The importance of fetal microchimerism to the field of fetal stem cell biology and issues of maternal contamination from perinatal tissues is highlighted and specific isolation strategies to overcome these translational obstacles are discussed.
Summary
Since the isolation of fetal stem cell populations from perinatal tissues, such as umbilical cord blood and placenta, interest has been growing in understanding their greater plasticity compared with adult stem cells and exploring their potential in regenerative medicine. The phenomenon of fetal microchimerism (FMC) naturally occurring during pregnancy through the transfer of fetal stem/progenitor cells to maternal blood and tissues has been integral in developing this dogma. Specifically, microchimeric mesenchymal stem cells and endothelial progenitors of fetal origin have now demonstrated a capacity for tissue repair in the maternal host. However, the use of similar fetal stem cells in therapy has been significantly hampered by the availability of clinically relevant cell numbers and/or contamination with cells of maternal origin, particularly when using the chorionic and decidual placenta. In the present prospective review, we highlight the importance of FMC to the field of fetal stem cell biology and issues of maternal contamination from perinatal tissues and discuss specific isolation strategies to overcome these translational obstacles.
Significance
Over the last decade, fetal stem cells from a variety of sources have been reported and have shown potential clinical applications. This study briefly reviews recent findings in the fetal stem cell arena, and particularly human term placenta as a robust cell source that harbors large quantities of both fetal and maternal stem cells of various types. It also appraises prospective isolation of large quantities of fetal endothelial progenitor cells and pure preparations of fetal or maternal mesenchymal stromal cells from the same placenta.
Introduction
Fetal stem/progenitor cells exhibit unique functional characteristics that make them an attractive source for future clinical application, such as enhanced differentiation and proliferation capacity [1–3]. To date, mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs), and hematopoietic stem cells (HSCs) have each been successfully harvested from various human fetal tissues, including the placenta, umbilical cord blood (UCB), bone marrow, and liver [4–8].
Although HSCs obtained from UCB are routinely used in transplantation, the benefit of other fetal stem cell populations is less clear. Fetal MSCs (fMSCs) have been the best characterized. fMSCs have enhanced immunoregulatory potential compared with their adult counterparts [9, 10]. They also display greater plasticity (bone/cartilage and extra lineage differentiation) and have superior proliferative capacity compared with adult bone marrow MSCs in vitro [1, 2]. In parallel, a growing body of data has emerged on the use of fetal EPCs. This coincided with the seminal description by Ingram et al. [11] of endothelial colony forming cells (ECFCs) isolated from UCB. Fetal ECFCs have a capacity for long-term in vitro culture and significant engraftment and paracrine actions when introduced into host ischemic tissues [6, 12, 13].
In the present report, we discuss the potency of fetal populations of mesenchymal or endothelial progenitors as witnessed by studies of fetal-maternal cell trafficking and also discuss the translational possibilities of such knowledge regarding prospective isolating strategies for future clinical application.
Fetal Cell Microchimerism: Progenitors of Fetal Origin Involved in Maternal Tissue Repair
An essential indicator that fetal placental stem cells might in fact have regenerative potential in vivo comes from the study of fetal cell microchimerism (FMC) during gestation [14, 15]. During normal pregnancy, fetal cells enter the maternal circulation and can persist for decades [16, 17]. This is referred to as naturally acquired FMC. Such FMC cells can be rapidly mobilized and will home to sites of maternal inflammation [18, 19]. Given the long-term persistence of FMC cells over decades, the fetal cells acquired by the mother during pregnancy have been assumed to be progenitors/stem cells. This has been further supported by the multilineage differentiation potential of FMC cells in different maternal tissues, adopting epithelial, hematopoietic, ectodermal (including neuronal), and endodermal (hepatocyte) fates [16, 20, 21]. Of specific significance is their lineage differentiation capacity into mesenchymal and endothelial tissues. In murine models of maternal skin wounds or maternal kidneys subjected to aristolochic acid toxicity, fetal cells were detected that expressed collagen I as part of the fibrotic process [22, 23]. Analogously, in parous women undergoing appendectomy, fetal cells expressing mesenchymal markers such as desmin could be identified [24]. This is consistent with the identification of fMSCs in the maternal circulation during the first trimester of pregnancy and their long-term presence and engraftment in the maternal bone marrow for decades [8, 25].
Similarly, in situations of maternal angiogenesis, such as inflammation [18], tumor [26], wounds [27], or myocardial infarction [28], fetal endothelial cells could be identified. These could form entire blood vessels that were connected to the maternal circulation. Analysis of mice subjected to wounds identified a circulating population of fetal cells expressing CD34. This is also consistent with reports of fetal CD34+ cells in the maternal blood during and after pregnancy [17, 29]. Fetal CD34+ cells could be detected in maternal intervillous blood space from term placental chorion, suggesting that their point of origin resided in the fetal villi in this tissue [30]. It was also shown that these CD34+ cells were not hematopoietic but had endothelial lineage characteristics. The placental origin of fetal cells with endothelial capacity was further supported by their expression of caudal-related homeobox 2, a trophoblast stem cell marker also expressed in early-stage placental development [28, 31].
Overall, microchimerism studies have supported the notion that the placenta harbors populations of fetal stem cells that, in this situation of natural transfer to the mother, have the capacity to integrate and contribute to various tissues and, most importantly, to the endothelium. This represents an important proof of concept for the clinical use of similar populations of fetal stem cells from the placenta.
The Elusive Fetal Stem Cell in Human Chorionic Placental Cultures
Human term placental MSCs (pMSCs) have been long described [3, 32, 33] and compared with other MSC sources, including adult and fetal bone marrow MSCs. They adhere to the strict in vitro MSC guidelines established by the International Society for Cellular Therapy (ISCT) in terms of cell surface expression and trilineage differentiation capacity [3, 32, 33]. Furthermore, the immunomodulatory properties of pMSCs are well described [10, 34]. In the past decade, pMSCs have been used extensively in clinical trials throughout the world in various diseases (available at http://clinicaltrials.gov) [35, 36]. However, the fetal or maternal origin of the placental cells isolated from the placenta’s chorionic plate and decidua is often unclear [33, 36, 37]. A recent systematic review by Heazlewood et al. [37] highlighted a 30% incidence of pure maternal origin in MSC cultures from the placental chorion but noted that more than 80% of reports of placental MSC have to date failed to document their maternal or fetal origin. Specifically, of 147 studies reviewed, only 26 evaluated the fetal and/or maternal origin of the cells, of which only 15 satisfied the ISCT MSC characterization criteria. Of these, 7 reported having pure fetal populations of cultures obtained from human chorionic tissue. To date, only 1 study reported taking tissue from the fetal or maternal aspect of the placenta that led to pure populations of each in culture [38]. This raises a significant question in the field, considering it has been widely assumed that the human chorion contains tissue merely fetal in origin. In addition, the assessment of mixed maternal/fetal MSCs has also pointed to the fact that maternal cells would almost be “exclusive” in a culture dish from passage three onward [33, 39–42]. Also, a growing body of evidence has suggested that the maternal and fetal MSCs have vastly different culture needs. Studies from others, and our group, have shown fMSCs from perinatal tissue (placenta and umbilical cord) grew only in culture medium such as endothelial growth medium-2, which are enriched with growth factors, and would fail to proliferate under standard MSC culture conditions, such as Dulbecco modified Eagle’s medium supplemented with 10% fetal bovine serum [43–45].
Although pMSCs are now broadly used in clinical trials, the origin of the cells is not always assessed. Maternal or fetal cells have the same infectious risk profile, but they can have different genetic traits and abnormalities that might only be symptomatic at a specific age or condition. For safety reasons, knowledge of the origin would help tailor donor screening questionnaires to fetal or maternal risks. Also, for ethical reasons, the discovery of cytogenetic abnormalities in the MSCs should be reported to the right donor. This underlines the need of continued refinement of isolation methods of stem cells from term placenta.
Fetal Tissue as a Robust Source of Endothelial Progenitor Cells
Since the discovery of the EPC concept in 1997 by Asahara et al. [46], interest in their potential for cell therapy in ischemic diseases such as myocardial infarction or critical leg ischemia has been great. Many clinical trials have been performed using a variety of cell types labeled as EPCs, with promising, but modest, results. The main difficulties were the lack of proper definition and the purity of the cells. In particular, hematopoietic contamination owing to shared cell surface markers such as CD31 or CD34 has always been problematic and a source of controversies [47, 48]. Ingram et al. introduced a new definition of EPCs and a hierarchy of endothelial cells in human UCB based on differences in clonogenic and proliferative potential [11]. They identified a population of EPCs in UCB as “endothelial colony-forming cells (ECFCs)” giving rise to highly proliferative colonies [11]. The self-renewal properties of ECFCs on replating and their ability to assist in angiogenic repair of ischemic sites when injected into a host were demonstrated [47, 49]. However, the number of colonies obtainable from peripheral blood and UCB samples is limited, necessitating the identification of another analogous perinatal source. Overall, ECFCs have significant translational potential and represent a pure population of fetal EPCs devoid of hematopoietic/myeloid contamination.
Human Term Placenta as a Source of Fetal EPCs and MSCs
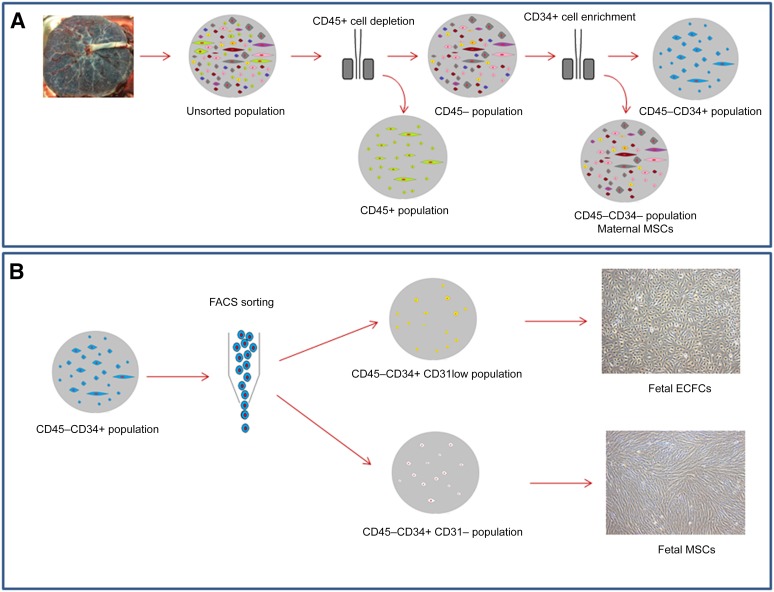
A major hindrance in the expansion of fetal stem cells to obtain relevant quantities for mainstream clinical applications is ethical constraints regarding their sourcing in utero, which typically involve invasive procedures, such as chorionic villous sampling, or products of pregnancy terminations. To overcome these barriers, we, and others, have used human term placenta, excluding all membranes, to develop isolation strategies for the copious quantities of fetal stem cells that reside there by separating them from maternal contaminating, presumably decidual origin, cells [5, 6, 38, 45]. From our findings in FMC paradigms and reports that in vivo fetal MSCs are actually CD34+ [27, 30, 50, 51], our group has developed novel strategies based on populations of cells in placental chorionic villous tissue that were CD34+ and CD45−, thus excluding hematopoietic cells (Fig. 1), without the need for detailed dissection of the chorionic villi.
Figure 1.
Schematic of isolation procedures for progenitors from human term placenta. (A): After villous enzymatic tissue digestion in agitation condition, the placental cells are depleted of CD45+ cells and then enriched for CD34+ cells by magnetic sorting [6]. Culture experiments showed that the CD45+ population gave rise to hematopoietic cells and CD34−CD45− cells displayed MSC characteristics and were maternal in origin. (B): On enrichment for CD45−CD34+ by magnetic-activated cell sorting, the cells were separated using FACS. The CD45−CD34+ cells were analyzed, and two populations separated by the expression levels of CD31 were sorted. The CD45−CD34+CD31− cells showed MSCs characteristics and were fetal in origin. The CD45−CD34+CD31low cells were also fetal in origin and resulted in ECFCs with high proliferative potential. Abbreviations: ECFCs, endothelial colony forming cells; FACS, fluorescence-activated cell sorting; MSCs, mesenchymal stem cells.
These placental CD45−CD34+ cells were further purified according to the cell surface expression of CD31, a panendothelial marker. A fluorescence-activated cell sorting strategy was applied directly on freshly isolated placental cells to harvest CD45−CD34+CD31low populations as a source of pure fetal placental ECFCs, such as can also be found in UCB [6]. Strikingly, using this sorting strategy, a same donor will have 27 times more high-proliferative-potential ECFCs in the placenta compared with the UCB when all the material is used. In parallel, plating the CD45−CD34+CD31− fraction of cells—defined strictly by the level of CD31 staining below the control (isotype or fluorescence minus one staining)—resulted in fMSC colonies. Plating the CD45−CD34− fraction of the placenta also allowed MSC colonies to be obtained; however, these proved to be maternal [45], determined by fluorescence in situ hybridization analysis in sex mismatch pregnancies. Both fMSCs and mMSCs satisfied the ISCT criteria for MSCs. They had the appropriate cell surface markers and differentiated adequately along osteogenic and adipogenic lineages. Fetal MSCs had enhanced mesodermal lineage differentiation.
This unique method is based on the assessment of known cell surface markers but also highlights how two stem cell populations can be separated just by their expression levels of CD31. Remarkably, this work also demonstrates the apparent importance, in particular, in the placenta and from studies of FMCs, of differentiating fetal and maternal cells according to CD34 expression.
Perspectives and Conclusion
The growing interest in using the human term placenta to obtain numerous types of fetal stem cells is increasingly matched with good rationale for the use of fetal stem cells and technical advances to isolate pure populations of fetal stem cells. Fetal microchimerism has been integral in developing our understanding of fetal stem cells in their plasticity and their capacity to respond to maternal injury in vivo. As our knowledge of the in vivo functional capacities of endogenous stem cell populations increases, their evaluation in clinical trials appears more feasible. Furthermore, the straightforward ability to obtain large amounts of placental fetal tissue is a significant advantage compared with other sources to conduct adequately designed clinical trials. However, owing to the amount of maternal involvement within the tissue, strict processes need to be developed to ensure that only pure fetal stem cells are isolated and used. With the recent development of these processes, major progress is expected in understanding the subtle differences in the endogenous cell biology of fetal and maternal cells, not only to further improve the isolation processes, but also to test their differential activity in transplantation settings.
Acknowledgments
This study was supported by the National Health and Medical Research Council (Project Grant 1023368). K.K. was supported by the National Health and Medical Research Council Career Development Fellowship (Grant 1023371). J.P. was supported by the National Heart Foundation of Australia Postdoctoral Fellowship.
Author Contributions
A.S.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; N.M.F. and D.W.H.: data analysis and interpretation, manuscript writing, final approval of manuscript; K.K. and J.P.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
N.M.F. has compensated expert testimony. K.K. has compensated research funding from National Health and Medical Research Council Career Development Fellowship. The other authors indicated no potential conflicts of interest.
References
- 1.Zhang ZY, Teoh SH, Chong MS, et al. Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells. 2009;27:126–137. doi: 10.1634/stemcells.2008-0456. [DOI] [PubMed] [Google Scholar]
- 2.Guillot PV, De Bari C, Dell’Accio F, et al. Comparative osteogenic transcription profiling of various fetal and adult mesenchymal stem cell sources. Differentiation. 2008;76:946–957. doi: 10.1111/j.1432-0436.2008.00279.x. [DOI] [PubMed] [Google Scholar]
- 3.Guillot PV, O’Donoghue K, Kurata H, et al. Fetal stem cells: Betwixt and between. Semin Reprod Med. 2006;24:340–347. doi: 10.1055/s-2006-952149. [DOI] [PubMed] [Google Scholar]
- 4.Ulrich C, Rolauffs B, Abele H, et al. Low osteogenic differentiation potential of placenta-derived mesenchymal stromal cells correlates with low expression of the transcription factors Runx2 and Twist2. Stem Cells Dev. 2013;22:2859–2872. doi: 10.1089/scd.2012.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapp BM, Saadatzedeh MR, Ofstein RH, et al. Resident endothelial progenitor cells from human placenta have greater vasculogenic potential than circulating endothelial progenitor cells from umbilical cord blood. Cell Medicine. 2012;2:85–96. doi: 10.3727/215517911X617888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel J, Seppanen E, Chong MS, et al. Prospective surface marker-based isolation and expansion of fetal endothelial colony-forming cells from human term placenta. Stem Cells Translational Medicine. 2013;2:839–847. doi: 10.5966/sctm.2013-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robin C, Bollerot K, Mendes S, et al. Human placenta is a potent hematopoietic niche containing hematopoietic stem and progenitor cells throughout development. Cell Stem Cell. 2009;5:385–395. doi: 10.1016/j.stem.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campagnoli C, Roberts IA, Kumar S, et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson H, Erkers T, Nava S, et al. Stromal cells from term fetal membrane are highly suppressive in allogeneic settings in vitro. Clin Exp Immunol. 2012;167:543–555. doi: 10.1111/j.1365-2249.2011.04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stubbendorff M, Deuse T, Hua X, et al. Immunological properties of extraembryonic human mesenchymal stromal cells derived from gestational tissue. Stem Cells Dev. 2013;22:2619–2629. doi: 10.1089/scd.2013.0043. [DOI] [PubMed] [Google Scholar]
- 11.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 12.Lin R-Z, Moreno-Luna R, Li D, et al. Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc Natl Acad Sci USA. 2014;111:10137–10142. doi: 10.1073/pnas.1405388111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouvard C, Gafsou B, Dizier B, et al. α6-Integrin subunit plays a major role in the proangiogenic properties of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:1569–1575. doi: 10.1161/ATVBAHA.110.209163. [DOI] [PubMed] [Google Scholar]
- 14.Seppanen E, Fisk NM, Khosrotehrani K. Pregnancy-acquired fetal progenitor cells. J Reprod Immunol. 2013;97:27–35. doi: 10.1016/j.jri.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Lee ES, Bou-Gharios G, Seppanen E, et al. Fetal stem cell microchimerism: Natural-born healers or killers? Mol Hum Reprod. 2010;16:869–878. doi: 10.1093/molehr/gaq067. [DOI] [PubMed] [Google Scholar]
- 16.Khosrotehrani K, Johnson KL, Cha DH, et al. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA. 2004;292:75–80. doi: 10.1001/jama.292.1.75. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi DW, Zickwolf GK, Weil GJ, et al. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93:705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen Huu S, Oster M, Uzan S, et al. Maternal neoangiogenesis during pregnancy partly derives from fetal endothelial progenitor cells. Proc Natl Acad Sci USA. 2007;104:1871–1876. doi: 10.1073/pnas.0606490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donoghue K, Sultan HA, Al-Allaf FA, et al. Microchimeric fetal cells cluster at sites of tissue injury in lung decades after pregnancy. Reprod Biomed Online. 2008;16:382–390. doi: 10.1016/s1472-6483(10)60600-1. [DOI] [PubMed] [Google Scholar]
- 20.Tan XW, Liao H, Sun L, et al. Fetal microchimerism in the maternal mouse brain: A novel population of fetal progenitor or stem cells able to cross the blood-brain barrier? Stem Cells. 2005;23:1443–1452. doi: 10.1634/stemcells.2004-0169. [DOI] [PubMed] [Google Scholar]
- 21.Zeng XX, Tan KH, Yeo A, et al. Pregnancy-associated progenitor cells differentiate and mature into neurons in the maternal brain. Stem Cells Dev. 2010;19:1819–1830. doi: 10.1089/scd.2010.0046. [DOI] [PubMed] [Google Scholar]
- 22.Roy E, Seppanen E, Ellis R, et al. Biphasic recruitment of microchimeric fetal mesenchymal cells in fibrosis following acute kidney injury. Kidney Int. 2014;85:600–610. doi: 10.1038/ki.2013.459. [DOI] [PubMed] [Google Scholar]
- 23.Seppanen E, Roy E, Ellis R, et al. Distant mesenchymal progenitors contribute to skin wound healing and produce collagen: Evidence from a murine fetal microchimerism model. Plos One. 2013;8:e62662. doi: 10.1371/journal.pone.0062662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos MA, O’Donoghue K, Wyatt-Ashmead J, et al. Fetal cells in the maternal appendix: A marker of inflammation or fetal tissue repair? Hum Reprod. 2008;23:2319–2325. doi: 10.1093/humrep/den261. [DOI] [PubMed] [Google Scholar]
- 25.O’Donoghue K, Chan J, de la Fuente J, et al. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364:179–182. doi: 10.1016/S0140-6736(04)16631-2. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen Huu S, Oster M, Avril MF, et al. Fetal microchimeric cells participate in tumour angiogenesis in melanomas occurring during pregnancy. Am J Pathol. 2009;174:630–637. doi: 10.2353/ajpath.2009.080566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nassar D, Droitcourt C, Mathieu-d’Argent E, et al. Fetal progenitor cells naturally transferred through pregnancy participate in inflammation and angiogenesis during wound healing. FASEB J. 2012;26:149–157. doi: 10.1096/fj.11-180695. [DOI] [PubMed] [Google Scholar]
- 28.Kara RJ, Bolli P, Karakikes I, et al. Fetal cells traffic to injured maternal myocardium and undergo cardiac differentiation. Circ Res. 2012;110:82–93. doi: 10.1161/CIRCRESAHA.111.249037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guetta E, Gordon D, Simchen MJ, et al. Hematopoietic progenitor cells as targets for non-invasive prenatal diagnosis: Detection of fetal CD34+ cells and assessment of post-delivery persistence in the maternal circulation. Blood Cells Mol Dis. 2003;30:13–21. doi: 10.1016/s1079-9796(03)00008-1. [DOI] [PubMed] [Google Scholar]
- 30.Parant O, Dubernard G, Challier J-C, et al. CD34+ cells in maternal placental blood are mainly fetal in origin and express endothelial markers. Lab Invest. 2009;89:915–923. doi: 10.1038/labinvest.2009.55. [DOI] [PubMed] [Google Scholar]
- 31.Beck F, Erler T, Russell A, et al. Expression of Cdx-2 in the mouse embryo and placenta: Possible role in patterning of the extra-embryonic membranes. Dev Dyn. 1995;204:219–227. doi: 10.1002/aja.1002040302. [DOI] [PubMed] [Google Scholar]
- 32.Kaviani A, Guleserian K, Perry TE, et al. Fetal tissue engineering from amniotic fluid. J Am Coll Surg. 2003;196:592–597. doi: 10.1016/s1072-7515(02)01834-3. [DOI] [PubMed] [Google Scholar]
- 33.Barlow S, Brooke G, Chatterjee K, et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008;17:1095–1107. doi: 10.1089/scd.2007.0154. [DOI] [PubMed] [Google Scholar]
- 34.Keelan JA, Blumenstein M, Helliwell RJ, et al. Cytokines, prostaglandins and parturition—A review. Placenta. 2003;24(suppl A):S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 35.Chambers DC, Enever D, Ilic N, et al. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology. 2014;19:1013–1018. doi: 10.1111/resp.12343. [DOI] [PubMed] [Google Scholar]
- 36.Ringdén O, Erkers T, Nava S, et al. Fetal membrane cells for treatment of steroid-refractory acute graft-versus-host disease. Stem Cells. 2013;31:592–601. doi: 10.1002/stem.1314. [DOI] [PubMed] [Google Scholar]
- 37.Heazlewood CF, Sherrell H, Ryan J, et al. High incidence of contaminating maternal cell overgrowth in human placental mesenchymal stem/stromal cell cultures: A systematic review. Stem Cells Translational Medicine. 2014;3:1305–1311. doi: 10.5966/sctm.2014-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Yang Y, Zhu Y, et al. Characterization of placenta-derived mesenchymal stem cells cultured in autologous human cord blood serum. Mol Med Rep. 2012;6:760–766. doi: 10.3892/mmr.2012.1000. [DOI] [PubMed] [Google Scholar]
- 39.Wulf GG, Viereck V, Hemmerlein B, et al. Mesengenic progenitor cells derived from human placenta. Tissue Eng. 2004;10:1136–1147. doi: 10.1089/ten.2004.10.1136. [DOI] [PubMed] [Google Scholar]
- 40.In ’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 41.Jaramillo-Ferrada PA, Wolvetang EJ, Cooper-White JJ. Differential mesengenic potential and expression of stem cell-fate modulators in mesenchymal stromal cells from human-term placenta and bone marrow. J Cell Physiol. 2012;227:3234–3242. doi: 10.1002/jcp.24014. [DOI] [PubMed] [Google Scholar]
- 42.Semenov OV, Koestenbauer S, Riegel M, et al. Multipotent mesenchymal stem cells from human placenta: Critical parameters for isolation and maintenance of stemness after isolation. Am J Obstet Gynecol. 2010;202:193.e191–193.e113. doi: 10.1016/j.ajog.2009.10.869. [DOI] [PubMed] [Google Scholar]
- 43.in ’t Anker PS, Noort WA, Scherjon SA, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88:845–852. [PubMed] [Google Scholar]
- 44.Kögler G, Sensken S, Wernet P. Comparative generation and characterization of pluripotent unrestricted somatic stem cells with mesenchymal stem cells from human cord blood. Exp Hematol. 2006;34:1589–1595. doi: 10.1016/j.exphem.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Patel J, Shafiee A, Wang W, et al. Novel isolation strategy to deliver pure fetal-origin and maternal-origin mesenchymal stem cell (MSC) populations from human term placenta. Placenta. 2014;35:969–971. doi: 10.1016/j.placenta.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 47.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Case J, Mead LE, Bessler WK, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Yoon C-H, Hur J, Park K-W, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: The role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 50.Sölder E, Böckle BC, Nguyen VA, et al. Isolation and characterization of CD133+CD34+VEGFR-2+CD45− fetal endothelial cells from human term placenta. Microvasc Res. 2012;84:65–73. doi: 10.1016/j.mvr.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 51.Mikhail MA, M’Hamdi H, Welsh J, et al. High frequency of fetal cells within a primitive stem cell population in maternal blood. Hum Reprod. 2008;23:928–933. doi: 10.1093/humrep/dem417. [DOI] [PubMed] [Google Scholar]