This study investigated the antiaging properties of bone marrow mesenchymal stem cells (BMSCs) and the underlying mechanism in a cellular model of cardiomyocyte senescence and a rat model of aging hearts. BMSC transplantation significantly prevented detrimental alterations and improved the impaired cardiac function in the aging rats. The present study therefore provides an alternative approach for the treatment of heart failure in the elderly population.
Keywords: Senescence, Bone marrow mesenchymal stem cells, Heart, Reactive oxygen species, p53
Abstract
Bone marrow mesenchymal stem cells (BMSCs) have been shown to offer a wide variety of cellular functions including the protective effects on damaged hearts. Here we investigated the antiaging properties of BMSCs and the underlying mechanism in a cellular model of cardiomyocyte senescence and a rat model of aging hearts. Neonatal rat ventricular cells (NRVCs) and BMSCs were cocultured in the same dish with a semipermeable membrane to separate the two populations. Monocultured NRVCs displayed the senescence-associated phenotypes, characterized by an increase in the number of β-galactosidase-positive cells and decreases in the degradation and disappearance of cellular organelles in a time-dependent manner. The levels of reactive oxygen species and malondialdehyde were elevated, whereas the activities of antioxidant enzymes superoxide dismutase and glutathione peroxidase were decreased, along with upregulation of p53, p21Cip1/Waf1, and p16INK4a in the aging cardiomyocytes. These deleterious alterations were abrogated in aging NRVCs cocultured with BMSCs. Qualitatively, the same senescent phenotypes were consistently observed in aging rat hearts. Notably, BMSC transplantation significantly prevented these detrimental alterations and improved the impaired cardiac function in the aging rats. In summary, BMSCs possess strong antisenescence action on the aging NRVCs and hearts and can improve cardiac function after transplantation in aging rats. The present study, therefore, provides an alternative approach for the treatment of heart failure in the elderly population.
Significance
This study demonstrates that bone marrow mesenchymal stem cells show antisenescence action on aging neonatal rat ventricular cells and heart and improve cardiac function in aging rats. The results provide a novel strategy for retarding the cardiac aging process in physiology and abnormal conditions or pathology. This study also provides an alternative approach for treatment of heart failure in the elderly population.
Introduction
Aging is a complex and multifactorial process resulting in damage to molecules, cells, and tissues leading to structural and functional declining of organs or organisms. Accumulating evidence has demonstrated that advanced age is closely associated with abnormalities of cardiac performance and structure, such as declines in left-ventricular relaxation and diastolic function [1, 2] and increases in left ventricle weight and cardiomyocyte size, as well as interstitial fibrosis [2]. Cardiac senescence is not only a physiological process during the natural aging process but also a pathological alteration in many cardiac conditions such as heart failure [3, 4]. Although it has been believed that delaying cardiomyocyte senescence is a vivid approach for the management of cardiac disease, complete understanding of the molecular mechanisms for aging physiology and pathology and efficient strategies for retarding the process are still lacking.
Senescence is a unique pathophysiological process determined by a variety of factors and pathways, such as telomere length [5], a number of genes [6, 7], oxidative stress [8], transcription factors, and pathways involved in aging. Reactive oxygen species (ROS), endogenous products from metabolism, are essential second messages in cell signaling in physiological conditions that regulate cell proliferation, cell cycle arrest, and cell death [9]. Furthermore, ROS is also a crucial determinant in cellular aging. Additionally, p53, p21Cip1/Waf1 [10, 11], and p16INK4a are also involved in the process of cellular senescence [12].
Because of the multifactorial nature of aging, gene therapy, although attractive, is facing great challenges. Additionally, cell therapy has demonstrated promising outcomes on aging. Bone marrow mesenchymal stem cells (BMSCs) are capable of self-renewal and can transdifferentiate under certain conditions into a variety of cell types such as cardiomyocytes, neurons, osteoblasts, and smooth muscle cells [13, 14]. It has been reported that BMSCs can improve stroke by secreting neurotrophic factors, supporting survival of neuroblasts, and promoting neurovascular remodeling and neocortical circuit restoration [15, 16]. BMSCs also have therapeutic effects in diabetes mellitus and kidney disease [17], reverse age-related degeneration of multiple organs [18], restore physical and cognitive functions of aged mice [19], and improve age-associated osteoporosis, Parkinson’s disease, and atherosclerosis [20].
Multiple lines of evidence have shown that BMSC transplantation produces significant improvement of cardiac performance owing to the myocardial regeneration, anti-inflammation, antifibrosis, and immunosuppressive actions evoked by these cells in animal studies and clinical trials [21]. It is reported that BMSC transplantation profoundly improves cardiac performance of infarcted rat hearts by differentiation into cardiomyocytes [13, 22], immunosuppressant activity [23], and secreting the protective factors or cytokines [23–25]. We have previously shown that BMSCs offer an antisenescence action on cardiomyocytes undergoing the pathological aging process induced by hypoxia/reoxygenation via blocking two important CDK inhibitors, p53 and p21Cip1/Waf1, which are crucial for turning on the premature senescence [26]. However, it is unclear whether these findings from a cellular model can be applied to more physiological conditions in whole animals. Further, although this previous study revealed the efficacy of BMSCs in fighting the hypoxia/reoxygenation-induced senescence of cardiomyocytes, it remains unknown whether BMSCs are also able to retard other pathological aging processes in cardiomyocytes and hearts.
To continue and expand our studies, and resolve the issues mentioned above, we conducted the present study looking at the effects of BMSCs on senescence-related phenotypes of the aging process in neonatal rat ventricular cells (NRVCs) and rat hearts. Our results demonstrated that BMSCs produce significant protective effects against senescence of cardiomyocytes and the aging rat heart.
Materials and Methods
Animals
Healthy male Sprague Dawley (SD) rats, 4 months (young) and 20 months (old) old, were used in the present study. The rats were kept under standard conditions (temperature 21°C ± 1°C; humidity 55%–60%) with food and water available ad libitum for 1 week before the experiment. All experimental procedures were in accordance with the guidelines and approved by the Institutional Animal Care and Use Committee of Harbin Medical University.
Isolation and Culture of Cells
NRVCs were isolated from 1- to 3-day-old neonatal SD rats. The heart tissue was digested into single cardiomyocytes by 0.25% trypsin. Pooled cell suspensions were centrifuged and resuspended in Dulbecco’s modified Eagle’s medium (DMEM) with 25 mM glucose (Hyclone, Logan, UT, http://www.hyclone.com) supplemented with 10% fetal bovine serum (FBS; Hyclone), 100 U/ml penicillin, and 100 μg/ml streptomycin. After the fibroblasts were removed, the NRVCs were plated into a 6-well plate at a density of 1 × 106 per well and incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
BMSCs were isolated from SD rats (male, 2 months old, body weight 70 ± 20 g) as previously described [25]. After rats were anesthetized with pentobarbital (40 mg/kg, i.v.), their femurs and tibias were taken, and bone marrow cells were flushed out into culture flasks with BMSC culture medium (Stem Cell Technologies Inc., Vancouver, BC, Canada, http://www.stemcell.com) and then cultured in BMSC medium supplemented with 10% FBS (Stem Cell Technologies Inc.) and 5% CO2 at 37°C. All experiments were performed with cells from the third passage.
Cardiomyocytes were cultured alone (monoculture) or cocultured with BMSCs at a ratio of 10:1 in the same culture dish with a semipermeable membrane to separate the two populations. Culture medium was changed every 48 hours.
Measurement of Senescence-Associated β-Galactosidase Activity
Activity of senescence-associated β-galactosidase (SA-β-gal) in cardiomyocytes was analyzed using senescence β-galactosidase staining kit (Genmed Scientifics Inc., Boston, MA, http://www.genmedoem.com), according to the manufacturer’s instructions. The cells were examined under a microscope, and SA-β-gal-positive cells were counted. Activity of SA-β-gal in heart tissue was analyzed with the tissue β-galactosidase activity assay kit (Genmed Scientifics Inc.). The absorbance was detected in 420 nm using a microplate reader, and activity of SA-β-gal was calculated in accordance with the manufacturer’s instructions.
Measurement of MDA Level and Activities of SOD and GSH-Px
The level of malondialdehyde (MDA) and activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in NRVCs and cardiac tissue were measured using MDA, SOD, and GSH-Px detection kits (Beyotime Institute of Biotechnology, Shanghai, China, http://www.beyotime.com), respectively, according to the manufacturer’s instructions.
Detection of ROS Level
The level of intracellular ROS was evaluated by reactive oxygen species assay kit (Beyotime Institute of Biotechnology). The fluorescence intensity was measured using a flow cytometer under an excitation wavelength of 488 nm.
Western Blotting
Total protein was extracted from the NRVCs and the left ventricle tissue of rats for immunoblotting analysis, respectively. Protein samples (60-80 μg) were separated in 15% SDS-PAGE and blotted to nitrocellulose membranes. After blocking, the membranes were probed with p16INK4a (Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com), p21Cip1/Waf1 (BD Pharmingen, Franklin lakes, NJ, http://www.bdbiosciences.com), p53 (Cell Signaling Technology, Danvers, MA, http://www.cellsignal.com), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies (Kangcheng Inc., Shanghai, China, http://kcbiochip.bioon.com.cn) incubated overnight at 4°C. IR fluorescent dye-labeled secondary antibody (Alexa Fluor; Molecular Probes, Eugene, OR, http://probes.invitrogen.com) was incubated with the membrane for 1 hour. Western blot bands were collected by using IR Imaging System (LI-COR Biosciences, Lincoln, NE, http://www.licor.com), and the band density was quantified using Odyssey 3.0 software for each group and normalized by GAPDH.
Acridine Orange/Ethidium Bromide Fluorescence Staining
The cells were incubated with acridine orange and ethidium bromide mixing solution for 5 minutes (Solarbio of Biotechnology, Beijing, China, http://solarbio.en.alibaba.com). Cellular morphological changes were examined by using fluorescence microscopy (×200). The percentage of apoptotic cells was calculated by the following formula: apoptotic rate (%) = number of apoptotic cells/number of all cells counted.
Transplantation of Stem Cells
The third generation of BMSCs (2 × 106 cells) was dispersed in 50 μl of PBS. After the rats had been anesthetized, a left-sided thoracotomy was performed, and the heart was exposed. The BMSCs were directly injected into the left-ventricular wall at five points in the old group. The young and the old control groups were injected with an equal volume of PBS. Subsequent experiments were conducted on the 3rd, 7th, and 15th day, respectively, following BMSC transplantation. The 5-ethynyl -2′-deoxyuridine (Edu; Ribo Inc., Guangzhou, China, http://www.ribobio.com) labeling method was used to detect the transplanted BMSCs in the recipient myocardium.
Histological Examination
The left ventricle of heart was cut into 5-µm-thick sections. Hematoxylin and eosin (H&E) staining was used to estimate the degree of cardiomyocyte hypertrophy. Masson’s trichrome staining was used to evaluate collagen deposition. Sections were imaged at ×200 magnification by bright-field microscopy. The extent of cardiac fibrosis in the region was assessed by calculating the collagen volume fraction. All quantitative evaluations were carried out by Image Pro Plus software.
Echocardiography
Cardiac function was evaluated by transthoracic echocardiography with an ultrasound machine Vevo2100 high-resolution imaging system (VisualSonics, Toronto, ON, Canada, http://www.visualsonics.com). The left-ventricular systolic diameter (LVSd) and left-ventricular diastolic diameter (LVDd) were measured, and left-ventricular end-diastolic volume (EDV), left-ventricular end-systolic volume (ESV), left-ventricular ejection fraction (EF), and fractional shortening (FS) were calculated from M-mode recording.
Statistical Analysis
The data are presented as means ± SEM or means ± SD. Statistical comparisons among multiple groups were performed by analysis of variance followed by Tukey’s multiple comparison test. Differences with values of p < .05 were regarded as statistically significant. Statistical values were calculated using the SPSS 19.0 software and illustrated using GraphPad Prism 5.0.
Results
BMSCs Attenuate Senescence-Associated Alterations in Cultured Neonatal Cardiomyocytes
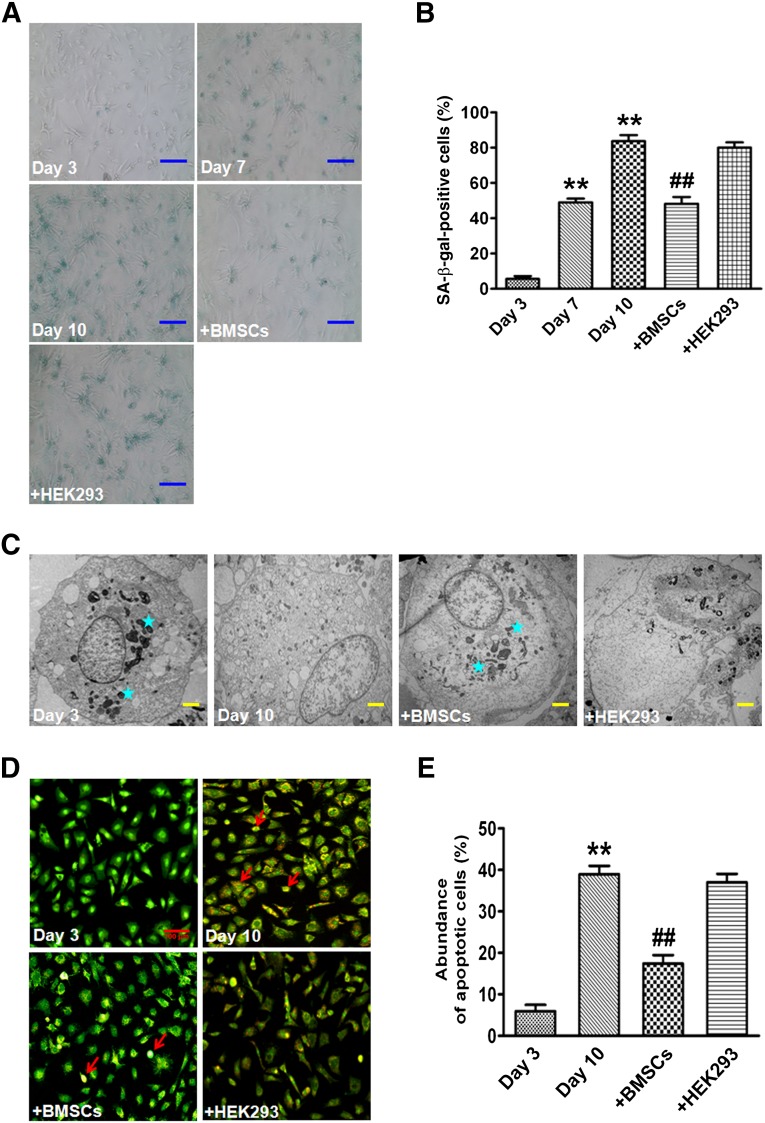
NRVCs were cultured in DMEM with 25 mM glucose that has been reported to induce cellular senescence [27, 28]. On days 7 and 10, the number of SA-β-gal-positive cells were markedly increased to 49.1% ± 3.5% and 83.7% ± 5.9% in NRVCs cultured alone (monoculture), respectively, compared with 5.6% ± 2.8% on day 3 as control (Fig. 1A, 1B). However, NRVCs cultured with BMSCs (coculture) presented a much smaller number of SA-β-gal-positive cells (48.2% ± 6.6%) than monocultured NRVCs on day 10. Of note, NRVCs cocultured with HEK293 cells did not show any significant change in the number of SA-β-gal-positive cells (Fig. 1A, 1B). In addition, cellular organelles were degraded, a common feature of senescence, in 10-day monocultured cardiomyocytes. However, cocultured NRVCs with BMSCs had less degradation of cellular organelles (Fig. 1C). Additionally, cardiomyocytes cultured for 10 days exhibited enhanced apoptosis (38.9% ± 3.4%) compared with those cultured for 3 days (5.9% ± 2.6%). Apoptotic cells were significantly reduced in cocultured cardiomyocytes with BMSCs (17.5% ± 3.5%) (Fig. 1D, 1E). This finding suggests that stem cells are able to enhance the antiapoptotic capacity of aging cardiomyocytes.
Figure 1.
BMSCs change the senescence-associated phenotype of cardiomyocytes. (A): Representative image of SA-β-gal staining of monocultured cardiomyocytes on days 3, 7, and 10 and cocultured cardiomyocytes with BMSCs or HEK293 on day 10, respectively. Scale bars = 100 μm. (B): Percentage of SA-β-gal-positive cells in different groups. (C): Micromorphological changes in cellular organelles examined by transmission electron microscopy. Stars indicate organelles. Scale bars = 2 μm. (D): Representative image of acridine orange/ethidium bromide staining of cardiomyocytes. Red arrows indicate apoptotic cells. Scale bar = 100 μm. (E): Percentage of apoptotic cells in four different groups. The data are expressed as means ± SEM, n = 3 for each group. ∗∗, p < .01 versus day 3; ##, p < .01 versus day 10. Abbreviations: β-gal, β-galactosidase; BSMC, bone marrow mesenchymal stem cell; SA, senescence-associated.
BMSCs Inhibit Oxidative Stress in Senescent Cardiomyocytes
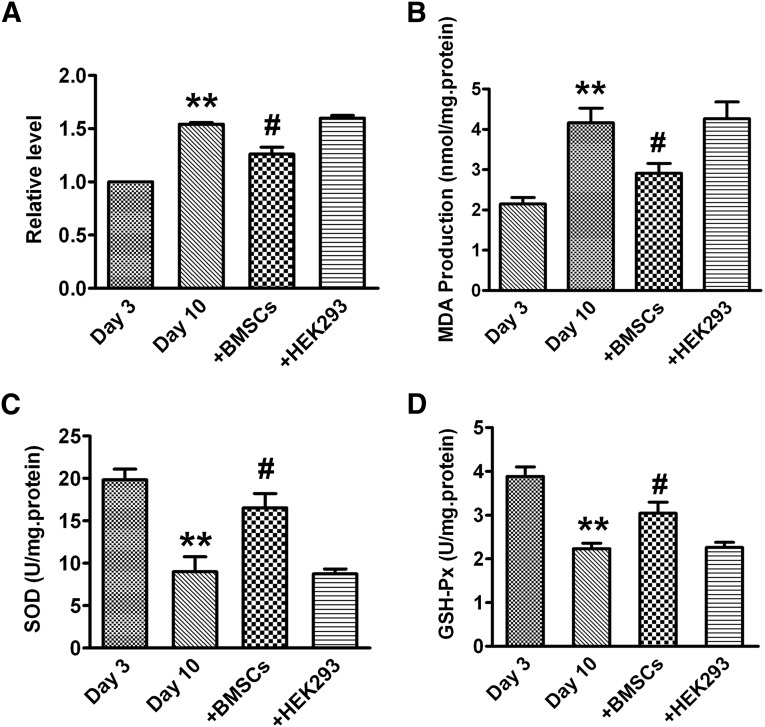
Levels of ROS and MDA were enhanced in 10-day monocultured cardiomyocytes compared with that in 3-day monocultured cells. However, the cardiomyocytes cocultured with BMSCs showed significant decreases in ROS and MDA levels. NRVCs cocultured with HEK293 cells did not show any significant changes of ROS and MDA levels (Fig. 2A, 2B). Additionally, the activities of SOD and GSH-Px were markedly reduced in 10-day monocultured cardiomyocytes, and the activities of SOD and GSH-Px were significantly increased in the cocultured group. As expected, HEK293 cells had no effect on the decreased SOD and GSH-Px activities (Fig. 2C, 2D).
Figure 2.
BMSCs improve the antioxidative capacity of cardiomyocytes. (A): Reactive oxygen species level. (B): MDA level. (C): SOD activity. (D): GSH-Px activity. The data are expressed as means ± SEM, n = 3 for each group; ∗∗, p < .01 versus day 3; #, p < .05 versus day 10. Abbreviations: BSMC, bone marrow mesenchymal stem cell; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase.
BMSCs Reduce Expression of p53 and p21Cip1/Waf1 in Senescent Cardiomyocytes
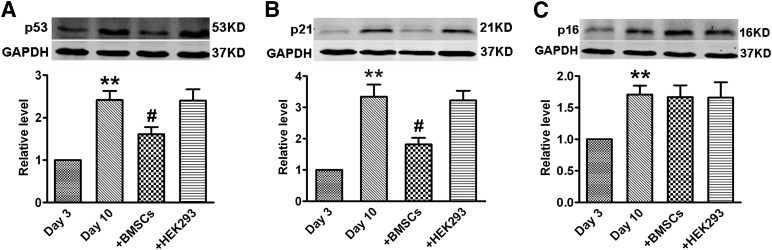
We further examined the expression of senescence-related proteins p53, p21Cip1/Waf1, and p16INK4a. Western blot assay showed that the expression of p53, p21Cip1/Waf1, and p16INK4a were significantly augmented in 10-day monocultured cardiomyocytes compared with 3-day monocultured cells (Fig. 3A–3C). The expression of p53 and p21Cip1/Waf1 was decreased in the cocultured cardiomyocytes with BMSCs compared with monocultured cells. As expected, HEK293 as a negative control had no effects on the increased expression of p53 and p21Cip1/Waf1 in the cardiomyocytes (Fig. 3A, 3B). Although increased p16INK4a expression was observed in 10-day monocultured cardiomyocytes, BMSCs did not affect this upregulation in the senescent NRVCs (Fig. 3C).
Figure 3.
BMSCs reduce the expression of p53 and p21Cip1/Waf1 in cardiomyocytes. Western blot analysis was used to evaluate the proteins expression. (A): p53 protein level. (B): p21Cip1/Waf1 protein level. (C): p16INK4a protein level. The data are expressed as means ± SEM, n = 3 for each group. ∗∗, p < .01 versus day 3; #, p < .05 versus day 10. Abbreviations: BSMC, bone marrow mesenchymal stem cell; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; KD, kilodalton.
Effects of BMSC Transplantation on SA-β-gal Activity, MDA Level, and SOD and GSH-Px Activities in In Vivo Study
To demonstrate the presence of transplanted BMSCs in the recipient myocardium, Edu, a DNA synthetic probe, was used to label BMSCs. Edu-positive BMSCs were consistently detected in the rat heart transplanted with BMSCs labeled with Edu after 72 hours of BMSCs injection, indicating survival of the transplanted BMSCs in the heart tissue in our experimental conditions (supplemental online Fig. 1; supplemental online data).
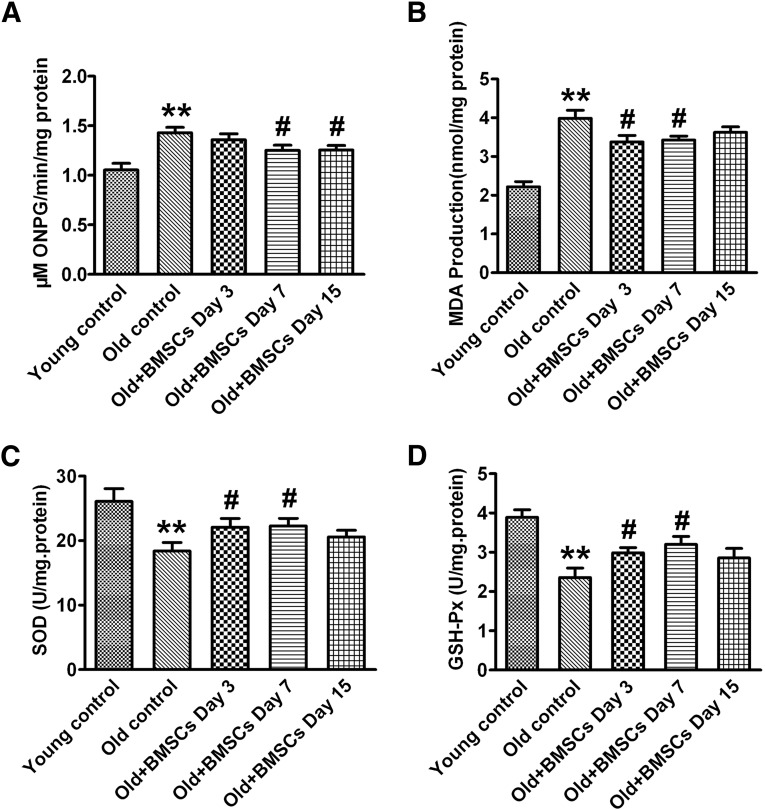
To confirm the above in vitro results, 20-month-old rats were used as an animal model of aging. We found that the SA-β-gal activity was markedly enhanced in the heart from aging rats compared with that from 4-month-old rats. The activity of SA-β-gal was significantly decreased on days 7 and 15 after implantation of BMSCs (Fig. 4A). Meanwhile, there was a substantial decline in MDA level on days 3 and 7 after BMSC implantation (Fig. 4B). Additionally, BMSC transplantation significantly normalized the decreased SOD (Fig. 4C) and GSH-Px activities (Fig. 4D) on days 3 and 7 after BMSC implantation.
Figure 4.
Transplantation of BMSCs decreases the senescence-associated (SA)-β-galactosidase (β-gal) activity and improves the antioxidant ability in the heart tissue at different time points. (A): SA-β-gal activity. (B): MDA level. (C): SOD activity. (D): GSH-Px activity. The data are expressed as means ± SEM, n = 6 for each group. ∗∗, p < .01 versus young control; #, p < .05 versus old control. Abbreviations: BSMC, bone marrow mesenchymal stem cell; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; ONPG, ortho-nitrophenyl-β-galactoside; SOD, superoxide dismutase.
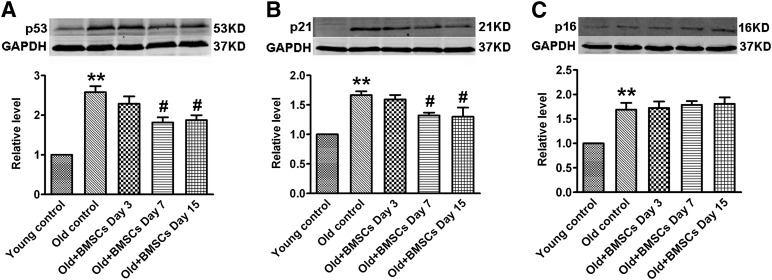
BMSC Transplantation Decreases Expression of p53 and p21Cip1/Waf1 but Not p16INK4a in the Aging Rat Hearts
To further confirm our in vitro results on the signaling mediators, we demonstrated that p53, p21Cip1/Waf1, and p16INK4a expression at the protein level was markedly upregulated in heart tissue from aging rats compared with that from young rats. BMSC transplantation significantly decreased the aberrantly upregulated p53 (Fig. 5A) and p21Cip1/Waf1 (Fig. 5B) on days 7 and 15 after BMSC implantation, but the expression of p16INK4a was not changed (Fig. 5C). These observations are consistent with our in vitro results.
Figure 5.
Transplantation of BMSCs reduces the expression of p53 and p21Cip1/Waf1 in the heart at different time points. (A): p53 protein level. (B): p21Cip1/Waf1 protein level. (C): p16INK4a protein level. The data are expressed as means ± SEM, n = 3 for each group. ∗∗, p <.01 versus young control; #, p < .05 versus old control. Abbreviations: BSMC, bone marrow mesenchymal stem cell; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; KD, kilodalton.
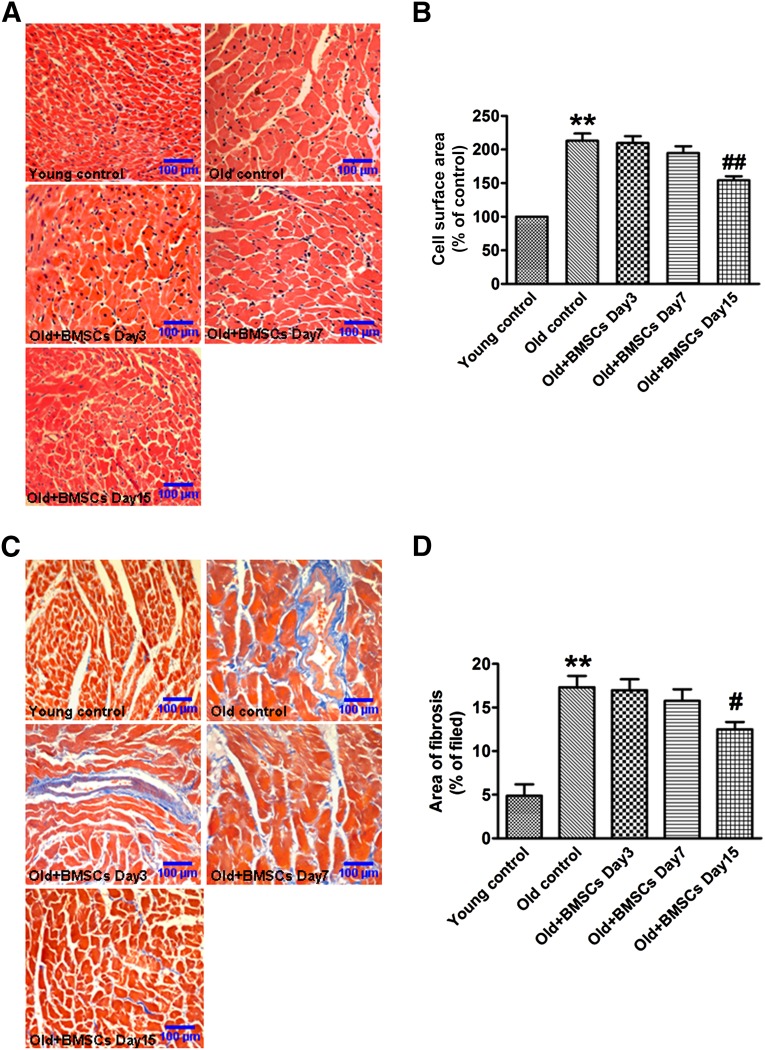
BMSC Transplantation Attenuates the Age-Associated Cardiac Hypertrophy and Fibrosis
To evaluate the effects of BMSCs on age-associated cardiac hypertrophy and fibrosis, H&E staining and Masson staining were used to analyze changes in the histological structure of the heart. Our data showed that the cross-sectional area of cardiac cells and the interstitial collagen content were significantly increased in the aging rats compared with young rats. On day 15 after stem cells transplantation, however, the cross-sectional area and the collagen content were significantly decreased compared with the old control group (Fig. 6A–6C).
Figure 6.
Transplantation of BMSCs reduces the age-associated cardiac hypertrophy and fibrosis. (A): Representative images of hematoxylin and eosin staining at a magnification of ×200. (B): Summarized data of cross-sectional area in different groups. (C): Representative sections of heart with Masson trichrome staining at a magnification of ×200. Fibrotic tissues were stained blue, and cardiomyocytes were stained red. (D): Collagen deposition was quantified with an automated image analyzer and is expressed as percentage of tissue area. The data are expressed as means ± SEM, n = 5 for each group. ∗∗, p < .01 versus young control; #, p < .05; ##, p < .01 versus old control. Abbreviation: BSMC, bone marrow mesenchymal stem cell.
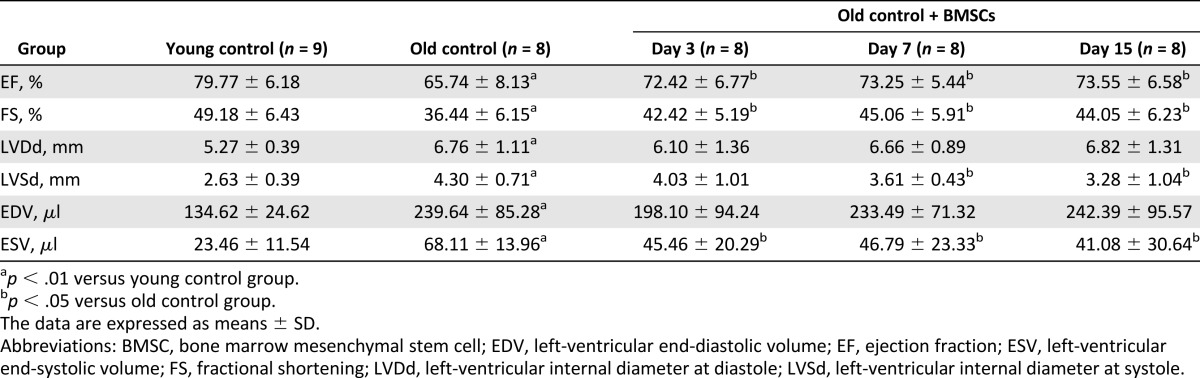
BMSC Transplantation Improves Cardiac Function in Aging Rats
Finally, we used echocardiography techniques to evaluate cardiac function in the rats. Our data showed that cardiac function was impaired in the aging rats, as reflected by the reduced EF and FS and the increased LVDd, LVSd, ESV, and EDV. Notably, BMSCs significantly ameliorated the impairment of cardiac function by increasing EF, FS, and ESV as early as on day 3 after BMSC transplantation (Table 1). Additionally, on days 7 and 15 after BMSC transplantation, the increased LVSd was significantly reduced.
Table 1.
BMSC transplantation improved the cardiac function in aging rats
Discussion
BMSCs possess a variety of unique biological properties that offer protective effects on damaged tissues or organs. We demonstrated here that BMSCs have a powerful antisenescence effect on the rat heart under both in vitro and in vivo conditions. There are several new findings in our study. First, NRVCs demonstrated a senescence process in a time-dependent manner, reflected by increased SA-β-gal activity, induced cell apoptosis and degradation of cellular organelles in senescent cells. Second, significant increases in ROS, MDA, p53, p21Cip1/Waf1, and p16INK4a and decreases in antioxidant enzymes SOD and GSH-Px were observed in both the senescent cardiomyocytes and the aging rat hearts. Third, BMSC transplantation significantly attenuated the aberrant alterations in ROS level, endogenous antioxidant activities, and expression of the molecular determinants of aging. Fourth, BMSC transplantation significantly improved cardiac function in aging rats. Our results suggest that the ROS/p53/p21Cip1/Waf1 signaling cascade is likely involved in cardiac senescence, and BMSCs offer protection against cardiac senescence in aging rats. Our study thus unravels the efficacy of BMSCs in fighting cardiac aging and provides a novel strategy for retarding the cardiac aging process.
In the present study, we demonstrated significant increases in ROS and MDA levels in aging cardiomyocytes, along with diminished activities of SOD and GSH-Px. This imbalance between the enhanced oxidative stress and the decreased antioxidant defense system is deemed to result in the aging process [29]. This aging process was attenuated in the cardiomyocytes cocultured with BMSCs. The antiaging effects of BMSCs could be ascribed to their antioxidative action. In addition, we also demonstrated that BMSCs attenuated apoptosis of cardiomyocytes, which is another piece of evidence for the antiaging effect of BMSCs.
It is well known that stem cells can differentiate into cardiomyocytes, smooth muscle cells, and other cell types after transplantation into the heart [13, 14]. However, it is commonly accepted that stem cells transplanted into the heart elicit their beneficial effects also via secreting a series of cytokines and chemokines including basic fibroblast growth factor, hepatocyte growth factor, and insulin growth factor I [30, 31]. In addition, the repair potential of stem cells is dependent on the age of the donors. Khan et al. [32] demonstrated that the repair effect of BMSCs from a young healthy donor would be better than with senescent infarcted myocardium from an aged donor.
In the present study, we demonstrate that BMSCs are able to rejuvenate the senescent NRVCs after prolonged culturing, and this antisenescence effect appears to be conferred by the antioxidant and anti-p53/p21Cip1/Waf1 efficacy of BMSCs. Most notably, BMSCs show a significant improvement on the cardiac function in aging hearts after BMSC transplantation for only 3 days. Our results are also in good agreement with the study by DeSantiago et al. [33], who demonstrated that stem cells are able to produce beneficial effects within 7 hours, including protecting cardiomyocytes from simulated ischemia/reperfusion injury, increasing intracellular calcium, and improving cell contractility [34]. In particular, BMSCs improve the activity of SERCA2a and uptake of intracellular calcium [21, 34]. We also found that BMSC transplantation significantly attenuated cardiac hypertrophy and fibrosis in the aging rat hearts, which is additional evidence for the antiaging properties of BMSCs. These findings indicate that attenuation of cardiac hypertrophy and fibrosis by stem cells transplantation may also be involved in the improvement of cardiac function in aging rats.
Oxidative stress is a causal factor of senescence and a potent inducer of p53, which in turn is able to transactivate many genes involved in cell cycle arrest and apoptosis [8, 9]. It has been reported that oxidative stress acts through the p53/p21Cip1/Waf1 pathway to induce senescence [8, 35]. On the other hand, p53 can also promote ROS production through enhancing the transcription of genes that increase ROS [36, 37]. This mutual upregulating effect between p53 and ROS, forming a positive feedback loop, can certainly induce and promote cell senescence. Our results show that BMSCs attenuated the increased levels of ROS, p53, and p21Cip1/Waf1 in the aging NRVCs and heart and strongly suggest that the ROS/p53/p21Cip1/Waf1 signaling pathway is likely involved in the senescence process of rat cardiomyocytes, and BMSCs produce the antisenescence effect probably by suppressing this signaling cascade. However, our study does not rule out the potential participation of other factors/pathways as mechanisms for the senescence process and for the observed antisenescence effects of BMSCs.
Conclusion
Our study indicates that BMSCs have strong antisenescence effects on the aging NRVCs and heart and improve the cardiac function in aging rats. These findings provide a promising therapeutic approach for the treatment of heart failure in the elderly population.
Supplementary Material
Acknowledgments
This work was supported in part by Major Program of National Natural Science Foundation of China Grant 81130088, 973 Program Earlier Research Project 2012CB723505, and National Natural Science Foundation of China Grants 81170219 and 81370245.
Author Contributions
M.Z.: collection and assembly of data, data analysis and interpretation, manuscript writing; D.L., S.L., Y.Z., R.L., F.S., W. Duan, W. Du, and T.Z.: collection and assembly of data; L.C., Y.W., and C.X.: data analysis and interpretation; Y.L.: manuscript writing, conception and design, final approval of manuscript, financial support.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Qin F, Siwik DA, Lancel S, et al. Hydrogen peroxide-mediated SERCA cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. J Am Heart Assoc. 2013;2:e000184. doi: 10.1161/JAHA.113.000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson TL, Tulis DA, Keeler BE, et al. The dopamine D3 receptor knockout mouse mimics aging-related changes in autonomic function and cardiac fibrosis. PLoS ONE. 2013;8:e74116. doi: 10.1371/journal.pone.0074116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shioi T, Inuzuka Y. Aging as a substrate of heart failure. J Cardiol. 2012;60:423–428. doi: 10.1016/j.jjcc.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Jugdutt BI. Aging and heart failure: Changing demographics and implications for therapy in the elderly. Heart Fail Rev. 2010;15:401–405. doi: 10.1007/s10741-010-9164-8. [DOI] [PubMed] [Google Scholar]
- 5.Campisi J. The biology of replicative senescence. Eur J Cancer. 1997;33:703–709. doi: 10.1016/S0959-8049(96)00058-5. [DOI] [PubMed] [Google Scholar]
- 6.Corbi G, Conti V, Scapagnini G, et al. Role of sirtuins, calorie restriction and physical activity in aging. Front Biosci (Elite Ed) 2012;4:768–778. doi: 10.2741/417. [DOI] [PubMed] [Google Scholar]
- 7.Vasto S, Scapagnini G, Bulati M, et al. Biomarkes of aging. Front Biosci (Schol Ed) 2010;2:392–402. doi: 10.2741/s72. [DOI] [PubMed] [Google Scholar]
- 8.Chen QM, Bartholomew JC, Campisi J, et al. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J. 1998;332:43–50. doi: 10.1042/bj3320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: Signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 10.Itahana K, Campisi J, Dimri GP. Mechanisms of cellular senescence in human and mouse cells. Biogerontology. 2004;5:1–10. doi: 10.1023/b:bgen.0000017682.96395.10. [DOI] [PubMed] [Google Scholar]
- 11.Campisi J. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivares EL, Ribeiro VP, Werneck de Castro JP, et al. Bone marrow stromal cells improve cardiac performance in healed infarcted rat hearts. Am J Physiol Heart Circ Physiol. 2004;287:H464–H470. doi: 10.1152/ajpheart.01141.2003. [DOI] [PubMed] [Google Scholar]
- 14.Sauer H, Sharifpanah F, Hatry M, et al. NOS inhibition synchronizes calcium oscillations in human adipose tissue-derived mesenchymal stem cells by increasing gap-junctional coupling. J Cell Physiol. 2011;226:1642–1650. doi: 10.1002/jcp.22495. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Zhang Y, Lin L, et al. Effects of bone marrow-derived mesenchymal stem cells on the axonal outgrowth through activation of PI3K/AKT signaling in primary cortical neurons followed oxygen-glucose deprivation injury. PLoS One. 2013;8:e78514. doi: 10.1371/journal.pone.0078514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song M, Mohamad O, Gu X, et al. Restoration of intracortical and thalamocortical circuits after transplantation of bone marrow mesenchymal stem cells into the ischemic brain of mice. Cell Transplant. 2013;22:2001–2015. doi: 10.3727/096368912X657909. [DOI] [PubMed] [Google Scholar]
- 17.Patel DM, Shah J, Srivastava AS. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int. 2013;2013:496218. doi: 10.1155/2013/496218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaza T, Miura Y, Akiyama K, et al. Mesenchymal stem cell-mediated ectopic hematopoiesis alleviates aging-related phenotype in immunocompromised mice. Blood. 2009;113:2595–2604. doi: 10.1182/blood-2008-10-182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Yin SJ, Chen YJ, et al. Transplanted bone marrow stromal cells improve cognitive dysfunction due to aging hypoperfusion in rats. Chin Med J (Engl) 2010;123:3620–3625. [PubMed] [Google Scholar]
- 20.Ikehara S, Li M. Stem cell transplantation improves aging-related diseases. Front Cell Dev Biol. 2014;2:16. doi: 10.3389/fcell.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Li T, Wei X, et al. Mesenchymal stem cell transplantation improves regional cardiac remodeling following ovine infarction. Stem Cells Translational Medicine. 2012;1:685–695. doi: 10.5966/sctm.2012-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li XH, Fu YH, Lin QX, et al. Induced bone marrow mesenchymal stem cells improve cardiac performance of infarcted rat hearts. Mol Biol Rep. 2012;39:1333–1342. doi: 10.1007/s11033-011-0867-2. [DOI] [PubMed] [Google Scholar]
- 23.Chung YC, Ma MC, Huang BY, et al. Protection of bone marrow-derived CD45+/CD34−/lin− stromal cells with immunosuppressant activity against ischemia/reperfusion injury in rats. Chin J Physiol. 2011;54:169–182. doi: 10.4077/cjp.2011.amm019. [DOI] [PubMed] [Google Scholar]
- 24.Lee BC, Hsu HC, Tseng WY, et al. Cell therapy generates a favourable chemokine gradient for stem cell recruitment into the infarcted heart in rabbits. Eur J Heart Fail. 2009;11:238–245. doi: 10.1093/eurjhf/hfn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benzhi C, Limei Z, Ning W, et al. Bone marrow mesenchymal stem cells upregulate transient outward potassium currents in postnatal rat ventricular myocytes. J Mol Cell Cardiol. 2009;47:41–48. doi: 10.1016/j.yjmcc.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Cai B, Zhu S, Li J, et al. Bone marrow-derived mesenchymal stem cells protected rat cardiomyocytes from premature senescence. Int J Cardiol. 2012;154:180–182. doi: 10.1016/j.ijcard.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 27.Yuan Q, Peng J, Liu SY, et al. Inhibitory effect of resveratrol derivative BTM-0512 on high glucose-induced cell senescence involves dimethylaminohydrolase/asymmetric dimethylarginine pathway. Clin Exp Pharmacol Physiol. 2010;37:630–635. doi: 10.1111/j.1440-1681.2010.05368.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang B, Cui S, Bai X, et al. SIRT3 overexpression antagonizes high glucose accelerated cellular senescence in human diploid fibroblasts via the SIRT3-FOXO1 signaling pathway. Age (Dordr) 2013;35:2237–2253. doi: 10.1007/s11357-013-9520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon YS, Wecker A, Heyd L, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagaya N, Kangawa K, Itoh T, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 32.Khan M, Mohsin S, Khan SN, et al. Repair of senescent myocardium by mesenchymal stem cells is dependent on the age of donor mice. J Cell Mol Med. 2011;15:1515–1527. doi: 10.1111/j.1582-4934.2009.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeSantiago J, Bare DJ, Banach K. Ischemia/reperfusion injury protection by mesenchymal stem cell derived antioxidant capacity. Stem Cells Dev. 2013;22:2497–2507. doi: 10.1089/scd.2013.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeSantiago J, Bare DJ, Semenov I, et al. Excitation-contraction coupling in ventricular myocytes is enhanced by paracrine signaling from mesenchymal stem cells. J Mol Cell Cardiol. 2012;52:1249–1256. doi: 10.1016/j.yjmcc.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Catalano A, Rodilossi S, Caprari P, et al. 5-Lipoxygenase regulates senescence-like growth arrest by promoting ROS-dependent p53 activation. EMBO J. 2005;24:170–179. doi: 10.1038/sj.emboj.7600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macip S, Igarashi M, Berggren P, et al. Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol Cell Biol. 2003;23:8576–8585. doi: 10.1128/MCB.23.23.8576-8585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polyak K, Xia Y, Zweier JL, et al. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.