suitable Transwells. TNFα was added to the medium at a final concentration of 10 ng/ml when the cells grew to 70% confluence. The chondrocytes were harvested for RNA preparation 48 hours after treatment.
Quantitative Real-Time Polymerase Chain Reaction
Total cellular RNA was isolated by using TRIzol (Invitrogen) as described in the manufacturer’s instructions. 2 µg of isolated RNA from each sample was reverse-transcribed into cDNA using Moloney murine leukemia virus reverse transcriptase (Invitrogen). Quantitative real-time PCR (qRT-PCR) was carried out to determine the mRNA levels of MMP13 (matrix metallopeptidase-13), ADAMTS-5 (a disintegrin and metalloproteinase with thrombospondin motifs 5), P65, and inducible nitric-oxide synthase (iNOS) with the SYBR Green Real-time PCR Master Mix kit (Takara Bio Inc., Seta 3-4-1, Otsu, Shiga, Japan, http://www.clontech.com/takara) in accordance with the manufacturer’s instructions. The PCR cycling consisted of 40 cycles of amplification of the template cDNA with primer annealing at 60°C. Then the relative level of expression of each target gene was calculated using the 2−ΔΔCt method. Target genes were normalized against the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The following primer sequences were used: MMP13, forward 5′-ATGCAGTCTTTCTTCGGCTTAG-3′, and reverse, 5′-ATGCCATCGTGAAGTCTGGT-3′; ADAMTS-5, forward 5′-ATCACCCAATGCCAAGG-3′, and reverse, 5′-AGCAGAGTAGGAGACAAC-3′; P65, forward, 5′- GAGCAGCGTGGGGACTAC-3′, and reverse, 5′-TGCCAGAGTTTCGGTTCA-3′; and iNOS, forward, 5′-GATGGCAGCATCAGAGGG-3′, and reverse, 5′- GGTCAGGTGGGATTTCGA-3′.
Western Blot Analysis
Total cellular protein was extracted with RIPA lysis buffer, and the protein concentration was determined by a BCA protein assay kit (Pierce, Rockford, IL, http://www.piercenet.com; catalog no. 23227). Subsequently, the extracted protein was loaded on SDS-polyacrylamide gel electrophoresis gels. After electrophoresis, proteins were transferred onto a polyvinylidene fluoride membrane and blocked in 5% (wt/vol) bovine serum albumin (BSA) for 1 hour at room temperature. Then the membrane was incubated with anti-HA-flag or GAPDH antibody overnight at 4°C. After washing in Tris-buffered saline with Tween (TBST), horseradish peroxidase (HRP) secondary antibody was diluted 1:10,000 with 5% (wt/vol) BSA and incubated with the membrane for 1.5 hours at room temperature. Excess secondary antibody was rinsed off the membrane with TBST, and a chemiluminiscent signal was generated by using Western blot detection reagents (ECL; Beyotime Institute of Biotechnology, Haimen, China, http://www.beyotime.com) according to the manufacturer’s protocol.
Surgically Induced Osteoarthritis Animal Model
All animals were treated according to standard guidelines approved by the Zhejiang University Ethics Committee (no. ZJU2013105002). In total, 44 female 6-8-week-old C57BL/6 mice were used. Osteoarthritis was induced by anterior cruciate ligament transection (ACLT) in 33 mice, and the other 11 mice were used as the normal group. Without any intervention, OA pathology developed gradually during the following 8 weeks. The 33 OA-induced mice were equally divided to three groups (OA, OA+MSC-Atsttrin, and OA+MSC-GFP) randomly. Intra-articular injections of MSC-Atsttrin or MSC-GFP (1 × 105 cells in 10 µl DMEM) were carried out at 1 week post-OA surgery with a 0.5-ml monoject (29-gauge) insulin syringe (BD Micro-Fine; Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com), and a proper pressure was chosen to guarantee less than 5% cell mortality. Before implantation, MSC-Atsttrin and MSC-GFP cells were stained with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Beyotime Institute of Biotechnology). To evaluate the survival of implanted cells within the defects, a noninvasive Kodak FX small animal imaging system Color CCD tracking system was used to image the implantation sites at 1 and 4 weeks after surgery [31]. Postoperatively, animals were allowed free cage activity. Then 2 mg/ml doxycycline (Biosharp, Hefei, China, http://www.biosharp.cn) was added in drinking water with 10 mg/ml sucrose (Sinopharm Chemical Reagent Co., http://shreagent.lookchem.com). Knee joints were collected 4 and 8 weeks after OA surgery for histological analysis, immunohistochemical staining, and RNA purification. The sham group was injected with 10 μl of DMEM.
Histological Analysis, Immunohistochemistry, and Immunofluorescence
The harvested knee joints at 4 weeks and 8 weeks postsurgery were fixed in 4% (vol/vol) neutral buffered formalin for 24 hours and decalcified in neutral 10% (wt/vol) EDTA solution for 1 month at room temperature. The samples were then dehydrated, cleared, and embedded in paraffin blocks sequentially. A microtome was used to produce histological sections (8 μm), and six representative sections of each joint from various depths were mounted on slides and subsequently stained with Safranin Orange. The stained sections were photographed digitally under a microscope. Human cartilage (n = 3) sections (8 μm) were prepared using freezing microtome. Mice samples (n = 6) from each group were evaluated by the Osteoarthritis Research Society International (OARSI) scoring according to the previous study [32]. The evaluation of OARSI scoring is as follows. A score of 0 stands for normal cartilage, 0.5 = loss of proteoglycan with an intact surface, 1 = superficial fibrillation without loss of cartilage, 2 = vertical clefts and loss of surface lamina (any % or joint surface area), 3 = vertical clefts/erosion to the calcified layer lesion for 1%–25% of the quadrant width, 4 = lesion reaches the calcified cartilage for 25%–50% of the quadrant width, 5 = lesion reaches the calcified cartilage for 50%–75% of the quadrant width, and 6 = lesion reaches the calcified cartilage for >75% of the quadrant width [32].
Normal and OA cartilage sections were incubated overnight with polyclonal mouse anti-TNFα antibody (Abcam, Cambridge, MA, http://www.abcam.com/; catalog no. ab66579), rabbit anti-ADAMTS-5 antibody (Abcam, catalog no. ab41037), and rabbit anti-MMP13 antibody (Anbobio; San Francisco, CA, http://www.anbobio.com; catalog no. C0265). The samples were then incubated with goat anti-mouse secondary antibodies conjugated with HRP (Beyotime Institute of Biotechnology) or with Alexa Fluor 488 or Alexa Fluor 546 fluorescent dyes (Invitrogen). The stained specimens were photographed digitally and viewed under confocal microscopy (Olympus, Tokyo, Japan, http://www.olympus-global.com; catalog no. BX61W1-FV1000).
Statistical Analysis
All quantitative data are presented as means ± SD of at least three samples from each group. Differences in numerical data between groups were analyzed using one-way analysis of variance, followed by post hoc analysis if the homogeneity of variances were significant, and p < .05 was considered as statistically significant. Significance level was presented as follows: *, p < .05; **, p < .01; or ***, p < .001.
Results
Expression of TNFα in Human and Mice OA Cartilages
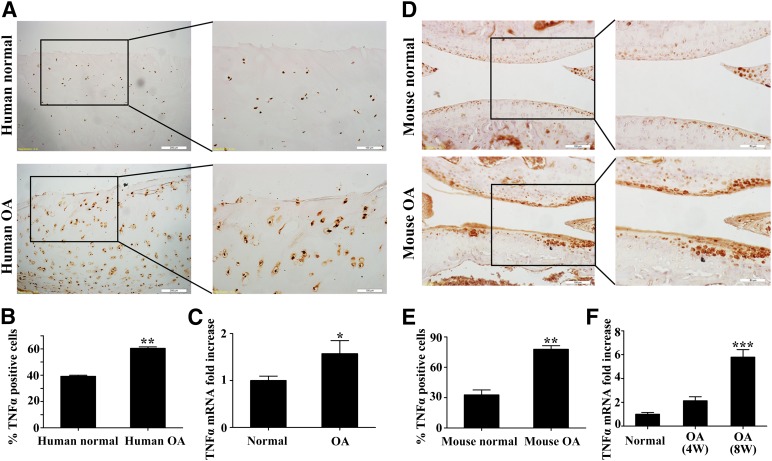
Human normal and OA cartilage samples were collected for the determination of TNFα expression by immunochemistry and quantitative real-time PCR. Compared with normal cartilage, the OA samples exhibited a significant increase of TNFα-positive cells (39.17% ± 1.4% vs. 60.47% ± 1.8%, p < .01) (Fig. 1A, 1B), which was consistent with the mRNA transcriptional level that was 1.57- ± 0.6-fold of the normal (p < .05) (Fig. 1C). Similarly, the TNFα positive cells (Fig. 1D, 1E) were increased in the mice OA group (32.75% ± 8.3% vs. 77.84% ± 5.9%, p < .001). Additionally, the corresponding mRNA expression levels exhibited an increasing trend with the progression of OA (Fig. 1F). These data collectively indicated that TNFα was up-regulated in both mice and human OA cartilage samples.
Figure 1.
TNFα expression levels evaluated in human and mice cartilage samples. (A): Immunohistochemical staining of TNFα in human normal and OA cartilage samples. Scale bars = 200 or 100 μm. (B): Quantification of TNFα-positive cells in human normal and OA cartilage. (C): Comparison of mRNA expression levels of TNFα between human normal and OA cartilages by quantitative real-time polymerase chain reaction (qRT-PCR). (D): Immunohistochemical staining of TNFα in mouse normal and OA cartilage. Scale bars = 100 or 50 μm. (E): Quantification of TNFα-positive cells in mouse normal and OA cartilage. (F): Comparison of mRNA expression levels of TNFα in mouse normal and OA cartilages by qRT-PCR. ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001. Abbreviations: 4W, 4 weeks; 8W, 8 weeks; OA, osteoarthritis; TNFα, tumor necrosis factor α.
Effects of MSC-Atsttrin on the Cocultured Chondrocytes
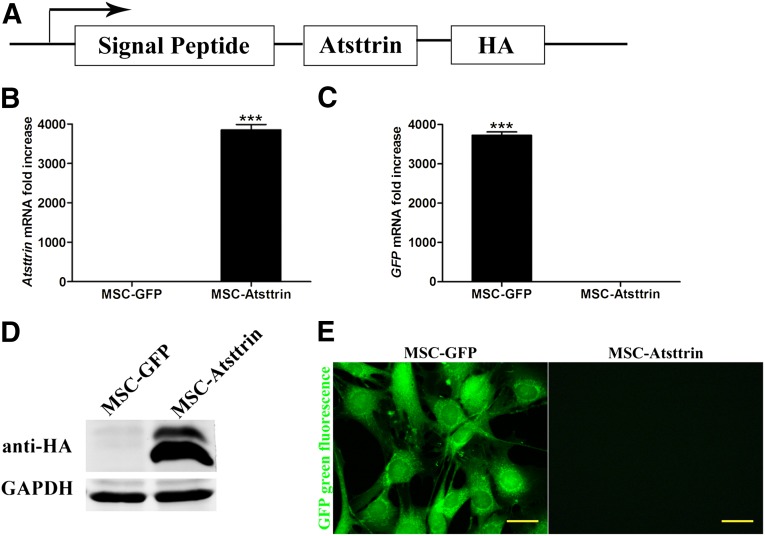
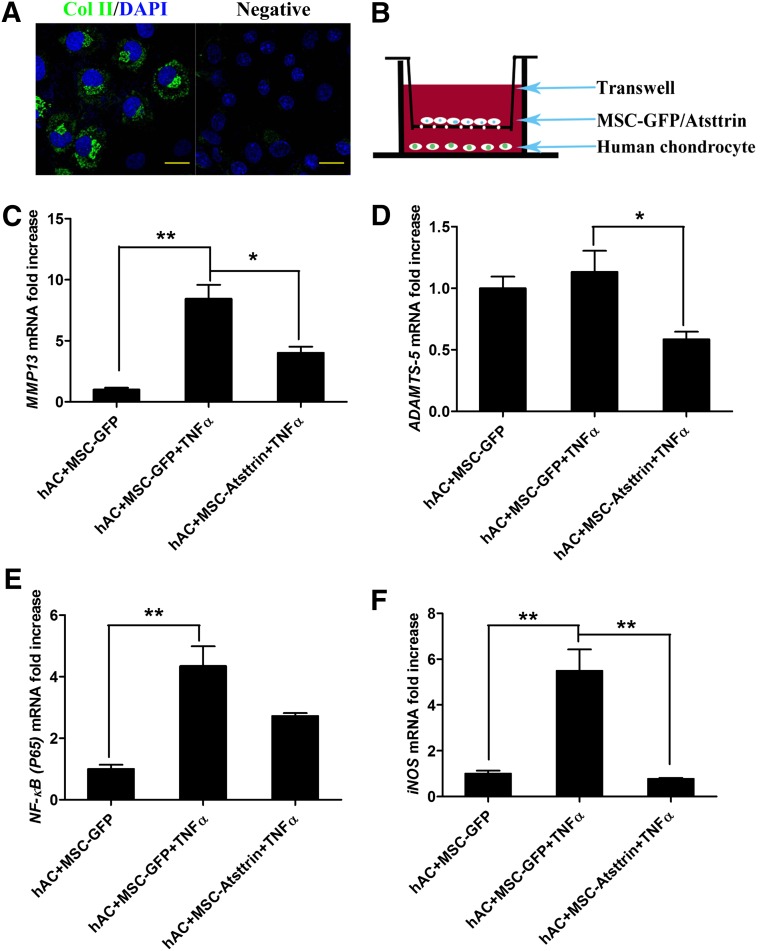
As indicated by quantitative RT-PCR, Western blots, and fluorescent microscopy in Figure 2B–2E, ectopic expression of Atsttrin and GFP in MSCs were successfully detected after induction with 10 µg/ml tetracycline. Based on Western blot analysis of the crude extracts from cell cultures, two bands (Fig. 2D) for MSC-Atsttrin appeared and as was expected; the higher molecular weight band corresponds to unprocessed Atsttrin protein (∼18.5 kDa), which contained the signal peptide (∼2 kDa), whereas the lower molecular weight band corresponds to the mature Atsttrin (∼16.5 kDa). Approximately half of a million human chondrocytes cell were obtained and were verified by immunostaining with the specific protein collagen II (Fig. 3A). As shown in Figure 3C and 3F, the up-regulation of MMP13 (8.45- ± 2.0-fold) and iNOS (5.49- ± 1.6-fold) induced by TNFα could be inhibited significantly by MSC-Atsttrin treatment, which was decreased to 4.02- ± 0.9-fold (p < .05) and 0.77- ± 0.1-fold (p < .01), respectively. Meanwhile, there is a tendency of P65 blocking (Fig. 3E). With regards to expression levels of ADAMTS-5 (Fig. 3D) that was not increased significantly by TNFα; a significant downregulation was observed in the MSC-Atsttrin group (p < .05). Hence, these results suggest that the expression of Atsttrin inhibited the TNFα-induced production of detrimental inflammatory factors on human chondrocytes.
Figure 2.
Stable expression of Atsttrin and GFP in MSCs. (A): Schematic map of the lentiviral vector. (B): mRNA expression levels of Atsttrin in MSC-GFP and MSC-Atsttrin cells by quantitative real-time polymerase chain reaction (qRT-PCR). (C): mRNA expression levels of GFP in MSC-GFP and MSC-Atsttrin cells by qRT-PCR. (D): Western blot analysis of MSC-GFP and MSC-Atsttrin cells with HA antibody. (E): Green fluorescent protein expressed by MSC-GFP. Scale bars = 20 µm. ∗∗∗, p < .001. Abbreviations: Col II, collagen II; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; HA, hemagglutinin; MSC, mesenchymal stem cell.
Figure 3.
Verification of isolated human articular chondrocytes and overexpression of Atsttrin decreased the expression of MMP13, ADAMTS-5, NF-κb (P65), and inducible nitric-oxide synthase (iNOS) induced by TNFα. (A): Col II staining of hAC. Scale bars = 20 µm. (B): Schematic representation of Transwell filter systems used in this study. (C–F): The effects of ectopic Atsttrin expression on mRNA transcript levels of MMP13, ADAMTS-5, NF-kb (P65), and iNOS in human articular chondrocytes after treatment with TNFα (10 ng/ml) for 48 hours. ∗, p < .05; ∗∗, p < .01 (n = 3). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; GFP, green fluorescent protein; hAC, human articular chondrocytes; MSC, mesenchymal stem cell; TNFα, tumor necrosis factor α.
Efficacy of MSC-Atsttrin Transplantation on the Repair of Surgically Induced OA in Mice
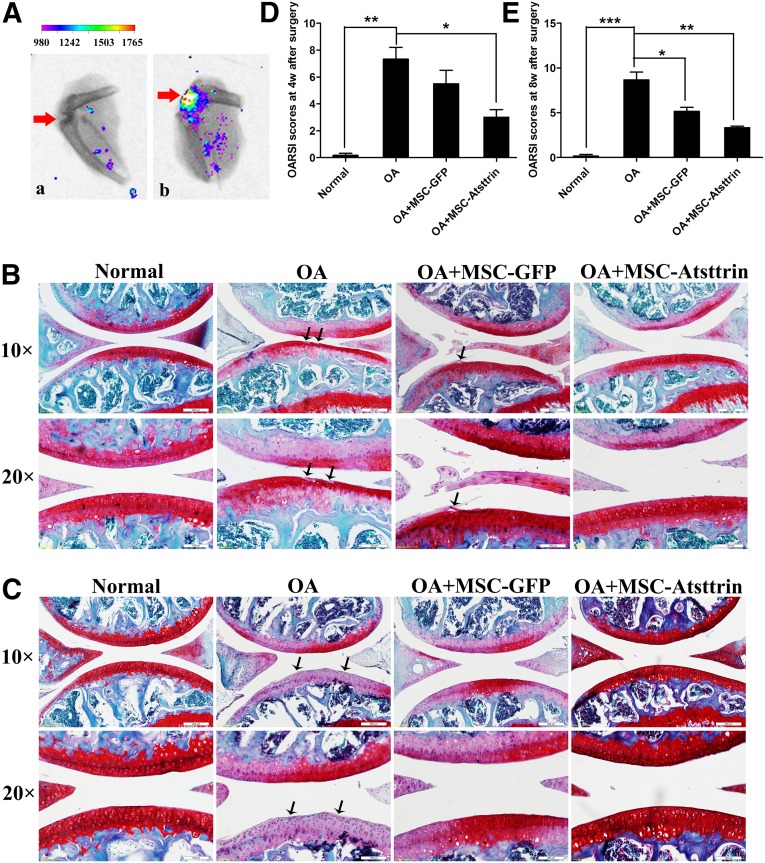
Intra-articular injection of MSC-Atsttrin was used to investigate whether transplantation of genetically modified MSCs could delay OA progression in mice. Immunohistochemical staining and OARSI scoring were used to evaluate mice joints harvested at 4 weeks and 8 weeks postsurgery. Compared with normal cartilage samples, the untreated samples at 4 weeks after ACLT (OA group) displayed the most serious defect in cartilage surface and the highest OARSI score (7.33 ± 1.5). Similar OA progression with a rough cartilage surface and decreased Safranin Orange staining was also observed in the MSC-GFP group, although a slightly better healing outcome was manifested by the relatively lower OARSI score (5.50 ± 1.7). Interestingly, intra-articular injection of MSC-Atsttrin significantly ameliorated OA progression, as evidenced by much less cartilage degradation, more Safranin Orange staining (Fig. 4B), and the lowest OARSI score (3.00 ± 1.0) among all the OA-related groups (Fig. 4D).
Figure 4.
Intra-articular injection of MSC-Atsttrin delayed OA cartilage degradation. (A): Cell tracking displayed positive orange fluorescent signal in the joint 1 week after cell injection by Color CCD. (a) represents the OA group without cell transplantation, and (b) represents the tracking image of OA+MSC-Atsttrin group (the color coding and grades showed the intensity of fluorescent signal). (B, C): Safranin Orange staining of cartilage samples at 4 weeks (B) and 8 weeks (C). Scale bars = 200 or 100 µm. (C): After anterior cruciate ligament transection, with arrows showing the defect area. Scale bars = 200 or 100 µm. (D, E): OARSI scores of samples at 4 (D) and 8 (E) weeks after OA induction. ∗ , p < .05; ∗∗, p < .01; ∗∗∗, p < .001 (n = 6). Abbreviations: GFP, green fluorescent protein; MSC, mesenchymal stem cell; OA, osteoarthritis; OARSI, Osteoarthritis Research Society International.
As shown in Figure 4C and 4E, the OA group at 8 weeks postsurgery exhibited an ossified surface, decreased Safranin O staining, and the highest OARSI score (8.67 ± 1.5). The injection of MSC-GFP resulted in better surface histology and a significantly lower OARSI score (5.17 ± 0.8). However, MSC-Atsttrin transplantation resulted in the best joint surface among all the experimental groups and was associated with the lowest OARSI score (3.33 ± 0.3). Taken together, these results suggest that transplantation of MSCs was beneficial in delaying the long-term progression of OA. Furthermore, Atsttrin enhanced the protective role of MSCs and delayed the onset of OA more efficaciously, as evidenced by the histology data.
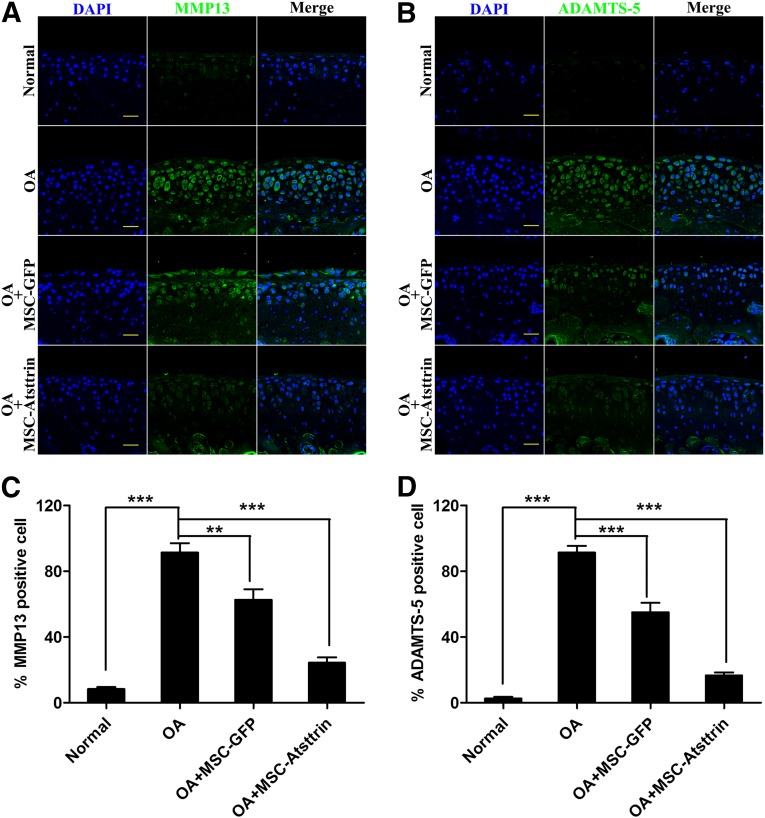
Immunohistochemistry was used to evaluate whether MSC-Atsttrin suppressed the production of detrimental cartilage extracellular matrix (ECM) degradation proteinases, MMP13 and ADAMTS-5, in all groups (Fig. 5). Compared with the untreated OA group, we found that both MMP13 (Fig. 5A) and ADAMTS-5 (Fig. 5B) were suppressed in the cell-transplanted groups at 8 weeks postsurgery. Additionally, the MSC-Atsttrin decreased the numbers of positive-stained cells further, which had a scattered distribution within the cartilage layers. Quantitative analysis affirmed these results. Transplantation of MSC-Atsttrin in mouse joints significantly decreased the percentages of MMP13- and ADAMTS-5-positive chondrocytes (Fig. 5C, 5D). With respect to MMP13, the ratios of positive cells were reduced from 91.33% ± 9.87% to 62.67% ± 11.02% and 24.33% ± 5.7% (p < .001) for the MSC-GFP and MSC-Atsttrin transplant groups, respectively. Similar results were observed with ADAMTS-5 expression, with the ratios of positive cells being reduced from 91.33% ± 7.1% to 55.00% ± 10.00% and 16.67% ± 3.1% (p < .001). Based on these data, we concluded that MSCs transplantation, particularly when Atsttrin was stably expressed, efficiently reduced the production of harmful ECM hydrolytes.
Figure 5.
Intra-articular injection of MSC-Atsttrin suppressed MMP13 and ADAMTS-5 expression during OA progression. (A, B): Comparison of MMP13 (A) and ADAMTS-5 (B) expression levels by immunohistochemical staining of cartilage samples at 8 weeks postsurgery. Scale bars = 100 µm. (C, D): Quantification of MMP13-positive (C) and ADAMTS-5-positive (D) cells within cartilage samples at 8 weeks postsurgery. ∗∗, p < .01; ∗∗∗, p < .001 (n = 3). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; GFP, green fluorescent protein; MSC, mesenchymal stem cell; OA, osteoarthritis.
Discussion
In this study, we demonstrated gene therapy for OA by intra-articular injection of Atsttrin-transduced MSCs (MSC-Atsttrin). Firstly, a mesenchymal stem cell line (C3H) was genetically modified with lentiviral vectors encoding Atsttrin under the transcriptional control of tetracycline-inducible promoters, which enabled efficient expression of the recombinant protein by MSCs. Secondly, the functions of the genetically engineered cells were evaluated by coculturing with human chondrocytes in the presence of TNFα, which led to downregulation of the production of inflammatory factors. The presence of MSC-Atsttrin could largely suppress the enhanced levels of MMP13, ADAMTS-5, and iNOS that were induced by TNFα, thereby exerting a chondro-protective effect. Thirdly, it was demonstrated that MSC-Atsttrin exhibited promising results when used to treat surgically induced osteoarthritis. Detrimental catabolic factors were suppressed in the mouse OA model, similar to what was observed in vitro. These data suggest that recombinant Atsttrin delivered by intra-articular transplantation of genetically modified stem cells can pave the way for innovative therapy of OA.
Based on previous findings with RA models, Atsttrin is expected to be therapeutically useful for multiple TNFα-associated conditions and pathologies [11]. Investigations of Atsttrin focused on two directions. The first line of investigation is to unravel the underlying mechanism of interaction between Atsttrin and TNFR family members. Screening the association of Atsttrin with other members of the TNFR subfamily demonstrated that TNFRSF25 was another interacting member besides TNFR1 and TNFR2 [19]. Additionally, it was found that alternating the order of the three fragments of Atsttrin did not influence anti-TNFα activities, implying that the three domains of Atsttrin functioned independently [20]. The second research direction is to evaluate whether Atsttrin treatment can be effective in other TNFα-associated inflammatory diseases, such as amelioration of pathology in dextran sulfate-induced colitis [19] and attenuation of inflammation in a mouse dermatitis model [16]. Our study investigated the potential use of Atsttrin to treat another inflammatory condition, osteoarthritis.
As an autocrine growth factor with cytokine-like properties, the naturally occurring TNFα antagonist progranulin also contributes to tumorigenesis in various cancers [33]. Atsttrin is considered to be safer than PGRN because each domain of the fused chimeric protein just maintains the binding affinity to TNFR while exhibiting less cytokine-like properties [11, 20]. Notably, no safety concerns of Atsttrin have been reported to date. Nevertheless, suppression of TNFα activity has been reported to increase opportunistic infections and even render increased susceptibility to cancer [34, 35]. In view of this potential detrimental side effect, we performed localized intra-articular injection of genetically modified MSCs and used controllable expression of the target gene.
Adult stem/progenitor cell transplantation has been shown to be an effective treatment strategy for a range of diseases. Transplanted stem cells contribute to the regeneration of injured cartilage through three distinct routes: differentiation into chondrocytes, secretion of growth factors, and suppression of inflammation [22]. However, the bottleneck limitation of this therapeutic strategy is the low cell survival rate (0.2%–10%) upon transplantation in vivo [36, 37]. That is, most of the transplanted cells die off in the short term, which may be due to environmental stress that the cells encounter after engraftment. In our study, the injected cells could be tracked after 1 week by DiI staining. However, after 4 weeks, no signal was detectable (data not shown) because of the low cell retention rate. The earlier therapeutic intervention is being carried out during OA progression, the better would be the treatment outcome achieved [38]. Hence, the survival of transplanted cells in the first few weeks is crucial to the inhibition of OA progression. In future studies, various measures may be used to extend cell survival so as to further enhance the treatment outcome. Another major limitation of this study is that the fate of the engrafted cells was not tracked successfully, although we have tried to detect the presence of positively stained cells with GFP-conjugated antibody in the joints (data not shown). The main reason for the low cell retention rate may be that diffusion takes place readily after intrajoint injection, as well as the low survival rate of the transplanted cells over the long term. Nevertheless, these limitations do not obscure the potential of MSC-based gene therapy approach to OA repair in the surgically induced mouse OA model.
Conclusion
In this study, it is demonstrated that MSC-Atsttrin plays a chondro-protective role and delays the progression of OA in vitro and in vivo by suppressing the expression of catabolic factors and enhancing the production of beneficial cartilage extracellular matrix. Taken together, our results highlight a novel potential clinical therapeutic approach to inhibiting TNFα and ameliorating OA development.
Acknowledgments
This work was supported by National Key Scientific Research Project 2012CB96660, the National Natural Science Fund (81125014, 81101356, 81201395, and 81472115), the Zhejiang province public welfare fund (2012C3112), the Zhejiang Provincial Natural Science Foundation of China (LY13C100001), the Technology Development Project (CXZZ201 30320172336579) of the Science Technology and Innovation Committee of Shenzhen Municipality, and the Postdoctoral Foundation of China (2013M531465), and was sponsored by Regenerative Medicine in Innovative Medical Subjects of Zhejiang Province and the Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents. We thank Huang Lu for providing the human OA cartilage joints from patients undergoing total knee replacement surgery. We also thank the Imaging Center and Core Facilities of Zhejiang University School of Medicine for technical assistance.
Author Contributions
Q.X.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; S.Z., Y.W., J.W., P.C., and J.L.: collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; Y.C.: final approval of manuscript; B.C.H.: manuscript writing, final approval of manuscript; H.W.O.: research design, final approval of manuscript; P.L.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, research design, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Chevalier X, Eymard F, Richette P. Biologic agents in osteoarthritis: Hopes and disappointments. Nat Rev Rheumatol. 2013;9:400–410. doi: 10.1038/nrrheum.2013.44. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7:43–49. doi: 10.1038/nrrheum.2010.197. [DOI] [PubMed] [Google Scholar]
- 4.Hunter DJ. Pharmacologic therapy for osteoarthritis: The era of disease modification. Nat Rev Rheumatol. 2011;7:13–22. doi: 10.1038/nrrheum.2010.178. [DOI] [PubMed] [Google Scholar]
- 5.Valdes AM, Spector TD. Genetic epidemiology of hip and knee osteoarthritis. Nat Rev Rheumatol. 2011;7:23–32. doi: 10.1038/nrrheum.2010.191. [DOI] [PubMed] [Google Scholar]
- 6.Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 7.Goldring MB. Osteoarthritis and cartilage: The role of cytokines. Curr Rheumatol Rep. 2000;2:459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 8.Westacott CI, Barakat AF, Wood L, et al. Tumor necrosis factor alpha can contribute to focal loss of cartilage in osteoarthritis. Osteoarthritis Cartilage. 2000;8:213–221. doi: 10.1053/joca.1999.0292. [DOI] [PubMed] [Google Scholar]
- 9.Stannus O, Jones G, Cicuttini F, et al. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18:1441–1447. doi: 10.1016/j.joca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Vangsness CT, Jr, Burke WS, Narvy SJ, et al. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis: A pilot study. Bull NYU Hosp Jt Dis. 2011;69:122–127. [PubMed] [Google Scholar]
- 11.Tang W, Lu Y, Tian QY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–484. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thurner L, Zaks M, Preuss KD, et al. Progranulin antibodies entertain a proinflammatory environment in a subgroup of patients with psoriatic arthritis. Arthritis Res Ther. 2013;15:R211. doi: 10.1186/ar4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goel N, Stephens S. Certolizumab pegol. MAbs. 2010;2:137–147. doi: 10.4161/mabs.2.2.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Güler-Yüksel M, Allaart CF, Watt I, et al. Treatment with TNF-α inhibitor infliximab might reduce hand osteoarthritis in patients with rheumatoid arthritis. Osteoarthritis Cartilage. 2010;18:1256–1262. doi: 10.1016/j.joca.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Liu CJ. Progranulin: A promising therapeutic target for rheumatoid arthritis. FEBS Lett. 2011;585:3675–3680. doi: 10.1016/j.febslet.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao YP, Tian QY, Liu CJ. Progranulin deficiency exaggerates, whereas progranulin-derived Atsttrin attenuates, severity of dermatitis in mice. FEBS Lett. 2013;587:1805–1810. doi: 10.1016/j.febslet.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu K, Zhang Y, Ilalov K, et al. Cartilage oligomeric matrix protein associates with granulin-epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferation. J Biol Chem. 2007;282:11347–11355. doi: 10.1074/jbc.M608744200. [DOI] [PubMed] [Google Scholar]
- 18.Guo F, Lai Y, Tian Q, et al. Granulin-epithelin precursor binds directly to ADAMTS-7 and ADAMTS-12 and inhibits their degradation of cartilage oligomeric matrix protein. Arthritis Rheum. 2010;62:2023–2036. doi: 10.1002/art.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C, Li XX, Gao W, et al. Progranulin-derived Atsttrin directly binds to TNFRSF25 (DR3) and inhibits TNF-like ligand 1A (TL1A) activity. PLoS One. 2014;9:e92743. doi: 10.1371/journal.pone.0092743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian Q, Zhao Y, Mundra JJ, et al. Three TNFR-binding domains of PGRN act independently in inhibition of TNF-alpha binding and activity. Front Biosci (Landmark Ed) 2014;19:1176–1185. doi: 10.2741/4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 22.Gupta PK, Das AK, Chullikana A, et al. Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther. 2012;3:25. doi: 10.1186/scrt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grogan SP, Miyaki S, Asahara H, et al. Mesenchymal progenitor cell markers in human articular cartilage: Normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11:R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koelling S, Kruegel J, Irmer M, et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4:324–335. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells. 2014;32:1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 26.Nöth U, Steinert AF, Tuan RS. Technology insight: Adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;4:371–380. doi: 10.1038/ncprheum0816. [DOI] [PubMed] [Google Scholar]
- 27.Gurusinghe S, Strappe P. Gene modification of mesenchymal stem cells and articular chondrocytes to enhance chondrogenesis. Biomed Res Int. 2014;2014:369528. doi: 10.1155/2014/369528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu P, Feng MG, Li WF, et al. Construction and characterization of a bifunctional fusion enzyme of Bacillus-sourced beta-glucanase and xylanase expressed in Escherichia coli. FEMS Microbiol Lett. 2006;261:224–230. doi: 10.1111/j.1574-6968.2006.00367.x. [DOI] [PubMed] [Google Scholar]
- 29.Lu P, Feng MG. Bifunctional enhancement of a beta-glucanase-xylanase fusion enzyme by optimization of peptide linkers. Appl Microbiol Biotechnol. 2008;79:579–587. doi: 10.1007/s00253-008-1468-4. [DOI] [PubMed] [Google Scholar]
- 30.Vierbuchen T, Ostermeier A, Pang ZP, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S, Jiang YZ, Zhang W, et al. Neonatal desensitization supports long-term survival and functional integration of human embryonic stem cell-derived mesenchymal stem cells in rat joint cartilage without immunosuppression. Stem Cells Dev. 2013;22:90–101. doi: 10.1089/scd.2012.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glasson SS, Chambers MG, Van Den Berg WB, et al. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 33.Ong CH, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell derived growth factor, acrogranin) in proliferation and tumorigenesis. Histol Histopathol. 2003;18:1275–1288. doi: 10.14670/HH-18.1275. [DOI] [PubMed] [Google Scholar]
- 34.Dinarello CA. Anti-inflammatory agents: Present and future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dinarello CA. Differences between anti-tumor necrosis factor-alpha monoclonal antibodies and soluble TNF receptors in host defense impairment. J Rheumatol Suppl. 2005;74:40–47. [PubMed] [Google Scholar]
- 36.Terrovitis JV, Smith RR, Marbán E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106:479–494. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooke MJ, Vulicb K, Shoichet MS. Design of biomaterials to enhance stem cell survival when transplanted into the damaged central nervous system. Soft Matter. 2010;6:4988–4998. [Google Scholar]
- 38.Altman RD. Early management of osteoarthritis. Am J Manag Care. 2010;16(Suppl Management):S41–S47. [PubMed] [Google Scholar]