Human umbilical cord mesenchymal stem cells (hucMSCs) and their exosomes have been considered as potential therapeutic tools for tissue regeneration; however, the underlying mechanisms are not well understood. The results of this study suggest that hucMSC-exosome-mediated Wnt4 induces β-catenin activation in endothelial cells and exerts proangiogenic effects, which could be an important mechanism for cutaneous wound healing.
Keywords: Exosomes, Angiogenesis, Regenerative medicine, Wnt4, β-Catenin
Abstract
Human umbilical cord mesenchymal stem cells (hucMSCs) and their exosomes have been considered as potential therapeutic tools for tissue regeneration; however, the underlying mechanisms are still not well understood. In this study, we isolated and characterized the exosomes from hucMSCs (hucMSC-Ex) and demonstrated that hucMSC-Ex promoted the proliferation, migration, and tube formation of endothelial cells in a dose-dependent manner. Furthermore, we demonstrated that hucMSC-Ex promoted wound healing and angiogenesis in vivo by using a rat skin burn model. We discovered that hucMSC-Ex promoted β-catenin nuclear translocation and induced the increased expression of proliferating cell nuclear antigen, cyclin D3, N-cadherin, and β-catenin and the decreased expression of E-cadherin. The activation of Wnt/β-catenin is critical in the induction of angiogenesis by hucMSC-Ex, which could be reversed by β-catenin inhibitor ICG-001. Wnt4 was delivered by hucMSC-Ex, and the knockdown of Wnt4 in hucMSC-Ex abrogated β-catenin nuclear translocation in endothelial cells. The in vivo proangiogenic effects were also inhibited by interference of Wnt4 expression in hucMSC-Ex. Taken together, these results suggest that hucMSC-Ex-mediated Wnt4 induces β-catenin activation in endothelial cells and exerts proangiogenic effects, which could be an important mechanism for cutaneous wound healing.
Significance
Human umbilical cord mesenchymal stem cells (hucMSCs) and their exosomes have been considered as potential therapeutic tools for tissue regeneration; however, the underlying mechanisms are still not well understood. In this study, it is reported that hucMSC-Ex-mediated Wnt4 induces β-catenin activation in endothelial cells and exerts proangiogenic effects, which could be one of the important mechanisms responsible for cutaneous wound healing.
Introduction
Mesenchymal stem cells (MSCs) are adult stem cells with unique characteristics including long-term ex vivo proliferation, multilineage differentiation potential, and immunomodulatory properties [1]. MSCs have been suggested as promising candidates for a variety of therapeutic applications, such as immune disorders including graft versus host disease and systemic lupus erythematosus, bone and cartilage regeneration, neurological diseases, hepatic injury, acute renal failure, and myocardial infarction [2–7]. Paracrine action but not transdifferentiation has been considered as the predominant mechanism for the role of MSCs in tissue repair [8, 9]. Cell-derived exosomes are emerging as a new mechanism in intercellular communication [10]. It has been proposed that MSC-derived exosomes are effective for reducing myocardial ischemia and reperfusion damage [11], alleviating liver fibrosis [12], and protecting against acute kidney injury [13]. Such suggestions indicate that MSC-derived exosomes may serve as a novel therapeutic tool for tissue repair; however, the mechanisms underlying these actions remain elusive.
Therapeutic angiogenesis has begun to make substantial inroads in the field of tissue engineering and wound healing [14, 15]. Angiogenesis involves a variety of coordinated events including degradation of the extracellular matrix surrounding the parent vessel, migration and proliferation of the endothelial cells and mural cells to assemble the new vessel, lumen formation, and construction of the mural cell layer of the vessel wall with associated pericytes and/or smooth muscle cells [16]. Extracellular vesicles derived from human bone marrow mesenchymal stem cells could promote angiogenesis in tissue regeneration [17, 18]. Exosomes enhance angiogenesis by delivering microRNAs, mRNAs, and active proteins [19–24]. We previously reported that exosomes from human umbilical cord mesenchymal stem cells (hucMSC-Ex) intensively enhance cutaneous wound healing; however, the effects of hucMSC-Ex on angiogenesis and the underlying mechanisms are not well characterized. Endothelial cells are the primary constituents of new vessels, and many endothelial cell functions are required for angiogenesis. In this study, we investigated the role of hucMSC-Ex in angiogenesis in vitro and in vivo. Our results showed that hucMSC-Ex promoted endothelial cell proliferation, migration, and tube formation. We found that hucMSC-Ex promoted angiogenesis to repair deep second-degree burn injury to skin by delivering Wnt4 and activating Wnt/β-catenin signaling in endothelial cells.
Materials and Methods
Cell Culture
HucMSCs were isolated and characterized, as described previously [25]. The cells in passages 3 and 4 were used for experiments. Human umbilical vein endothelial cell-derived cell line EA.hy926 and human lung fibroblasts (HFL1) were purchased from the cell bank of the Chinese Academy of Sciences (Beijing, People’s Republic of China, http://english.cas.cn) and maintained in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS).
Exosome Purification and Characterization
HucMSC-Ex and HFL1-Ex were purified by differential centrifugation, as described previously and with modifications [26]. Briefly, hucMSCs and HFL1 were cultured in serum-free DMEM (ExCell Bio, Shanghai, People’s Republic of China, http://www.excellbio.com) for 48 hours. When the cells reached 50% confluence, the conditioned medium was collected and centrifuged at 300g for 10 minutes, at 2,000g for 10 minutes, and at 10,000g for 30 minutes to remove cells and cell debris. The clarified supernatant was then concentrated with 100-kDa molecular weight cutoff (MWCO) hollow fiber membrane (Millipore, Billerica, MA, http://www.emdmillipore.com) at 1,000g for 30 minutes. The concentrated supernatant was loaded onto a 30% sucrose/D2O cushion (5 ml, density 1.210 g/cm3) and then ultracentrifuged at 100,000g for 3 hours (Optima L-90K; Beckman Coulter, Brea, CA, https://www.beckmancoulter.com). The exosome-enriched fraction was collected from the bottom of the tube and washed 3 times with phosphate-buffered saline (PBS) by centrifugation at 1,500g for 30 minutes with 100-KDa MWCO. Final exosomes were passed through a 0.22-μm filter and stored at −70°C. The protein content of the concentrated exosomes was determined using a bicinchoninic acid (BCA) protein assay kit (CWBIO, Shanghai, People’s Republic of China, http://www.cwbiotech.bioon.com.cn/), and exosomal markers CD9 and HSP70 were determined using Western blot. Purified exosomes were identified by transmission electron microscopy. A drop of exosomes (20 μl) was pipetted onto a grid which was coated with formvar and carbon, standing for 5 minutes at room temperature. The excess fluid was removed with a piece of filter, and the sample was negatively stained with 3% (wt/vol) phosphotungstic acid (pH 6.8) for 5 minutes. After air drying under an electric incandescent lamp, the sample was analyzed by transmission electron microscopy (FEI Tecnai 12; FEI, Hillsboro, OR, http://www.fei.com). The diameter of exosomes was determined with the NanoSight LM10 instrument (NanoSight, Amesbury, U.K., http://www.nanosight.com). The samples were serially diluted in PBS to reach a particle concentration suitable for analysis with nanoparticle tracking analysis (NTA; 1.0 × 108 to 2.5 × 109 particles per milliliter; NanoSight) and then injected into the LM10 unit (approximately 500 μl) with a 1-ml sterile syringe. Videos were collected and analyzed using the NTA software (version 2.3, build 0006 BETA2; NanoSight). The protein content, as the quantification of exosomes, was determined by using a BCA protein assay kit (CWBIO). The purified exosomes were stored at −70°C until use. The final concentration of exosomes used for treating skin cells in vitro was 80 or 160 μg/ml, and a total of 200 μg of exosomes were applied to treat each animal.
Exosome Labeling and Internalization
HucMSC-Ex were labeled with the crosslinkable membrane dye CM-DiI (red), according to the manufacturer’s protocol (Molecular Probes; Thermo Fisher Scientific, Waltham, MA, http://www.lifetechnologies.com/us/en/home/brands/molecular-probes.html). The labeled exosome suspension was filtered through a 100-kDa MWCO hollow fiber membrane (Millipore), and PBS was used as a negative control. EA.hy926 cells (5 × 103 per well) were seeded in 96-well plates and incubated with labeled exosomes at 37°C for 4 hours. The cells were then washed with PBS and fixed in 4% paraformaldehyde. The nuclei were counterstained with Hoechst 33342 (1:200, σ). Confocal images were sequentially acquired with ArrayScan VTI (Thermo Fisher Scientific).
Cell Viability Assay
Cell viability was determined by using the MTT (3-[4,5-dimethylthiazol-2-yl]- 2,5-diphenyltetrazolium bromide) assay, as described previously [27]. Briefly, EA.hy926 cells were seeded in 96-well plates at 3 × 103 cells per well and incubated at 37°C for 12 hours. After synchronization with 2% FBS for 24 hours, cells were cocultured with exosomes (80 and 160 μg/ml) or exosome diluent (PBS) for 24, 48, 72, and 96 hours, and then 10 μl of the MTT solution (5 mg/ml; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) was added into each well. The supernatant was then removed, and the formazan crystals were dissolved in 100 μl of dimethyl sulfoxide. The optical density was measured at 490 nm on a multiwell plate reader (Bio-TEK, Winooski, VT, http://www.biotek.com), and all samples were assayed with five copies.
Cell-Counting Assay
A total of 3 × 103 EA.hy926 cells were seeded in each well of the 96-well plate and incubated at 37°C for 12 hours. After synchronization with 2% FBS for 24 hours, cells were cocultured with exosomes (80 and 160 μg/ml) or exosome diluent (PBS) for 24, 48, 72, and 96 hours. Cell numbers were counted at the indicated time points, and all samples were repeated in triplicate.
Cell Migration Assay
The migration of EA.hy926 cells was assayed by Transwell (Corning, Corning, NY, http://www.corning.com) chemotaxis chambers (8-μm-pore filters) and wound-healing assay. Briefly, after synchronization with 2% FBS for 24 hours, EA.hy926 cells (5 × 104 in 200 μl) were suspended in serum-free medium and added to the upper chamber. Next, 600 μl of media with 2% FBS containing hucMSC-Ex (80 and 160 μg/ml) or PBS was added to the lower chamber. Cells on the upper layer of the membrane were wiped up with swabs after incubation for 12 hours at 37°C. Cells that had migrated through the membrane were fixed with 4% paraformaldehyde and stained with crystal violet. The stained cells were observed under a microscope, and at least six fields of cells were assayed for each group.
For the wound-healing assay, when the EA.hy926 cells were grown to monolayer, wounds were scratched with a sterile plastic 10-μl micropipette tip, washed, and incubated with or without hucMSC-Ex for 12 hours. Images were taken with a digital camera at 0, 12, and 24 hours and measured manually with ImageJ software (NIH, Bethesda, MD, http://imagej.nih.gov/ij/). The data were reported as the ratio of migration relative to the control.
Endothelial Tube Formation Assay
The endothelial tube-formation assay was performed following the manufacturer’s protocol (BD Biosciences, Franklin Lakes, NJ, https://www.bdbiosciences.com). Matrigel (50 μl) was added to each well of a 96-well plate and allowed to polymerize. HucMSC-Ex (80 and 160 μg/ml) or PBS was added to the synchronized EA.hy926 cells (2 × 104) plated on Matrigel. After incubation for 12 hours at 37°C, the cells were viewed under a microscope and photographed. Tube length was measured with ImageJ software.
Western Blot
Cells were collected, washed, and lysed with RIPA buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EGTA, 0.1% SDS, 1 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 1 mg/ml aprotinin, 1 mg/ml leupeptin). The protein concentration of each sample was determined using a BCA protein assay kit. Overall, 80 μg protein were subjected to 12% SDS-polyacrylamide gel electrophoresis gels and then transferred onto polyvinylidene fluoride membranes. After being blocked with 5% skim milk for 1 hour, the membranes were incubated with primary and horseradish peroxidase-conjugated secondary antibodies and detected by the Amersham ECL detection system (GE Healthcare Life Sciences, Little Chalfont, U.K., http://www.gelifesciences.com) and quantitated using a Molecular Dynamics densitometer (Sage Creation Science, Beijing, People’s Republic of China, http://www.sagecreation.com.cn/en/) with MD ImageQuant software(GE Healthcare Life Sciences). Primary antibodies used in the experiments were as follows: CD9 (1:500; Bioworld Technology, St. Louis Park, MN, http://bioworlde.com), HSP70 (1:300; Bioworld Technology), CD63 (1:300; Bioworld Technology), CD81 (1:300; Epitomics, Burlingame, CA, http://www.epitomics.com), N-cadherin(1:400; Bioworld Technology), E-cadherin (1:200; Santa Cruz Biotechnology, Dallas, TX, http://www.scbt.com), proliferating cell nuclear antigen (PCNA; 1:500; Bioworld Technology), cyclin D3 (1:200; Bioworld Technology), glyceraldehyde-3-phosphate dehydrogenase (1:2,000; CWBIO), Wnt4 (1:200, Santa Cruz Biotechnology), and histone (1:500; Signalway Antibody, College Park, MD, http://www.sabbiotech.com). The secondary antibodies were horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse antibodies (1:2,000; CWBIO).
Immunofluorescence Analysis
EA.hy926 cells were fixed in 4% paraformaldehyde for 20 minutes, permeabilized for 3 minutes with 0.1% Triton-X100, blocked with 5% bovine serum albumin, and incubated with rabbit monoclonal anti-β-catenin (1:200; Cell Signaling Technology, Beverly, MA, http://www.cellsignal.com) and CD31 (1:50; Bioworld Technology) antibodies overnight at 4°C, followed by incubation with Cy3-labeled anti-rabbit IgG secondary antibody (1:800, σ) at 37°C for 45 minutes. The nuclei were counterstained with Hoechst 33342 (1:200; Sigma-Aldrich). Images were acquired sequentially with a fluorescent microscope (Nikon, Tokyo, Japan, http://www.nikon.com).
Rat Skin Wound Model and Treatment
A rat model of skin-deep second-degree burn wound was established, as described previously [28, 29]. Subsequently, 200 μg exosomes (hucMSC-Ex and HFL1-Ex) suspended in 200 μl PBS were injected subcutaneously at 3 sites. The normal group had no treatment. The animals were housed individually. At 1 week and 2 weeks after treatment, the rats were sacrificed, and the wound area was cut for further analysis.
Hematoxylin and Eosin Staining
The wound skin and surrounding skin (4 mm2) were fixed in 4% paraformaldehyde (pH 7.4) and gradually dehydrated, embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin and eosin for light microscopy.
Lentiviral Knockdown of Wnt4 in HucMSCs
The Wnt4 short hairpin RNA (shRNA) lentiviral expression vector was specifically selected for Wnt4 gene silencing, which was defined as Lenti-Wnt4-shRNA, and Lenti-GFP-shRNA was the negative control. The Wnt4 shRNA lentivirus vectors were generated by ligating the vector Tet-pLKO-puro. Wnt4 shRNA oligonucleotide sequences are as follows: forward, 5′-CCGGCCCAAGAGATACTGGTTGTATCTCGAGATACAACCAGTATCTCTTGGGTTTTTG-3′; reverse, 5′-AATTCAAAAACCCAAGAGATACTGGTTGTATCTCGAGATACAACCAGTATCTCTTGGG-3′. The sequences of control shRNA are as follows: forward, 5′-CCGGGCAAGCTGACCCTGAAGTTCATCTCGAGATGAACTTCAGGGTCACGTTGCTTTTTG-3′; reverse, 5′-AATTCAAAAAGCAAGCTGACCCTGAAGTTCATCTCGAGATGAACTTCAGGGTCACGTTGC-3′. The recombinant lentivirus was produced by cotransfecting HEK293T cells with PLKO-GFP-shRNA or PLKO-Wnt4-shRNA, PU1562, and PU1563 plasmid by using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific). The virus-containing supernatant was harvested at 48 and 72 hours after transfection. HucMSCs were transduced with the prepared lentivirus (Lenti-Wnt4-shRNA or Lenti-GFP-shRNA). Stable cell lines were obtained after selection with 1 μg/ml puromycin (Invitrogen) for 15 days. The expression of shRNA was induced by the addition of 80 μg/ml doxycycline for 2 days. The efficiency of Wnt4 knockdown was evaluated using quantitative reverse transcription polymerase chain reaction and Western blot. The stable cell lines were cultured in serum-free medium for 48 hours, and then the supernatants were collected and exosomes isolated for further study.
Cytoplasm and Nuclear Fractionation
Cytoplasm and nuclear fractionation was performed according to the manufacturer’s instructions (HiScript II First Strand cDNA Synthesis Kit, R211-02; Vazyme Biotech, Nanjing, Jiangsu, People’s Republic of China, http://www.vazyme.com). Briefly, cells were harvested and washed once with cold phosphate-buffered saline. Cells were then suspended in isolation buffer A mixed with protease inhibitors and rotated at 4°C for 1 minute. After 12,000g centrifugation at 4°C for 5 minutes, supernatant was collected containing the cytoplasm fraction. The remaining cell debris was then suspended in isolation buffer B mixed with protease inhibitors and rotated at 4°C for 1 minute and repeated 3 times every 10 minutes. Cytoplasm and nuclear fractionation was stored at −80°C to prepare for Western blot detection.
Statistical Analysis
All data were expressed as mean ± SD. Statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA, http://www.graphpad.com). The means of different treatment groups were compared by one-way analysis of variance. A p value <.05 was considered statistically significant.
Results
Exosome Characterization and Internalization
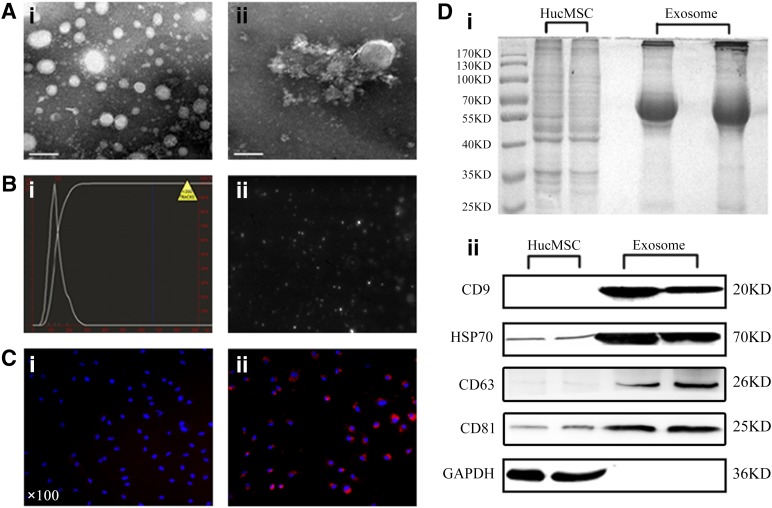
HucMSC-Ex were successfully isolated using the differential centrifugation method and purified on a 30% sucrose/D2O cushion. The morphology of purified exosomes was observed by using transmission electron microscopy. As shown in Figure 1A and 1B, the exosomes had a characteristic saucer-like shape that was limited by a lipid bilayer with a diameter ranging from 30 to 100 nm. A comparison of the protein profiles of cell lysates and exosomes revealed that the component of cell lysates differed from that of exosomes (Fig. 1Di). The results of Western blot showed that hucMSC-Ex expressed exosomal markers such as CD9 and HSP70, and these proteins were more abundant in exosome fractions (Fig. 1Dii). To test the internalization of exosomes by endothelial cells, the CM-DiI-labeled exosomes were added into culture medium and incubated with EA.hy926 cells for 4 hours at 37°C. We found that red fluorescence was detected in the EA.hy926 cells, whereas the PBS group had no fluorescence, suggesting the internalization of labeled exosomes by EA.hy926 cells (Fig. 1C).
Figure 1.
Exosome characterization and internalization. (Ai, Aii): Morphologic analysis of hucMSC-derived exosomes (hucMSC-Ex) by transmission electron microscopy (scale bar = 100 nm). (B): Size distribution measurements under flow conditions by nanoparticle tracking (Bi) with the corresponding video frame (Bii). (C): Phosphate-buffered saline was used as a control (×100) (Ci), and CM-Dil-labeled exosomes were taken up by EA.hy926 cells (×100) (Cii). (Di): SDS-polyacrylamide gel electrophoresis analysis of the protein profiles of cell lysates and exosomes. (Dii): Detection of exosomal marker expression in hucMSC-Ex by Western blot. Abbreviation: hucMSC, human umbilical cord mesenchymal stem cells.
HucMSC-Ex Prompted the Proliferation and Migration of Endothelial Cells
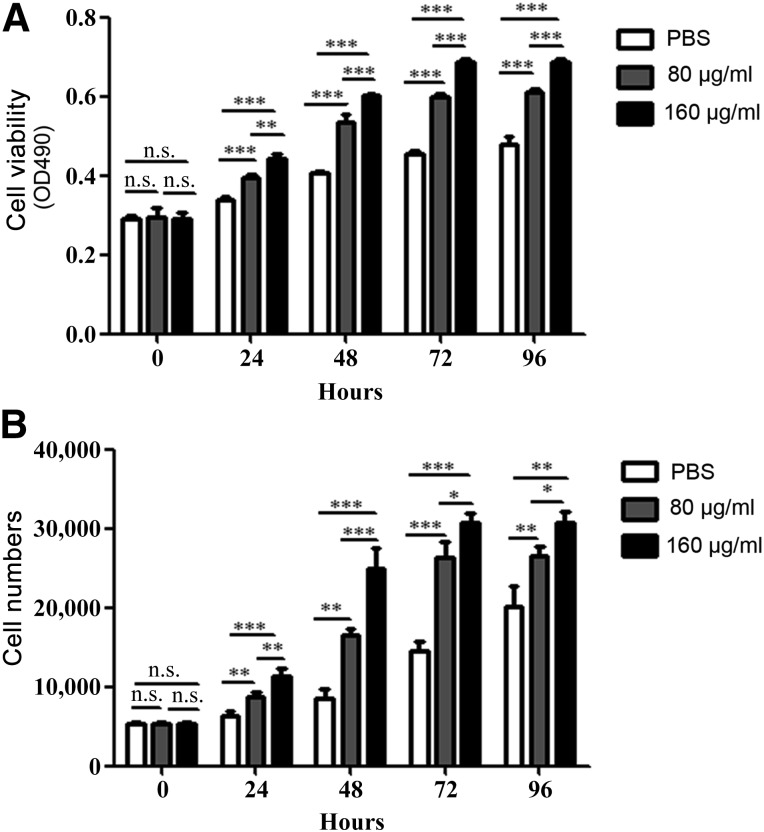
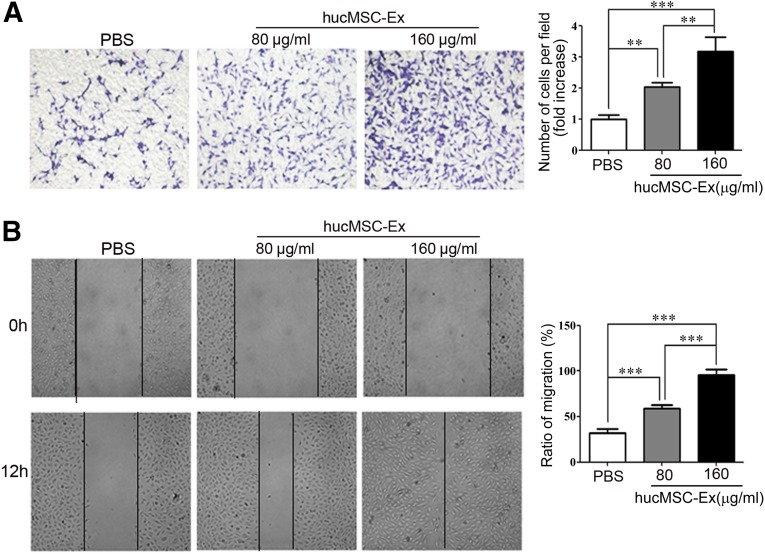
To determine the effects of hucMSC-Ex on endothelial cell growth, we evaluated the viability of EA.hy926 cells treated with various concentrations of exosomes (80 and 160 μg/ml) for different times (24, 48, 72, and 96 hours). The results of the MTT assay showed that hucMSC-Ex promoted EA.hy926 cell growth in a dose-dependent manner (Fig. 2A), and this finding was further confirmed by the results of the cell-counting assay (Fig. 2B). Endothelial cell migration is critical for angiogenesis [30]. To analyze whether the migratory ability of EA.hy926 cells was affected by hucMSC-Ex, EA.hy926 cells were incubated with different concentrations of hucMSC-Ex (80 and 160 μg/ml) for 12 hours. The results of both the transwell migration assay and the wound-healing assay showed that hucMSC-Ex incubation induced an enhanced migratory capacity in EA.hy926 cells relative to the control in a dose-dependent manner (Fig. 3).
Figure 2.
Exosomes from human umbilical cord mesenchymal stem cells (HucMSC-Ex) prompted the proliferation of endothelial cells. (A): MTT assay for the proliferating ability of EA.hy926 cells with or without hucMSC-Ex treatment. (B): Cell-counting assay for the proliferating ability of hucMSC-Ex-treated EA.hy926 cells. ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001. Abbreviations: n.s., not significant; PBS, phosphate-buffered saline.
Figure 3.
HucMSC-Ex prompted the migration of endothelial cells. (A): EA.hy926 cells were treated with different concentrations of exosomes (80, 160 μg/ml) or PBS for 12 hours. Transwell migration assay was performed to analyze the migratory ability of the cells (×100). Cells that migrated to the bottom were counted. ∗∗, p < .01; ∗∗∗, p < .001. (B): EA.hy926 cells treated with different concentrations of exosomes (80, 160 μg/ml) were subjected to a wound-healing assay for 12 hours (×100), and the percentage of closure of the wounded areas was measured. ∗∗, p < .01; ∗∗∗, p < .001. Abbreviations: hucMSC-Ex, exosomes from human umbilical cord mesenchymal stem cells; PBS, phosphate-buffered saline.
HucMSC-Ex Improved the Tube-Formation Ability of Endothelial Cells In Vitro and Promoted Angiogenesis in a Cutaneous Burn Model In Vivo
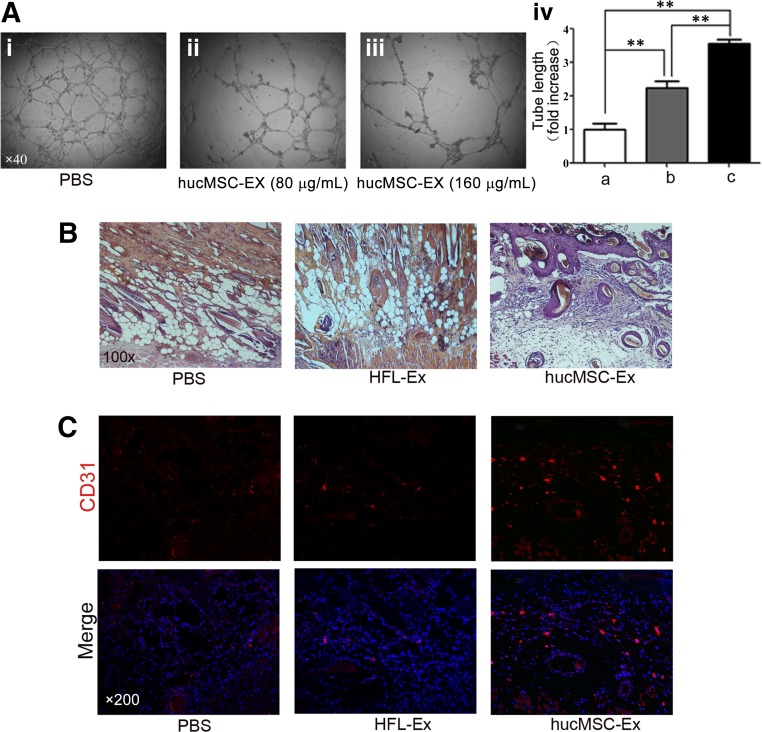
The ability of hucMSC-Ex to promote angiogenesis in vitro was evaluated by using the endothelial tube formation assay. As shown in Figure 4A, hucMSC-Ex increased the tube length more efficiently than control in a dose-dependent manner. To study the effects of hucMSC-Ex on angiogenesis in vivo, we established a skin-deep second-degree burn model in rats. The results of histological evaluation of wounds at 1 week after infusion showed that the number of epidermal and dermal cells significantly increased in hucMSC-Ex-treated wounds (Fig. 4B), whereas wounds treated with PBS and control exosomes (HFL1-Ex) were still in a second-degree burn-injury state (Fig. 4B). The results of immunofluorescence staining for vascular endothelial cell marker CD31 showed that the hucMSC-Ex group had more CD31-positive cells in the wound area than the PBS and HFL1-Ex groups at both 1 week and 2 weeks after infusion (Fig. 4C). Taken together, these results revealed that hucMSC-Ex improved the function of endothelial cells in vitro and promoted angiogenesis in a cutaneous burn model in vivo.
Figure 4.
HucMSC-Ex improved the tube-formation ability of endothelial cells and vascular density in cutaneous burn model. (Ai–Aiii): Exosomes increased the tube length in a dose-dependent manner (×40). (Aiv): Measurement of the tube length by ImageJ software. ∗∗, p < .01. (B): Representative micrographs of wound histological images (hematoxylin and eosin staining) at 1 week after treatment with hucMSC-Ex, HFL1-Ex, or PBS (×100). (C): Representative immunofluorescence images of CD31 expression in the wound area after treatment with hucMSC-Ex, HFL1-Ex, or PBS (×200). Abbreviations: HFL-Ex, exosomes from human lung fibroblast; hucMSC-Ex, exosomes from human umbilical cord mesenchymal stem cells; PBS, phosphate-buffered saline.
HucMSC-Ex Promoted Angiogenesis by Activating Wnt/β-Catenin Signaling
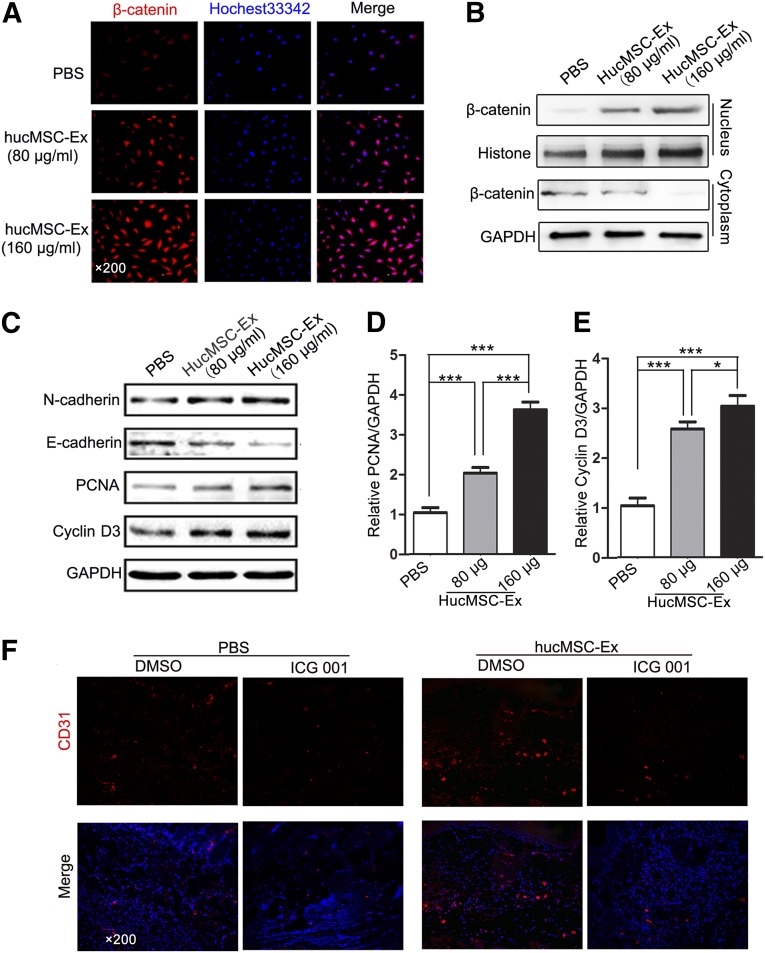
Many studies have proved that the Wnt signaling pathway plays important roles in angiogenesis [31–33]. We hypothesized that Wnt/β-catenin signaling might be involved in the biological effects of hucMSC-Ex on angiogenesis. We found that hucMSC-Ex improved the stability and nuclear accumulation of β-catenin in a concentration-dependent manner (Fig. 5A, 5B). EA.hy926 cells were treated with different amounts of hucMSC-Ex (80 and 160 μg/ml) for 12 hours, and the whole-cell lysates were collected. The results of Western blot showed that PCNA and cyclin D3 protein expression was increased in a dose-dependent manner (Fig. 5C–5E). To investigate whether alteration of cell junctional components could be responsible for the increase in cell motility, we detected the expression of N-cadherin and E-cadherin in EA.hy926 cells. We found that hucMSC-Ex treatment increased N-cadherin expression and decreased E-cadherin expression in endothelial cells (Fig. 5C). We next determined the importance of β-catenin activation in hucMSC-Ex-mediated angiogenesis in vivo. Coinjection of β-catenin inhibitor ICG-001 significantly reversed the increase of vascular density in the wound area from hucMSC-Ex (Fig. 5F). In summary, hucMSC-Ex activates Wnt/β-catenin signaling to enhance angiogenesis in the process of wound healing.
Figure 5.
HucMSC-Ex prompted angiogenesis by activating Wnt/β-catenin signaling. (A): β-Catenin expression in EA.hy926 cells after exosome treatment (80 or 160 μg/ml) treatment was detected by immunofluorescence staining (×200). ∗∗, p < .01. (B): Cytoplasm and nuclear fractions were prepared from EA.hy926 cells treated with indicated concentrations of hucMSC-Ex or PBS. β-Catenin protein levels were determined using Western blot. (C): EA.hy926 cells were treated with different concentrations of exosomes (80 or 160 μg/ml). The expression of N-cadherin, E-cadherin, PCNA, and cyclin D3 proteins was detected using Western blot. (D): Densitometric analysis of the PCNA Western blot bands (∗∗∗, p < .001) compared with the PBS group (n = 3). (E): Densitometric analysis of the cyclin D3 Western blot bands (∗, p < .05 and ∗∗∗, p < .001) compared with the PBS group (n = 3). (F): The cutaneous-burn rat models were injected with hucMSC-Ex with or without ICG-001 (1 mg per rat). PBS and DMSO were used as controls for hucMSCs and ICG-001, respectively. Representative immunofluorescence images of CD31 expression in the wound area are shown (×200). Abbreviations: DMSO, dimethyl sulfoxide; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hucMSC-Ex, exosomes from human umbilical cord mesenchymal stem cells; PBS, phosphate-buffered saline; PCNA, proliferating cell nuclear antigen.
HucMSC-Ex-Delivered Wnt4 Was Critical for Angiogenesis In Vitro and In Vivo
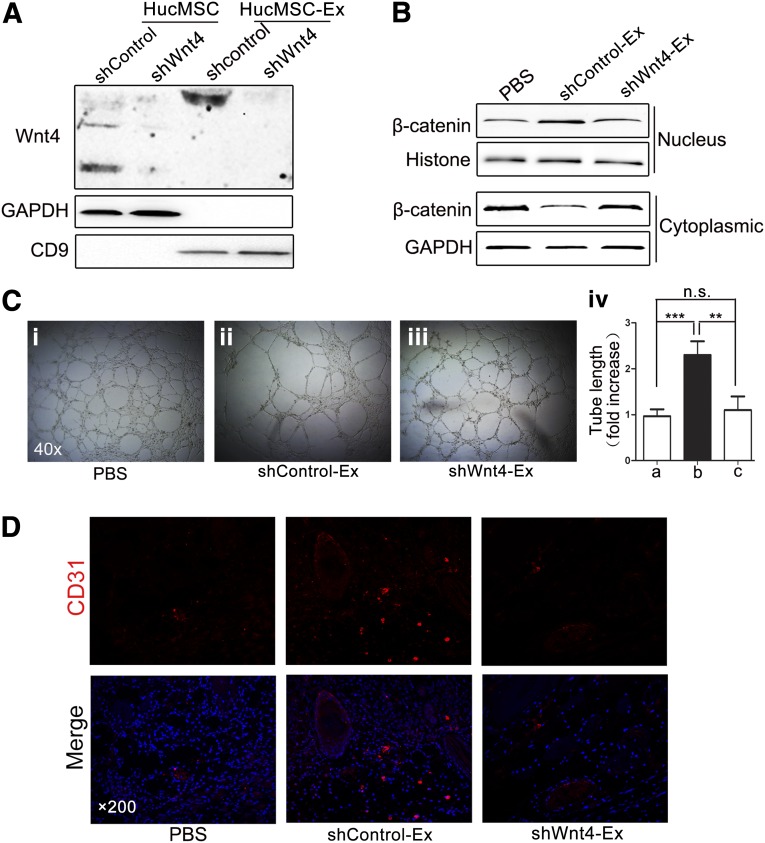
Because of the special molecular structure of Wnt, it is believed that Wnt can be delivered by exosome in tumor metastasis [34] and tissue development [35]. Our recent study also discovered that Wnt4 is highly expressed in hucMSC-Ex and is required for hucMSC-Ex mediated cutaneous regeneration [29]. In this study, we wanted to explore the role of hucMSC-Ex-delivered Wnt4 in wound angiogenesis. We knocked down Wnt4 in hucMSCs by using shRNA (Fig. 6A). The enhanced nuclear translocation of β-catenin by hucMSC-Ex was abrogated by Wnt4 knockdown in EA.hy926 cells (Fig. 6B). Furthermore, hucMSC-Ex-induced formation of the longer pipes was also abolished by Wnt4 knockdown (Fig. 6C). Wnt4 knockdown delayed the expression of angiogenesis marker CD31 induced by hucMSC-Ex in wound healing (Fig. 6D). These results indicate that Wnt4 plays an important role in hucMSC-Ex-mediated angiogenesis in cutaneous wound healing.
Figure 6.
HucMSC-Ex-delivered Wnt4 was critical for angiogenesis in vitro and in vivo. (A): HucMSCs were transfected with lentivirus Wnt4-shRNA or control-shRNA. The expression of Wnt4 in hucMSCs and hucMSC-Ex was determined using Western blot. (B): Cytoplasm and nuclear fractions were prepared from EA.hy926 cells treated with exosomes from Wnt4-shRNA or control-shRNA-transfected hucMSCs. β-catenin protein levels were determined using Western blot. (Ci–Ciii): The tube-formation ability of EA.hy926 cells treated with PBS, shWnt4-Ex, or shControl-Ex was determined (×40). (Civ): Measurement of the tube length by Image J software. ∗∗, p < .01; ∗∗∗, p < .001. (D): The rat wound models were treated with PBS, shControl-Ex, or shWnt4-Ex for 1 week. The expression of CD31 in the wound area was determined using immunofluorescence staining (×200). Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hucMSCs, human umbilical cord mesenchymal stem cells; hucMSC-Ex, exosomes from human umbilical cord mesenchymal stem cells; n.s., not significant; PBS, phosphate-buffered saline; PCNA, proliferating cell nuclear antigen; shControl, control-short hairpin RNA lentiviral expression vector; shControl-Ex, exosomes from control-short hairpin RNA-transfected hucMSCs; shWnt4, Wnt4-short hairpin RNA lentiviral expression vector; shWnt4-Ex, exosomes from Wnt4-short hairpin RNA-transfected hucMSCs.
Discussion
MSCs have been reported to be ideal candidates for tissue repair. Our previous studies demonstrated that MSCs derived from human umbilical cords could efficiently repair ischemia and reperfusion-induced acute kidney injury and carbon tetrachloride-injured hepatic injury [7, 8, 36]. MSCs have been shown to perform their therapeutic roles by promoting angiogenesis [37–39]. It is generally accepted that transplanted MSCs promote angiogenesis mainly through paracrine mechanisms [40]. Cell-derived exosomes have been described as the most important effective ingredients that play an important role in cell-to-cell communication [41, 42]. In this study, we successfully isolated exosomes from hucMSCs and characterized hucMSC-Ex. Membrane dye labeling showed that hucMSC-Ex could be incorporated into EA.hy926 endothelial cells. This is consistent with reports previously showing the incorporation of exosomes into renal tubular epithelial cells [16]; however, the underlying mechanisms of internalization remain unclear. Camussi and colleagues suggest that adhesion molecules such as CD29, α4 integrin might contribute to this process [43, 44]. Our flow cytometry detection showed that hucMSC-Ex were also positive for CD29 (data not shown). The effects of hucMSC-Ex on proliferation, migration, and tube formation of endothelial cells together contribute to new vessel formation [21]. Furthermore, our previous studies proved that hucMSC-Ex could alleviate liver fibrosis [15], protects against acute kidney injury [16], and enhance cutaneous wound closure [29]. Based on these findings, we proposed that hucMSC-Ex might achieve therapeutic effects by promoting angiogenesis; however, it remains largely unknown which component in hucMSC-Ex contributes to the proangiogenic activity.
Active mRNAs, microRNAs, and proteins can be transported by exosomes [45, 46]. Many factors delivered by exosome have been found to play an important role in angiogenesis [19–23, 43]. Endothelial cells that secrete exosomes containing miR-214 induce angiogenesis in human and mouse endothelial cells [19]. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells by mediating miR-210 delivery [21]. Exosomal miR-135b from hypoxic multiple myeloma cells enhanced endothelial tube formation under hypoxia via the HIF-FIH signaling pathway [22]. Exosomes can reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells by delivering matrix metalloproteinases, IL-8, platelet-derived growth factors (PDGFs), caveolin 1, and lysyl oxidase during tumor development [23]. Endothelial progenitor cell-derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNAs, such as mRNAs associated with the PI3K/AKT signaling pathway [43]. All of these findings suggest that exosomes may promote angiogenesis by transporting active molecules to endothelial cells.
The Wnt signaling pathway is critically involved in angiogenesis through the modulation of endothelial cell proliferation, migration, vascular sprouting, remodeling, and vascular system maturation [36–38]. Taverna et al. reported that treatment with exosomes from chronic myelogenous leukemia caused an increase in endothelial cell motility accompanied by a loss of VE-cadherin and β-catenin from the endothelial cell surface [47]. We previously demonstrated that hucMSC-Ex strongly activated Wnt/β-catenin signaling [29]. Wnt/β-catenin signaling inhibitor ICG-001 reversed the angiogenesis induced by hucMSC-Ex in an animal model. The previous studies suggested that exosomal proteins such as sonic hedgehog [48], HSP20 [49], IL-8, and PDGFs [23] might contribute to the proangiogenic activity. In addition, recent studies have reported that exosomes and extracellular vesicles could carry Wnt on their surface to induce Wnt signaling activity in target cells [34, 35, 50]. Because of lipid modification, Wnt cannot spread over a distance among cells [51]. Consequently, the range of Wnt signaling is narrowed. Panáková et al. suggested that exosome-like particles are the main carriers of Wnt molecules [52]. Exosomes promote the growth of Drosophila wings by delivering Wnt and activating Wnt signaling [34]. Cancer-associated fibroblast-secreted exosomes mediate cancer cell Wnt11 autocrine production to promote tumor metastasis [35]. Menck et al. found that two different types of extracellular vesicles were involved in Wnt5a induction and transportation during the process of macrophage-mediated malignant invasion [50]. Our recent study also indicated that hucMSC-Ex Wnt4 prompts wound healing by activating Wnt/β-catenin signaling [29]. Consequently, we explored the role of hucMSC-Ex Wnt4 in angiogenesis during tissue regeneration. Our data showed that Wnt4 knockdown delayed the expression of CD31 induced by hucMSC-Ex in wound healing. In conclusion, hucMSC-Ex could promote tube formation of endothelial cells in vitro and angiogenesis in a rat injury model. Our findings showed that hucMSC-Ex enhanced angiogenesis by delivering Wnt4 to activate Wnt/β-catenin in endothelial cells, which provides a mechanism for tissue repair and shows promise for tissue-engineering applications in regenerative therapy.
Conclusion
Our results have clearly demonstrated that hucMSC-Ex enhance angiogenesis in the repair of skin second-degree burn injury and that knockdown of Wnt4 in hucMSC-Ex delays tube formation of endothelial cells in vitro and the expression of CD31 in vivo.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grants 31340040, 81272481, 81270214), Jiangsu Province for Outstanding Sci-tech Innovation Team in Colleges and Universities (Grant SJK2013-10), Jiangsu Province’s Outstanding Medical Academic Leader and Sci-tech Innovation Team Program (Grant LJ201117), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and Jiangsu Province's Doctoral Innovation Fund (Grant CXZZ13_0703).
Author Contributions
B.Z. and X.W.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; X.Z.: conception and design, collection and/or assembly of data, data analysis and interpretation; Y.S, Y.Y., H.S., and Y.Z.: collection and/or assembly of data; L.W., Z.P., and W.Z.: conception and design, data analysis; H.Q. and W.X.: conception and design, data analysis and interpretation, financial support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Batsali AK, Kastrinaki MC, Papadaki HA, et al. Mesenchymal stem cells derived from Wharton’s jelly of the umbilical cord: Biological properties and emerging clinical applications. Curr Stem Cell Res Ther. 2013;8:144–155. doi: 10.2174/1574888x11308020005. [DOI] [PubMed] [Google Scholar]
- 2.Yoo HS, Yi T, Cho YK, et al. Mesenchymal stem cell lines isolated by different isolation methods show variations in the regulation of graft-versus-host disease. Immune Netw. 2013;13:133–140. doi: 10.4110/in.2013.13.4.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun L, Wang D, Liang J, et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Wang Y, Gou W, et al. Role of mesenchymal stem cells in bone regeneration and fracture repair: A review. Int Orthop. 2013;37:2491–2498. doi: 10.1007/s00264-013-2059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laroni A, Novi G, Kerlero de Rosbo N, et al. Towards clinical application of mesenchymal stem cells for treatment of neurological diseases of the central nervous system. J Neuroimmune Pharmacol. 2013;8:1062–1076. doi: 10.1007/s11481-013-9456-6. [DOI] [PubMed] [Google Scholar]
- 6.Yan Y, Xu W, Qian H, et al. Mesenchymal stem cells from human umbilical cords ameliorate mouse hepatic injury in vivo. Liver Int. 2009;29:356–365. doi: 10.1111/j.1478-3231.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- 7.Cao H, Qian H, Xu W, et al. Mesenchymal stem cells derived from human umbilical cord ameliorate ischemia/reperfusion-induced acute renal failure in rats. Biotechnol Lett. 2010;32:725–732. doi: 10.1007/s10529-010-0207-y. [DOI] [PubMed] [Google Scholar]
- 8.Tögel F, Weiss K, Yang Y, et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 9.Camussi G, Deregibus MC, Tetta C. Paracrine/endocrine mechanism of stem cells on kidney repair: Role of microvesicle-mediated transfer of genetic information. Curr Opin Nephrol Hypertens. 2010;19:7–12. doi: 10.1097/MNH.0b013e328332fb6f. [DOI] [PubMed] [Google Scholar]
- 10.Bang C, Thum T. Exosomes: New players in cell-cell communication. Int J Biochem Cell Biol. 2012;44:2060–2064. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res (Amst) 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Li T, Yan Y, Wang B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Xu H, Xu W, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4:34. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhandari V, Elias JA. The role of angiopoietin 2 in hyperoxia-induced acute lung injury. Cell Cycle. 2007;6:1049–1052. doi: 10.4161/cc.6.9.4229. [DOI] [PubMed] [Google Scholar]
- 15.Dragneva G, Korpisalo P, Ylä-Herttuala S. Promoting blood vessel growth in ischemic diseases: Challenges in translating preclinical potential into clinical success. Dis Model Mech. 2013;6:312–322. doi: 10.1242/dmm.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo SY, Kwon SM. Angiogenesis and its therapeutic opportunities. Mediators Inflamm. 2013;2013:127170. doi: 10.1155/2013/127170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bian S, Zhang L, Duan L, et al. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl) 2014;92:387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 18.Pascucci L, Alessandri G, Dall’Aglio C, et al. Membrane vesicles mediate pro-angiogenic activity of equine adipose-derived mesenchymal stromal cells. Vet J. 2014;202:361–6. doi: 10.1016/j.tvjl.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 19.van Balkom BW, de Jong OG, Smits M, et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006, S1–S15. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Zhao L, Li D, et al. Microvesicle-delivery miR-150 promotes tumorigenesis by up-regulating VEGF, and the neutralization of miR-150 attenuate tumor development. Protein Cell. 2013;4:932–941. doi: 10.1007/s13238-013-3092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tadokoro H, Umezu T, Ohyashiki K, et al. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem. 2013;288:34343–34351. doi: 10.1074/jbc.M113.480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umezu T, Tadokoro H, Azuma K, et al. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124:3748–3757. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kucharzewska P, Christianson HC, Welch JE, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci USA. 2013;110:7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mineo M, Garfield SH, Taverna S, et al. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion. Angiogenesis. 2012;15:33–45. doi: 10.1007/s10456-011-9241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao C, Xu W, Zhu W, et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol Int. 2008;32:8–15. doi: 10.1016/j.cellbi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Zhu W, Huang L, Li Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315:28–37. doi: 10.1016/j.canlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Qian H, Zhang X, Xu W, et al. Lentivirus-modified human umbilical cord mesenchymal stem cells maintain their pluripotency. Biotechnol Appl Biochem. 2010;55:53–62. doi: 10.1042/BA20090210. [DOI] [PubMed] [Google Scholar]
- 28.Nacer Khodja A, Mahlous M, Tahtat D, et al. Evaluation of healing activity of PVA/chitosan hydrogels on deep second degree burn: Pharmacological and toxicological tests. Burns. 2013;39:98–104. doi: 10.1016/j.burns.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Zhang B, Wang M, Gong A, et al. HucMSC-exosome mediated -Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2014 doi: 10.1002/stem.1771. in press. [DOI] [PubMed] [Google Scholar]
- 30.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 31.Franco CA, Liebner S, Gerhardt H. Vascular morphogenesis: A Wnt for every vessel? Curr Opin Genet Dev. 2009;19:476–483. doi: 10.1016/j.gde.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Reis M, Liebner S. Wnt signaling in the vasculature. Exp Cell Res. 2013;319:1317–1323. doi: 10.1016/j.yexcr.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Pate KT, Stringari C, Sprowl-Tanio S, et al. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014;33:1454–1473. doi: 10.15252/embj.201488598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luga V, Zhang L, Viloria-Petit AM, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 35.Gross JC, Chaudhary V, Bartscherer K, et al. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Qian H, Zhu W, et al. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev. 2011;20:103–113. doi: 10.1089/scd.2009.0495. [DOI] [PubMed] [Google Scholar]
- 37.Ma XL, Liu KD, Li FC, et al. Human mesenchymal stem cells increases expression of α-tubulin and angiopoietin 1 and 2 in focal cerebral ischemia and reperfusion. Curr Neurovasc Res. 2013;10:103–111. doi: 10.2174/1567202611310020003. [DOI] [PubMed] [Google Scholar]
- 38.Kong P, Xie X, Li F, et al. Placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki (GK) rats. Biochem Biophys Res Commun. 2013;438:410–419. doi: 10.1016/j.bbrc.2013.07.088. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda K, Falkenberg KJ, Woods AA, et al. Adipose-derived stem cells promote angiogenesis and tissue formation for in vivo tissue engineering. Tissue Eng Part A. 2013;19:1327–1335. doi: 10.1089/ten.tea.2012.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 41.Simons M, Raposo G. Exosomes—vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Février B, Raposo G. Exosomes: Endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 44.Fonsato V, Collino F, Herrera MB, et al. Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells. 2012;30:1985–1998. doi: 10.1002/stem.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 46.Vella LJ, Sharples RA, Nisbet RM, et al. The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur Biophys J. 2008;37:323–332. doi: 10.1007/s00249-007-0246-z. [DOI] [PubMed] [Google Scholar]
- 47.Taverna S, Flugy A, Saieva L, et al. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int J Cancer. 2012;130:2033–2043. doi: 10.1002/ijc.26217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benameur T, Soleti R, Porro C, et al. Microparticles carrying Sonic hedgehog favor neovascularization through the activation of nitric oxide pathway in mice. PLoS One. 2010;5:e12688. doi: 10.1371/journal.pone.0012688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Wang X, Zhu H, et al. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS One. 2012;7:e32765. doi: 10.1371/journal.pone.0032765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menck K, Klemm F, Gross JC, et al. Induction and transport of Wnt 5a during macrophage-induced malignant invasion is mediated by two types of extracellular vesicles. Oncotarget. 2013;4:2057–2066. doi: 10.18632/oncotarget.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willert K, Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol. 2012;4:a007864. doi: 10.1101/cshperspect.a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panáková D, Sprong H, Marois E, et al. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]