An optimized protocol enabled efficient reprogramming that can be applied to a variety of patients for developing induced pluripotent stem cell (iPSC)-based models of cardiac diseases. The dual optical recording using R-GECO1 and ArcLight fluorescent indicators is a robust approach to examine physiological phenotypes and to test drug candidates in long QT syndrome iPSC-derived cardiomyocytes.
Keywords: Human induced pluripotent stem cells, Disease modeling, Cardiac arrhythmia, Optical recording, Drug test
Abstract
Reprogramming of human somatic cells to pluripotency has been used to investigate disease mechanisms and to identify potential therapeutics. However, the methods used for reprogramming, in vitro differentiation, and phenotyping are still complicated, expensive, and time-consuming. To address the limitations, we first optimized a protocol for reprogramming of human fibroblasts and keratinocytes into pluripotency using single lipofection and the episomal vectors in a 24-well plate format. This method allowed us to generate multiple lines of integration-free and feeder-free induced pluripotent stem cells (iPSCs) from seven patients with cardiac diseases and three controls. Second, we differentiated human iPSCs derived from patients with Timothy syndrome into cardiomyocytes using a monolayer differentiation method. We found that Timothy syndrome cardiomyocytes showed slower, irregular contractions and abnormal calcium handling compared with the controls. The results are consistent with previous reports using a retroviral method for reprogramming and an embryoid body-based method for cardiac differentiation. Third, we developed an efficient approach for recording the action potentials and calcium transients simultaneously in control and patient cardiomyocytes using genetically encoded fluorescent indicators, ArcLight and R-GECO1. The dual optical recordings enabled us to observe prolonged action potentials and abnormal calcium handling in Timothy syndrome cardiomyocytes. We confirmed that roscovitine rescued the phenotypes in Timothy syndrome cardiomyocytes and that these findings were consistent with previous studies using conventional electrophysiological recordings and calcium imaging with dyes. The approaches using our optimized methods and dual optical recordings will improve iPSC applicability for disease modeling to investigate mechanisms underlying cardiac arrhythmias and to test potential therapeutics.
Significance
This study found that dual optical recording using genetically encoded fluorescent indicators is a useful approach for identifying new lead chemical compounds in human induced pluripotent stem (iPS) cell-based models of not only cardiac diseases but also neuronal disorders. It will facilitate drug development and personalized medicine using iPS technology.
Introduction
To study the molecular and cellular mechanisms underlying a variety of human genetic diseases and identify novel treatments, human stem cell-based models have been developed using patient-specific induced pluripotent stem cells (iPSCs) because patient somatic cells can be reprogrammed to pluripotency and differentiated into target cells, such cardiomyocytes and neurons [1–5]. However, the time and cost requirements are still major limitations to scale up iPSC-based disease models to develop new platforms for drug testing. We report newly optimized methods in two key steps: (a) to reprogram fibroblasts and keratinocytes using single lipofection of the episomal vectors to generate feeder-free, integration-free, and virus-free iPSCs; and (b) to investigate phenotypes in excitation-contraction coupling in cardiomyocytes using two genetically encoded fluorescent indicators, ArcLight [6] and R-GECO1 [7], for voltage and intracellular calcium ions, respectively.
Materials and Methods
Cell Culture
Feeder-free iPSC lines were cultured in Essential 8 (E8) medium [8] with penicillin and streptomycin (P.S., all from Life Technologies, Carlsbad, CA, http://www.lifetechnologies.com) on plates or dishes (Corning Enterprises, Corning, NY, http://www.corning.com) coated with Geltrex (Life Technologies).
Transfection
When we tested the lipofection reagents in IMR90 cells, no major differences were present between Opti-MEM and Dulbecco’s modified Eagle’s medium (DMEM) (P.S. free), with 10% fetal bovine serum (FBS; HyClone; catalog no. SH30071.03; Thermo Scientific, Wilmington, DE, http://www.thermoscientific.com) for TransFectin (Bio-Rad, Hercules, CA, http://www.bio-rad.com) and Fugene HD (Promega, Madison, WI, http://www.promega.com). Procedures using Xfect (Clontech, TaKaRa Bio, Shiga, Japan, http://www.takara-bio.com), Lipofectamine 2000, and Lipofectamine LTX (both from Life Technologies) were followed according to the manufacturer’s instructions. pGW1-YFP [9] and pCXLE-enhanced green fluorescent protein (EGFP) (catalog no. 27082; Addgene, Cambridge, MA, http://www.addgene.org) were used to examine transfection efficiency.
iPSC Generation
The episomal vectors (Addgene) established by Okita et al. [10] were used for reprogramming. In brief, ∼1.25 × 104 cells per well were plated in 24-well plates using 20% FBS-containing DMEM (high glucose, GlutaMAX I, with P.S.; Life Technologies). The next day, lipofection was performed using Lipofectamine LTX (2 μl per well) with the vector DNA (0.9 μg per well) and 0.9 μl of Plus Reagent in Opti-MEM (all from Life Technologies). The media were completely changed to fresh DMEM supplemented with 20% FBS 30 minutes after lipofection. At day 5 or 6, before the fibroblasts became 100% confluent, the cells in a well in the 24-well plates were plated on a Geltrex-coated well in a 6-well plate. The next day, the culture media were switched to E8 media under hypoxia. Additional media changes were performed daily until picking colonies at days 22–27.
R-GECO1 and ArcLight Preparation and Imaging
The plasmids of R-GECO1 and ArcLight (both from Addgene) were used for subcloning into the lentiviral vector that was prepared from LV-SD-Cre (Addgene) with EcoRI and XhoI sites. The procedures were performed in accordance with the National Institutes of Health guidelines for recombinant DNA research. To prepare the lentiviruses, the purified LV-SD-ArcLight/R-GECO1 vectors were transfected together with the pCMV-dR8.2 dvpr and pCMV-VSV-G (Addgene) in HEK293T cells. At 3–5 days after infection, cardiomyocytes were placed in glass bottom culture dishes (MatTek Corp., Ashland, MA, http://www.mattek.com) coated with Geltrex. To conduct dual optical recordings for action potentials and calcium handling, a Nikon Ti-U epifluorescent microscope connected to an EMCDD camera (Photometrics, Tucson, AZ, http://www.photometrics.com) was used with an light-emitting diode light source (Lumencor, Inc., Renton, WA, http://www.lumicor.com) operated by MetaFluor (Molecular Devices, Sunnyvale, CA, http://www.moleculardevices.com).
Results
Single Lipofection for Reprogramming Into Pluripotency
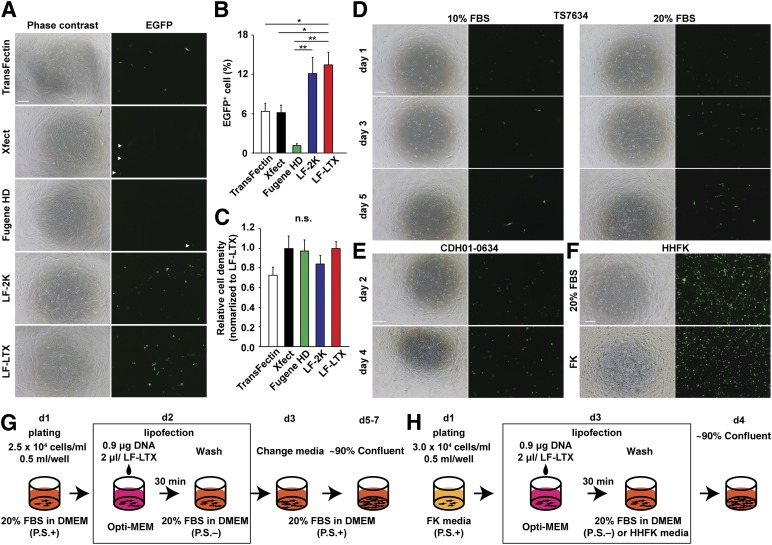
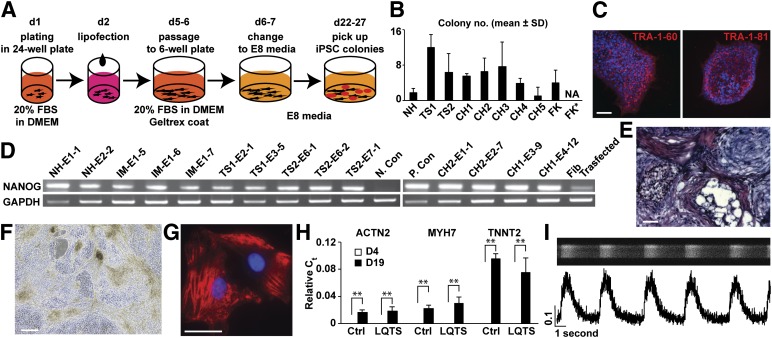
Gene transduction using electroporation is widely used for reprogramming human fibroblasts with the episomal vectors to generate integration/virus-free iPSCs [10, 11]. However, electroporation requires equipment, expertise, and millions of cells to minimize toxicity and optimize the efficiency of gene transduction in each patient sample. In contrast, lipofection is a relatively simple procedure compared with electroporation, although repeated lipofection of modified RNA is required for reprogramming [12]. Therefore, developing a simple and inexpensive protocol for reprogramming to pluripotency is a priority. To address this concern, we first optimized the lipofection conditions in IMR90 human embryonic lung fibroblasts in 24-well plates using Lipofectamine 2000 (LF-2K). LF-2K has been used for gene transduction in mammalian primary cells such as cortical neurons [9]. We found that a 30-minute exposure of IMR90 cells to LF-2K-DNA complexes in Opti-MEM provided greater expression of yellow fluorescent protein (YFP) and less cell death from toxicity of lipofection in cases using 20% FBS in culture media for recovery after lipofection (supplemental online Fig. 1). To determine whether single lipofection using LF-2K is sufficient for reprogramming of IMR90 to iPSCs, we next tested this method using the established episomal vectors [10]. We found that iPSC lines could be derived using a 24-well plate under hypoxia [13] using E8 medium [8] (supplemental online Fig. 2). However, we found that 1 of 4 wells in a 24-well plate failed to generate iPSCs. In addition, IMR90 embryonic fibroblasts are known to be reprogrammed easily compared with human adult skin fibroblasts [3]. Therefore, we further optimized the conditions and reagents to use lipofection in patient fibroblasts. After testing several lipofection reagents to transfect an episomal vector, pCXLE-EGFP, we found that human fibroblasts transfected using Lipofectamine LTX (LF-LTX) showed significantly higher EGFP expression compared with those transfected with other lipofection reagents (Fig. 1A–1C). Therefore, we used LF-LTX to transfect the episomal vectors in the control and patient fibroblasts in a 24-well plate (Fig. 1D, 1E), and we generated multiple independent lines of control and patient-specific iPSCs from patients with long QT syndrome (LQTS, type 8, Timothy syndrome) [3] and congenital heart disease (CHD) (Fig. 2A, 2B; supplemental online Table 1). We characterized these lines and confirmed that the iPSCs showed pluripotency, using immunocytochemistry, karyotyping, a teratoma formation assay, gene expression profiles, and in vitro differentiation (Fig. 2C–2E; supplemental online Figs. 3–7). Next, we examined whether the method is generally applicable to other cell types, especially to less invasive sources of cell such as hair follicle keratinocytes. Therefore, we used LF-LTX to transfect the vectors into human hair follicle keratinocytes on the same scale (Fig. 1F, 1H). We found that we could generate iPSCs from the keratinocytes using the same recovery media used for human fibroblasts on days 2–6 after lipofection. In contrast, no iPSCs were generated in cases using keratinocyte-specific media on days 2–6 after lipofection (Fig. 2B). These results demonstrate that the optimized lipofection protocol for reprogramming is applicable to a variety of disease models using patient skin fibroblasts and hair keratinocytes.
Figure 1.
Optimization of single lipofection in human skin fibroblasts and hair follicle keratinocytes. (A): Phase contrast images (left) and fluorescent images (right) of IMR90 cells transfected with TransFectin, Xfect, Fugene HD, LF-2K, and LF-LTX. An episomal vector pCXLE-EGFP was used as a reporter to examine gene transduction in IMR90 cells. Arrowheads show EGFP-positive cells with Xfect and Fugene HD because of lower expression. EGFP-positive cell number (B) and relative cell density (C) after transfection at day 5 were examined. These results revealed that LF-LTX shows higher transfection efficiency and lower toxicity than did other candidates in human fibroblasts. ∗, p < .05, ∗∗, p < .01, with one-way analysis of variance (multiple comparisons). (D): LF-LTX and pCXLE-EGFP episomal vector were used for transfection in patient fibroblasts to examine whether this protocol works for patient skin fibroblasts of TS7634 in 24-well plates. After lipofection, culture media was changed to 10% or 20% FBS-containing DMEM without antibiotics. (E): An identical condition of lipofection was tested in the fibroblasts of 1 patient, CDH01-0634. DMEM containing 20% FBS was used after lipofection. (F): LF-LTX and pCXLE-EGFP episomal vector were used for transfection in HHFKs to examine whether this protocol works for keratinocytes in 20% FBS-containing DMEM (top) and FK (bottom). (G): Outline of lipofection using LF-LTX in human skin fibroblasts. (H): Outline of lipofection using LF-LTX in HHFKs. Scale bars = 250 μm. Abbreviations: ACTN2, actinin 2; d, day; DMEM, Dulbecco’s modified Eagle’s medium; EGPP, enhanced green fluorescent protein; FBS, fetal bovine serum; FK, keratinocyte-specific media; HHFK, human hair follicle keratinocyte; LF-2K, Lipofectamine 2000; LF-LTX, Lipofectamine LTX; TS7634, long QT syndrome; P.S., penicillin and streptomycin.
Figure 2.
Cellular reprogramming with single lipofection and monolayer cardiac differentiation of iPSCs. (A): Outline of reprogramming of skin fibroblasts into iPSCs using lipofection with Lipofectamine LTX in 24-well plate scale. (B): The number of iPSC-like colonies generated from human skin fibroblast and FKs plated in 24-well plate scale (n = 4–8 in 2 independent experiments). (C): Immunocytochemistry of congenital heart disease (CHD) patient iPSCs (CH1-E4-12) using antibodies to TRA-1-60 (red, left) and TRA-1-81 (red, right) and Hoechst 33285 stain (blue) for nuclei. Scale bar = 50 μm. (D): Expression of pluripotent marker Nanog and housekeeping gene GAPDH in iPSC lines, H9 line (P. Con), untreated patient Fib, N. Con, and transfected fibroblasts using the episomal vectors (collected at day 5 after lipofection). (E): Section of teratoma formed by control iPSC line (IM-E1-7) stained using hematoxylin and eosin stain. Scale bar = 100 μm. (F): Phase contrast image of LQTS beating cardiomyocytes (day 13, TS1-E3-5; supplemental online Video 1). Scale bar = 250 μm. (G): Immunocytochemistry of control cardiomyocytes using the antibody to sarcomeric α-actinin. Scale bar = 25 μm. (H): Gene expression of cardiac myofilaments, ACTN2, MYH7, and TNNT2, in control and LQTS cells at days 4 and 19 using quantitative reverse transcription-polymerase chain reaction, normalized to GAPDH expression. ∗∗, p < .01, with Student’s t test between days 4 and 19. (I): Imaging of calcium transients in control single cardiomyocytes using confocal microscope. Line scan image (top, black/white) and calcium transients (bottom) are shown (n = 102). Abbreviations: Ctrl, control; d, day; DMEM, Dulbecco’s modified Eagle’s medium; E8, Essential 8; FBS, fetal bovine serum; Fib, fibroblasts; FK, hair keratinocyte; FK*, no iPSC-like colonies (NA) were found in cases that used keratinocyte media at days 2–6; LQTS, long QT syndrome; N. Con, negative control; NA, not applicable; P. Con, positive control.
Monolayer Differentiation Into Cardiomyocytes
For mesodermal differentiation of human pluripotent stem cells, human recombinant growth factors such as activin A, bone morphogenetic protein 4, and Wnt3a have been widely used [14, 15]. However, using recombinant protein is not ideal to scale up cell production because of its cost and instability. In addition, for future automation of cardiac cell production, monolayer differentiation seems to be promising compared with embryoid body formation because changing the media and cell dissociation are efficient and the cells are uniformly exposed to the differentiation factors in culture media. We thus used small chemical compounds to replace the recombinant factors and conducted monolayer cardiac differentiation of the generated iPSC lines, in accordance with previous reports [14, 16, 17]. We observed spontaneously beating cardiomyocytes at day 11 and found that LQTS cardiomyocytes showed slower and irregular contractions (Fig. 2F; supplemental online Video 1; supplemental online data). In addition, immunocytochemistry demonstrated that sarcomere formation was observed in control and LQTS cells, and fluorescence-activated cell sorting analyses using the antibody to sarcomeric α-actinin indicated that approximately 60% of the cells were positive for the marker at day 19 in multiple lines from control and patient iPSCs (Fig. 2G; supplemental online Fig. 8). Gene expression profiles using quantitative reverse transcription-polymerase chain reaction demonstrated that the expression of cardiac myofilament genes, ACTN2, MYH7 and TNNT2, were significantly upregulated at day 19, but expression was not observed at day 4 (Fig. 2H). The correlations between cardiac marker expression in the control and patient cells were significant (supplemental online Fig. 8C). To examine calcium handling in the control and patient cardiomyocytes, we next conducted confocal calcium imaging to find spontaneous calcium transients with dye (FluoForte; Enzo Life Sciences, Inc., Enzo Biochem, Inc., Farmingdale, NY, http:www.enzolifesciences.com). We observed normal calcium handling in the control cells (Fig. 2I). In contrast, LQTS iPSC-derived cardiomyocytes stopped beating and/or showed slow and irregular calcium transients that can be rescued by roscovitine, as previously reported [3] (supplemental online Fig. 8E). We confirmed that LQTS cardiomyocytes had phenotypes in contraction and calcium handling compared with the controls. The results were consistent with previous reports using retroviral methods for reprogramming and methods with embryoid body formation for cardiac differentiation [3]. These results demonstrate that we successfully generated control and patient cardiomyocytes from control and patient feeder-free integration-free iPSC lines generated using the optimized reprogramming method with single lipofection.
Dual Optical Recordings of Action Potentials and Calcium Handling in iPSC-Derived Cardiomyocytes
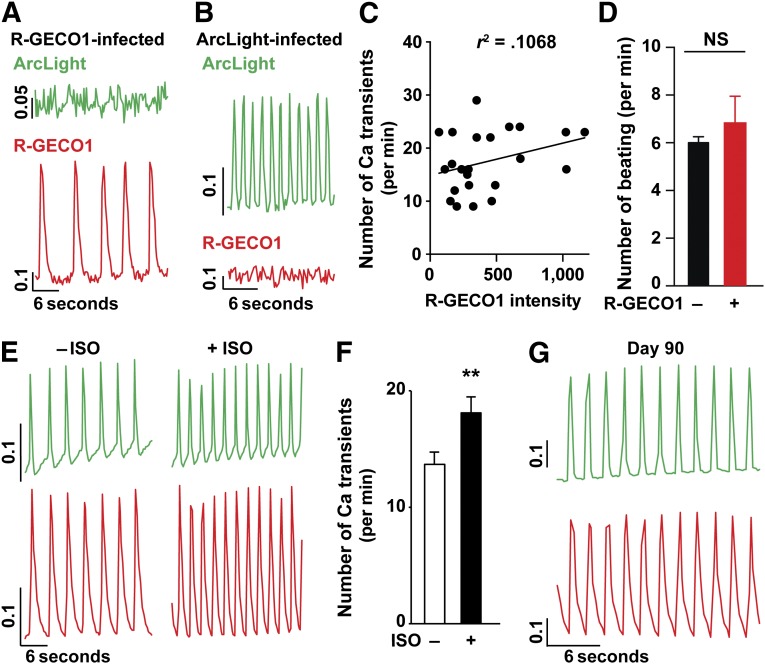
To examine cardiac excitation-contraction coupling in disease models, calcium imaging using dyes and electrophysiological recordings using a patch clamp have been widely used [18, 19]. However, the recorded cells are no longer reusable after the experiments. Therefore, it is difficult to time the experiments at intervals when cellular phenotypes will be observed in disease models. To address this limitation, we examined whether two genetically encoded fluorescent indicators, R-GECO1 for intracellular calcium ions [7] and ArcLight for voltage [6, 20], could be used to improve the efficiency of cellular phenotyping in cardiomyocytes derived from patients. First, to examine whether ArcLight and R-GECO1 proteins can be expressed in a single lentiviral construct using self-cleaved 2A peptide sequences, we prepared and tested ArcLight-2A-R-GECO1 and R-GECO1-2A-ArcLight in NIH 3T3 cells. We found that neither construct was fully functional owing to protein aggregations and mislocalization (supplemental online Fig. 9A–9D). Therefore, we used both R-GECO1- and ArcLight-expressing lentiviruses together for dual optical recordings for action potentials and calcium handling. Using R-GECO1 and ArcLight lentiviruses, we conducted dual optical recordings in iPSC-derived cardiomyocytes using an epifluorescent microscope. For method validation, we examined whether any crossover occurred between the fluorescent signals using ArcLight and R-GECO1, and we ensured that any signal crossover between ArcLight and R-GECO1 was not detected in the dual optical recordings (Fig. 3A, 3B), although the signals of red calcium dye Rhod-FF AM and ArcLight overlapped (data not shown). Next, because the calcium indicator R-GECO1 is calmodulin-based, we analyzed the correlation between the beating rate and R-GECO1 expression in control cardiomyocytes to examine the effects of a permanent chelation of calcium ions by R-GECO1 on cardiac function. For this analysis, we found no effect of R-GECO1 on spontaneous contraction in generated cardiomyocytes (Fig. 3C). In addition, we compared R-GECO1-infected LQTS cardiomyocytes with noninfected ones and found that the slow beating phenotype in LQTS cardiomyocytes was not changed by R-GECO1 (Fig. 3D), revealing that R-GECO1 does not have a significant effect on cardiac function.
Figure 3.
Dual optical recordings for action potentials and calcium handling using ArcLight and R-GECO1 in human induced pluripotent stem cell-derived cardiomyocytes. Testing the crossover of emission signals between ArcLight (top, green) and R-GECO1 (bottom, red) using R-GECO1-infected (A) and ArcLight-infected (B) cardiomyocytes. No crossover was detected between R-GECO1 and ArcLight (n = 7). (C): No correlation was found between R-GECO1 expression and the number of spontaneous calcium transients in control cardiomyocytes. (D): No effect was found of R-GECO1 expression on the cellular phenotype, slower beating, in long QT syndrome cardiomyocytes (n = 10). (E): Representative traces of calcium and voltage in spontaneously beating monolayer of cardiomyocytes expressing ArcLight (top) and R-GECO1 (bottom) before (ISO−, left) and after (ISO+, right, n = 21) adding isoproterenol. (F): ISO significantly increased the rate of calcium transients. ∗∗, p < .01, with paired Student’s t test (mean ± SEM). (G): Dual optical recordings using ArcLight (top) and R-GECO1 (bottom) were available at day 90 after coinfection was performed at day 15 (n = 10, 2 control lines). The values of the y-axis are relative fluorescent signal (ΔF/F0) for R-GECO1 and −ΔF/F0 for ArcLight. Abbreviations: ISO, isoproterenol; NS, not significant.
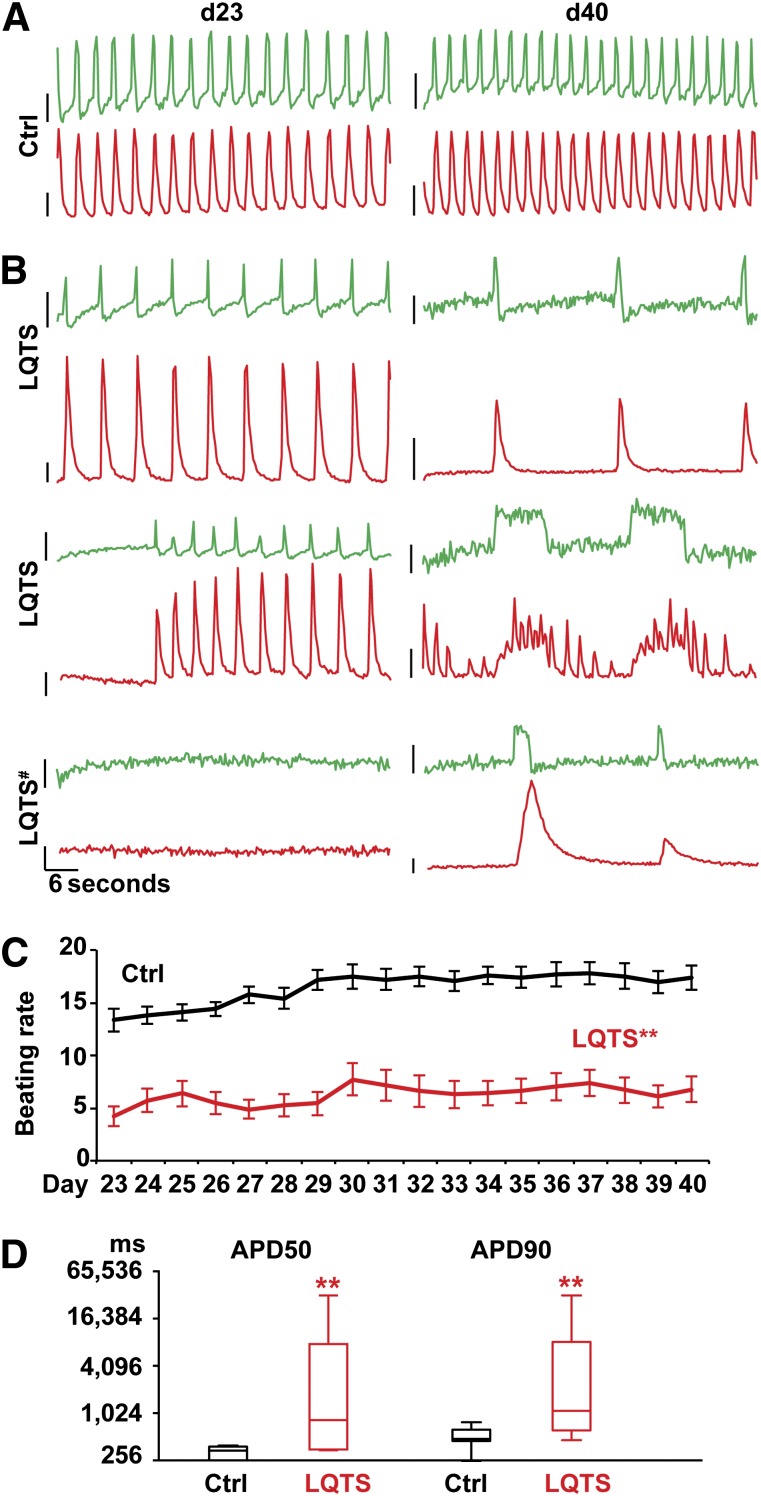
As a proof of principle, we tested the β-agonist, isoproterenol (ISO), on control cardiomyocytes to determine the effect of ISO on the contraction rate, calcium handling, and action potentials. ISO significantly increased the beating rate, calcium transients, and action potentials (Fig. 3E, 3F). Next, we infected control cardiomyocytes with R-GECO1 and ArcLight at day 15 to determine how long after lentiviral infection it was possible to record the calcium and voltage signals. We found that the dual optical recording was feasible in the infected cells until day 90 (75 days total after the infection; Fig. 3G). Next, to examine the physiological phenotypes in LQTS cardiomyocytes, we infected LQTS cardiomyocytes using the viruses and conducted daily optical recordings. The imaging demonstrated irregular calcium transients and prolonged action potentials in LQTS cardiomyocytes compared with the control cardiomyocytes (Fig. 4). These results were consistent with previous reports using conventional recordings with calcium dyes and a whole cell patch clamp [3]. These results demonstrate that the dual optical recording is a useful approach to examine contraction, calcium handling, and action potentials in iPSC-derived cardiomyocytes. Next, to test whether dual optical recording can be used to visualize physiological phenotypes in single cardiomyocytes derived from control and LQTS patients, we dissociated a monolayer of infected cardiac cells into single cardiomyocytes and performed additional dual optical recordings (Fig. 5). We found that LQTS cardiomyocytes showed irregular and prolonged action potentials, abnormal calcium transients, and irregular contraction, and we confirmed that roscovitine could restore electrical activity, calcium handling, and spontaneous beating in LQTS cardiomyocytes. Additional analyses also demonstrated that phenotypes in calcium handling and electrical activities were observed in LQTS ventricular-like cardiomyocytes but not in nodal-like and atrial-like cells (supplemental online Fig. 9E), which had been reported in previous findings [3]. These results demonstrate that dual optical recording using ArcLight and R-GECO1 is a useful approach to examine contraction, calcium handling, and action potentials in iPSC-derived cardiomyocytes.
Figure 4.
Dual optical recordings for action potentials and calcium handling in patient cardiomyocytes. Representative traces of dual optical recordings using ArcLight (top) and R-GECO1 (bottom) in Ctrl (n = 27, [A]) and LQTS cardiomyocytes (n = 32, [B]) at days 23 and 40. Dynamic changes of cellular phenotypes were observed in LQTS cardiomyocytes. #, Contraction was observed at days 12–18 and the cardiomyocytes stopped beating at day 23. The values of y-axis are 0.1 (relative fluorescent signal, ΔF/F0) for R-GECO1 and −0.1 (−ΔF/F0) for ArcLight. (C): The beating rate (per minute) during daily optical recording in Ctrl and LQTS cardiomyocytes at days 23–40. ∗∗, p < .01, with Student’s t test at each time point (mean ± SEM). (D): Action potential values in Ctrl and LQTS cardiomyocytes at day 40. APDs are shown with Student’s t test between Ctrl and LQTS (∗∗, p < .01; box and whiskers, minimum to maximum). Abbreviations: APD50, action potential duration 50% from peak; APD90, action potential duration 90% from peak; Ctrl, control; d, day; LQTS, long QT syndrome.
Figure 5.
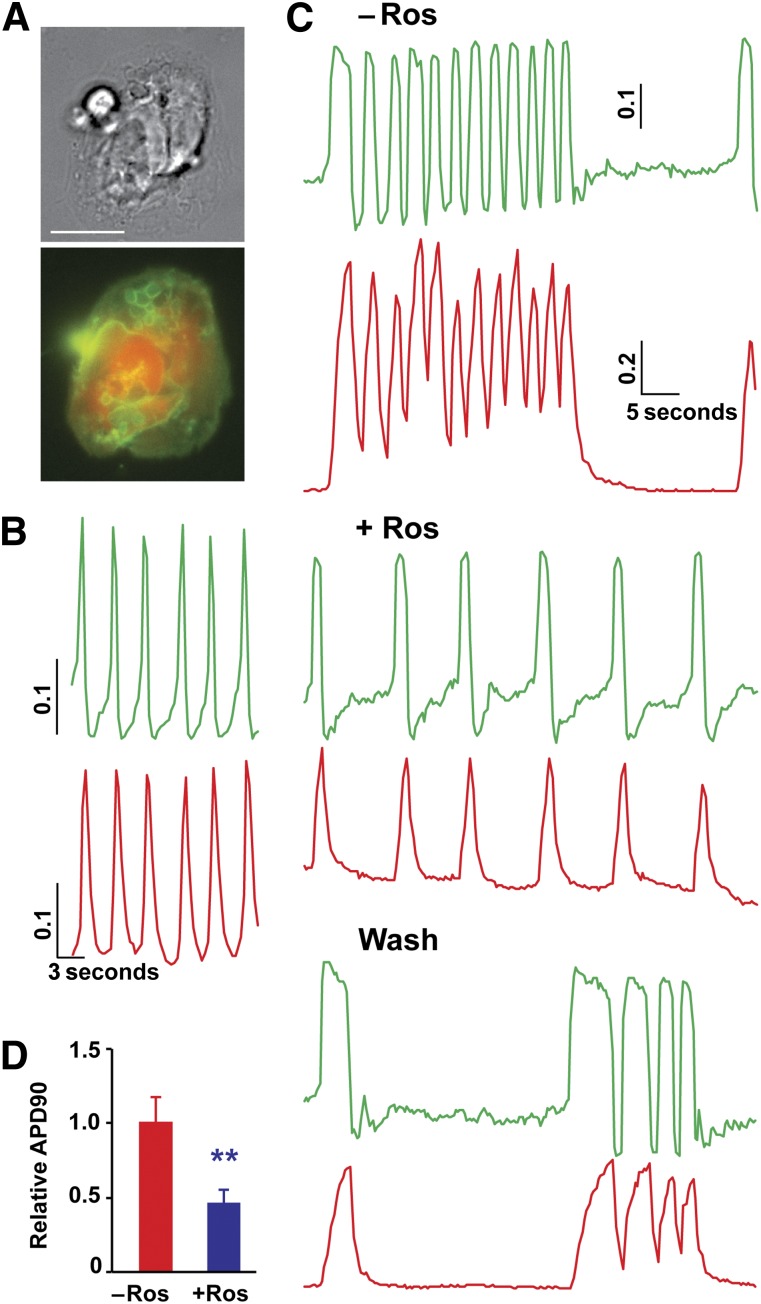
Dual optical recordings for action potentials and calcium handling in single patient cardiomyocytes. (A): Phase contrast image (top) and fluorescent image (bottom) of a control single cardiomyocyte with ArcLight (green) and R-GECO1 indicators (red). Scale bar = 10 μm. (B): Representative traces of voltage (top) and calcium ions (bottom) in control single cardiomyocytes. (C): Dual optical recording for action potentials and calcium handling in LQTS cardiomyocytes before, during, and after treatment with 5 μM Ros. Ros prevented irregular calcium handling and electrical activities observed in LQTS cardiomyocytes. (D): Ros rescued prolonged action potentials in LQTS cardiomyocytes. ∗∗, p < .01, with Student’s t test (n = 12; 4 lines from 2 patients with LQTS). The values of y-axis are relative fluorescent signal (ΔF/F0) for R-GECO1 and −ΔF/F0 for ArcLight. Abbreviations: APD90, action potential duration 90% from peak; LQTS, long QT syndrome; Ros, roscovitine.
Discussion
In the present study, we optimized and improved several experimental conditions to generate integration- and feeder-free iPSC lines efficiently, although we used the original E8 medium [8] and episomal vectors [10, 21]. Single transfection was sufficient for feeder-free reprogramming of human keratinocytes in our protocol, although previous studies required repeated gene transductions on feeder cultures [22]. In addition, this protocol using single lipofection does not require installation and the use of an electroporation system. In contrast, previous studies needed an electroporator to introduce the episomal vectors into the cells. The protocol will enable us to handle multiple patient fibroblasts and keratinocytes at the same time with a 24-well plate to generate multiple independent lines of feeder-free, integration-free, and virus-free iPSCs efficiently and reproducibly. Gene expression profiling demonstrated that the expression of EBNA1, a component of episomal vectors, was not detected in all established lines used for the additional experiments and cardiac differentiation (supplemental online Fig. 6B), suggesting that no active episomal vectors were present in the generated iPSCs. Although the current efficiency is sufficient to generate several independent iPSC lines from a patient using this protocol with minimal steps and inexpensive reagents, the efficiency in reprogramming using single lipofection could be further improved by additional factors that were recently reported [23–25].
A variety of genetically encoded fluorescent indicators for calcium and voltage have been developed, validated, and compared with conventional recordings using calcium imaging with dyes and a patch clamp [6, 7]. However, no study has used these indicators for recording voltage and calcium simultaneously in human patient iPSC-derived cardiomyocytes to examine cellular phenotypes. The approach using the dual optical recordings for action potentials and calcium handling allowed us to repeat experiments in the same cardiomyocytes daily or weekly to identify the cellular phenotypes associated with cardiac arrhythmias. Although lentiviral expression enables cellular phenotyping in patient-specific cardiomyocytes, as well as drug testing, stable expression of R-GECO1 and ArcLight using the adeno-associated virus S1 integration site in human iPSCs would be ideal to address the remaining questions regarding human cardiovascular development, function, and disease and to use human pluripotent stem cell-derived cardiomyocytes for regenerative medicine [26]. In addition, dual optical recording for action potentials and calcium handling would be a useful tool to study cellular phenotypes in neural progenitors and neurons for iPSC modeling of psychiatric diseases, autism, and developmental disorders.
Conclusion
Our optimized protocol enables efficient reprogramming that can be applied to a variety of patients for developing iPSC-based models of cardiac diseases. Dual optical recording using R-GECO1 and ArcLight fluorescent indicators is a robust approach to examine physiological phenotypes and test drug candidates in LQTS iPSC-derived cardiomyocytes.
Supplementary Material
Acknowledgments
We thank J. Feng for the helpful discussion and FACS analyses and B. Corneo and G. Vunjak-Novakovic for the helpful discussion. We are grateful to I. Minami for advice in cardiac differentiation, T. Swayne for confocal calcium imaging, S.H. Ho for fluorescence-activated cell sorting analyses, W. Froehlich-Santino, J. Bernstein, J. Hallmayer, and R. E. Dolmetsch for sharing long QT syndrome patient fibroblasts, J. Wynn for congenital heart disease (CHD) study coordination, and P. Lanzano for CHD patient fibroblast preparations. Funding was provided by NIH Grants U01HL098163 and R01HD057036 to W.K.C. and the Leona M. and Harry B. Helmsley Charitable Trust stem cell starter grant, Columbia Core Usage Funding Program (for confocal microscopy), NIH Pathway to Independent Award (Grant R00HL11345), and startup funds from the Columbia Stem Cell Initiative and Department of Rehabilitation and Regenerative Medicine to M.Y. D.W.A. is currently affiliated with the Rowan University School of Osteopathic Medicine, Stratford, NJ.
Author Contributions
L.S.: collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; D.W.A., E.Y.H., E.U.-A., and S.-H.E.P.: collection and assembly of data, data analysis and interpretation; Y.A.Y: data collection; W.K.C.: provision of study materials, manuscript writing; M.Y.: conception and design, collection and assembly of data, data analysis and interpretation, financial support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Moretti A, Bellin M, Welling A, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 2.Itzhaki I, Maizels L, Huber I, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471:225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 3.Yazawa M, Hsueh B, Jia X, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471:230–234. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paşca SP, Portmann T, Voineagu I, et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat Med. 2011;17:1657–1662. doi: 10.1038/nm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellin M, Casini S, Davis RP, et al. Isogenic human pluripotent stem cell pairs reveal the role of a KCNH2 mutation in long-QT syndrome. EMBO J. 2013;32:3161–3175. doi: 10.1038/emboj.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin L, Han Z, Platisa J, et al. Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron. 2012;75:779–785. doi: 10.1016/j.neuron.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Araki S, Wu J, et al. An expanded palette of genetically encoded Ca2+ indicators. Science. 2011;333:1888–1891. doi: 10.1126/science.1208592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G, Gulbranson DR, Hou Z, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krey JF, Paşca SP, Shcheglovitov A, et al. Timothy syndrome is associated with activity-dependent dendritic retraction in rodent and human neurons. Nat Neurosci. 2013;16:201–209. doi: 10.1038/nn.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okita K, Matsumura Y, Sato Y, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 11.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida Y, Takahashi K, Okita K, et al. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 15.Kattman SJ, Witty AD, Gagliardi M, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Lian X, Zhang J, Azarin SM, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minami I, Yamada K, Otsuji TG, et al. A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine- and xeno-free conditions. Cell Rep. 2012;2:1448–1460. doi: 10.1016/j.celrep.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Ma J, Guo L, Fiene SJ, et al. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol. 2011;301:H2006–H2017. doi: 10.1152/ajpheart.00694.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itzhaki I, Rapoport S, Huber I, et al. Calcium handling in human induced pluripotent stem cell derived cardiomyocytes. PLoS One. 2011;6:e18037. doi: 10.1371/journal.pone.0018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leyton-Mange JS, Mills RW, Macri VS, et al. Rapid cellular phenotyping of human pluripotent stem cell-derived cardiomyocytes using a genetically encoded fluorescent voltage sensor. Stem Cell Rep. 2014;2:163–170. doi: 10.1016/j.stemcr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakagawa M, Taniguchi Y, Senda S, et al. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep. 2014;4:3594. doi: 10.1038/srep03594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piao Y, Hung SS, Lim SY, et al. Efficient generation of integration-free human induced pluripotent stem cells from keratinocytes by simple transfection of episomal vectors. Stem Cells Translational Medicine. 2014;3:787–791. doi: 10.5966/sctm.2013-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rais Y, Zviran A, Geula S, et al. Deterministic direct reprogramming of somatic cells to pluripotency. Nature. 2013;502:65–70. doi: 10.1038/nature12587. [DOI] [PubMed] [Google Scholar]
- 24.Shinagawa T, Takagi T, Tsukamoto D, et al. Histone variants enriched in oocytes enhance reprogramming to induced pluripotent stem cells. Cell Stem Cell. 2014;14:217–227. doi: 10.1016/j.stem.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotency in human somatic cells via a transient state resembling primitive streak-like mesendoderm. Nat Commun. 2014;5:3678. doi: 10.1038/ncomms4678. [DOI] [PubMed] [Google Scholar]
- 26.Chong JJ, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.